Abstract
Background
Spontaneous hemopneumothorax is a rare condition that can be life-threatening if not promptly diagnosed and treated. We report a case of early treatment with transcatheter arterial embolization and video-assisted thoracoscopic surgery.
Case presentation
A 19-year-old Japanese male was diagnosed with left pneumothorax and underwent chest tube drainage. A total of 10 hours after admission, the patient developed dyspnea, chest pain, and sudden massive bloody effusion. Contrast-enhanced computed tomography revealed contrast extravasation near the left lung apex, and spontaneous hemopneumothorax was diagnosed. Angiography revealed bleeding from a branch of the subscapular artery and transcatheter arterial embolization was performed. The patient underwent video-assisted thoracoscopic surgery and recovered uneventfully.
Conclusions
Anesthesiologists involved in urgent surgeries must be aware that a patient with spontaneous pneumothorax can develop a hemopneumothorax, even when full lung expansion has been obtained following chest tube drainage, owing to latent aberrant artery disruption. Interprofessional team engagement is essential for spontaneous hemopneumothorax management.
Similar content being viewed by others
Background
Although spontaneous pneumothorax is a common condition, spontaneous hemopneumothorax (SHP) is rare, occurring in only 5% of patients with spontaneous pneumothorax [1,2,3,4,5,6,7]. SHP is a life-threatening disease, if not diagnosed and treated promptly, causing hemorrhagic shock in 13.3–50% of patients [2, 7,8,9]. Anesthesiologists must also recognize this rare condition, as a delay in initiating appropriate management can be fatal. Here, we report a case of SHP that was successfully treated with early treatment using transcatheter arterial embolization (TAE) and video-assisted thoracoscopic surgery (VATS).
Case presentation
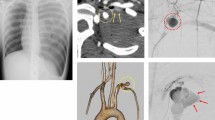
A 19-year-old Japanese male presented at our hospital with a 2-day history of fever. Plain chest radiographs taken at the first hospital revealed a left pneumothorax (Fig. 1a). A small-pore chest tube (12-Fr) was inserted via the fourth intercostal space (Fig. 1b), which was then connected under suction (−10 hPa), and air leaking ceased soon thereafter. No bloody discharge was observed. A repeat chest computed tomography (CT) scan confirmed full lung expansion, with no collection of pleural effusion observed (Fig. 1c). Ten hours after admission (6 hours post-tube thoracotomy), dyspnea and chest pain developed without any apparent trigger. Over the next 3 hours, approximately 600 mL of bloody pleural effusion was drained. Plain chest radiographs and CT scans showed decreased permeability with a massive left pleural effusion in the left lung fields (Fig. 1d, e). A diagnosis of hemothorax was made. This young man was an active smoker with a history of a parotid tumor but reported no recent history of trauma. Despite massive bloody pleural drainage, the patient showed no respiratory symptoms and was hemodynamically stable. Based on the clinical course and the hemothorax onset of the patient, its clinical presentation did not appear typical of iatrogenic vessel injury related to chest tube insertion. The patient was referred to the emergency unit at our hospital for treatment of active hemothorax of unknown etiology. Its general condition remained stable. Laboratory findings showed a hemoglobin level of 14.4 g/dL, white blood cell count of 11,800/μL, and CRP of 3.7 mg/dL. An additional thoracostomy tube (24-Fr) was placed via the sixth intercostal space. After approximately 500 mL of fresh bloody effusion had drained, the chest tube was clamped. Contrast-enhanced computed tomography (CT) revealed contrast extravasation near the left pulmonary apex (Figure. 2a–c). Our patient underwent an angiographic evaluation approximately 1 hour after referral, which established that the bleeding was derived from a branch of the subscapular artery (Fig. 2d). Transcatheter arterial embolization (TAE) at a torn branch of the subscapular artery was performed under local anesthesia by a cardiologist to effectively control the bleeding (Fig. 2e, f). While no bloody pleural effusion was observed post-TAE, a large hematoma remained in the left thoracic cavity, and the left upper lobe collapsed. The total blood loss from the time of hemothorax onset was estimated to be approximately 1500 mL. Following consultation with a thoracic surgeon concerning this unfamiliar onset and the indication for surgical intervention, we scheduled an elective surgery for the retained hemothorax. The patient was admitted to the operating room 6 hours after arriving at the hospital. During the interval between its arrival and the time of surgery, the patient received 2500 mL of extracellular fluid. Following the induction of general anesthesia, a 37-Fr double lumen endotracheal tube for left lung isolation was intubated. The position of the endotracheal tube tip was confirmed using a bronchial fiber. After the patient was placed in the right lateral position, the tube was checked again, following which the surgical procedure was initiated under right one-lung ventilation. Subsequently, after the placement of two ports, the patient underwent VATS. The retained clot (weight 175 g) in the left pleural cavity was removed. No vessel injuries to the intercostal artery/vein, heart, aorta, or pulmonary arteries were observed. Neither the chest wall nor the diaphragm was injured. An apical bulla was observed in the left upper lobe with a torn aberrant vessel at the distal end near the lung apex, along with a subpleural hematoma (Fig. 3a). Hemostasis was confirmed at the proximal end of the torn vessel (i.e., an embolized branch of the subscapular artery) on the cupula pleurae (Fig. 3b). The apical bulla was excised, hemostasis was confirmed, and surgery was completed. The postoperative hemoglobin level of the patient was 8.4 g/dL. After transfusion with four units of red blood cells, the hemoglobin level of the patient improved to 9.1 g/dL. The patient recovered uneventfully without any major complications and was discharged on postoperative day four.
Radiographic images taken on presentation at the first hospital. a Chest computed tomography on arrival. b, c Plain chest radiographs and chest computed tomography scans taken post-chest tube insertion. While no obvious evidence of hemothorax is observed, a left pneumothorax is confirmed. d, e Plain chest radiography and computed tomography scans taken 10 hour postadmission showed decreased permeability with massive left pleural effusion in the left lung field.
Radiographic images taken at our hospital. a, b, c Chest contrast-enhanced computed tomography and three-dimensional computed tomography. Extravasation of the contrast agent is observed in the thoracic cavity at the left lung apex (white arrow). d Digital subtraction angiography showing contrast agent flowing into the thoracic cavity at the left lung apex from an abnormal vessel that developed from the left subscapular artery and subclavian artery (white arrowhead). e, f Digital subtraction angiography and chest radiography after hemostasis show a coil to a branch of the subscapular artery (blue arrowhead).
Discussion and conclusion
SHP predominantly occurs in young males [10, 11] and often follows primary spontaneous pneumothorax [8, 9]. SHP is more often associated with chest pain than spontaneous pneumothorax but is not associated with smoking [5]. The definition of SHP is unclear, with some authors defining it as the presence of > 400 mL of blood in the pleural cavity in cases of primary spontaneous pneumothorax [12]. Hemorrhagic shock due to SHP occurs in 12.9–33.3% of hospitalizations and requires blood transfusion in 21–50% to improve circulation [2, 13, 14]. In the present case, our young male patient developed acute anemia requiring a blood transfusion. The patient presented with chest pain at the onset and had a history of smoking. The main cause of bleeding in SHP is the disruption of aberrant vessels between the parietal pleura and adhesion cysts due to lung collapse caused by a pneumothorax [15, 16]. Abnormal vessels causing bleeding often branch from the upper intercostal artery but sometimes arise from the subclavian artery [4]. Other potential causes include rupture of the parietal pleura and the lung parenchyma [16, 17]. The cause of bleeding, in this case, was the rupture of an abnormal vessel from the subscapular artery. Although the patient had no previous history of pneumothorax, cysts were detected in its lungs, based on which, we speculated that the patient may have had an asymptomatic pneumothorax. We considered that the aberrant vessels were generated by angiogenesis due to wound healing. A chest CT image taken after insertion of chest tube drainage showed that that tip was directed toward the anterior mediastinum and was not in contact with the pulmonary apex where the subpleural hematoma was located. Additionally, intraoperative VATS revealed the absence of bleeding in the intercostal region surrounding the site of chest drain insertion. Consequently, we could rule out the possibility of an iatrogenic hemorrhage.
Two factors prevent hemostasis in SHP. First, the pleural cavity must be negatively pressurized for treatment. Hemostasis is achieved by an increase in pleural cavity pressure due to a pneumothorax. However, thoracic drainage decreases pleural cavity pressure, potentially causing rebleeding. Hemorrhagic shock developing after thoracic drainage has been reported in many cases [18]. Second, the lack of muscle tissue around abnormal vessels in SHP may result in continued bleeding after rupture because vascular constriction cannot be achieved [19]. In this case, prior to thoracic drainage, the thoracic cavity was under positive pressure and the bleeding stopped. However, after thoracic drainage, the thoracic cavity was under negative pressure, and we accordingly speculated that the patient’s chest pain was associated with a rupture of an aberrant vessel, which caused a hemorrhage. Treatment options for SHP include conservative management, open surgery, and VATS. Conservative management may lead to complications such as restrictive disorders and empyema due to residual hematoma [16, 20, 21]. Some patients require open surgery because of difficulty in identifying the bleeding point with persistent arterial bleeding [21]. Compared with open chest surgery, early VATS results in fewer blood transfusions, less analgesic use, and shorter drainage and hospital stay [2, 16, 22, 23]. Similar to this case, detection of bleeding from the SHP using contrast-enhanced CT, followed by successful treatment using TAE for hemostasis and subsequent VATS, has been reported [24]. TAE for hemostasis is an effective treatment for patients with stable circulation without evidence of hemorrhagic shock or high risk for general anesthesia or one-lung ventilation. Even in fatal SHP with hemorrhagic shock, hemostasis with TAE can still be considered a treatment option, given the surgical risk [25]. In this case, aggressive TAE was successful in identifying the bleeding site followed by surgery. We believe that control of bleeding with TAE improves surgical safety and allows more time to prepare for surgery, further reducing the risk to the patient and the stress on the healthcare provider.
If an SHP is diagnosed, aggressive hemostasis is required. Aggressive treatment with TAE after identification of the bleeding site is considered a promising new treatment for SHP. Anesthesiologists involved in urgent surgeries must be aware that thoracic drainage for the treatment of spontaneous pneumothorax may contribute to hemorrhage, resulting in a life-threatening SHP. From the start of treatment, all healthcare professionals involved in the patient's care should understand the causes of pneumothorax, the patient's background, drain management, and the presence of adherent vessels that, should they fail, could result in a fatal outcome. We consider that better management could then be provided to such patients.
From the onset of treatment, all healthcare professionals involved in the patient’s care should understand the causes of pneumothorax, the patient’s medical history, drain management, and the presence of adherent vessels that, if disrupted, could result in a fatal outcome. We believe that improved management strategies can be implemented for such patients.
Availability of data and materials
Please contact the author for data requests.
Abbreviations
- SHP:
-
Spontaneous hemopneumothorax
- TAE:
-
Transcatheter arterial embolization
- VATS:
-
Video-assisted thoracoscopic surgery
- CT:
-
Computed tomography
References
Hart SR, Willis C, Thorn A, Barfoot L. Spontaneous haemopneumothorax: are guidelines overdue? Emerg Med J. 2002;19:273–4.
Hsu NY, Shih CS, Hsu CP, Chen PR. Spontaneous hemopneumothorax revisited: clinical approach and systemic review of the literature. Ann Thorac Surg. 2005;80:1859–63.
Homma T, Sugiyama S, Kotoh K, Doki Y, Tsuda M, Misaki T. Early surgery for treatment of spontaneous hemopneumothorax. Scand J Surg. 2009;98:160–3.
Haciibrahimoglu G, Cansever L, Kocaturk CI, Aydogmus U, Bedirhan MA. Spontaneous hemopneumothorax: is conservative treatment enough? Thorac Cardiovasc Surg. 2005;53:240–2.
Onuki T, Goto Y, Kuramochi M, Inagaki M, Sato Y. Spontaneous hemopneumothorax: epidemiological details and clinical features. Surg Today. 2014;44:2022–7.
Cheng YL, Huang TW, Lin CK, Lee SC, Tzao C, Chen JC, et al. The impact of smoking in primary spontaneous pneumothorax. J Thorac Cardiovasc Surg. 2009;138:192–5.
Kakaris S, Athanassiadi K, Vassilikos K, Skottis I. Spontaneous hemopneumothorax: a rare but life-threatening entity. Eur J Cardiothorac Surg. 2004;25:856–8.
Mendogni P, Vannucci J, Ghisalberti M, Anile M, Aramini B, Congedo MT, et al. Epidemiology and management of primary spontaneous pneumothorax: a systematic review. Interact Cardiovasc Thorac Surg. 2020;30:337–45.
Hsu CC, Wu YL, Lin HJ, Lin MP, Guo HR. Indicators of haemothorax in patients with spontaneous pneumothorax. Emerg Med J. 2005;22:415–7.
Ho C, Ismail AR. Spontaneous haemothorax: a case report. Méd J Malays. 2014;69:234–5.
Chen Y, Guo Z. Unusual case of primary spontaneous hemopneumothorax in a young man with atypical tension pneumothorax: a case report. J Med Case Rep. 2018;12:188.
Ohmori K, Ohata M, Narata M, Iida M, Nakaoka Y, Irako M, et al. [28 cases of spontaneous hemopneumothorax]. Zasshi J Nihon Kyobu Geka Gakkai Zasshi. 1988;36:1059–64.
Igai H, Sawabata N, Obuchi T, Matsutani N, Kadokura M. A retrospective multi-institutional survey of characteristics of surgically treated spontaneous hemopneumothorax patients. Gen Thorac Cardiovasc Surg. 2023;71:240–50.
Chong K, Qureshi SA, Badea G, Lok S. Spontaneous haemopneumothorax. BMJ Case Rep. 2011. https://doi.org/10.1136/bcr.04.2011.4065.
Chang YT, Dai ZK, Kao EL, Chuang HY, Cheng YJ, Chou SH, et al. Early video-assisted thoracic surgery for primary spontaneous hemopneumothorax. World J Surg. 2007;31:19–25.
Tam A, Ong B, Koh T, Lim C. Surgical management of primary spontaneous haemopneumothorax. Heart Lung Circ. 2018;27:S589–90.
Muraguchi T, Tsukioka K, Hirata S, Fukuda S, Mizugami K, Kishi A, et al. Spontaneous hemopneumothorax with aberrant vessels found to be the source of bleeding: report of two cases. Surg Today. 1993;23:1119–23.
Shinohara T, Mitumori N, Nojiri T, Asakura J, Doi N, Miyoshi I, et al. Two cases of shocked spontaneous hemopneumothorax after chest tube drainage. Nihon Rinsho Geka Gakkai Zasshi (Journal of Japan Surgical Association). 1999;60:2086–90.
Wu YC, Lu MS, Yeh CH, Liu YH, Hsieh MJ, Lu HI, et al. Justifying video-assisted thoracic surgery for spontaneous hemopneumothorax. Chest. 2002;122:1844–7.
Hwong TMT, Ng CSH, Lee TW, Wan S, Sihoe ADL, Wan IYP, et al. Video-assisted thoracic surgery for primary spontaneous hemopneumothorax. Eur J Cardiothorac Surg. 2004;26:893–6.
Luh SP, Tsao TC. Video-assisted thoracic surgery for spontaneous haemopneumothorax. Respirology. 2007;12:443–7.
Nose N, Mori H, Yonei A, Maeda R, Ayabe T, Tomita M, et al. A case of spontaneous hemopneumothorax in which the condition worsened after chest drainage. J Surg Case Rep. 2018. https://doi.org/10.1093/jscr/rjy217.
Patrini D, Panagiotopoulos N, Pararajasingham J, Gvinianidze L, Iqbal Y, Lawrence DR. Etiology and management of spontaneous haemothorax. J Thorac Dis. 2015;7:520–6.
Gorter EA, Dubois EFL, Guicherit OR, Urlings TAJ, de Mol van Otterloo van JC. Embolisation in spontaneous haemopneumothorax. Ned Tijdschr Geneeskd. 2012;156:A4425.
Ono Y, Kariya S, Nakatani M, et al. Transarterial embolization for life-threatening spontaneous hemopneumothorax. Intervent Radiol. 2018;3:84–7. https://doi.org/10.22575/interventionalradiology.2017-0020.
Acknowledgements
We gratefully acknowledge all medical personnel involved in the treatment of this patient. ChatGPT was used for the English translation, for which we performed a detailed validation.
Funding
This research received no specific grants from funding agencies in the public, commercial, or not-for-profit sectors.
Author information
Authors and Affiliations
Contributions
MY was the primary author of the manuscript. MY, TB, RD, SI, and MS treated the patient, participated in the collection of data, and assisted in the preparation of the manuscript by conducting a literature search and providing research advice. All authors read and approved the final manuscript.
Corresponding author
Ethics declarations
Ethics approval and consent to participate
Informed consent for participation was obtained from the patient.
Consent for publication
Written informed consent was obtained from the patient for the publication of this case report and any accompanying images. A copy of the written consent is available for review by the Editor-in-Chief of this journal.
Competing interests
The authors declare that they have no competing interests.
Additional information
Publisher’s Note
Springer Nature remains neutral with regard to jurisdictional claims in published maps and institutional affiliations.
Rights and permissions
Open Access This article is licensed under a Creative Commons Attribution-NonCommercial-NoDerivatives 4.0 International License, which permits any non-commercial use, sharing, distribution and reproduction in any medium or format, as long as you give appropriate credit to the original author(s) and the source, provide a link to the Creative Commons licence, and indicate if you modified the licensed material. You do not have permission under this licence to share adapted material derived from this article or parts of it. The images or other third party material in this article are included in the article’s Creative Commons licence, unless indicated otherwise in a credit line to the material. If material is not included in the article’s Creative Commons licence and your intended use is not permitted by statutory regulation or exceeds the permitted use, you will need to obtain permission directly from the copyright holder. To view a copy of this licence, visit http://creativecommons.org/licenses/by-nc-nd/4.0/.
About this article
Cite this article
Nishikawa, M., Shimizu, M., Banno, T. et al. Spontaneous hemopneumothorax causing life-threatening hemorrhage: a case report. J Med Case Reports 18, 375 (2024). https://doi.org/10.1186/s13256-024-04715-9
Received:
Accepted:
Published:
DOI: https://doi.org/10.1186/s13256-024-04715-9