Abstract
Background
Sodium glucose cotransporter-2 (SGLT-2) inhibitors are known to reduce hospitalization and cardiovascular mortality in various heart failure (HF) populations, potentially through enhanced excretion of water and sodium. However, there are concerns regarding the risk of acute kidney injury (AKI) associated with their use. This meta-analysis aimed to unravel the effects of SGLT-2 inhibitors on risk of AKI in a variety of patients with HF.
Methods
This study conducted a comprehensive literature search using PubMed, EMBASE, Cochrane Library, and clinicaltrials.gov for studies published up to January 1, 2024. Data were analyzed using both random-effects or fixed-effects models to estimate the overall relative risk (RR) with a 95% confidence interval (CI).
Results
Our analysis included 25,172 patients with HF from 16 randomized controlled trials. Treatment with SGLT-2 inhibitors led to a 28% reduction in the risk of AKI progression compared to placebo (RR 0.72, 95% CI 0.61–0.85, p<0.0001), without an increased risk of hypotension (RR 1.21, 95% CI 0.87–1.70, p = 0.26) and hypovolemia (RR 2.26, 95% CI: 0.70–7.33, p = 0.17). Notably, SGLT-2 inhibitors significantly decreased AKI in specific subgroups, including patients with HF with reduced ejection fraction (RR 0.59, 95% CI 0.43–0.80, p = 0.0007), those treated with empagliflozin (RR 0.70, 95% CI 0.57–0.88, p = 0.002) or dapagliflozin (RR 0.74, 95% CI 0.57–0.98, p = 0.04), in studies with a follow-up of at least 1 year (RR 0.67, 95% CI 0.55–0.82, p = 0.0001), and in patients aged 65 years or older (RR 0.72, 95% CI 0.61–0.85, p < 0.0001).
Conclusion
Use of SGLT-2 inhibitors did not increase the incidence of AKI regardless of the ejection fraction environment (chronic and acute), type of SGLT-2 inhibitors, or patient age.
Similar content being viewed by others
Introduction
Despite significant advances in the treatment of heart failure (HF), its incidence and mortality rates remain high [1]. Sodium-glucose cotransporter 2 (SGLT-2) inhibitors have been demonstrated to reduce both hospitalization rates [2] and cardiovascular mortality [3] in patients with HF with reduced ejection fraction (HFrEF), irrespective of their type 2 diabetes mellitus (T2DM) status. Recent studies have also explored the efficacy of SGLT-2 inhibitors in patients with HF with preserved ejection fraction (HFpEF) [4, 5] and acute HF (AHF) [6, 7] demonstrating a reduction in hospitalization rates in these patients.
SGLT-2 inhibitors were originally developed as antidiabetic agents in patients with T2DM, and they act by inhibiting the reabsorption of sodium and glucose in the proximal tubules of the kidney. In recent years, the cardioprotective effect of SGLT-2 inhibitors has been increasingly recognized: consistent cardiovascular benefits have been observed in studies on HFrEF treatment, suggesting that its therapeutic mechanism of action is not related to hypoglycemic effects, but may be related to water and sodium excretion [8]. However, the same mechanism raises concerns about potentially lowering blood pressure and renal perfusion, which could lead to acute kidney injury (AKI) [9]. Recent large-scale randomized controlled trials (RCTs) have assessed the incidence of AKI in patients with HF treated with SGLT-2 inhibitors [10,11,12]. However, uncertainties persist concerning the renal safety of these drugs, particularly across different SGLT-2 inhibitors, varied ejection fractions, and older patient populations.
This systematic review and meta-analysis assesses the relative risk of AKI in patients with HF treated with SGLT-2 inhibitors in RCTs. Additionally, factors such as hypotension and hypovolemia were taken into account to determine whether the risk associated with SGLT-2 inhibitors was consistent across various SGLT-2 inhibitors drugs, different ejection fraction groups, and age. The outcome of this analysis will assist clinicians in deciding whether to prescribe SGLT-2 inhibitors for patients with HF, thereby addressing a significant clinical concern.
Methods
The methodologies employed in this study rigorously comply with the Preferred Reporting Items for Systematic Reviews and Meta-Analyses (PRISMA) guidelines [13]. This systematic review protocol is registered in the PROSPERO database under registration number CRD42024508011.
Data sources and search strategy
The search encompassed four principal medical databases—PubMed, Embase, Cochrane Library, and ClinicalTrials.gov —spanning records from their inception through January 1, 2024. This study imposed no limitations on the date or language of publication. The specific search strings utilized are detailed in Additional file 1: Table S1-S4. Additionally, the reference lists of included studies and related meta-analysis were examined to uncover additional qualifying studies. Duplicate articles were excluded using EndNoteX9.2 software. Two researchers, XHW and MHH, independently screened the titles and abstracts using pre-established inclusion and exclusion criteria to pinpoint pertinent studies, followed by a full-text examination to establish relevance. Any differences in opinion were resolved by consulting a third researcher, CCS, to achieve consensus.
Outcomes
The primary endpoint was defined as the incidence rate of AKI. Secondary endpoints included the incidence rates of hypotension and hypovolemia In existing literature, these conditions are categorized as serious adverse events. These rates were collated from the ClinicalTrials.gov registry and published studies.
Study selection
Inclusion criteria for the studies in our analysis were as follows: (1) RCTs; (2) participants diagnosed with HF, irrespective of ejection fraction (EF) or whether the condition was chronic or acute; (3) interventions involving SGLT-2 inhibitors and a placebo; (4) outcomes that included the incidence rates of AKI, hypotension, or hypovolemia. The exclusion criteria were (1) duplicate publications, conference abstracts, case reports, review articles, and animal studies; (2) other drug interventions besides SGLT-2 inhibitors; (3) RCTs with incomplete or unreported results.
Data extraction and quality assessment
Two researchers, XHW and MHH, performed the data extraction. They sourced the study data from the published manuscripts or the results listed on ClinicalTrials.gov. The extracted data included the following: (1) the RCT name, authors, ClinicalTrials.gov unique identifier, year of publication, and sample size; (2) participant baseline characteristics such as age and gender; (3) baseline health conditions and comorbidities, including the incidence of T2DM and prediabetes, average left ventricular ejection fraction, and the use of medications for HF; (4) details of the treatment including the regimen, dosage, and duration; (5) the duration of follow-up; (6) the primary outcomes measured; (7) reported serious adverse events, specifically the number of participants who experienced AKI, hypotension, and hypovolemia from the start to the conclusion of the study.
The Cochrane Collaboration’s risk of bias tool was employed to evaluate the methodological quality of the included studies [14]. Two researchers independently assessed the risk of bias at the levels of study, intervention, and outcome for each included study. Additionally, the GRADE method was utilized to determine the evidence quality of the summary results [15]. The domains assessed encompass bias risk, inconsistency, indirectness, imprecision, and risk of publication bias. The evidence quality was categorized into four grades: high, moderate, low, and very low. Any disagreement was resolved by consensus among the authors or by consulting a third author, CCS.
Statistical analyses
Data analysis was conducted using RevMan 5.3 (The Cochrane Collaboration, Oxford, England) and Stata 12.0 (StataCorp, Texas, USA) software. The results were presented as relative risk (RR) and 95% confidence intervals (CI), with the placebo serving as the reference for assessing the association between SGLT-2 inhibitors and clinical outcome measures. The significance of the overall results and RRs was determined using the Mantel-Haenszel method and the Z-test. Heterogeneity was assessed using the I2 statistic, considering an I2 value over 50% or a corresponding p-value less than 0.05 to indicate significant heterogeneity among studies, prompting the utilization of a random-effects model for meta-analysis. Conversely, when I2 ≤ 50%, p ≥ 0.05, a fixed-effects model was employed. Funnel plots and Egger’s test were used to assess potential publication bias. To further assess the robustness of results, subgroup analyses exploring the influence of variables such as the type of SGLT-2 inhibitors, HF classification, follow-up duration, and patient age were conducted. Furthermore, sensitivity analyses were conducted to assess the influence of individual studies on the aggregate effect size. Given that all randomized controlled trials incorporated in the analysis administered an identical dosage of SGLT-2 inhibitors, additional dose-related subgroup analyses were deemed unnecessary. A p-value of < 0.05 was considered statistically significant.
Results
Search results and baseline characteristics
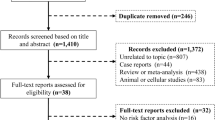
The process of literature retrieval is illustrated in Fig. 1. A total of 1782 articles or studies were identified as potentially relevant publications. After screening and excluding duplicated studies, 993 articles remained. These articles were screened based on the inclusion and exclusion criteria. Ultimately, 16 RCTs were included in our analysis [2, 3, 5, 7, 9, 16,17,18,19,20,21,22,23,24,25]. The total number of patients was 25,172, with 12,581 patients receiving SGLT-2 inhibitors and 12,591 patients receiving placebo. The follow-up period ranged from 60 to 1410 days, and the publication years ranged from 2020 to 2023. All studies compared the use of SGLT-2 inhibitors with placebo, eight trials used empagliflozin [2, 5, 9, 19, 20, 22, 23, 25], six used dapagliflozin [3, 16,17,18, 21], one used canagliflozin [24], and one used sotagliflozin [7]. Six trials included patients with HFrEF (EF ≤ 40%) [2, 3, 9, 18, 20], five trials included patients with HFpEF (EF ≥ 40% or 45%) [5, 16, 17, 19, 21], three trials included patients with AHF [7, 22, 23], and two trials included patients with any type of HF, irrespective of baseline ejection fraction [24, 25]. Most studies required patients to receive standard HF treatment. The key clinical characteristics of the included studies are presented in Table 1 and Additional file 1: Table S5.
Risk of bias assessment
The risk of bias assessment for all included trials is summarized in Additional file: Figure S1. The majority of RCTs demonstrated adequate random sequence generation and allocation concealment, indicating a generally low risk of bias.
Primary outcome
The impact of SGLT-2 inhibitors on AKI in patients with HF
A meta-analysis of these 16 RCTs was conducted to assess the incidence of AKI in patients with HF. Among the 12,581 patients treated with SGLT-2 inhibitors, 228 AKI events were observed. However, in the placebo group consisting of 12,591 participants, 319 AKI events were observed. The meta-analysis demonstrates that SGLT-2 inhibitors significantly reduce the risk of AKI in patients with HF compared to placebo (RR 0.72, 95% CI 0.61–0.85, p < 0.0001) as shown in Fig. 2. Additionally, no significant heterogeneity was observed among the trials (p = 0.83; I2 = 0%). The funnel plot showed no notable asymmetry (Fig. 3), the Egger’s test did not indicate significant publication bias (p = 0.85). Furthermore, sensitivity analysis conducted by sequentially excluding each study, showed that individual studies did not change the overall outcome, thus confirming the robustness of the results (Additional file 1: Figure S2).
Subgroup analyses
Subgroup analyses were performed based on different types of HF, categorized mainly into HFrEF, HFpEF, AHF, and overall HF. Among patients with HFrEF, those treated with SGLT-2 inhibitors exhibited a significantly reduced risk of AKI (RR 0.59, 95% CI 0.43–0.80, p = 0.0007, I2 = 0%). However, RCTs for patients with HFpEF (RR 0.82, 95% CI 0.66–1.02, p = 0.07, I2 = 0%), AHF (RR 0.64, 95% CI 0.38–1.08, p = 0.09, I2 = 0%), and overall HF (RR 0.43, 95% CI 0.06–2.82, p = 0.38, I2 = 0%) did not show increased risk of AKI with the use of SGLT-2 inhibitors as shown in Fig. 4A and Table S7.
Subgroup analysis was performed based on the type of SGLT-2 inhibitors, which revealed that the use of empagliflozin (RR 0.70, 95% CI 0.57–0.88, p = 0.002, I2 = 0%) and dapagliflozin (RR 0.74, 95% CI 0.57–0.98, p = 0.04, I2 = 24%) significantly reduced the risk of AKI. However, sotagliflozin (RR 0.69, 95% CI 0.32–1.48, p = 0.35) and canagliflozin (RR 0.34, 95% CI 0.01–8.39, p = 0.51, I2 = 0%) did not exhibit increased risk of AKI with the use of SGLT-2 inhibitors (Fig. 4B and Table S7).
Additionally, among the analyzed RCTs, 4 had a follow-up period exceeding one year, whereas the remaining 12 had a follow-up period of less than one year. Subgroup analysis based on follow-up time revealed that for HF patients, the risk of AKI remained significantly decreased when the follow-up duration was more than one year (RR 0.67, 95% CI 0.55–0.82, p = 0.0001, I2 = 0%). However, no significant differences were observed in trials with a follow-up period of less than 1 year (RR 0.83, 95% CI 0.62–1.11, p = 0.20, I2 = 0%; Fig. 4C and Table S7).
Further subgroup analysis was conducted based on age. The risk of AKI was significantly reduced in patients aged ≥ 65 years (RR 0.72, 95% CI 0.61–0.85, p = 0.0001, I2 = 0%). In contrast, no significant increase in AKI risk was observed in patients under 65 years of age (RR 0.61, 95% CI 0.08–4.62, p = 0.63, I2 = 0%; Fig. 4D and Table S7).
Secondary outcomes
Effect of SGLT-2 inhibitors on hypotension rate in patients with HF
The data on hypotension were reported in 12 RCTs [2, 3, 5, 7, 16,17,18, 21, 23,24,25], involving a total of 24,171 HF patients. Among these patients, 131 reported hypotension events, with 72 patients in the SGLT-2 inhibitors group and 59 patients in the placebo group. The analysis showed no significant difference in the occurrence of hypotension between the SGLT-2 inhibitors group and placebo group (RR 1.21, 95% CI 0.87–1.70, p = 0.26; Fig. 5). Additionally, no heterogeneity was observed among the studies (p = 0.61; I2 = 0%). The funnel plot was visually basically symmetrical (Fig. 6) and Egger’s test did not reveal significant publication bias (p = 0.80). Furthermore, sensitivity analysis that sequentially excluded each study showed that individual studies did not change the overall findings (Additional file 1: Figure S3), confirming the robustness of the results.
Further subgroup analyses were also performed based on the type of SGLT-2 inhibitor, HF type, age, and follow-up time. Consistent findings were shown across all subgroup analyses, and there was no increase in the incidence of hypotension in patients with HF irrespective of SGLT-2 inhibitors type, HF type, age, or follow-up time (Fig. 7 and Additional file 1:Table S8).
Effect of SGLT-2 inhibitors on hypovolemia rate in patients with HF
Only four RCTs reported data on hypovolemia [17, 18, 21, 23]. Among these studies, 3,679 patients were treated with SGLT-2 inhibitors and 3,685 patients with a placebo. No significant heterogeneity was observed between the studies (p = 0.53; I2 = 0%) (Fig. 8). The analysis showed no significant difference in the occurrence of hypovolemia between the SGLT-2 inhibitors and placebo groups (RR = 2.26, 95% CI: 0.70–7.33, p = 0.17), as shown in Fig. 8. The results indicate that compared to placebo, SGLT-2 inhibitors does not increase the risk of hypovolemia events in HF patients. Sensitivity analysis yielded consistent results (Additional file 1: Figure S4). Due to the inclusion of fewer than 10 studies, publication bias was not further assessed using funnel plot methods and the Egger’s test.
Furthermore, additional subgroup analyses were performed based on the type of SGLT-2 inhibitors, type of HF, age, and duration of follow-up. These analyses consistently showed no increased risk of hypovolemia across all subgroups, regardless of these factors (Fig. 9 and Additional file 1:Table S9).
GRADE investigates the quality of evidence results
For the primary outcome, AKI, and the secondary outcome, hypovolemia, the quality of evidence is rated as “high”. However, for the secondary composite outcome, including hypotension, the quality of evidence is rated as “moderate” due to issues of imprecision (Additional file 1:Table S10A-C).
Discussion
The results of this systematic review and meta-analysis indicate that SGLT-2 inhibitors compared with placebo, significantly reduce the risk of AKI in patients with HFrEF and do not increase the risk of AKI in HFrEF and AHF. Subgroup analysis revealed that the use of empagliflozin and dapagliflozin significantly decreased the risk of AKI in patients with HF. Additionally, treatment with SGLT-2 inhibitors with a follow-up exceeding one year is also significantly associated with a reduced risk of AKI. It is noteworthy that increasing age in patients with HF receiving SGLT-2 inhibitors did not lead to an increase in the incidence rate of AKI. Furthermore, SGLT-2 inhibitors did not increase the risk of hypotension or hypovolemia events in patients with HF.
To the best of our knowledge, this is the most extensive and comprehensive meta-analysis to date on the effect of SGLT-2 inhibitors on the risk of AKI among patients with HF, incorporating twice the number of AKI events compared with the findings in previous meta-analyses [26]. These findings provide further evidence supporting the safety of SGLT-2 inhibitors in patients with HF, thereby suggesting that SGLT-2 inhibitors may be a valuable therapeutic option to prevent the risk of AKI.
AKI is a common event in HF patients, and influencing factors include hemodynamic status, and a low cardiac output or congestive status. As expected, the incidence of AKI increased in patients with eGFR < 60 mL/min/1.73 m2, and the greater the decrease in eGFR, the higher the incidence and severity of AKI. In previous meta-analyses, the relationship between SGLT-2 inhibitors and AKI has been discussed, and the results indicate that SGLT-2 inhibition not only reduces the progression of chronic kidney disease and the probability of AKI occurring during hospitalization or non-hospitalization treatment, but also has a preventive effect on AKI [27]. In other studies, it was found that SGLT-2 inhibitors can reduce the risk of kidney disease progression in non-diabetes patients by 37% and the risk of AKI by 23% [28]. The underlying mechanism involved may be related to reducing water and sodium excretion and reducing renal perfusion. Due to the low renal function reserve generated by HF, or the increased risk of renal function deterioration due to age and comorbidities, there are concerns that the use of SGLT-2 inhibitors in HF patients may lead to AKI. Our study further showed that regardless of the ejection fraction of HF patients, the use of SGLT-2 inhibitors did not increase the occurrence of AKI.
Accumulating evidence increasingly substantiates the beneficial effects of SGLT-2 inhibitors in preventing progressive renal injury. Our research findings align with the SOLOIST-WHF [29] and EMPULSE [30] trials conducted in patients with deteriorating HF, which investigated the protective effect of SGLT-2 inhibitors in patients with HF exacerbation and the significant reduction in the risk of hospitalization due to HF and renal disease progression. In addition, our findings confirmed that regardless of the ejection fraction and whether the setting was chronic and acute. Clinicians can be confident in initiating SGLT-2 inhibitors therapy, as it does not augment the incidence of AKI. Although SGLT-2 inhibitors usage in HF can preserve the estimated glomerular filtration rate (eGFR) over time [8], our study goes further by demonstrating a reduction in AKI risk. A randomized study has indicated that early initiation of SGLT-2 inhibitors in patients with HFrEF, regardless of diabetes or early chronic kidney disease presence, is safe and well tolerated, with no adverse effects on renal function [9]. A recent meta-analysis on AHF supports our findings, showing no increased risk of AKI or hypotension with SGLT-2 inhibitors, which aligns with our results [31].
It is noteworthy that only empagliflozin and dapagliflozin were significantly associated with a decreased risk of AKI events in HF patients compared to placebo. However, a limited number of studies was included involving the use of canagliflozin and sotagliflozin in HF patients in the included research, with only one study each for these two medications, the impact of these two SGLT-2 inhibitors, thus on the risk of AKI in such patients needs to be further determined. Recent large-scale clinical studies have shown that dapagliflozin provides long-term cardiovascular and renal protection benefits without increasing the risk of severe renal adverse events [32, 33]. Previous meta-analyses have demonstrated that empagliflozin and dapagliflozin significantly reduce the risk of composite renal endpoints in patients with HFrEF [34]. These findings are consistent with our research findings. In addition, our study has demonstrated that the use of SGLT-2 inhibitors in HFpEF and AHF populations is safe and does not increase the risk of serious renal adverse events. In addition, most RCTs in our study had more than 50% of patients who received treatment with renin-angiotensin system inhibitors (RASis), and the proportion of patients receiving RASis treatment was similar to the proportion in the SGLT-2 inhibitors group and the placebo group (Additional file 1: Table S5). SGLT-2 inhibitors can reduce the risk of AKI in HF patients regardless of whether they receive ACEI/ARB treatment at the same time, thereby indicating that the beneficial effect on the kidney is obvious.
Further analysis revealed that the use of SGLT-2 inhibitors has slightly different effects on AKI in patients with HF who belonged to different age groups, with a more significant reduction in the incidence of AKI observed in patients older than 65 years. Considering the challenges associated with polypharmacy and the increased mortality risk in older patients—coupled with their underutilization of guideline-recommended treatments—physicians may worry about the diminished efficacy and safety of SGLT-2 inhibitors treatment in this demographic [35, 36]. However, our findings suggest that SGLT-2 inhibitors do not lead to an increased risk of AKI with advancing age, affirming their safety and effectiveness for older patients with HF. Moreover, in the DELIVER trial, the efficacy of SGLT-2 inhibitors was not compromised by advancing age in patients with either HFrEF or HFpEF [10]. In contrast to younger patients, many older individuals with HFpEF exhibit smaller left ventricular size, higher estimated ejection fractions, and evident cardiac remodeling patterns, suggesting that therapeutic benefits of SGLT-2 inhibitors in HF are not reduced by increasing age.
Notably, clinicians often hesitate to prescribe SGLT-2 inhibitors to patients with lower baseline systolic blood pressure (SBP) due to fears of adverse hemodynamic effects. Our meta-analysis indicates that the use of SGLT-2 inhibitors in HF patients is not associated with an increased risk of hypotension or hypovolemia compared to placebo. In previous studies, it was found that the reduction in SBP in patients with heart failure treated with SGLT-2 inhibitors therapy after initiation is clinically insignificant [37]. The DELIVER trial demonstrated that dapagliflozin had a minor effect on blood pressure and did not increase the risk of severe hypovolemia-related adverse events [33]. Although empagliflozin can lower SBP in patients with HF, its effect is slight in those with already low SBP [38], and it has beneficial effects on renal function that are independent of baseline SBP values [39].
The underlying mechanisms through which SGLT-2 inhibitors exert their benefits in patients with HF have not been fully elucidated. However, it appears that their effects are not directly related to glucose control, likely stem from direct cardioprotective and nephroprotective actions. These effects might involve modulation of sodium balance, enhancement of energy homeostasis, and alleviation of cellular stress, or they could be triggered by alterations in renal hemodynamics [8, 40]. Additionally, SGLT-2 inhibitors may also potentially improve renal function indirectly by reducing activation of the sympathetic nervous system, alleviating inflammation, and ameliorating oxidative stress [41]. They have also been suggested to boost erythropoietin production, inhibit peritubular inflammation and fibrosis—thereby safeguarding the renal tubules [42], and induce changes in the tubules that reduce their sensitivity to AKI [43]. Further research is needed to delineate the beneficial mechanisms of SGLT-2 inhibitors therapy in HF.
This study has several limitations that need to be considered. Firstly, no trial has identified the risk of AKI as a primary endpoint of a study, which may result in discrepancies between our conclusions and reality. Secondly, the drug sotagliflozin, used in the SOLOIST-WHF trial, inhibits both SGLT-2 and SGLT-1 receptors, which may affect the specificity of the results. Thirdly, SGLT-2 inhibitors are hypoglycemic agents mainly used in patients with type 2 diabetes and its associated kidney disease, and there is still no indication for the treatment of HF in some countries. Fourthly, trials were unable to stratify based on comorbidities since not all trials reported baseline prevalence of diabetes, chronic kidney disease, or coronary artery disease. Further research is needed to validate and extend these findings. Fifthly, there is a lack of clarity in the definition and measurement of endpoints potentially biasing our results. Lastly, none of the included studies reported staging of AKI, creating challenges in the analysis of the effect of SGLT-2 inhibitors on different severity levels of AKI. In the future, further validation can be achieved by designing large-scale clinical studies with varying degrees of AKI as the primary outcome.
Conclusions
In summary, this meta-analysis indicates that the use of SGLT-2 inhibitors does not increase the occurrence of AKI and has no impact on hypotension and hypovolemia, regardless of the ejection fraction environment (chronic and acute), type of SGLT-2 inhibitors, or patient age. These results provide substantial evidence for the use of SGLT-2 inhibitors in patients with HF.
Data availability
No datasets were generated or analysed during the current study.
References
Conrad N, et al. Temporal trends and patterns in heart failure incidence: a population-based study of 4 million individuals. Lancet. 2018;391(10120):572–80.
Packer M, et al. Cardiovascular and renal outcomes with Empagliflozin in Heart failure. N Engl J Med. 2020;383(15):1413–24.
McMurray JJV, et al. Dapagliflozin in patients with heart failure and reduced ejection fraction. N Engl J Med. 2019;381(21):1995–2008.
Solomon SD, et al. Dapagliflozin in Heart failure with mildly reduced or preserved ejection fraction. N Engl J Med. 2022;387(12):1089–98.
Anker SD, et al. Empagliflozin in Heart failure with a preserved ejection fraction. N Engl J Med. 2021;385(16):1451–61.
Damman K, et al. Randomized, double-blind, placebo-controlled, multicentre pilot study on the effects of empagliflozin on clinical outcomes in patients with acute decompensated heart failure (EMPA-RESPONSE-AHF). Eur J Heart Fail. 2020;22(4):713–22.
Bhatt DL, et al. Sotagliflozin in patients with diabetes and recent worsening heart failure. N Engl J Med. 2021;384(2):117–28.
Petrie MC, et al. Effect of Dapagliflozin on worsening heart failure and Cardiovascular Death in patients with heart failure with and without diabetes. JAMA. 2020;323(14):1353–68.
Anker SD, et al. Kidney function after initiation and discontinuation of Empagliflozin in patients with heart failure with and without type 2 diabetes: insights from the EMPERIAL trials. Circulation. 2021;144(15):1265–7.
Peikert A, et al. Efficacy and safety of Dapagliflozin in Heart failure with mildly reduced or preserved ejection Fraction according to age: the DELIVER Trial. Circ Heart Fail. 2022;15(10):e010080.
Butt JH, et al. Efficacy and safety of Dapagliflozin according to Frailty in Heart failure with reduced ejection fraction: a Post Hoc Analysis of the DAPA-HF trial. Ann Intern Med. 2022;175(6):820–30.
Butler J, et al. Safety and Efficacy of Empagliflozin and Diuretic Use in patients with heart failure and preserved ejection fraction: a Post Hoc Analysis of the EMPEROR-Preserved trial. JAMA Cardiol. 2023;8(7):640–9.
Page MJ, et al. The PRISMA 2020 statement: an updated guideline for reporting systematic reviews. BMJ. 2021;372:n71.
Higgins JP, et al. The Cochrane collaboration’s tool for assessing risk of bias in randomised trials. BMJ. 2011;343:d5928.
Guyatt GH, et al. GRADE: an emerging consensus on rating quality of evidence and strength of recommendations. BMJ. 2008;336(7650):924–6.
McMurray JJV, et al. Effect of Dapagliflozin Versus placebo on symptoms and 6-Minute Walk Distance in patients with heart failure: the DETERMINE Randomized clinical trials. Circulation. 2024;149(11):825–38.
Nassif ME, et al. The SGLT2 inhibitor dapagliflozin in heart failure with preserved ejection fraction: a multicenter randomized trial. Nat Med. 2021;27(11):1954–60.
Nassif ME, et al. Dapagliflozin effects on biomarkers, symptoms, and functional status in patients with heart failure with reduced ejection fraction: the DEFINE-HF Trial. Circulation. 2019;140(18):1463–76.
Abraham WT, et al. Rationale and design of the EMPERIAL-Preserved and EMPERIAL-Reduced trials of empagliflozin in patients with chronic heart failure. Eur J Heart Fail. 2019;21(7):932–42.
Lee MMY, et al. Effect of Empagliflozin on Left ventricular volumes in patients with type 2 diabetes, or Prediabetes, and heart failure with reduced ejection fraction (SUGAR-DM-HF). Circulation. 2021;143(6):516–25.
Mc Causland FR, et al. Dapagliflozin and kidney outcomes in patients with heart failure with mildly reduced or preserved ejection fraction: a prespecified analysis of the DELIVER Randomized Clinical Trial. JAMA Cardiol. 2023;8(1):56–65.
Voorrips SN, et al. Longitudinal changes in circulating ketone body levels in patients with Acute Heart failure: a Post Hoc Analysis of the EMPA-Response-AHF trial. J Card Fail. 2023;29(1):33–41.
Kosiborod MN, et al. Effects of Empagliflozin on symptoms, Physical limitations, and quality of life in patients hospitalized for Acute Heart failure: results from the EMPULSE Trial. Circulation. 2022;146(4):279–88.
Spertus JA, et al. The SGLT2 inhibitor canagliflozin in heart failure: the CHIEF-HF remote, patient-centered randomized trial. Nat Med. 2022;28(4):809–13.
Nassif ME, et al. Empagliflozin effects on Pulmonary Artery pressure in patients with heart failure: results from the EMBRACE-HF trial. Circulation. 2021;143(17):1673–86.
Vukadinovic D, et al. Side effects and treatment initiation barriers of sodium-glucose cotransporter 2 inhibitors in heart failure: a systematic review and meta-analysis. Eur J Heart Fail. 2022;24(9):1625–32.
Menne J, et al. Acute kidney injury and adverse renal events in patients receiving SGLT2-inhibitors: a systematic review and meta-analysis. PLoS Med. 2019;16(12):e1002983.
Nuffield Department of Population Health Renal Studies, G. and, Consortium SiM-AC-RT. Impact of diabetes on the effects of sodium glucose co-transporter-2 inhibitors on kidney outcomes: collaborative meta-analysis of large placebo-controlled trials. Lancet. 2022;400(10365):1788–801.
Pitt B, et al. Effect of Sotagliflozin on early mortality and heart failure-related events: a Post Hoc Analysis of SOLOIST-WHF. JACC Heart Fail. 2023;11(8 Pt 1):879–89.
Voors AA, et al. Renal effects of empagliflozin in patients hospitalized for acute heart failure: from the EMPULSE trial. Eur J Heart Fail. 2022;24(10):1844–52.
Salah HM, et al. Efficacy and safety of sodium-glucose cotransporter 2 inhibitors initiation in patients with acute heart failure, with and without type 2 diabetes: a systematic review and meta-analysis. Cardiovasc Diabetol. 2022;21(1):20.
Jhund PS, et al. Efficacy of Dapagliflozin on renal function and outcomes in patients with heart failure with reduced ejection fraction: results of DAPA-HF. Circulation. 2021;143(4):298–309.
Chatur S, et al. Renal and blood pressure effects of dapagliflozin in recently hospitalized patients with heart failure with mildly reduced or preserved ejection fraction: insights from the DELIVER trial. Eur J Heart Fail. 2023;25(7):1170–5.
Zannad F, et al. SGLT2 inhibitors in patients with heart failure with reduced ejection fraction: a meta-analysis of the EMPEROR-Reduced and DAPA-HF trials. Lancet. 2020;396(10254):819–29.
Lazzarini V, et al. Heart failure in elderly patients: distinctive features and unresolved issues. Eur J Heart Fail. 2013;15(7):717–23.
Stolfo D, et al. Use of evidence-based therapy in heart failure with reduced ejection fraction across age strata. Eur J Heart Fail. 2022;24(6):1047–62.
Serenelli M, et al. Effect of dapagliflozin according to baseline systolic blood pressure in the Dapagliflozin and Prevention of adverse outcomes in heart failure trial (DAPA-HF). Eur Heart J. 2020;41(36):3402–18.
Bohm M, et al. Heart failure and renal outcomes according to baseline and achieved blood pressure in patients with type 2 diabetes: results from EMPA-REG OUTCOME. J Hypertens. 2020;38(9):1829–40.
Patel RB, et al. Kidney function and outcomes in patients hospitalized with heart failure. J Am Coll Cardiol. 2021;78(4):330–43.
Inzucchi SE, et al. How does Empagliflozin reduce Cardiovascular Mortality? Insights from a mediation analysis of the EMPA-REG OUTCOME Trial. Diabetes Care. 2018;41(2):356–63.
Lopaschuk GD, Verma S. Mechanisms of Cardiovascular benefits of Sodium glucose co-transporter 2 (SGLT2) inhibitors: a state-of-the-art review. JACC Basic Transl Sci. 2020;5(6):632–44.
Ito M, Tanaka T. The Anticipated Renoprotective effects of Sodium-glucose cotransporter 2 inhibitors. Intern Med. 2018;57(15):2105–14.
Dekkers CCJ, et al. Effects of the SGLT-2 inhibitor dapagliflozin on glomerular and tubular injury markers. Diabetes Obes Metab. 2018;20(8):1988–93.
Acknowledgements
None.
Funding
None.
Author information
Authors and Affiliations
Contributions
XW and MH contributed to the literature database search, data collection, data extraction, data analysis, and writing of the manuscript. CS, and DJ performed data analysis of the results. HL reviewed and revised this article. All authors read and approved the final manuscript.
Corresponding author
Ethics declarations
Ethics approval and consent to participate
Not applicable.
Consent for publication
Not applicable.
Competing interests
The authors declare no competing interests.
Additional information
Publisher’s Note
Springer Nature remains neutral with regard to jurisdictional claims in published maps and institutional affiliations.
Electronic supplementary material
Below is the link to the electronic supplementary material.
Rights and permissions
Open Access This article is licensed under a Creative Commons Attribution-NonCommercial-NoDerivatives 4.0 International License, which permits any non-commercial use, sharing, distribution and reproduction in any medium or format, as long as you give appropriate credit to the original author(s) and the source, provide a link to the Creative Commons licence, and indicate if you modified the licensed material. You do not have permission under this licence to share adapted material derived from this article or parts of it. The images or other third party material in this article are included in the article’s Creative Commons licence, unless indicated otherwise in a credit line to the material. If material is not included in the article’s Creative Commons licence and your intended use is not permitted by statutory regulation or exceeds the permitted use, you will need to obtain permission directly from the copyright holder. To view a copy of this licence, visit http://creativecommons.org/licenses/by-nc-nd/4.0/.
About this article
Cite this article
Wang, X., He, M., Jin, D. et al. Effect of SGLT-2 inhibitors on acute kidney injury in patients with heart failure: a systematic review and meta-analysis. Diabetol Metab Syndr 16, 207 (2024). https://doi.org/10.1186/s13098-024-01446-1
Received:
Accepted:
Published:
DOI: https://doi.org/10.1186/s13098-024-01446-1