Abstract
Background
MIER1α is a transcriptional regulator that interacts with estrogen receptor α and inhibits estrogen-stimulated growth of breast carcinoma cells. Interestingly, analysis of MIER1α subcellular localization in breast samples revealed a stepwise shift from the nucleus to the cytoplasm during progression to invasive carcinoma. Previously, we demonstrated that MIER1α is nuclear in MCF7 cells yet it does not contain a nuclear localization signal. Instead MIER1α is targeted to the nucleus through interaction and co-transport with HDAC 1 and 2.
Results
In this study, we demonstrate that treatment of MCF7 breast carcinoma cells with either insulin or insulin-like growth factor affects the subcellular localization of MIER1α. Both factors reduce the percentage of cells with nuclear MIER1α from 81 and 89 to 41 and 56 %, respectively. Treatment with 17β-estradiol, on the other hand, had no effect and MIER1α remained nuclear.
Conclusions
Our data demonstrate that insulin and IGF-1 can contribute to loss of nuclear MIER1α in the MCF7 breast carcinoma cell line.
Similar content being viewed by others
Background
MIER1α is a transcriptional repressor [1, 2] that has been implicated as a tumour suppressor in breast cancer [3]. It interacts with ERα and inhibits estrogen-stimulated anchorage-dependent growth of breast carcinoma cells [3]. Moreover, analysis of patient breast biopsies revealed a dramatic reduction in nuclear MIER1α during progression, from 75 % nuclear MIER1α in normal samples to 51 % nuclear in ductal carcinoma in situ to 4 % nuclear in invasive ductal carcinoma [3]. Thus loss of nuclear MIER1α is associated with breast cancer progression.
MIER1 represses transcription through several distinct mechanisms: it can recruit histone deacetylase (HDAC) 1 and 2 to the promoter of responsive genes [1]; it can bind Creb binding protein (CBP) and inhibit its histone acetyltransferase activity [4]; finally, it can interact directly with transcription factors such as Sp1 and displace them from their cognate site on target gene promoters [2]. All of these functions are dependent on localization of MIER1α in the nucleus, yet it does not contain a functional NLS [5]. Instead, translocation into the nucleus is dependent on interaction and co-transport with HDAC1 and 2 [6]. In this report, we show that localization of MIER1α in the nucleus of MCF7 cells is significantly reduced by treatment with insulin or IGF-1, but not by 17β-estradiol (E2). This suggests that insulin or IGF-1 could attenuate MIER1α’s transcriptional repressor/chromatin modifying functions in MCF7.
Methods
The MCF7 breast carcinoma cell line was obtained from the ATCC and cultured in DMEM (GIBCO) containing 10 % serum [7.5 % calf serum (CS) + 2.5 % fetal bovine serum (FBS)] (GIBCO), in a humidified 37 °C incubator with 5 % CO2. Insulin was purchased from Life Technologies and used at a concentration of 10 ug/ml. IGF-1 was purchased from PeproTech and used at a concentration of 10 ng/ml. 17β-estradiol was purchased from Sigma-Aldrich and used at a concentration of 10−8 M. For experiments using 17β-estradiol, cells were cultured in phenol red-free DMEM (GIBCO) supplemented with 10 % charcoal-stripped FBS (Hyclone). Cells were treated for 4 h with insulin, IGF-1 or 17β-estradiol prior to fixation. Construction of the human mier1α sequence (GenBank: AY124188) in the CS3 + MT vector has been described previously [1]. Transient transfection, confocal microscopy, antibodies used and Z-stack analysis were performed as described in [6, 7]. Subcellular localization was scored as ‘nuclear’ if the nucleus was intensely stained, with little or no cytoplasmic staining; ‘cytoplasmic’ if staining was primarily in the cytoplasm, with little or no staining in the nucleus; ‘whole cell’ if both the nucleus and cytoplasm were stained [6]. Statistical analysis was performed using a two-sided Fisher’s exact test.
Results and discussion
Insulin alters nuclear localization of MIER1α in MCF7 cells
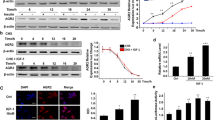
We have shown previously that MIER1α is targeted to the nucleus in MCF7 cells despite the lack of an intrinsic NLS [6, 7]. In those studies, cells were cultured in DMEM containing 10 % CS/FBS. Several laboratories, including the ATCC, add 10 ug/ml insulin to culture media for MCF7 cells, however when we added insulin, we noticed a change in the subcellular localization pattern of MIER1α. To investigate this effect more thoroughly, we analysed mier1α-transfected MCF7 cells by confocal microscopy. In the presence of insulin, only 41 % of cells had exclusively nuclear MIER1α (Fig. 1Ad–f, B), compared to 81 % of cells in the absence of insulin (Fig. 1Aa–c, B). The percentage cells with MIER1α in both the nucleus and cytoplasm (whole cell staining) increased in the presence of insulin, from 18 to 42 % (Fig. 1B). Likewise, the proportion of cells with exclusively cytoplasmic MIER1α increased over tenfold, from 1 to 17 % (Fig. 1B). These results demonstrate that in the presence of insulin, localization of MIER1α in MCF7 cells is shifted from the nucleus to the cytoplasm.
Insulin treatment reduces nuclear localization of MIER1α. MCF7 cells were transfected with myc-tagged mier1α and treated 24 h later with either vehicle (panels a–c) or 10 ug/ml insulin (panels d–f). Cells were fixed 4 h after the addition of insulin and localization was analyzed by confocal microscopy. Random fields were selected and the staining pattern of each cell within the field was scored as ‘nuclear’, ‘whole cell’ or ‘cytoplasmic’, using sequential Z-stage scanning. A Shown are Z-stacks compiled into individual images, illustrating stained nuclei (DAPI; panels a, d), MIER1α localization (9E10 anti-myc tag antibody and an AlexaFluor-488 secondary antibody; panels b, e) and merged channels (panels c, f); arrowheads show examples of nuclear staining and arrows show whole cell staining. B Histogram showing the results of 3 independent experiments; the MIER1α localization pattern of >1000 cells was scored. Plotted is the percentage of cells in each category ±SD; Asterisk indicates that the difference was statistically significant, p < 0.05 (Fisher’s exact test)
Our previous research demonstrated that MIER1α localizes to the nucleus through interaction and co-transport with HDAC1/2. Therefore we investigated whether insulin also affected localization of HDAC1/2. Confocal analysis demonstrated that while insulin reduces nuclear accumulation of MIER1α (Fig. 2Ab, f, j), it does not affect localization of HDAC1 or 2 (Fig. 2Ac, g, k) and both were 100 % nuclear (Fig. 2B).
HDAC1 and 2 localization are not affected by insulin. Cells were transfected, treated with insulin and prepared for confocal microscopy as described in Fig. 1. A Compiled Z-stacks showing nuclei (DAPI; panels a, e, i), MIER1α (AlexaFluor-488, panels b, f, j), HDAC1 (AlexaFluor-647, panels c, g), HDAC2 (AlexaFluor-647, panel k) and merged 488 and 647 channels (panels d, h, l); arrowheads show examples of nuclei and arrows show whole cell staining. B Histogram showing the localization of HDAC1 and HDAC2 in untreated (Con) and in insulin treated MCF7 cells; plotted is the percentage of cells in each category ±SD from three independent experiments
Nuclear accumulation of MIER1α is affected by IGF-1, but not by E2
IGF-1 is closely related to insulin and both can interact with the insulin and IGF receptors, albeit with differing affinities [8]. In addition, there is a wealth of evidence implicating IGF-1 in breast cancer development and progression (reviewed in [9] ) and it has been shown to increase invasiveness of MCF7 cells [10]. Since MCF7 cells express receptors for both insulin and IGFs [11], we explored the possibility that IGF-1 also affects localization of MIER1α. As expected, confocal analysis demonstrated that IGF-1 had a similar effect on nuclear accumulation of MIER1α (Fig. 3Ab, f, j, B). IGF-1 reduced the percentage of cells with nuclear MIER1α from 89 to 56 % and increased the percentage with ‘whole cell’ staining from 10 to 40 %. The percent with ‘cytoplasmic’ MIER1α was also increased from 0.3 to 4 %. Thus, both insulin and IGF-1 have similar effects on the subcellular localization of MIER1α in MCF7.
IGF-1 reduces nuclear localization of MIER1α. MCF7 cells were transfected with myc-tagged mier1α and treated 24 h later with either vehicle (panels a–d) or 10 ng/ml IGF-1 (panels e–l). Cells were fixed 4 h after the addition of IGF-1 and localization was analyzed by confocal microscopy, as in Fig. 1. A Compiled Z-stacks showing nuclei (DAPI; panels a, e, i), MIER1α (AlexaFluor-488, panels b, f, j), HDAC1 (AlexaFluor-647, panels c, g), HDAC2 (AlexaFluor-647, panel k) and merged 488 and 647 channels (panels d, h, l). B Histogram showing the results of two independent experiments; the MIER1α localization pattern of >1000 cells was scored. Plotted is the percentage of cells in each category ±SD; Asterisk indicates that the difference was statistically significant, p < 0.05 (Fisher’s exact test). Note that nuclear localization of HDAC1 and HDAC2 was unaffected by IGF-1
Insulin and IGFs are potent mitogens for MCF7 cells [12], leading to the question of whether changes in nuclear accumulation of MIER1α are related to the fact that the cells are proliferating. We therefore examined MIER1α localization in cells treated with E2, a classic mitogen for ER + breast carcinoma cells. Unlike insulin and IGF-1, E2 had no significant effect on nuclear accumulation of MIER1α (Fig. 4). In the presence of E2, 77 % of cells displayed nuclear MIER1α (Fig. 4Af, j, B) compared to 80 % of untreated cells (Fig. 4Ab, B). Likewise there was no significant difference in the percentage of cells with ‘whole cell’ or ‘cytoplasmic’ staining (Fig. 4B). These results demonstrate that the shift in MIER1α localization directed by insulin and IGF-1 is not the non-specific result of cell proliferation.
E2 has no effect on subcellular localization of MIER1α. MCF7 cells were transfected with myc-tagged mier1α and 24 h later, either vehicle (panels a–d) or 10−8M E2 (panels e–l) was added. Cells were fixed 4 h later and localization was analyzed by confocal microscopy, as in Fig. 1. A Compiled Z-stacks showing nuclei (DAPI; panels a, e, i), MIER1α (AlexaFluor-488, panels b, f, j), HDAC1 (AlexaFluor-647, panels c, g), HDAC2 (AlexaFluor-647, panel k) and merged 488 and 647 channels (panels d, h, l). B Histogram showing the results of two independent experiments; the MIER1α localization pattern of >500 cells was scored. Plotted is the percentage of cells in each category ±SD; there was no statistically significant difference between the percentage of control and E2 treated cells in each of the three categories, p > 0.05 (Fisher’s exact test)
More than likely activation of one of the insulin/IGF signalling pathways is responsible for the altered MIER1α localization in MCF7. This type of effect has been observed for the FOXO family of transcription factors. For example, FOXO is driven out of the nucleus by insulin as well as other growth factors [13] and by Src signalling [14]. In C. elegans, activation of DAF-2, the nematode ortholog of the IGF-1 receptor, prevents nuclear accumulation of the DAF-16 (FOXO) transcription factor [15].
The data presented here demonstrate that for MCF7 the culture conditions, specifically the common practice of including insulin in the medium, can have important consequences for studies of nuclear proteins like MIER1α.
Abbreviations
- CBP:
-
creb binding protein
- CS:
-
calf serum
- E2:
-
17-β estradiol
- ERα:
-
estrogen receptor alpha
- FBS:
-
fetal bovine serum
- HDAC:
-
histone deacetylase
- IGF-1:
-
insulin-like growth factor 1
- NLS:
-
nuclear localization signal
References
Ding Z, Gillespie LL, Paterno GD. Human MI-ER1 alpha and beta function as transcriptional repressors by recruitment of histone deacetylase 1 to their conserved ELM2 domain. Mol Cell Biol. 2003;23:250–8.
Ding Z, Gillespie LL, Mercer FC, Paterno GD. The SANT domain of human MI-ER1 interacts with Sp1 to interfere with GC box recognition and repress transcription from its own promoter. J Biol Chem. 2004;279:28009–16.
McCarthy PL, Mercer FC, Savicky MW, Carter BA, Paterno GD, Gillespie LL. Changes in subcellular localisation of MI-ER1 alpha, a novel oestrogen receptor-alpha interacting protein, is associated with breast cancer progression. Br J Cancer. 2008;. doi:10.1038/sj.bjc.6604518.
Blackmore TM, Mercer CF, Paterno GD, Gillespie LL. The transcriptional cofactor MIER1-beta negatively regulates histone acetyltransferase activity of the CREB-binding protein. BMC Res Notes. 2008;. doi:10.1186/1756-0500-1-68.
Post JN, Gillespie LL, Paterno GD. Nuclear localization signals in the Xenopus FGF embryonic early response 1 protein. FEBS Lett. 2001;502(1–2):41–5.
Li S, Paterno GD, Gillespie LL. Nuclear localization of the transcriptional regulator MIER1alpha requires interaction with HDAC1/2 in breast cancer cells. PLoS One. 2013;. doi:10.1371/journal.pone.0084046.
Clements JA, Mercer FC, Paterno GD, Gillespie LL. Differential splicing alters subcellular localization of the alpha but not beta isoform of the MIER1 transcriptional regulator in breast cancer cells. PLoS One. 2012;. doi:10.1371/journal.pone.0032499.
Werner H, Weinstein D, Bentov I. Similarities and differences between insulin and IGF-I: structures, receptors, and signalling pathways. Arch Physiol Biochem. 2008;. doi:10.1080/13813450801900694.
Christopoulos PF, Msaouel P, Koutsilieris M. The role of the insulin-like growth factor-1 system in breast cancer. Mol Cancer. 2015;. doi:10.1186/s12943-015-0291-7.
Walsh LA, Damjanovski S. IGF-1 increases invasive potential of MCF 7 breast cancer cells and induces activation of latent TGF-beta1 resulting in epithelial to mesenchymal transition. Cell Commun Signal CCS. 2011;. doi:10.1186/1478-811X-9-10.
Cullen KJ, Yee D, Sly WS, Perdue J, Hampton B, Lippman ME, et al. Insulin-like growth factor receptor expression and function in human breast cancer. Cancer Res. 1990;50(1):48–53.
Bentel JM, Lebwohl DE, Cullen KJ, Rubin MS, Rosen N, Mendelsohn J, et al. Insulin-like growth factors modulate the growth inhibitory effects of retinoic acid on MCF-7 breast cancer cells. J Cell Physiol. 1995;. doi:10.1002/jcp.1041650124.
Greer EL, Brunet A. FOXO transcription factors at the interface between longevity and tumor suppression. Oncogene. 2005;. doi:10.1038/sj.onc.1209086.
Bulow MH, Bulow TR, Hoch M, Pankratz MJ, Junger MA. Src tyrosine kinase signaling antagonizes nuclear localization of FOXO and inhibits its transcription factor activity. Sci Rep. 2014;. doi:10.1038/srep04048.
Lin K, Hsin H, Libina N, Kenyon C. Regulation of the Caenorhabditis elegans longevity protein DAF-16 by insulin/IGF-1 and germline signaling. Nat Genet. 2001;. doi:10.1038/88850.
Authors’ contributions
SL performed the experiments. LLG analysed the data, prepared the Figures and wrote the manuscript. LLG and GDP participated in the design of the experiments and interpretation of the data. All authors were involved in the revisions. All authors read and approved the final manuscript.
Acknowledgements
This work was supported by a grant from the Canadian Institutes of Health Research and the Canadian Breast Cancer Foundation-Atlantic Chapter to LLG and GDP.
Compliance with ethical guidelines
Competing interests The authors declare that they have no competing interests.
Author information
Authors and Affiliations
Corresponding author
Rights and permissions
Open Access This article is distributed under the terms of the Creative Commons Attribution 4.0 International License (http://creativecommons.org/licenses/by/4.0/), which permits unrestricted use, distribution, and reproduction in any medium, provided you give appropriate credit to the original author(s) and the source, provide a link to the Creative Commons license, and indicate if changes were made. The Creative Commons Public Domain Dedication waiver (http://creativecommons.org/publicdomain/zero/1.0/) applies to the data made available in this article, unless otherwise stated.
About this article
Cite this article
Li, S., Paterno, G.D. & Gillespie, L.L. Insulin and IGF-1, but not 17β-estradiol, alter the subcellular localization of MIER1α in MCF7 breast carcinoma cells. BMC Res Notes 8, 356 (2015). https://doi.org/10.1186/s13104-015-1336-0
Received:
Accepted:
Published:
DOI: https://doi.org/10.1186/s13104-015-1336-0