Abstract
Background
This study aimed to determine whether a simultaneous diagnosis of main components of metabolic syndrome (MetS) (hypertension, diabetes mellitus, and dyslipidemia) plays a mediator between income level and stroke.
Methods
We used the National Health Insurance Service National Sample Cohort database from 2006 to 2015. The mediator variables were the number of main MetS components diagnosed simultaneously (two or more/three or more). We used a weighting approach method of causal mediation analysis to apply counterfactual frameworks to the Cox proportional hazards regression model.
Results
A total of 213,526 people were included with 1,690,665.3 person-years of followed up. Compared with the high-income group, the risk of being diagnosed with two or more components of MetS significantly increased in all other income groups [middle-income OR 1.05 (95% CI 1.02–1.08); low-income OR 1.09 (95% CI 1.05–1.12); Medical Aid beneficiaries OR 1.39 (95% CI 1.32–1.47)]. A lower level of income was significantly associated with a higher risk of stroke compared with the high-income group [middle-income HR 1.15 (95% CI 1.07–1.25); low-income HR 1.19 (95% CI 1.10–1.29); Medical Aid beneficiaries HR 1.63 (95% CI 1.48–1.80)]. In the Medical Aid beneficiaries, simultaneous diagnosis of the main metabolic components acted as a significant mediator between income levels and stroke incidence, with 26.6% mediated when diagnosed with two or more diseases and 21.1% when diagnosed with all three.
Conclusions
Co-diagnosis of MetS components played a significant mediator role between income level and stroke incidence.
Similar content being viewed by others
Background
Socioeconomic determinants are essential factors in the occurrence of stroke. Several previous studies have reported that low socioeconomic status, such as low income, increases the risk of stroke.
MetS (Metabolic syndrome) is a set of co-occurring diseases that increase the risk of cardiovascular disease [1]. MetS is known to increase the risk of stroke [2,3,4]. While there are several criteria for definitions of MetS, the most commonly used criteria used throughout the world include following components; abdominal obesity, insulin resistance, dyslipidemia, and hypertension [4]. These components are often diagnosed together, and they share mutual risk factors such as aging, obesity, and smoking [5]. It is controversial whether having two or more of these components at the same time increases the risk of stroke. Some studies reported that having those diseases simultaneously at the same time has little or no excess risk on stroke occurrence [6, 7]. On the other hand, other studies announced that simultaneous diagnosis of these diseases had a higher risk of stroke [5, 8].
Several previous studies reported that lower socioeconomic status increases the risk of MetS and its associated risk factors and diseases, such as hypertension, dyslipidemia, and type 2 diabetes [9,10,11,12]. While we consider the link between income level, diseases belonging to the MetS, and stroke, it is expected that the incidence of diseases belonging to the MetS plays as a mediator between income level and stroke. However, there have been no studies on this to date. Moreover, because the diseases belonging to MetS are often diagnosed concurrently, it is expected that the mediation rate may be higher if two or more diseases are diagnosed simultaneously.
This study aimed to determine whether a simultaneous diagnosis of hypertension, diabetes mellitus, and dyslipidemia, which belong to the diagnosis criteria of MetS, plays a mediator between income level and stroke.
Methods
Study setting
South Korea has a universal health care (UHC) system that covers all citizens. The UHC in South Korea is comprised of National Health Insurance (NHI) and Medical Aid.
NHI populations are divided into "Insured Employees" and "Insured Self-employed" [13]. Workers and employers of all workplaces are registered as representatives of households of the Insured Employees. The Insured Employee category also includes the representatives' dependents and immediate family, including siblings and parents from both sides of the family. The rest of the people who are not registered as the Insured Employee category are included in the Insured Self-employed category. The NHI receives monthly premiums from the representative of each household. Thus the representative of each household and family members who registered as dependents are considered to have the same income. The Insured Employee's dependents do not need to live together with the representative. Unlike Insured Employees, Insured Self-employed cannot register dependent of the household representative unless they live together. Therefore, the Insured Self-employed category reflects the individual's income level more accurately than the Insured Employee category [14]. For the Insured Self-employed category, monthly NHI premiums are determined by household income, wealth, standard of living, and economic activity participation rate. In addition, all NHI members pay a portion of the cost when using medical services designated by the NHI-approved Services. In South Korea, 57.7% of the total population is the Insured employee and 38.6% is the Insured Self-employed. Around 3.7% of the total population is Medical Aid beneficiaries [13].
Medical Aid is a public assistance program aimed at securing a minimum livelihood for low-income households by providing medical services nearly free [15]. Medical Aid beneficiaries do not pay health insurance premiums. Because the South Korean government pays expenses for their medical services, Medial Aid beneficiaries pay little or no cost for using NHI-approved medical services. [13]
Data source
In this study, the National Health Insurance Service National Sample Cohort (NHIS-NSC) database of South Korea’s NHI Service from 2006 to 2015 was used [16]. This cohort database consists of a sample cohort of over one million people randomly selected from among 48,222,537 people of South Korea. This database provides each individual’s medical service usage history based on the subject's medical billing data from 2002 to 2015. In this study, we used the data from 2006 to 2015 because this dataset did not include the Medical Aid beneficiaries’ medical usage information from 2002 to2005.
Study population
The subjects of this study were adults 18 years of age or older who were registered as Insured Self-employed and Medical Aid beneficiaries in the NHIS-NSC in 2008. As we mentioned in the study setting section, because the Insured Self-employed category reflects the individual's income level more accurately than the Insured Employee category, we excluded the Insured Employees and their dependents. Medical Aid beneficiaries generally have lower incomes than low-income households of the NHI. A mandatory requirement for Medical Aid is that all household members are not working. Thus, Medical Aid beneficiaries were included in the study as a group with a lower income than the low-income group of Insured Self-employed category.
We excluded the patients diagnosed with hypertension, diabetes mellitus, dyslipidemia, and stroke during the washout period (2006–2007). In addition, patients with a medical history of cardiovascular disease and cerebrovascular diseases such as transient ischemic attacks were excluded. Each disease was classified using 10th version of the International Classification of Diseases (ICD-10) diagnostic codes recorded in the medical record diagnosis. The ICD-10 codes for each disease were defined as below; I10-I15 for hypertension, E10-14 for diabetes mellitus, E78 for dyslipidemia, I60-I63 for stroke, I20-I25 for cardiovascular disease, and G45-46 for cerebrovascular disease [17]. We also excluded the cases that could not confirm the income level of individuals.
Variables
The independent variable of this study was income level. We used the income level of 2008, based on the assumption that the income level in 2008 had not changed. In the NHIS-NSC, the income level of the Insured Self-employees is divided into deciles according to household income. Medical Aid beneficiaries are classified separately. In this study, income levels were classified into four groups; High-income (the top 1–3 decile in the Insured Self-employees), middle-income (the top 4–6 decile in the Insured Self-employees), low-income (the top 7–10 decile in the Insured Self-employees), and Medical Aid beneficiaries.
We validated the volatility of the income level to test the assumption that the income level at a specific point in time could represent the entire observation period. Among total 1,105,369 individuals in the NHIS-NSC dataset, we analyzed 514,148 people who belonged to the Insured Self-employed category/Medical Aid beneficiaries at least twice over a 10-year period from 2006 to 2015. The income level variability was confirmed as the standard deviation using this study's classification criteria, setting the high-income level as 1, the middle-income level as 2, the low-income level as 3, and Medical Aid beneficiaries as 4 as continuous variables. There was no change in the income level in 276,633 people (53.6% of the subjects), and the 75% quartile of the standard deviation was 0.52 with the maximum value of 2.12 (Additional file 2: Fig. S1). Considering the variability identified above, we were able to assume that for most of the study subjects the income level classification at a specific point in time did not show large fluctuations during the follow-up period, thus we concluded that the income level in the first year of the study could be used as an exposure variable.
The dependent variable was the occurrence of stroke.
The mediator variables were number of main MetS components (hypertension, diabetes mellitus, and dyslipidemia) diagnosed simultaneously (two or more/three or more). There are several criteria for the clinical diagnosis of MetS. The criteria most commonly used is the definition developed by the American Heart Association. In this definition, MetS is diagnosed when more than three of below five constitutes are satisfied; elevated waist circumference, elevated triglycerides, reduced HDL-C, elevated blood pressure, and elevated fasting glucose [18]. We regarded the three components—hypertension, diabetes mellitus, and dyslipidemia—as “MetS diseases.”
In addition, gender, age, region of residence, degree of disability, comorbidity, diagnosis of cardiovascular disease, and diagnosis of cerebrovascular disease such as transient ischemic attack were considered for confounding variables. Age was adjusted as a continuous variable. Residential areas were classified into four categories based on each individual’s residential area in 2008, those living: (1) Seoul, (2) metropolitan cities, (3) Gyeonggi-do (nearby Seoul), and (4) other provinces. The degree of physical disability was classified as severe and moderate, mild, and no disability. For comorbidities, the Charlson Comorbidity Index (CCI) was used. CCI was measured using the ICD codes of medical service records diagnosis in 2006–2007. [19]
Statistical analysis
Baseline characteristics were described with frequency and percentage.
A multivariate logistic regression model was used to verify the association between income level (the independent variable) and the number of MetS diseases diagnosed simultaneously (the mediator variable).
Traditional mediation analysis had calculated the proportion of mediating effects by comparing the estimates of the model without a mediator and the model with the mediator [20]. However, previous studies have found that the traditional approach may overestimate or underestimate the actual effects of mediators because of the below reasons: (1) mediator-outcome confounding, (2) exposure-mediator interaction, and (3) mediator-outcome confounding affected by the exposure [21,22,23]. Therefore, we used a causal mediation analysis method based on a counterfactual framework to overcome these flaws. In this study, survival analysis was performed using the Cox proportional hazards regression model. In order to apply the counterfactual framework to this model, a weighting approach method was used [24].
In each mediation analysis, the mediation proportion was obtained by modifying the equation used by VanderWeele and Vansteelandt, replacing an odds ratio to a hazard ratio [25].
where, aHR: adjusted Hazard Ratio, NDE: Natural Direct Effect, NIE: Natural Indirect Effect.
Analysis was performed using SAS Enterprise Guide version 7.1(SAS Institute Inc., Cary, NC, USA). In the logistic regression analysis and survival analysis, confounding variables such as sex, age, region of residence, degree of disability, CCI, and diagnosis of cardiovascular disease and cerebrovascular disease were adjusted.
Ethics statement
This study was approved by the Institutional Review Board of Seoul National University (IRB No. E1808/003-002).
Results
In the NHIS-NSC, adults 18 years of age or older in 2008 were 807,162. We excluded 27,931 people diagnosed with stroke before 2008 and 252,078 people diagnosed with hypertension, diabetes mellitus, dyslipidemia, cardiovascular disease, or cerebrovascular disease before 2008. We also excluded 313,553 people in the Insured Employee category and 74 persons whose income decile could not be verified. Finally, 213,526 people were enrolled, and a total of 1,690,665.3 person-years were followed up.
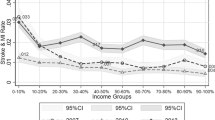
Table 1 shows the demographics of the study population by income level. The number of the diagnosed MetS diseases was different by income level. In the high-income group, 62.5% were not diagnosed with any of the MetS diseases, and 4.4% with all three of the MetS diseases. In the Medical Aid beneficiaries, 49.0% were not diagnosed with any of the MetS diseases, and 10.4% with all three of MetS diseases. Overall, among the MetS diseases, the diagnosis rate of dyslipidemia was the highest (29.2%). The stroke incidence rate was 1.7% in the high-income group, 1.9% in the middle-income group, 2.1% in the low-income group, and 6.3% in the Medical Aid beneficiaries.
Table 2 shows the multivariate logistic regression analysis results on the risk of a simultaneous diagnosis of MetS diseases varies by income level. Compared with the high-income group, the risk of being diagnosed with two or more MetS diseases significantly increased in all other income groups. The aOR (adjusted odds ratio) for the middle-income group was 1.05 (95% CI 1.02–1.08), and the aOR for the low-income group was 1.09 (95% CI 1.05–1.12). The aOR for Medical Aid beneficiaries was 1.39 (95% CI 1.32–1.47). The risk of being diagnosed with all three MetS diseases was significantly higher in all income groups compared with the high-income group. The aOR was 1.08 (95% CI 1.03–1.14) for the middle-income group, 1.20 (95% CI 1.14–1.27) for the low-income group, and 1.50 (95% CI 1.38–1.63) for Medical Aid beneficiaries.
Table 3 shows survival analysis results using the Cox proportional hazards model for stroke incidence according to the income level. Model 1 is the result of adjusting for age, sex, residential area, CCI, disability severity, and diagnosis of cardiovascular and cerebrovascular diseases. Model 2 results were obtained by adding adjustment of the simultaneous diagnosis of two or more MetS diseases to Model 1. In Model 2, all income groups had a significantly higher risk of stroke than the high-income group. The middle-income group had 1.15 times (95% CI 1.07–1.25) higher risk of stroke than the high-income group, and the low-income group had 1.19 times (95% CI 1.10–1.29), and Medical Aid beneficiaries had 1.63 times (95% CI 1.48–1.80). The HR (hazard ratio) for the simultaneous diagnosis of two or more MetS diseases was 5.44 (95% CI 5.10–5.80). Model 3 adds adjustment of the simultaneous diagnosis of all three MetS diseases to Model 1. All of the HRs of income levels decreased in Model 3 compared to Models 1 and 2. It was 1.14 (95% CI 1.05–1.23) for the middle-income group, 1.17 (95% CI 1.08–1.26) for the low-income group, and 1.61 (95% CI 1.46–1.78) for Medical Aid beneficiaries. The HR for the simultaneous diagnosis of all three MetS diseases was 4.50 (95% CI 4.21–4.81).
Table 4 shows the results of the weighted approach mediation analysis based on the counterfactual framework. It was found that the natural indirect effects of a simultaneous diagnosis of MetS diseases were significant only for Medical Aid beneficiaries. When the diagnosis of two or more MetS diseases was a mediator, the mediation rate was 26.6%, and when the diagnosis of three MetS diseases was a mediator, the mediation rate was 21.1% (Fig. 1).
The mediation effect of the simultaneous diagnosis of MetS diseases between the Medical Aid beneficiaries and the stroke incidence. MetS metabolic syndrome, aHR adjusted hazard ratio, CI confidence interval, CCI Charlson comorbidity index, IHD Ischemic heart disease, CVD cerebrovascular disease. MetS diseases: hypertension, diabetes mellitus, dyslipidemia
Discussion
In this study, the lower-income groups had a higher risk of a simultaneous diagnosis of MetS diseases than the high-income group. The risk of simultaneous diagnosis of MetS diseases was significantly higher in all the other income groups than in the high-income group. Also, as the number of diagnosed diseases increased, the hazard risks also increased. In addition, the risk of stroke incidence was significantly higher in all the other income groups than in the high-income group. Simultaneous diagnosis of MetS diseases was a significant mediator between income level and stroke occurrence in the Medical Aid beneficiaries. The mediation proportion was 26.6% when the diagnosis of two or more MetS diseases was a mediator. In the case of diagnosis of three MetS diseases, the mediation proportion was 21.1%.
In previous studies, non-communicable diseases, especially diseases included in diagnostic criteria for metabolic syndrome such as hypertension, diabetes mellitus, and dyslipidemia, were closely related to income levels [9,10,11,12]. There was also a close association between income level and stroke incidence [14, 26,27,28]. However, there were no studies on the mediating effect of a simultaneous diagnosis of MetS diseases between income level and stroke incidence. This study found that co-diagnosis of major diseases belonging to MetS diagnostic criteria plays a significant mediator role between income level and stroke incidence with high mediation proportions (26.6% for two or more and 21.1% for three MetS diseases) in the Medical Aid beneficiaries group.
In this study, we did not directly use the diagnostic definition of MetS proposed by the American Heart Association in 2005. Instead, for the mediator variable, we made a new definition of "MetS diseases" and defined a mediator as two or three of "MetS diseases" are diagnosed simultaneously. The NHIS-NSC provides the subjects' national health check-up results, and abdominal obesity is a component of the health check-up list. However, using the health check-up data is concerned about serious selection bias because national health check-up is not mandatory, and the health check-up rate rapidly decreases as income decreases [29]. When we checked the proportion of the health check-up by the income level with this dataset, it was confirmed that the proportion of people who received a health check-up before diagnosing a stroke decreased according to their income level (the high-income group: 63.5%, the middle-income group: 60.7%, the low-income group: 54.1%, and Medical Aid beneficiaries: 46.7%). Also, since it is a one-time examination, the figures may be temporarily inaccurate. Therefore, the health check-up results were not used for this analysis, and the medical record of outpatient and inpatient treatment were used. In general, abdominal obesity does not require outpatient and inpatient treatment. For this reason, abdominal obesity was not included in the elements for the "MetS diseases." However, we assume that using this definition would be similar to using an exact definition of metabolic syndrome. Because, first, two of the five diagnostic criteria for MetS are diagnostic criteria for dyslipidemia. Furthermore, in the sensitivity analysis performed using health check-up data, there was no significant difference between the results using the definition used in this study and the result of adding abdominal obesity for an element (Additional file 1: Tables S1–S5). Therefore, we believe that the results of this study provide evidence that MetS has a mediating effect between income level and stroke.
In our study, the mediating effect of the simultaneous diagnosis of MetS diseases was not significant in the middle- and low-income groups; however, it was significant in the Medical Aid beneficiaries group. We also found the higher risk of both simultaneous diagnosis of MetS diseases and stroke in the Medical Aid beneficiaries group than other income groups. Since MetS diseases are multifaceted health problems, they cannot be treated with a single agent. Therefore, the management of MetS diseases cannot be resolved with medical expense support alone, but should be accompanied by multiple interventions, including lifestyle modification, high medication compliance, and relieving mental stress, which is one of the main challenges particularly for Medical Aid beneficiaries [9, 30,31,32]. This relationship among the independent variable, the mediator variable, and the outcome variable would be a reason that the mediating effect of the Medical Aid beneficiaries was significant with a high proportion compared to other income groups. Furthermore, in a previous study, Medical Aid beneficiaries had more outpatient visits than NHI members, and there was no difference in the number of outpatient visits according to income within the Medical Aid beneficiaries; because they receive treatment of diseases such as hypertension, diabetes mellitus, or dyslipidemia treatment for free or near to free [33]. Thus, there are low chances of misclassifying a MetS disease patient as a nonpatient in the Medical Aid beneficiaries group, which may have led more accurate measure of the mediating effect in the Medical Aid beneficiaries group in this study.
Considering these results, to prevent inequity in stroke occurrence, it is necessary to prevent hypertension, diabetes mellitus, and dyslipidemia, especially among Medical Aid beneficiaries. Management of health behaviors such as smoking cessation, abstinence of binge drinking, encouragement of physical activity, and mental stress management are essential [34]. Management of newly diagnosed patients with MetS will also be critical. Medication management and removal of risk factors that aggravate the disease are required.
The mediating effect was significant only in the Medical Aid beneficiaries. However, all the other income groups had a higher risk than the high-income group to diagnose MetS diseases simultaneously, and the magnitude of the hazard ratios increased as the income level decreased. The same trend was observed for the occurrence of stroke, which suggests that income inequity for diagnosis of MetS and stroke has a gradient pattern, and the health of all the population is affected by the inequity. It provides evidence that policies for health inequity should focus on the entire population, not just the most disadvantaged population [35].
The finding that income level acts as a significant risk factor for poor health outcomes means that this is not a matter of individual will but a social context that makes this prevention challenging for the lower-income class. For the lower-income patients, unhealthy health behaviors such as smoking, drinking alcohol, poor eating habits, and lack of physical activity often increase the risk of metabolic syndrome and stroke [34]. In previous studies, people with a low income have a tendency to focus more on the present situation than on long-term investments in future life. They also had a higher possibility of choosing unhealthy behavior because of the opportunity cost of obtaining good health behavior, pessimism about the future, and the influence of family and neighbors with similar environments [36]. Therefore, there is a need for a policy that goes beyond simply carrying out health behavior promotion projects and makes it easier for people with low incomes to choose healthy habits.
In this study, the mediation rate was the highest when two or more MetS diseases were diagnosed than when all three were diagnosed. When we performed an additional analysis using the diagnosis of one of the three diseases as a mediator, the mediating effect was significant in Medical Aid beneficiaries, and the mediation rate was 19.5% (Additional file 1: Table S6). We can explain it below: In the mediation analysis, the mediation rate is higher when: (1) the greater the effect of the mediator on the outcome, and (2) the greater proportion of the population with the mediator. In this study we observed: (1) the greater the number of diagnosed MetS diseases, the more significant contribution they had on the occurrence of stroke (outcome), and (2) the proportion of the patients with the mediator decreased as the number of the diagnosed MetS diseases increased. As shown in Table 3, as the number of diagnosed MetS diseases increased, the risk of income level on stroke decreased. Therefore, though the mediation rate was higher when two or more MetS diseases were diagnosed, more attention should be paid to people with more diseases(those with 3 MetS diseases) when applying it in actual policy.
As mentioned in the Methods section, we tried to avoid underestimating or overestimating mediating effects in this study by applying the causal mediation analysis method. We compared our result and the result of the traditional mediation analysis by performing additional analysis. In the traditional mediation analysis, we found that in both cases- the mediator was a diagnosis of two or more MetS diseases, or all three MetS diseases- mediated effects were significant in all the other income groups compared with the high-income group. When applying traditional mediation analysis, the magnitudes of mediating effects in Medical Aid beneficiaries were higher than when calculated through causal mediation analysis (Additional file 1: Table S7).
This study has several strengths. First, the mediating effect was measured more accurately using the causal mediation analysis, overcoming the weaknesses of the traditional mediation analysis. Second, the data we used can represent the entire country’s population. Third, as only 3599 people, or 1.7% of the total enrolled population, were censored before 2015, the last year, the completeness of the data is excellent.
This study has several limitations. First, we classified the income level by the income level in 2008, assuming that the income would not change. Thus there is a possibility of errors due to the change in income. However, as we described in the Method section, in the results of testing the income level volatility, we found that most people did not experience a change or experienced subtle changes in their income level during a 10-year period. Thus, we assume that the bias from the change in the income level is minimal. Second, there is an opportunity for a difference between the diagnosis and the actual prevalence because the case of diagnosis was confirmed based on the medical usage record. Third, since the duration of having the Mets diseases was not reflected, the effect of the accumulation of diseases could not be reflected. Fourth, there is a possibility of unmeasured confounding effects, such as pharmacological treatment status of the patients (drug interactions). In addition, we did not use the official diagnostic criteria for MetS directly.
Conclusions
In this study, low income was a significant risk factor for simultaneous diagnosis of main MetS components and stroke. Co-diagnosis of MetS components played a significant mediator role between income level and stroke incidence. Therefore, there is a need for a policy to prevent and manage metabolic syndrome, especially for low-income patients.
Availability of data and materials
The data used in this study are provided by the National Health Insurance Service(NHIS). Any researcher who wishes to access the dataset should obtain permission from the NHIS and pay of appropriate license fee.
Abbreviations
- MetS:
-
Metabolic syndrome
- UHC:
-
Universal health care
- NHI:
-
National Health Insurance
- NHIS-NSC:
-
National Health Insurance Service National Sample Cohort
- ICD-10:
-
10Th version of the International Classification of Diseases
- CCI:
-
Charlson comorbidity index
- aOR:
-
Adjusted odds ratio
References
Huang PL. A comprehensive definition for metabolic syndrome. Dis Model Mech. 2009;2:231–7.
Tang XN, Liebeskind DS, Towfighi A. The role of diabetes, obesity, and metabolic syndrome in stroke. Semin Neurol. 2017;37:267–73.
Mottillo S, Filion KB, Genest J, Joseph L, Pilote L, Poirier P, et al. The metabolic syndrome and cardiovascular risk a systematic review and meta-analysis. J Am Coll Cardiol. 2010;56:1113–32.
Cornier M-A, Dabelea D, Hernandez TL, Lindstrom RC, Steig AJ, Stob NR, et al. The metabolic syndrome. Endocr Rev. 2008;29:777–822.
Lu S, Bao M-Y, Miao S-M, Zhang X, Jia Q-Q, Jing S-Q, et al. Prevalence of hypertension, diabetes, and dyslipidemia, and their additive effects on myocardial infarction and stroke: a cross-sectional study in Nanjing, China. Ann Transl Med. 2019;7:436–436.
Rawshani A, Rawshani A, Franzén S, Sattar N, Eliasson B, Svensson A-M, et al. Risk factors, mortality, and cardiovascular outcomes in patients with type 2 diabetes. N Engl J Med. 2018;379:633–44.
Sehestedt T, Hansen TW, Li Y, Richart T, Boggia J, Kikuya M, et al. Are blood pressure and diabetes additive or synergistic risk factors? Outcome in 8494 subjects randomly recruited from 10 populations. Hypertens Res. 2011;34:714–21.
Zhang Y, Jiang X, Bo J, Yin L, Chen H, Wang Y, et al. Risk of stroke and coronary heart disease among various levels of blood pressure in diabetic and nondiabetic Chinese patients. J Hypertens. 2018;36:93–100.
Blanquet M, Legrand A, Pélissier A, Mourgues C. Socio-economics status and metabolic syndrome: a meta-analysis. Diabetes Metab Syndr Clin Res Rev. 2019;13:1805–12.
Leng B, Jin Y, Li G, Chen L, Jin N. Socioeconomic status and hypertension: a meta-analysis. J Hypertens. 2015;33:221–9.
Wu H, Meng X, Wild SH, Gasevic D, Jackson CA. Socioeconomic status and prevalence of type 2 diabetes in mainland China, Hong Kong and Taiwan: a systematic review. J Glob Health. 2017;7:11103.
Nam GE, Cho KH, Park YG, Do Han K, Choi YS, Kim SM, et al. Socioeconomic status and dyslipidemia in Korean adults: the 2008-2010 Korea National Health and Nutrition Examination Survey. Prev Med. 2013;57:304–9.
Song YJ. The South Korean health care system. Japan Med Assoc J. 2009;52:206–9.
Seo SR, Kim SY, Lee SY, Yoon TH, Park HG, Lee SE, et al. The incidence of stroke by socioeconomic status, age, sex, and stroke subtype: a nationwide study in Korea. J Prev Med Public Heal. 2014;47:104–12.
Hwang KT, Ju YW, Kim YA, Kim J, Oh S, Jung J, et al. Prognostic influence of Korean public medical insurance system on breast cancer patients. Ann Surg Treat Res. 2019;96:58–69.
Lee J, Lee JS, Park SH, Shin SA, Kim KW. Cohort profile: The National Health Insurance Service-National Sample Cohort (NHIS-NSC), South Korea. Int J Epidemiol. 2017;46: e15.
Rha J-H, Koo J, Cho KH, Kim E-G, Oh GS, Lee SJ, et al. Two-year direct medical costs of stroke in Korea: a multi-centre incidence-based study from hospital perspectives. Int J Stroke. 2012;8:186–92.
Grundy SM, Cleeman JI, Daniels SR, Donato KA, Eckel RH, Franklin BA, et al. Diagnosis and management of the metabolic syndrome: an American Heart Association/National Heart, Lung, and Blood Institute scientific statement. Circulation. 2005;112:2735–52.
Thygesen SK, Christiansen CF, Christensen S, Lash TL, Sørensen HT. The predictive value of ICD-10 diagnostic coding used to assess Charlson comorbidity index conditions in the population-based Danish National Registry of Patients. BMC Med Res Methodol. 2011;11:2–7.
Baron RM, Kenny DA. The moderator-mediator variable distinction in social psychological research: conceptual, strategic, and statistical considerations. J Pers Soc Psychol. 1986;51:1173–82.
Richiardi L, Bellocco R, Zugna D. Mediation analysis in epidemiology: methods, interpretation and bias. Int J Epidemiol. 2013;42:1511–9.
Imai K, Keele L, Tingley D. A general approach to causal mediation analysis. Psychol Methods. 2010;15:309–34.
VanderWeele TJ. Mediation analysis: a practitioner’s guide. Annu Rev Public Health. 2016;37:17–32.
Lange T, Vansteelandt S, Bekaert M. A simple unified approach for estimating natural direct and indirect effects. Am J Epidemiol. 2012;176:190–5.
VanderWeele TJ, Vansteelandt S. Odds ratios for mediation analysis for a dichotomous outcome. Am J Epidemiol. 2010;172:1339–48.
Avan A, Digaleh H, Di Napoli M, Stranges S, Behrouz R, Shojaeianbabaei G, et al. Socioeconomic status and stroke incidence, prevalence, mortality, and worldwide burden: an ecological analysis from the Global Burden of Disease Study 2017. BMC Med. 2019;17:191.
Zhou W, Chen R, Hopkins A, Wang Y, Tang J, Chen X, et al. Association between socioeconomic status and incident stroke in China. J Epidemiol Community Health. 2020;74:519–26.
Kerr GD, Slavin H, Clark D, Coupar F, Langhorne P, Stott DJ. Do vascular risk factors explain the association between socioeconomic status and stroke incidence: a meta-analysis. Cerebrovasc Dis. 2011;31:57–63.
Shin H-Y, Kang H-T, Lee JW, Lim H-J. The association between socioeconomic status and adherence to health check-up in Korean adults, based on the 2010–2012 Korean National Health and Nutrition Examination Survey. Korean J Fam Med. 2018;39:114–21.
Baum A, Garofalo JP, Yali AM. Socioeconomic status and chronic stress. Does stress account for SES effects on health? Ann N Y Acad Sci. 1999;896:131–44.
Lee H, Park JH, Floyd JS, Park S, Kim HC. Combined effect of income and medication adherence on mortality in newly treated hypertension: nationwide study of 16 million person-year. J Am Heart Assoc. 2019. https://doi.org/10.1161/JAHA.119.013148.
Allen L, Williams J, Townsend N, Mikkelsen B, Roberts N, Foster C, et al. Socioeconomic status and non-communicable disease behavioural risk factors in low-income and lower-middle-income countries: a systematic review. Lancet Glob Heal. 2017;5:e277–89.
Kong NY, Kim DH. Factors influencing health care use by health insurance subscribers and medical aid beneficiaries: a study based on data from the Korea welfare panel study database. BMC Public Health. 2020;20:1133.
Wang Y, Tu R, Yuan H, Shen L, Hou J, Liu X, et al. Associations of unhealthy lifestyles with metabolic syndrome in Chinese rural aged females. Sci Rep. 2020;10:1–8.
Braveman PA, Cubbin C, Egerter S, Williams DR, Pamuk E. Socioeconomic disparities in health in the United States: what the patterns tell us. Am J Public Health. 2010;100(Suppl):S186–96.
Pampel FC, Krueger PM, Denney JT. Socioeconomic disparities in health behaviors. Annu Rev Sociol. 2010;36:349–70.
Acknowledgements
Not applicable.
Funding
Not applicable.
Author information
Authors and Affiliations
Contributions
SJ suggested the study; SJ and SIC participated in the design of the study and developed the study protocol; SJ performed the statistical analysis; SJ drafted the manuscript; all authors contributed to critical revision of manuscript; All authors read and approved the final manuscript.
Corresponding author
Ethics declarations
Ethics approval and consent to participate
This study was approved by the Institutional Review Board of Seoul National University (IRB No. E1808/003-002). The study involves no prospectively collected data so there is no access to patients or opportunity to seek informed consent.
Consent for publication
Not applicable.
Competing interests
The authors declare that they have no competing interests.
Additional information
Publisher's Note
Springer Nature remains neutral with regard to jurisdictional claims in published maps and institutional affiliations.
Supplementary Information
Additional file 1:
Table S1. Results with the definition used in this study as a mediator (diagnosis of two or more MetS diseases) in the health check-up data. Table S2. Results with the new definition as a mediator (diagnosis of two or more among "hypertension, diabetes mellitus, dyslipidemia, and abdominal obesity") in the health check-up data. Table S3. Results with the definition used in this study as a mediator (diagnosis of three of MetS diseases) in the health check-up data. Table S4. Results with the new definition as a mediator (diagnosis three or more among "hypertension, diabetes mellitus, dyslipidemia, and abdominal obesity") in the health check-up data. Table S5. Results with the new definition as a mediator (diagnosis of four among "hypertension, diabetes mellitus, dyslipidemia, and abdominal obesity") in the health check-up data. Table S6. Causal mediation analysis result: the mediator was a diagnosis of one or more MetS diseases. Table S7. Result of the traditional mediation analysis.
Additional file 2:
Fig. S1. The volatility of the income level in 514,148 subjects in the dataset. X-axis: Standard deviation of the changes in the income level in each individual. Y-axis: % of the subjects.
Rights and permissions
Open Access This article is licensed under a Creative Commons Attribution 4.0 International License, which permits use, sharing, adaptation, distribution and reproduction in any medium or format, as long as you give appropriate credit to the original author(s) and the source, provide a link to the Creative Commons licence, and indicate if changes were made. The images or other third party material in this article are included in the article's Creative Commons licence, unless indicated otherwise in a credit line to the material. If material is not included in the article's Creative Commons licence and your intended use is not permitted by statutory regulation or exceeds the permitted use, you will need to obtain permission directly from the copyright holder. To view a copy of this licence, visit http://creativecommons.org/licenses/by/4.0/. The Creative Commons Public Domain Dedication waiver (http://creativecommons.org/publicdomain/zero/1.0/) applies to the data made available in this article, unless otherwise stated in a credit line to the data.
About this article
Cite this article
Jeong, S., Cho, Si. & Kong, S.Y. Effect of income level on stroke incidence and the mediated effect of simultaneous diagnosis of metabolic syndrome diseases; a nationwide cohort study in South Korea. Diabetol Metab Syndr 14, 110 (2022). https://doi.org/10.1186/s13098-022-00882-1
Received:
Accepted:
Published:
DOI: https://doi.org/10.1186/s13098-022-00882-1