Abstract
Background
Posterior cortical atrophy (PCA) is a rare condition characterized by early-onset and progressive visual impairment. Individuals with PCA have relatively early-onset and progressive dementia, posing certain needs for early detection. Hence, this study aimed to investigate the association of alterations in outer retinal and choroidal structure and microvasculature with PCA neuroimaging and clinical features and the possible effects of apolipoprotein E(APOE) ε4 allele on outer retinal and choroidal alterations in participants with PCA, to detect potential ocular biomarkers for PCA screening.
Methods
This cross-sectional study included PCA and age- and sex-matched healthy control participants from June 2022 to December 2023. All participants with PCA completed a comprehensive neurological evaluation. All participants were recorded baseline information and underwent an ophthalmic evaluation. Quantitative analyses were performed using swept-source optical coherence tomography (SS-OCT) and angiography (SS-OCTA). Adaptive optics scanning laser ophthalmoscopy (AO-SLO) was performed in some patients. In participants with PCA, the influence of APOE ε4 on outer retinal and choroidal alterations and the correlation of outer retinal and choroidal alterations with PCA neuroimaging and clinical features in participants with PCA were investigated.
Results
A total of 28 participants (53 eyes) with PCA and 56 healthy control participants (112 eyes) were included in the current study. Compared with healthy control participants, participants with PCA had significantly reduced outer retinal thickness (ORT) (p < 0.001), choriocapillaris vessel density (VD) (p = 0.007), choroidal vascular index (CVI) (p = 0.005) and choroidal vascular volume (CVV) (p = 0.003). In participants with PCA, APOE ε4 carriers showed thinner ORT (p = 0.009), and increased choriocapillaris VD (p = 0.004) and CVI (p = 0.004). The PCA neuroimaging features were positively associated with the ORT, CVI and CVV. Furthermore, differential correlations were observed of PCA clinical features with the CRT, CVV and CVI.
Conclusions
Our findings highlighted the association of outer retinal and choroidal alterations with PCA neuroimaging and clinical features in participants with PCA. Noninvasive SS-OCT and SS-OCTA can provide potential biomarkers for the diagnosis and management of PCA, improving awareness of PCA syndrome among ophthalmologists, neurologists, and primary care providers.
Similar content being viewed by others
Explore related subjects
Discover the latest articles, news and stories from top researchers in related subjects.Introduction
Posterior cortical atrophy (PCA), also known as a visual variant of Alzheimer’s disease (AD), is a rare condition characterized by early-onset and progressive visual impairment, with remarkable pathology in the parietal and occipital lobes involved in high-order visual processing [1, 2]. While most commonly associated with AD, PCA can also be attributable to different underlying causes with relatively decreasing frequency, including Lewy body disease (LBD), corticobasal degeneration (CBD) and prion disease [1, 2]. The exact incidence and prevalence of PCA remain unknown. Previous studies indicated that PCA may account for approximately 5-13% of AD cases [3, 4]. Individuals with PCA frequently experience considerable delays in diagnosis and management owing to their relatively young age at onset and atypical symptoms during presentation as compared to typical AD [2, 5]. In some cases, individuals with PCA may present to the ophthalmology department at the first visit, complaining of visual dysfunction but with a normal basic eye examination and relatively preserved cognitive function [6]. The PCA cognitive profile is characterized by posterior cortical impairment, while other cognitive domains remain relatively intact [7]. Individuals with PCA usually present with mild dementia at diagnosis, highlighting the importance of early detection [2].
The current consensus on the diagnosis of PCA highlights the role of clinical and cognitive features, with supportive neuroimaging biomarkers if available [7], such as structural atrophy on magnetic resonance imaging (MRI) or functional abnormalities on positron emission tomography (PET) in the posterior cortex [7]. Visual symptoms are commonly reported by individuals with PCA at their initial visit. Consequently, they are often first referred to the ophthalmology department for evaluation of potential ocular abnormalities [5].
The Apolipoprotein E (APOE) ε4 allele is the strongest genetic risk factor for late-onset AD [8]. It is associated with AD pathologies, including stimulating amyloid β (Aβ) production, reducing their clearance, and increasing tau pathology and neurodegenerative patterns [9,10,11,12]. The frequency of the APOE ε4 allele is relatively lower in PCA compared to typical AD [13]. Recent studies have suggested an association between APOE and an increased risk of PCA [14]. Singh and colleagues found that APOE ε4 is associated with a greater predisposition in the medial temporal cortex, as well as brain connectivity in individuals with PCA [10, 15]. These findings imply that APOE ε4 plays an important role in the pathophysiology of PCA but raise the question of whether APOE ε4 also influences the eyes, particularly the retina and choroid.
As an extension of the central nervous system (CNS), the eye provides a unique window for detecting CNS disorders. Given the rapid development of ophthalmic multimodal imaging techniques, including noninvasive, convenient, and relatively low-cost swept-source optical coherence tomography (SS-OCT) and angiography (SS-OCTA), fundus examination has been explored as a new research approach for studying possible CNS disorders in neurodegenerative diseases [16,17,18,19]. Moreover, previous studies have identified the pathological features of AD in the retina, suggesting that the impairment of the visual pathway may be present even in the early phase of AD with low-level Aβ [20,21,22]. Thinning of the retina and impaired retinal microvasculature have been reported in individuals with PCA [23]. However, Den Haan and colleagues found no significant difference in retinal thickness between individuals with PCA and typical AD, and controls [24]. These inconsistencies suggest that further large-scale research is needed to precisely investigate retinal changes in individuals with PCA and to determine their exact value in the management of PCA. Additionally, the correlation between impaired retinal microvasculature and the decline in cognitive function was investigated in a recent study [23]. Furthermore, some researchers have investigated the similarities between AD and age-related macular degeneration (AMD) pathogenesis, potentially expanding our exploration of CNS and ocular neurodegenerative diseases [25,26,27]. Drusen is a biomarker for the development of AMD [28]. Recent studies found the association of typical AD and PCA with hard drusen formation, which indicated that drusen might be a potential biomarker for AD [29, 30]. Taken together, these findings revealed that the pathological processes of outer retinal dysfunction may share similarities between AMD and AD [27]. However, a detailed assessment of the outer retinal and choroidal alterations and their potential association with the PCA pathological features is lacking.
In the present study, we aimed to characterize the alterations in the outer retina and choroid in participants with PCA using SS-OCT, SS-OCTA and adaptive optics scanning laser ophthalmoscopy (AO-SLO). Subsequently, we detected the potential effects of APOE ε4 on the severity of outer retinal and choroidal alterations in participants with PCA. Furthermore, we investigated the potential correlation of alterations in the outer retina and choroid with PCA neuroimaging and clinical features. We hypothesize that the APOE ε4 influences the outer retina and choroid in PCA and that ocular alterations are associated with PCA neuroimaging and clinical features.
Methods
This cross-sectional study was approved by the Ethics Committee on Biomedical Research, West China Hospital of Sichuan University, and was conducted in accordance with the Declaration of Helsinki. All the participants were recruited in this study from June 2022 to December 2023. Written informed consent was obtained from all the enrolled participants.
Study participants
Participants with PCA and age- and sex-matched healthy control participants were recruited from West China Hospital (the details are shown in Supplementary Methods). All participants with PCA completed a comprehensive neurological evaluation, including a clinical history interview, physical examination, and neuropsychological assessment. The clinical diagnosis of PCA was confirmed in accordance with previously proposed clinical diagnostic criteria [7]. Baseline information, including age, sex, and educational level, was recorded for all the participants, and the participants underwent a complete ophthalmic evaluation, including slit-lamp biomicroscopy, fundus examination, SS-OCT, and SS-OCTA. AO-SLO with a 2.4 × 2.4 field of view was performed in some patients (Supplementary Fig. 1). All evaluations and examinations were conducted over 1 month (Supplementary Methods).
Neurological evaluation and examination
A detailed medical history was obtained in all participants with PCA, and they underwent extensive neurological evaluation, including the following: Montreal Cognitive Assessment (MoCA), and Mini-Mental State Examination (MMSE), and Clinical Dementia Rating (CDR)assessment for general cognitive function, and APOE genotyping. 18 F-AV45 PET/MRI was performed using the GE 3T scanner (SIGNA PET/MR; GE Healthcare, Chicago, IL, USA) to identify the Aβ deposition in the brain (Fig. 1).
Representative sagittal 18 F-AV45 PET/MRI images. (A) A participant with PCA with significant cortical amyloid-β (Aβ) deposition (white arrows) and occipital-parietal atrophy of right hemisphere in brain MRI (yellow circle). (B) An age and sex matched healthy control participant with typical negative 18 F AV45-PET scan and MRI scan indicates no significant occipital-parietal atrophy
A visual assessment of PET images was performed by two experienced nuclear medicine physicians in a collaborative session, during which they were required to provide a binary diagnosis (positive or negative). To mitigate potential bias, the order of image readings was randomized, and all participant information was concealed. The criteria for identifying positive 18 F-AV45 PET images were based on visual rating guidelines for interpreting amyloid PET [31].
Swept-source OCT, OCTA and AO
All participants underwent SS-OCT (VG200, SVision Imaging, Ltd., Luoyang, China) with a central wavelength of 1,050 nm, scanning speed of 200,000 A-scans/s, scanning depth of 2.7 mm in tissue, and sampling spacing of 12 mm [17]. The scanning models included (1) 6 mm × 6mm2,512 × 512 pixels, R4, centered on the fovea, (2) 33 scan lines, R16, centered on the fovea. Both SS-OCT and SS-OCTA images with signal strengths > 7/10 without severe motion artefacts were obtained and included in this study.
The SS-OCT structure parameters were automatically measured in a 6 mm × 6mm2 diameter region centered on the fovea, including the outer retinal thickness (ORT), choroidal thickness (CRT), retinal nerve fiber layer (RNFL) and ganglion cell inner plexiform layer (GCIPL). The SS-OCTA flow parameters were automatically assessed in a scanning mode of 6 mm × 6 mm,512 × 512 pixels, R4, and centered on the fovea, which included the choriocapillaris vessel density (VD), choroidal vascular volume (CVV) and choroidal vascular index (CVI). Both the SS-OCT structural parameters and SS-OCTA flow parameters were automatically measured using the Early Treatment Diabetic Retinopathy Study (ETDRS) circle, including 0–1 mm, 1–3 mm, 3–6 mm and total (0–6 mm) circles.
Two participants with PCA and two healthy control participants underwent AO-SLO scanning (Mona II, Robotrak Technologies, Nanjing, China), the field of 2.4 × 2.4° (approximately 700 × 700 μm) view on the macula. Cone segmentation is automatically generated and cone morphology properties, including cell density, cell dispersion and cell regularity, were automatically analyzed.
The Cone cell density is defined as the average cone cell number per square millimeter. The cone cell regularity refers to the uniformity of cone distribution, measured by the ratio of cells with a precise number of closest cells within a certain range. The cone cell dispersion is defined as the extent of cell dispersed or clustered, quantified by the proportion of the average distance between cone cells [32] (Supplementary Fig. 1).
The outer retinal and choroidal alterations, and thickness of RNFL and GCIPL were compared between the enrolled eyes of PCA and age- and sex-matched healthy control participants. Accordingly, subgroup comparisons between ε4 carriers and ε4 non-carriers were performed to detect the effects of APOE ε4 on the retina and choroid in participants with PCA. To investigate the potential association between the ocular alterations and the PCA features: (1) the PCA neuroimaging features, including cortical volume and cortical thickness of the occipital, parietal, and temporal lobes; (2) the PCA clinical features, including cognitive function, assessed by MMSE, MoCA and CDR examinations were assessed.
Statistical analysis
Demographic and clinical variables were analyzed using an independent t-test for continuous variables and χ 2 test for categorical variables. Generalized estimating equation (GEE) models were used to compare the differences in the SS-OCT structure and SS-OCTA flow parameters between participants with PCA and healthy control participants while adjusting for inter-eye dependencies, age, sex and signal strengths. Bonferroni method was applied for multiple comparisons. Partial correlation analyses were used to detect correlations between SS-OCT structure SS-OCTA flow parameters and PCA pathologic characteristics. All P values were performed on 2-tailed tests, and a P-value < 0.05 was considered significant. All statistical analyses were performed using IBM SPSS Statistics version 23.
Results
Demographic and clinical characteristics
Of the 31 participants with PCA and 56 healthy control participants recruited in this study, nine eyes of participants with PCA were excluded, including five with drusen, two with epiretinal membrane, one with pigment epithelial detachment and one with vitreous opacity (Supplementary Fig. 2). Therefore, 28 participants (53 eyes) with PCA (19[67.9%] women and 9[32.1%] men; mean age [SD], 60.21 [7.38] years) and 56 healthy control participants (112 eyes) (35 [62.5%] women and 21[37.5%] men; mean age [SD], 60.11 [7.92] years) were included. The demographic and clinical characteristics of the enrolled participants are demonstrated in Table 1. There were no significant differences in sex (p = 0.629), age (p = 0.687) and educational level (p = 0.804) between participants with PCA and healthy control participants. The participants with PCA had lower MoCA, MMSE, CDR-G, and CDR-SB scores than the healthy control participants (all p < 0.001). All participants with PCA were Aβ PET positive. Blood collection for APOE testing was done in 24 participants with PCA.
Comparison of structural and flow parameters
In the comparison of outer retinal and choroidal structures between participants with PCA and healthy control participants, the ORT in the 1–3 mm (168.35 [10.27] vs. 173.53[10.10] µm, p = 0.002), 3–6 mm (143.53 [9.28] vs. 149.57 [8.35] µm, p < 0.001), and total (150.31 [9.04] vs. 156.02 [8.23] µm, p < 0.001) ETDRS circles were significantly decreased in participants with PCA while there was no significant difference in CRT (all p > 0.0125). Regarding flow parameters, participants with PCA had significantly decreased choriocapillaris VD in 1–3 mm ETDRS circles (67.39 [5.29] vs. 69.85 [5.41] %, p = 0.007), CVI in the 1–3 mm ETDRS circles(0.35 [0.05] vs. 0.38 [0.06], p = 0.005) and CVV in the 0–1 mm(0.08 [0.03] vs. 0.09 [0.03], p = 0.002), 1–3 mm(0.62 [0.23] vs. 0.72 [0.22], p = 0.009),3–6 mm (1.69 [0.67] vs. 2.49 [0.63], p = 0.002), and total (2.39 [0.92] vs. 2.85 [0.87], p = 0.003) ETDRS circles (Table 2; Fig. 2). AO-SLO image analysis showed that the participants with PCA had relatively reduced cone cell density (Supplementary Fig. 1 and Supplementary Table 1).
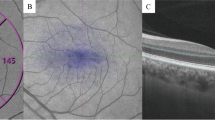
Representative SS-OCTA images in a 6 × 6 -mm region. The Early Treatment Diabetic Retinopathy Study (ETDRS) circle was used for quantification. The choriocapillaris (CC) is defined and automatically segmented as 20 μm below Bruch’s membrane. (A) From a participant with posterior cortical atrophy. (B) From an age and sex-matched healthy control participant
Meanwhile, participants with PCA had thinner GCIPL in the 0–1 mm (24.54 [6.70] vs. 28.89 [6.34] µm, p = 0.003), 1–3 mm (83.13 [9.23] vs. 88.52 [8.79] µm, p < 0.001) and total (61.49 [7.39] vs. 64.93 [6.88] µm, p = 0.002) ETDRS circles, compared with healthy control. There was no significant difference in the thickness of RNFL between PCA and healthy control participants (all p > 0.05) (Supplementary Table 2).
Effects of APOE ε4 on the outer retina and choroid
The effects of APOE ε4 on the outer retinal and choroidal alterations were investigated. The APOE ε4 carriers (n = 11) had thinner ORT in the 1–3 mm (165.14 [6.83] vs. 171.84 [6.82] µm, p = 0.005) and total ETDRS circles (147.91 [5.72] vs. 154.05 [7.97] µm, p = 0.009) and increased choriocapillaris VD in the 0–1 mm ETDRS circles (70.56 [3.03] vs. 65.66 [5.80] %, p = 0.004) and 1–3 mm (67.95 [4.15] vs. 61.93 [6.84] %, p = 0.004) ETDRS circles and CVI in the 0–1 mm (0.37 [0.05] vs. 0.30 [0.10], p = 0.004) ETDRS circles than APOE ε4 noncarriers (n = 13) (Fig. 3 and Supplementary Table 3).
The Effects of APOE ε4 on the outer retinal and choroidal alterations between apolipoprotein E (APOE) ε4 noncarriers (n = 13) and carriers (n = 11) in participants with posterior cortical atrophy. (A) Subregional comparison of outer retina thickness (ORT). There were significant differences in ORT in the total and 1–3 mm ETDRS circles between APOE ε4 noncarriers and carriers. (B) Subregional comparison of choriocapillaris vessel density (VD). The APOE ε4 carriers had significant increased choriocapillaris VD in the 0–1 mm and 1–3 mm ETDRS circles. (C) Subregional comparison of choroidal vascular index (CVI). The APOE ε4 carriers had significant increased CVI in the 0–1 mm ETDRS circle
Additionally, the APOE ε4 carriers had thicker RNFL in the 3–6 mm (45.74 [5.35] vs. 40.70 [5.47] µm, p = 0.010) ETDRS circles than APOE ε4 noncarriers. There was no significant difference in the thickness of GCIPL between APOE ε4 carriers and noncarriers (all p > 0.05) (Supplementary Table 4).
Correlation between ocular biomarkers and PCA pathological features
Partial correlation analyses were performed to detect the association between alterations in the outer retina and choroid and the severity of disease while controlling for sex and age. The results of partial correlation analysis of the association of outer retinal and choroidal structural and flow parameters with PCA neuroimaging features are shown in Fig. 4. The right temporal lobe cortical volume, right parietal and temporal lobes cortical thickness were positively associated with the ORT in the 0–1 mm ETDRS circle in the left eye (r = 0.480, p = 0.015; r = 0.408, p = 0.043; r = 0.489, p = 0.013). We observed positive correlations of the left parietal lobe cortical volume with CVV in the 0–1 mm ETDRS circle(r = 0.433, p = 0.030), of the left parietal and temporal lobes cortical volume, and temporal lobe cortical thickness with CVV in the 1–3 mm ETDRS circle in the left eye (r = 0.439, p = 0.028; r = 0.417, p = 0.038; r = 0.403, p = 0.046), and of the left temporal lobe cortical volume with the CVV in the 0–1 mm ETDRS circle in the right eye (r = 0.410, p = 0.047). The cortical volume of the parietal and temporal lobes and cortical thickness of the parietal lobe in the left cerebral hemisphere were positively associated with CVI in the 0–1 mm ETDRS circle in the right eye (r = 0.445, p = 0.029; r = 0.449, p = 0.028; r = 0.419, p = 0.042) (Supplementary Tables 5–8).
Association of outer retinal and choroidal alterations with posterior neuroimaging features. (A) Association of CVI in the 0–1 mm ETDRS circle in the right eye with left parietal lobe cortical volume in participants with posterior cortical atrophy (PCA). (B) Association of CVI in the 0–1 mm ETDRS circle in the right eye with left temporal lobe cortical volume in participants with PCA. (C) Association of CVI in the 0–1 mm ETDRS circle in the right eye with left parietal lobe cortical thickness in participants with PCA. (D) Association of CVV in the 0–1 mm ETDRS circle in the right eye with left temporal lobe cortical volume in participants with PCA. (E) Association of CVV in the 0–1 mm ETDRS circle in the left eye with left parietal lobe cortical volume in participants with PCA. (F) Association of CVV in the 1–3 mm ETDRS circle in the left eye with left parietal lobe cortical volume in participants with PCA. (G) Association of CVV in the 1–3 mm ETDRS circle in the left eye with left temporal lobe cortical volume in participants with PCA. (H) Association of CVV in the 1–3 mm ETDRS circle in the left eye with left temporal lobe cortical thickness in participants with PCA. (I) Association of ORT in the 0–1 mm ETDRS circle in the left eye with right temporal lobe cortical volume in participants with PCA. (J) Association of ORT in the 0–1 mm ETDRS circle in the left eye with right parietal lobe cortical volume in participants with PCA. (K) Association of ORT in the 0–1 mm ETDRS circle in the left eye with right temporal lobe cortical thickness in participants with PCA. CVI, choroidal vascular index; CVV, choroidal vascular volume; ORT, outer retinal thickness
Meanwhile, we found positive correlations of the right parietal and temporal lobes cortical volume, and the right parietal lobes cortical thickness with RNFL in the 0–1 mm ETDRS circle in the left eye (r = 0.407, p = 0.043; r = 0.464, p = 0.019; r = 0.424, p = 0.035) and of the right temporal lobe cortical volume with GCIPL in the 0–1 mm ETDRS circle in the right eye (r = 0.425, p = 0.039). Negative associations were observed between the left occipital lobe cortical volume with GCIPL in the 1–3 mm ETDRS circle in the left eye (r = -0.450, p = 0.024) and between the right occipital lobe cortical thickness with GCIPL in the 3–6 mm ETDRS circle in the right eye (r = -0.413, p = 0.045) (Supplementary Tables 9–12).
Furthermore, we observed positive relations of MoCA score with CRT in the 0–1 mm ETDRS circle (r = 0.423, p = 0.031) and of MoCA and MMSE scores with the CVV in the 0–1 mm ETDRS circle (r = 0.433, p = 0.027; r = 0.485; p = 0.012). Moreover, we found negative associations between the CDR-G score and CVV in the 0–1 mm and 1–3 mm ETDRS circles (r = -0.469, p = 0.016; r = -0.427, p = 0.030). The CDR-SB score was negatively correlated to CVV in the 0–1 mm ETDRS circle (r = -0.446; p = 0.022) (Table 3). There were no significant associations of RNFL and GCIPL with PCA clinical features (all p > 0.05) (Supplementary Table 13).
Discussion
Our cross-sectional study demonstrated that outer retinal and choroidal alterations may occur in participants with posterior cortical atrophy. Moreover, APOE ε4 carriers had significantly thinner ORT and increased choriocapillaris VD and CVI in participants with PCA, suggesting the effects of the APOE ε4 allele on the outer retina and choroid. Furthermore, we investigated the association between outer retinal and choroidal alterations and the disease severity. Our findings indicated that these alterations may had differential correlations with PCA neuroimaging and clinical features.
We observed abnormalities in both the outer retina and choroid in participants with PCA, which have been poorly reported in previous studies. Sun and colleagues found individuals with PCA had impaired retinal structure and microvasculature, including the thinning of RNFL, GCIPL and INL, and impairments in the inner retinal microvasculature, compared to healthy controls [23]. However, Den Haan and colleagues reported that no significant difference in pRNFL and mRT was found in PCA and controls [24]. Our study indicated that participants with PCA had a thinner GCIPL compared to healthy controls, while no significant difference in RNFL was found between the two groups. These inconsistencies may be due to the heterogeneity of PCA and the varying conditions of individuals with PCA included in the studies. AD is the most common underlying cause of PCA [2, 7]. Several studies have described AD-specific pathology in the retinas of individuals with AD, which is similar to AD brains, including accumulation of Aβ deposits [33] and pTau [34, 35]. Proteomic analysis of AD retinas has revealed inhibition of photoreceptor-related pathways [21]. Fascinatingly, we observed that participants with PCA had reduced cone cell density measured on AO-SLO images compared with healthy control participants, suggesting potential cone loss. The outer retina, with high demands of nutrition and oxygen, is comprised of cell bodies and synapses of photoreceptors, which is mainly supported by the choroidal microvasculature. As color vision is generated through the rate of quantum catches signals emitted by different classes of cones [36, 37], our findings suggested that the deficits in photoreceptors may have ramifications for the concomitant color vision deficiency in PCA and the outer retinal and choroidal alterations may reflect the underlying neurodegenerative process in PCA.
In the current study, we described the first detailed assessment of the difference in outer retinal and choroidal structure and microvasculature between APOE ε4 carriers and APOE ε4 noncarriers in participants with PCA. Our findings indicated that APOE ε4 carriers exhibited significantly thinner ORT, increased choriocapillaris VD and CVI, and thicker RNFL than noncarriers. APOE, as a risk factor of PCA [14], is correlated with the severity of PCA-related pathology. A recent study suggested that APOE ε4 carries demonstrated greater volume loss in the medial temporal lobe in PCA [10]. APOE ε4 influences brain connectivity in PCA, with an increase in connectivity in the salience within-network and reduced connectivity in between-network [15]. In addition, the association of alterations in inner retinal structure and microvasculature with the APOE ε4 allele has been investigated in previous studies. Sheriff and colleagues reported that older adults with the APOE ε4 allele exhibited thinner RNFL and a smaller foveal avascular zone area [38]. Furthermore, in individuals with AD, reduced vascular density of the deep capillary plexus has also been observed in APOE ε4 carriers [39]. Photoreceptor loss has been identified in retinal degenerative diseases, which may be caused by pathological high oxygen consumption and related oxidative stress [37]. Hence, the influence of APOE ε4 on alterations in the outer retina and choroid may be driven by PCA-related pathologies, resulting in the impairment of photoreceptors and synapses in PCA. Accordingly, the thinner ORT and increased choriocapillaris VD in APOE ε4 carriers may reflect the retina and CNS undergo a similar neurodegenerative process.
The moderate evidence of the association of alterations in the outer retina and choroid with PCA neuroimaging and clinical features extends previous work. Significantly, few studies have explored the possible association between outer retinal and choroidal impairments and PCA characteristics. Sun and colleagues observed a weak correlation of retinal microvasculature with MMSE and MoCA scores in participants with PCA [23]. Our current study highlighted the correlation between PCA characteristics and ocular biomarkers. However, the underlying mechanism remains unclear. Progressive neurodegeneration in the parietal, occipital, and occipitotemporal cortices was highlighted in individuals with PCA, resulting in a progressive decline in visuoperceptual, visuospatial and other posterior cortical skills [2, 40]. Although these associations were weaker than the connections between brain regions (Supplementary Fig. 3 and Fig. 4), we provided new evidence of possible associations between ocular biomarkers and alterations in significant pathological regions in the brains of participants with PCA. The impairments in the retina and choroid may result in anterograde and retrograde neurodegeneration, and/or reflecting parallel pathological processes in both the retina and brain [41]. Fascinatingly, we found that the decline in cognitive ability, reflected by reduced MMSE and MoCA scores, and increased CDR-G and CDR-SB scores were significantly associated with the ocular manifestation in PCA. Given the similarities between the human retina and the rest of the CNS, our results indicated that investigating alterations in the anterior and posterior visual pathways and the possible association between these alterations offers an opportunity to learn more about the pathophysiological mechanisms of PCA as a whole.
This study has several strengths. First, all participants with PCA were diagnosed based on clinical, cognitive and neuroimaging features and pathological biomarkers on PET or/and cerebrospinal fluid biomarkers. Second, we used SS-OCT, SS-OCTA, and AO-SLO to detect the outer retinal and choroidal structure and microvasculature in participants with PCA. With a scanning speed of 200,000 A-scans per second and a central wavelength of 1050 nm, SS-OCT and SS-OCTA allow for deeper tissue penetration, facilitating our study of the outer retina and choroid. Meanwhile, AO-SLO enables non-invasive visualization of the human retina in vivo with single-cell resolution, particularly for evaluating the outer retina. Third, we were the first to investigate the different severities of outer retinal and choroidal alterations in APOE ε4 carriers and noncarriers among participants with PCA. Our findings could help to unravel the potential effects of APOE ε4 on the eye. Finally, this study fills a gap in the data regarding the association of the ocular structure and microvasculature with PCA pathological features, including neuroimaging biomarkers, cognitive dysfunction and neuropsychiatric symptom severity, which integrates neuropathology and biomarkers on both the eye and brain as a whole and again highlights the potential role of the eye as an ideal window to study the CNS. However, it is worth noting that these biomarkers may not be specific to PCA. Outer retinal and choroidal impairments have also been reported in pathological myopia [42], AMD [43, 44], glaucoma [45], Parkinson’s disease [46], early diabetes mellitus [47] and other conditions. Retinal imaging techniques are still in the early stages of the study of PCA. Given the rapid advancements in ophthalmic imaging technologies, further research is needed to clarify the potential applications of these novel retinal imaging techniques in PCA and other central nervous system diseases.
The current study has some limitations. First, the sample size was relatively small. However, as a rare syndrome, PCA only accounts for 5% of people with AD [3]. This low prevalence may be a reasonable explanation. Second, this was a cross-sectional study, and we were unable to investigate longitudinal alterations in these individuals. Third, detailed APOE phenotypes were not available in healthy control participants. As the presence of APOE ε4 carriers in the general population [48], the potential effects of APOE ε4 on the retina and choroid in PCA should be interpreted with caution. Long-term studies with larger sample sizes and comprehensive information on both PCA and healthy control participants are warranted to determine whether these ocular findings can serve as biomarkers for detecting the PCA progression and investigating the underlying pathophysiological mechanisms.
Conclusions
In conclusion, individuals with PCA, the visual variant of AD, often present early visual symptoms and visit the ophthalmology department. In this cross-sectional study, we found that outer retinal and choroidal alterations were significant associated with PCA neuroimaging and clinical features and that APOE ε4 carriers displayed more severe outer retinal alteration and increased choriocapillaris VD and CVI than noncarriers in PCA. Alterations in the outer retina and choroid detected by noninvasive and direct SS-OCT and SS-OCTA can be biomarkers early in the PCA.
Data availability
Data collected in this study are available from the corresponding author to the public with reasonable request through appropriate data sharing protocols.
Abbreviations
- AD:
-
Alzheimer’s disease
- AMD:
-
Age-related macular degeneration
- AO-SLO:
-
Adaptive optics scanning laser ophthalmoscopy
- APOE:
-
Apolipoprotein E
- Aβ:
-
Amyloid β
- CBD:
-
Corticobasal degeneration
- CDR:
-
Clinical dementia rating
- CDR-G:
-
Clinical Dementia Rating-Global
- CDR-SB:
-
Clinical Dementia Rating- Sum of Boxes
- CNS:
-
Central nervous system
- CRT:
-
Choroidal thickness
- CVI:
-
Choroidal vascular index
- CVV:
-
Choroidal vascular volume
- ETDRS:
-
Early Treatment Diabetic Retinopathy Study
- GCIPL:
-
Ganglion cell inner plexiform layer
- GEE:
-
Generalized estimating equation
- LBD:
-
Lewy body disease
- MoCA:
-
Montreal Cognitive Assessment
- MMSE:
-
Mini-Mental State Examination
- MRI:
-
Magnetic resonance imaging
- ORT:
-
Outer retinal thickness
- PCA:
-
Posterior cortical atrophy
- PET:
-
Positron emission tomography
- RNFL:
-
Retinal nerve fiber layer
- SS-OCT:
-
Swept-source optical coherence tomography
- SS-OCTA:
-
Swept-source optical coherence tomography angiography
- VD:
-
Vessel density
References
Benson DF, Davis RJ, Snyder BD. Posterior cortical atrophy. Arch Neurol. 1988;45:789–93. https://doi.org/10.1001/archneur.1988.00520310107024.
Crutch SJ, Lehmann M, Schott JM, et al. Posterior cortical atrophy. Lancet Neurol. 2012;11:170–8. https://doi.org/10.1016/S1474-4422(11)70289-7.
Snowden JS, Stopford CL, Julien CL, et al. Cognitive phenotypes in Alzheimer’s disease and genetic risk. Cortex. 2007;43:835–45. https://doi.org/10.1016/s0010-9452(08)70683-x.
Koedam EL, Lauffer V, van der Vlies AE, et al. Early-versus late-onset Alzheimer’s disease: more than age alone. J Alzheimers Dis. 2010;19:1401–8. https://doi.org/10.3233/JAD-2010-1337.
Chapleau M, La Joie R, Yong K, et al. Demographic, clinical, biomarker, and neuropathological correlates of posterior cortical atrophy: an international cohort study and individual participant data meta-analysis. Lancet Neurol. 2024;23:168–77. https://doi.org/10.1016/S1474-4422(23)00414-3.
Bejanin A, Villain N. Posterior cortical atrophy: new insights into treatments and biomarkers for Alzheimer’s disease. Lancet Neurol. 2024;23:127–8. https://doi.org/10.1016/S1474-4422(23)00501-X.
Crutch SJ, Schott JM, Rabinovici GD, et al. Consensus classification of posterior cortical atrophy. Alzheimers Dement. 2017;13:870–84. https://doi.org/10.1016/j.jalz.2017.01.014.
van der Flier WM, Schoonenboom SN, Pijnenburg YA, et al. The effect of APOE genotype on clinical phenotype in Alzheimer disease. Neurology. 2006;67:526–7. https://doi.org/10.1212/01.wnl.0000228222.17111.2a.
Liu CC, Zhao N, Fu Y et al.,. ApoE4 Accelerates Early Seeding of Amyloid Pathology. Neuron 2017;96:1024-32 e3https://doi.org/10.1016/j.neuron.2017.11.013.
Singh NA, Tosakulwong N, Graff-Radford J, et al. APOE epsilon4 influences medial temporal atrophy and tau deposition in atypical Alzheimer’s disease. Alzheimers Dement. 2022. https://doi.org/10.1002/alz.12711.
Baek MS, Cho H, Lee HS, et al. Effect of APOE epsilon4 genotype on amyloid-beta and tau accumulation in Alzheimer’s disease. Alzheimers Res Ther. 2020;12:140. https://doi.org/10.1186/s13195-020-00710-6.
Koutsodendris N, Nelson MR, Rao A, Huang Y, Apolipoprotein E. Alzheimer’s Disease: findings, hypotheses, and potential mechanisms. Annu Rev Pathol. 2022;17:73–99. https://doi.org/10.1146/annurev-pathmechdis-030421-112756.
Carrasquillo MM, Khan Q, Murray ME, et al. Late-onset Alzheimer disease genetic variants in posterior cortical atrophy and posterior AD. Neurology. 2014;82:1455–62. https://doi.org/10.1212/WNL.0000000000000335.
Schott JM, Crutch SJ, Carrasquillo MM, et al. Genetic risk factors for the posterior cortical atrophy variant of Alzheimer’s disease. Alzheimers Dement. 2016;12:862–71. https://doi.org/10.1016/j.jalz.2016.01.010.
Singh NA, Martin PR, Graff-Radford J, et al. APOE epsilon4 influences within and between network functional connectivity in posterior cortical atrophy and logopenic progressive aphasia. Alzheimers Dement. 2023;19:3858–66. https://doi.org/10.1002/alz.13059.
Gao Y, Kwapong WR, Zhang Y, et al. Retinal microvascular changes in white matter hyperintensities investigated by swept source optical coherence tomography angiography. BMC Ophthalmol. 2022;22:77. https://doi.org/10.1186/s12886-021-02143-7.
Gao Y, Liu Y, Zhang Y, et al. Assessment of Outer Retina and Choroid using swept source optical coherence tomography and angiography in patients with multiple sclerosis. J Neuroophthalmol. 2023. https://doi.org/10.1097/WNO.0000000000001970.
Snyder PJ, Alber J, Alt C, et al. Retinal imaging in Alzheimer’s and neurodegenerative diseases. Alzheimers Dement. 2021;17:103–11. https://doi.org/10.1002/alz.12179.
Zhang Y, Yang L, Gao Y, et al. Choroid and choriocapillaris changes in early-stage Parkinson’s disease: a swept-source optical coherence tomography angiography-based cross-sectional study. Alzheimers Res Ther. 2022;14:116. https://doi.org/10.1186/s13195-022-01054-z.
Gupta VB, Chitranshi N, den Haan J, et al. Retinal changes in Alzheimer’s disease- integrated prospects of imaging, functional and molecular advances. Prog Retin Eye Res. 2021;82:100899. https://doi.org/10.1016/j.preteyeres.2020.100899.
Koronyo Y, Rentsendorj A, Mirzaei N, et al. Retinal pathological features and proteome signatures of Alzheimer’s disease. Acta Neuropathol. 2023;145:409–38. https://doi.org/10.1007/s00401-023-02548-2.
Origlia N, Capsoni S, Cattaneo A, et al. Abeta-dependent inhibition of LTP in different intracortical circuits of the visual cortex: the role of RAGE. J Alzheimers Dis. 2009;17:59–68. https://doi.org/10.3233/JAD-2009-1045.
Sun Y, Zhang L, Ye H, et al. Potential ocular indicators to distinguish posterior cortical atrophy and typical Alzheimer’s disease: a cross-section study using optical coherence tomography angiography. Alzheimers Res Ther. 2024;16:64. https://doi.org/10.1186/s13195-024-01431-w.
den Haan J, Csinscik L, Parker T, et al. Retinal thickness as potential biomarker in posterior cortical atrophy and typical Alzheimer’s disease. Alzheimers Res Ther. 2019;11:62. https://doi.org/10.1186/s13195-019-0516-x.
Wen LY, Wan L, Lai JN, et al. Increased risk of Alzheimer’s disease among patients with age-related macular degeneration: a nationwide population-based study. PLoS ONE. 2021;16:e0250440. https://doi.org/10.1371/journal.pone.0250440.
Sutton SS, Magagnoli J, Cummings TH, et al. Alzheimer Disease Treatment with acetylcholinesterase inhibitors and Incident Age-Related Macular Degeneration. JAMA Ophthalmol. 2024;142:108–14. https://doi.org/10.1001/jamaophthalmol.2023.6014.
Bergen AA, Arya S, Koster C, et al. On the origin of proteins in human drusen: the meet, greet and stick hypothesis. Prog Retin Eye Res. 2019;70:55–84. https://doi.org/10.1016/j.preteyeres.2018.12.003.
Hageman GS, Luthert PJ, Victor Chong NH, et al. An integrated hypothesis that considers drusen as biomarkers of immune-mediated processes at the RPE-Bruch’s membrane interface in aging and age-related macular degeneration. Prog Retin Eye Res. 2001;20:705–32. https://doi.org/10.1016/s1350-9462(01)00010-6.
Csincsik L, MacGillivray TJ, Flynn E, et al. Peripheral retinal imaging biomarkers for Alzheimer’s Disease: a pilot study. Ophthalmic Res. 2018;59:182–92. https://doi.org/10.1159/000487053.
Csincsik L, Quinn N, Yong KXX, et al. Retinal phenotyping of variants of Alzheimer’s disease using ultra-widefield retinal images. Alzheimers Dement (Amst). 2021;13:e12232. https://doi.org/10.1002/dad2.12232.
Lundeen TF, Seibyl JP, Covington MF, et al. Signs and artifacts in amyloid PET. Radiographics. 2018;38:2123–33. https://doi.org/10.1148/rg.2018180160.
Fang D, Liang J, Diao Y, et al. Morphologic and functional Assessment of photoreceptors in Laser-Induced Retinopathy using adaptive Optics scanning laser Ophthalmoscopy and Microperimetry. Am J Ophthalmol. 2024;265:61–72. https://doi.org/10.1016/j.ajo.2024.03.021.
Koronyo Y, Biggs D, Barron E, et al. Retinal amyloid pathology and proof-of-concept imaging trial in Alzheimer’s disease. JCI Insight. 2017;2. https://doi.org/10.1172/jci.insight.93621.
Hart de Ruyter FJ, Morrema THJ, den Haan J, et al. Phosphorylated tau in the retina correlates with tau pathology in the brain in Alzheimer’s disease and primary tauopathies. Acta Neuropathol. 2023;145:197–218. https://doi.org/10.1007/s00401-022-02525-1.
den Haan J, Morrema THJ, Verbraak FD, et al. Amyloid-beta and phosphorylated tau in post-mortem Alzheimer’s disease retinas. Acta Neuropathol Commun. 2018;6:147. https://doi.org/10.1186/s40478-018-0650-x.
Bazan NG, Calandria JM, Serhan CN. Rescue and repair during photoreceptor cell renewal mediated by docosahexaenoic acid-derived neuroprotectin D1. J Lipid Res. 2010;51:2018–31. https://doi.org/10.1194/jlr.R001131.
Simunovic MP. Acquired color vision deficiency. Surv Ophthalmol. 2016;61:132–55. https://doi.org/10.1016/j.survophthal.2015.11.004.
Sheriff S, Shen T, Saks D, et al. The Association of APOE epsilon4 allele with retinal layer thickness and microvasculature in older adults: Optic nerve decline and cognitive change study. J Clin Med. 2023;12. https://doi.org/10.3390/jcm12196219.
Shin JY, Choi EY, Kim M, et al. Changes in retinal microvasculature and retinal layer thickness in association with apolipoprotein E genotype in Alzheimer’s disease. Sci Rep. 2021;11:1847. https://doi.org/10.1038/s41598-020-80892-z.
Lehmann M, Crutch SJ, Ridgway GR, et al. Cortical thickness and voxel-based morphometry in posterior cortical atrophy and typical Alzheimer’s disease. Neurobiol Aging. 2011;32:1466–76. https://doi.org/10.1016/j.neurobiolaging.2009.08.017.
Lawlor M, Danesh-Meyer H, Levin LA, et al. Glaucoma and the brain: trans-synaptic degeneration, structural change, and implications for neuroprotection. Surv Ophthalmol. 2018;63:296–306. https://doi.org/10.1016/j.survophthal.2017.09.010.
Ye J, Shen M, Huang S, et al. Visual acuity in pathological myopia is correlated with the photoreceptor myoid and Ellipsoid Zone Thickness and affected by Choroid Thickness. Invest Ophthalmol Vis Sci. 2019;60:1714–23. https://doi.org/10.1167/iovs.18-26086.
Pappuru RR, Ouyang Y, Nittala MG, et al. Relationship between outer retinal thickness substructures and visual acuity in eyes with dry age-related macular degeneration. Invest Ophthalmol Vis Sci. 2011;52:6743–8. https://doi.org/10.1167/iovs.10-6723.
Owsley C, McGwin G Jr., Swain TA, et al. Outer retinal thickness is Associated with cognitive function in normal aging to Intermediate Age-Related Macular Degeneration. Invest Ophthalmol Vis Sci. 2024;65:16. https://doi.org/10.1167/iovs.65.5.16.
Choi SS, Zawadzki RJ, Lim MC, et al. Evidence of outer retinal changes in glaucoma patients as revealed by ultrahigh-resolution in vivo retinal imaging. Br J Ophthalmol. 2011;95:131–41. https://doi.org/10.1136/bjo.2010.183756.
Chrysou A, Heikka T, van der Zee S, et al. Reduced thickness of the retina in de novo Parkinson’s Disease shows a distinct pattern, different from Glaucoma. J Parkinsons Dis. 2024;14:507–19. https://doi.org/10.3233/JPD-223481.
Zheng G, Li J, Zhou Y et al. Outer retina and choroid as potential imaging markers for evaluation of neural impairment in early type 2 diabetic patients. Eur J Ophthalmol 202411206721241258637. https://doi.org/10.1177/11206721241258637
Regy M, Dugravot A, Sabia S, et al. The role of dementia in the association between APOE4 and all-cause mortality: pooled analyses of two population-based cohort studies. Lancet Healthy Longev. 2024;5:e422–30. https://doi.org/10.1016/S2666-7568(24)00066-7.
Funding
This work was supported by 1.3.5 Project of West China Hospital of Sichuan University (2023HXFH043), the Foundation of Science and Technology Department of Sichuan Province (2023NSFSC1668), The National Natural Science Foundation of China (No. 82071203, No. U23A20422) and STI2030-Major Projects (No. 2022ZD0213600).
Author information
Authors and Affiliations
Contributions
Conception and design of the study: MZ, QC, HBY, YZG and RHW; Drafting of the original manuscript: YZG; Revising the manuscript: YZG and RHW; Acquisition and analysis of data: YZG, RHW, KFM, YFZ, HYX, YLL, FY, XYW and LB; Curation and visualization of data: YXG and HBY; Resources: JZ; Funding acquisition: MZ, YZG and YXG. All authors read and approved the final manuscript.
Corresponding authors
Ethics declarations
Ethical approval and consent to participate
This study was audited and approved by Ethics Committee on Biomedical Research, West China Hospital of Sichuan University. All participants signed an informed consent form before the examination according to the Declaration of Helsinki.
Consent for publication
Not applicable.
Competing interests
The authors declare no competing interests.
Additional information
Publisher’s Note
Springer Nature remains neutral with regard to jurisdictional claims in published maps and institutional affiliations.
Electronic supplementary material
Below is the link to the electronic supplementary material.
Rights and permissions
Open Access This article is licensed under a Creative Commons Attribution-NonCommercial-NoDerivatives 4.0 International License, which permits any non-commercial use, sharing, distribution and reproduction in any medium or format, as long as you give appropriate credit to the original author(s) and the source, provide a link to the Creative Commons licence, and indicate if you modified the licensed material. You do not have permission under this licence to share adapted material derived from this article or parts of it. The images or other third party material in this article are included in the article’s Creative Commons licence, unless indicated otherwise in a credit line to the material. If material is not included in the article’s Creative Commons licence and your intended use is not permitted by statutory regulation or exceeds the permitted use, you will need to obtain permission directly from the copyright holder. To view a copy of this licence, visit http://creativecommons.org/licenses/by-nc-nd/4.0/.
About this article
Cite this article
Gao, Y., Wang, R., Mou, K. et al. Association of outer retinal and choroidal alterations with neuroimaging and clinical features in posterior cortical atrophy. Alz Res Therapy 16, 187 (2024). https://doi.org/10.1186/s13195-024-01551-3
Received:
Accepted:
Published:
DOI: https://doi.org/10.1186/s13195-024-01551-3