Abstract
In recent years, biologists and clinicians have witnessed prominent advances in in vitro 3D culture techniques related to biomimetic human/animal tissue analogs. Numerous data have confirmed that unicellular and multicellular (tumoroids) tumor spheroids with dense native cells in certain matrices are sensitive and valid analytical tools for drug screening, cancer cell dynamic growth, behavior, etc. in laboratory settings. Angiogenesis/vascularization is a very critical biological phenomenon to support oxygen and nutrients to tumor cells within the deep layer of solid masses. It has been shown that endothelial cell (EC)-incorporated or -free spheroid/tumoroid systems provide a relatively reliable biological platform for monitoring the formation of nascent blood vessels in micron/micrometer scales. Besides, the paracrine angiogenic activity of cells within the spheroid/tumoroid systems can be monitored after being treated with different therapeutic approaches. Here, we aimed to collect recent advances and findings related to the monitoring of cancer angiogenesis using unicellular and multicellular tumor spheroids. Vascularized spheroids/tumoroids can help us in the elucidation of mechanisms related to cancer formation, development, and metastasis by monitoring the main influencing factors.
Similar content being viewed by others
Introduction
Inside the body, cells reside in a specific three-dimensional (3D) microenvironment which is known as a niche. The close interaction between cells and the environment within the defined 3D space can regulate each cell's morphology and function [1]. From the past to the present time, cell culture has been widely used to understand the participation of various molecular pathways, cell behaviors, and the mechanism of diseases and pathological conditions [2]. In this regard, the conventional two-dimensional (2D) culture system is a basic method for the cultivation, expansion, and conduction of several experiments in most laboratories [3]. As a common belief, the 2D culture system is a relatively low-cost and friendly-use in vitro model for large-scale production and harvesting of human and animal cells [2]. In this system, adherent cells are directly attached to a plastic surface predominantly composed of polystyrene-based materials [4]. While the non-adherent cells are expanded without the necessity to attach to the bottom of culturing flasks [5]. Despite these advantages, several issues and limitations restrict the generalization of in vitro data to in vivo conditions. It is postulated that 2D culture systems could not completely reflect the in vivo conditions because of the lack of accurate tissue model structures, cellular heterogeneity, and real-time pathological changes [6]. Generally, the monolayer cell culture systems produce flattened cells with unlimited access to micro-, macro-elements, metabolites, signaling molecules, and growth factors, leading to uncontrolled cell proliferation and expansion, and loss of specified morphologies and functions [7, 8]. Of course, the subcellular localization of organelles, paracrine activity, and stimulation of certain signaling pathways are also changed in cells cultured in 2D systems [9, 10]. Under such conditions, the proteomic, metabolic, and genomic profiles of cells are also changed compared to cells of the same lineages inside the body [11]. Due to the easy distribution of soluble factors in 2D culture systems, even trivial concentrations of therapeutics can evenly affect the cultured cells which is not comparable to the in vivo conditions while in the in vivo microenvironment, there is a competition between the cells to access nutrients and other signaling molecules [12]. Besides, diffusible molecules are delivered to various tissues in a concentration gradient manner [13]. The absence of extracellular matrix (ECM), multiple signals, and mechanical cues inhibit or blunt the activity of certain molecular effectors inside the cells, resulting in low-rate predictability [14]. High-rate proliferation and expansion of cells in 2D culture systems can result in the existence of numerous dead cells with diverse waste products which can affect the physiology of co-expanded cells [15,16,17]. To overcome these limitations, 3D in vitro cell culture systems have been advanced for basic and pre-clinical cancer research [18]. In this regard, Petersen and Bissell presented the 3D organotypic structures to mimic healthy and cancerous breast tissue niches [19]. It is believed that in vitro organoid systems can in part, but not completely, provide the key elements of in vivo conditions via the regulation of cell morphologies, juxtacrine and paracrine activities, and cell-to-ECM interaction [20]. Using various supporting ECM components at different ratios, organoid (tumoroid) microstructure can be developed similar to the native tissues [18]. Despite the existence of different limitations, 3D culture settings provide valuable information about cell behavior for clinical use compared to 2D culture settings [21, 22].
As above-mentioned, the development of optimized biomimetic culture systems with high similarity to in vivo conditions can yield more valid and comparable data [23]. To date, several 3D culture systems have been developed to recapitulate the physiological and pathological conditions (Table 1). Among several 3D culture systems, organoids/spheroids are engineered in vitro micro/macrostructures composed of stem cells, cancer cells, and/or mature cells and have been used for theranostics, large‐scale drug screening, hereditary diseases, and personalized medicine purposes (Table 2) [24, 25]. In this review article, the recent advances in 3D culture systems with a focus on organoids/spheroids will be discussed in terms of stem cell biology and tumor cells.
Different organoid/spheroid systems in biomedical fields
Terms and general features
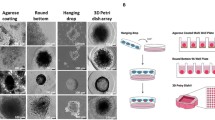
The advent and development of 3D culture systems have helped biologists in the conduction of numerous animal-free studies for basic biology and clinical research [26]. Irrespective of ethical issues, data from animal studies are relatively inefficient in understanding the physiopathological conditions of human counterparts and could not be completely applied to clinical settings [27]. The cost of drugs and therapeutic applications is high on different animal models. The reproducibility and reliability analyses on animals are under question as different animal strains are treated using rigorous protocols and conditions [28]. Of note, most of the drugs examined in animals fail to yield relatively similar responses in human counterparts because of differences in animal models, strain, dose of therapeutics, and administration route [28]. To be specific, organoids and spheroids are 3D miniaturized tissue structures with either stem cells, mature cells, or even cancer cells to recapitulate the in vivo-like microenvironments and efficient pre-clinical platforms for drug screening and validation [1]. Carcinogenesis and promotion of anaplastic changes coincide with the activation of several sequential processes that provoke cancer cells and non-cancer cells into the tumor stroma [29]. To date, several techniques and approaches have been used for the fabrication of organoids/spheroids, and other 3D culture systems (Table 1 and Fig. 1). Data have confirmed that cells maintained and expanded within the organoid/spheroid/tumoroid system exhibited relatively similar patterns of in vivo dynamic growth and response to therapeutics compared to conventional 2D culture systems [30]. Progress and advancement in 3D culture systems like organoids have led to the compensation of gaps and shortcomings between the 2D culture system and animal models [16, 17]. In this scenario, the 3D culture system exhibits more valuable data about the physiology of cells because of reciprocal cell-to-cell and cell-to-ECM interaction [16, 17].
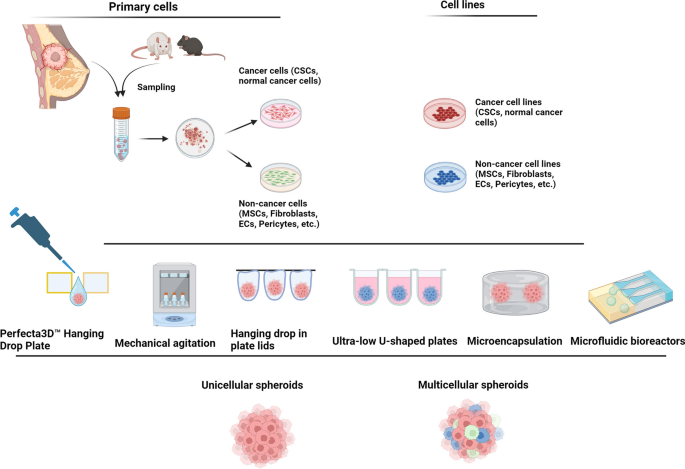
Different approaches are used for the development of unicellular (homotypic) and multicellular (heterotypic) tumor spheroids in in vitro conditions. Several studies have been performed to generate tumor spheroids by using different cells such as freshly isolated or cultured primary cells, and cancer and non-cancer cell lines. The cancer cell aggregate is composed of single cancer cells (spheroids) or different cell lineages (multicellular spheroids or tumoroids) using the supporting matrix to maintain the cells within certain sizes. Designed by using BioRender's web-based software
In cancer biology, several tumoroid models have been used for the evaluation of therapeutics as follows; 3D tumor cell culture, 3D tumor slice, patient-derived organoids, vascularized tumoroid, spheroid, and organoid culture models [31,32,33,34,35,36,37]. Most often there is an overlap between the terms spheroids and organoids concerning cellular sources and generation procedure [33]. Spheroids have less complexity and are common 3D structures for preliminary monitoring of anti-cancer drugs while organoids have higher similarities in terms of genetic patterns and histological features to the source tissues and tumors [1]. In the context of cancers, assembling disassociated tumor cells using suitable ECM components generates organoids which are known as tumoroids [31]. It has been proposed that 3D culture systems like organoids are valid tools to simulate tiny tumor masses using several cell types with relatively in vivo-like growth and treatment patterns [16, 17]. To the best of our knowledge, unicellular tumor spheroids have been extensively utilized for cancer cell biology, invasion, and drug screening. However, they do not accurately mimic the intricate biological and clinical characteristics of primary tumor tissues, thus restricting their effectiveness in predicting individualized responses to chemotherapy. In contrast, organoids (known also tumoroids) exhibited more valuable tools for drug screening, disease modeling, and personalized medicine. Tumor organoids can be developed from primary patient samples within a defined 3D framework. It is thought that tumoroids have relatively structural and functional cues like in vivo tumor masses, with a reliable platform for clinical decision-making [38]. Unlike tumoroids, single-cell spheroids lack cell polarity, heterogenicity, and complexity and function of cancer cells within the cancer masses. Thus, organoids reflect more accurately the cellular diversity and physiological roles found in organs [39]. Besides, in stem cell research, organoids composed of tissue-specific stem cells with other specific cell lineages have been used to mimic the architecture and function of the target tissues [40]. Stem cell organoids are effective in vitro modeling tools in the establishment of studies related to developmental processes (organogenesis), regenerative defects, and certain pathophysiological conditions. Retention inside the defined microenvironments helps the stem cells to preserve their stemness while it simultaneously helps us to use naïve or genetically manipulated stem cells for specific regenerative purposes [40].
For conventional organoid/tumoroid development, three main elements including broad-ranging cells (either homogenous or heterogeneous cell type), supporting scaffolds, and endogenous/exogenous signaling molecules can be modulated according to the purpose and objective of the study [1]. In several studies, both natural and synthetic substrates have been used for the generation of organoids/tumoroids to provide a platform for cell attachment, proliferation, and juxtacrine within a 3D structure [41] (Table 1). For generation of organoid/tumoroid structure using natural ECM components, the existence of specific motifs such as Arg-Gly-Asp (RGD) can be useful in providing an in vivo-like microenvironment. Despite the several advantages (toxicity↓, biocompatibility↑, bioactivity↑, and biodegradability↑) related to the application of natural substrates, the final organoids/tumoroids lack appropriate mechanical stability and integrity in response to the changes of certain parameters such as pH and incubation temperature [42]. In line with these comments, the addition of synthetic substrates such as polyurethane (PU)-, polyethylene glycol (PEG)-, poly (lactic-co-glycolic acid) (PLGA)-based composites can yield organoids/tumoroids with higher mechanical properties and resistance to environmental parameters. However, the lack of essential bioactive motifs like RGD, etc. restricts their bulk application in organoid/tumoroid fabrication [43].
Mechanisms related to tumoroid formation
Due to the complexity of the tumor microenvironment (TME) and the involvement of different cell types, it seems that an intricate molecular network is involved in the formation of tumor colonies [44]. Like in vivo conditions, in vitro protocols should be performed with a focus on the stimulation of such molecular interactions and specific cell behavior to promote cell aggregation within distinct dimensions and geometries. In a common belief, the loosely cell-to-cell connection is promoted in in vitro conditions mainly via the attachment of integrins and cadherins to ECM components, leading to the formation of preliminary cell aggregates [45, 46]. The initial spheroid units undergo compaction via the maintenance of homotypic cadherin binding [45]. By the promotion of homotypic (homophilic) cadherin interaction, cells are juxtaposed in contact areas while the surface tension is also diminished [47]. In this regard, E and N-cadherins are the most important cadherin types and they participate in homophilic cell-to-cell attachment [48]. However, the possibility of heterophilic interaction between N and E-cadherins is also possible via physical interactions between the cadherins ectodomains [48]. Upon induction of cadherin-to-cadherin interaction, intracellular actomyosin contractility is stimulated, resulting in cell aggregate morphogenesis [49]. Of course, the type and level of cadherins are associated with the cell lineage. For instance, E-cadherin is actively involved in juxtacrine interaction between the cancer cells rather than cancer stem cells (CSCs). Powan and co-workers indicated that the lack of cell-to-cell connection via cadherin can increase the possibility of apoptosis (p53↑, phosphorylated FAK↓), leading to loss of integrity in tumor cell aggregates [50]. Despite the existence of heterophilic cadherin interaction between the CSC N-cadherin and non-CSC E-cadherin, it seems that this interaction is not highly stable when compared to the homophilic E-cadherin-to-E-cadherin connection. Besides, the conversion of E-cadherin to N-cadherin during the anaplastic changes can reduce the possibility of colony formation and increase the metastatic behavior, leading to the disintegration of multicellular components within the tumoroid systems [51, 52]. Previous data showed the co-precipitation of cytoskeletal proteins like actin with N-cadherin, indicating the ability of N-cadherin to modulate specific cell morphologies between the juxtaposed cells [46]. As above-mentioned, the activity of ECM components along with cell surface adherens can promote the integrity of cell-to-cell junction and thus tumoroid stability. For instance, fibronectin with fibrillar strands can recruit tensin, and α5β1 integrins and strengthen the physical contacts between the cells. The loss of fibronectin can result in N-cadherin ligation recruited α5β1 integrins, tensin, and β-catenin [46]. These data show that cells tend to preserve their juxtacrine interactions using different mechanisms even in the absence of certain ECM components.
Of note, the application of several cells within the tumoroid system can stimulate several cell-to-cell junction pathways. It has been shown that CAFs can secret different fibroblast growth factor members within the ovarian tumor mass [53]. In the presence of FGF-2, the stability of VE-cadherin increases between the juxtaposed ECs via controlling SHP2 [54]. These features can promote blood support into tumor parenchyma and facilitate tumor cell growth and expansion. According to these data, one can hypothesize that the integrity of cells via juxtacrine interaction can be induced via the application of multiple cells within the tumoroid system rather than of unicellular units. Within the tumor parenchyma, ECM is remodeled with the balances between the production and degradation. For example, several cell types such as tumor associate macrophages (TAMs) and CAFs can release various MMPs (MMP2, 7, 9, and 12) along with cytokines such as VEGF and FGF-2 into tumor parenchyma, leading to bulk remodeling of ECM and angiogenesis [55]. Collagen is the most abundant ECM component with the potential to induce cancer fibrosis. The mutual tumor cell-collagen interaction is done via the engagement of several signaling pathways such as transforming growth factor-β (TGF-β)/Smad axis, etc. Collagen fibers in proximity to epithelial cells generate a stiff fibrotic layer via reciprocal interaction between the Smad4 and phosphorylated myosin light chain 2 [56]. Inside tumor parenchyma, several collagen types are produced with diverse biological activities. The orientation, alignment, and stroma stiffness are related to the size and fiber type of collagen in which the long collagen fibers are closely aligned to each other and stabilize cell localization and organization. There is a physical contact between cell surface integrins α (1, 2, 3, 4, 10, 11)/β1 subunits with ECM collagen fibers. Unlike long collagen fibers, short fibers can increase the permeability of tumor niches [57]. In terms of angiogenesis, it was suggested that collagen stiffness can influence microvascular integrity and development. Of note, there is an inverse relationship between the microvascular network distribution and collagen fiber density. On the other hand, the increase of inter-collagen fibril branching and spatial arrangement (especially type IV collagen) promotes vascular density and number [57]. In in vitro conditions, the application of collagen with basal membrane components takes approximately five days to generate relatively compact cell aggregates with certain viscoelastic behavior [58]. Therefore, the existence of supporting ECM can support simultaneously reciprocal cell-to-cell and cell-to-ECM interactions.
Tumoroids/spheroids and angiogenesis potential
Tumor angiogenesis
It has been indicated that angiogenesis or neo-vascularization is a critical phenomenon for the development, and expansion of tumor cells within the solid cancer parenchyma [59]. Angiogenesis is the formation of new blood vessels from the pre-existing vascular network while vascularization is the phenomenon of in situ differentiation and expansion of vascular units with the participation of endothelial progenitor cells (EPCs) [60]. Angiogenesis with different signaling pathways, and cell components, especially endothelial cells (ECs) supports blood and oxygen demand to cells exposed to hypoxic conditions [60]. Considering the urgent need for establishing rapid, reproducible, and valid in vitro cancer angiogenesis models for the evaluation of various pro-, and anti-angiogenesis compounds, it seems that the selection of appropriate assays such as tumoroid/spheroid-based assays can help us to understand angiogenesis mechanisms better than conventional cell-based assays [61]. Most commonly used in vitro angiogenesis assays are based on the culture and expansion of ECs on the plastic surface or certain substrates (Table 3) [62]. Notably, 2D culture settings and numerous 3D culture assays can lead to the loss of typical function and phenotype of ECs [63]. Based on previously published data, cultured ECs in 2D culture systems exhibited prominent changes in karyotype, cell-surface antigens, and dynamic growth activity [64]. To achieve suitable angiogenesis potential, ECs must be in a quiescent state to stimulate the juxtaposition of several vascular cells with simultaneous activation of certain pro-angiogenesis factors [65]. Of note, there is no 2D gold-standard and definite angiogenesis assay for the detection of cellular and molecular events [65]. Thus, more angiogenesis-related assays are mandatory to achieve reliable results. It should not be forgotten that most assays use ECs as the only cellular source for angiogenesis assessment while different cell lineages with a high degree of heterogeneity (either structural or functional diversity) participate in blood vessel formation [66]. Noteworthy, the shape and size of ECs, and the complexity of junctions are significantly altered in 2D culture platforms compared to the in vivo conditions [64]. Commensurate with these descriptions, the development of novel angiogenesis protocols is highly recommended. To date, several 3D assays based on tumoroid/spheroid generation have been conducted to evaluate the angiogenesis in different tumor cells (Table 4) [67]. As mentioned above, it is thought that unicellular spheroids and multicellular tumoroids are valid avascular units and can mimic relatively in vivo cytoarchitectural features compared to the 2D culture systems.
Impact of hypoxia on angiogenesis
Inside the body, solid tumor cells are exposed to hypoxia when O2 levels drop below 21% which can worsen the prognosis of cancer individuals [68]. Due to micron-size dimensions, tumoroids/spheroids can provide diffusional limitations and radial gradients in terms of O2, nutrients, micro- and macroelements, and waste byproducts [69]. Cells located at the deeper layers of organoid/tumoroid microstructures are more prone to hypoxic changes because of an imbalance between O2 consumption and the limited distribution of nutrients [70]. It has been indicated that hypoxia is a potent angiogenesis stimulator via the regulation of several pro-angiogenic pathways. Besides the critical role of angiogenesis factors in different aspects of EC biology, hypoxic conditions can also influence vascular cell arrangement, and functions [68]. In response to hypoxic conditions, the activation of hypoxia-inducible factors (HIFs) can per se switch on the expression of multiple angiogenesis factors, leading to angiogenesis transition via direct interaction with cognate receptors on tumor cells, ECs, and other vascular cells [71]. HIFs consist of HIF-1α, -1β, -2α, and -3α with the potential (especially HIF-1α) to simultaneously alter the transcription of other genes such as vascular endothelial growth factor (VEGF), and erythropoietin following the attachment to hypoxia response elements (HREs) [72]. HIF-1α is inhibited via factor inhibiting HIF (FIH), and prolyl-hydroxylases (PHDs) when O2 contents return to basal levels. In contrast, these factors (PHDs, and FIH) are inactive under hypoxic conditions which in turn promote the translocation of HIF-α into the nucleus and its interaction with HIF-β [73]. Hypoxic conditions result in excessive cytosolic hyperacidification due to the accumulation of waste byproducts, including lactate, pyruvate, and acetic acid in the central zone [74]. Of course, the intensity of the pH value is blunted from the central zone to the outer layers. The activation of HIF-1α triggers the enzymes of the anaerobic glycolysis pathway and heightens the intracellular levels of lactate (Fig. 2) [70, 75, 76]. In the presence of HIF-1α, the function of the Na+/H+ exchanger is altered to efflux accumulated proton ions and regulate intracellular pH homeostasis, leading to the reduction of ECM pH values [77]. Acidic conditions can increase the solubility of angiogenesis factors such as VEGF and activate relevant signaling pathway effectors such as MAPK/ERK in target cells. It should not be forgotten that excessive acidic conditions beyond the cell threshold can inhibit the angiogenic behaviors of ECs, leading to the promotion of necrotic cell death [78, 79]. In support of this statement, Mena et al. found that acid preconditioning (pH = 6.6) of endothelial colony-forming cells promotes vasculogenesis, and increases the adhesion, and cell resistance in response to hyperglycemic conditions and pro-inflammatory status [61]. Of course, the intensity of the pH value is blunted from the central zone to the outer layers.
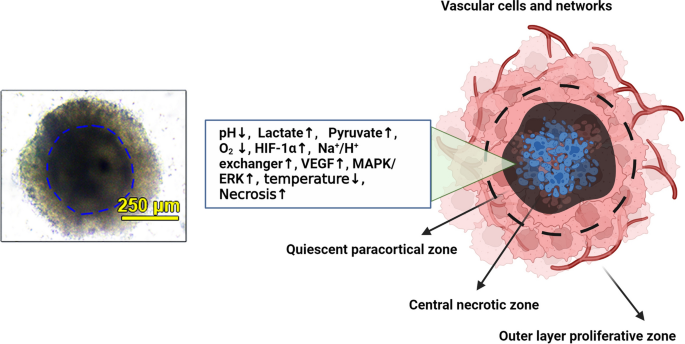
Copyright 2023. [79]. Cancer Cell International (Springer Nature Publishing Group). In the right panel, the schematic of tumor spheroid cell layers is indicated. Due to hyperacidification, lack of enough O2, and other parameters, most of the cells in the central zone undergo apoptotic and necrotic changes. In response to these features, juxtaposed to the central zone can release HIF-1α along with several angiogenesis factors to stimulate the formation of blood vessels. These vascular units are indicated by typical capillary sprouts in the tumor mass periphery and anastomosis within the stroma. The existence of hypoxic conditions is an essential element for the promotion of angiogenesis in tumor spheroids/tumoroids. The right panel was designed by using BioRender's web-based software
Typical tumor spheroid structure. In the left panel, the multicellular tumor spheroid is composed of human adenocarcinoma colorectal HT-29 cells, HUVECs, and HFFF2 fibroblasts. Spheroids have an inner dark compact core area and are enclosed by several cells in the periphery (outer layers). The connection of cells is loose in the external layers, indicating higher proliferation capacity.
In an experiment conducted by Taghavi Narmi et al., they developed colon cancer tumoroids using human HT-29 adenocarcinoma cells, HFFF2 fibroblasts, and umbilical cord vein ECs (HUVECs) using hanging drop embedded in 2.5% methylcellulose solution [79]. Based on histological analysis, central cells exhibit typical features of necrotic changes with prominent pyknotic changes [79]. These conditions promoted the production of angiogenesis factors, especially endocan in the outer layers [79]. Schmitz et al. indicated a significant reduction of O2 levels in mesenchymal stem cell (MSC) spheroids using CPOx-orange polystyrene microbeads, and OPAL Optical O2 measurement system by monitoring UnaG hypoxia reporter protein [80]. Data indicated O2 levels dropped below 1% (v/v) in spheroids containing about 3 × 104 MSCs while these levels were near 2.5% (v/v) in a 2D culture system [80, 81]. Of course, it should be kept in mind that local O2 levels are variable in cell microaggregates developed using different fabrication platforms. For instance, the O2 tension is higher in the deep layer of MSC spheroids induced by hanging drop methods using Terasaki plates when compared to spheroids fabricated by direct culture on ultra-low attachment plates [80]. The activation of vascularization and accumulation of metabolic byproducts can lead to differences in temperature values compared to the surrounding tissues [82]. It seems that the active pro-angiogenesis properties increase the local temperature within the solid tumor masses while the progression of necrotic changes, accumulation of metabolic byproducts, and reduction of metabolic activity reduce the local cancer mass temperature compared to the healthy niches [82]. In an interesting experiment conducted by Kumar et al., they proved a temperature difference between the 3D tumoroid core and periphery composed of human HCT-8 colon cancer cells and NIH3T3 fibroblast cells using the fluorescent polymeric nano-thermometers. Data indicated fewer temperature values (~ 2.9 °C) in the core zone compared to the outer layers of the tumoroid system [83]. Therefore, one can hypothesize that the active metabolic state along with higher temperature values can help us in the evaluation of pro-angiogenesis status within the tumor parenchyma in in vitro conditions. Hypothermic conditions in the core of tumor mass are highly related to the lack of angiogenesis signaling and prominent necrotic changes as seen in real tumor masses. Besides, these features can stimulate specific signaling molecular pathways. For instance, several signaling pathways related to inflammation, angiogenesis, and epithelial-mesenchymal transition (EMT) were indicated in hepatocellular organoids [84]. Of note, prolonged hypoxic conditions can result in the expression of angiogenesis-related factors such as VEGF within the organoid systems [85]. Regarding the fact that most tumor cell types prefer glycolysis over oxidative phosphorylation, it seems that the lack of suitable oxygen levels can educate these cells to behave similarly to the in vivo-like conditions. In organoids exposed to hypoxic conditions, the proliferation of ECs and differentiation of stem cells and progenitors toward endothelial lineage is increased [86]. Each cell type can select specific spatial localization within the organoid/tumoroid structure. For example, ECs prefer the periphery of spheroids/organoids in the early days after development while the formation of vascular units and elongation of these cells help them to penetrate the deeper layers (Fig. 3) [87]. In line with this statement, in heterotypic spheroids consisting of ECs and MSCs, green fluorescent ECs can be detected in the periphery of these structures over time and promotion of angiogenesis response increases the EC migration into the central zone according to gradient density of angiogenesis factors [87]. These features highlight the importance of EC location within the tumoroid/spheroid system which can pre-determines the angiogenesis capacity. It is postulated that the poor access of innermost cells of tumoroids to O2 can initiate the pro-angiogenesis signaling pathways while new vessel formation is promoted by the stimulation of outer layer ECs. It was suggested that ECs in the periphery of unicellular spheroids are more potent in generating capillary sprouts and outgrowth within the collagen gel [65]. Inside the multicellular spheroids composed of human endothelial and osteoblast lineages, ECs can generate the CD31+ microvessels in in vitro conditions and these vascular units can interconnect with surrounding capillaries after being transplanted into the target tissues [88]. Using appropriate ECM components and suitable cell types, the angiogenic potential of vascular cells can be stimulated [89]. In spheroids composed of ECs and smooth muscle cells (SMCs) within the methacrylated hyaluronic acid with fibrinogen, the expression of angiogenesis factors such as stromal-derived factor-1 alpha, HIF-1α, and angiopoietin 1 and migration capacity were in the maximum levels in EC-SMC spheroids compared to EC, and SMC spheroids [89]. These data indicate that heterotypic interaction can promote the function of ECs and thereby angiogenesis potential inside the tumoroids/spheroids. In breast cancer tumoroids consisting of MDA-MB-231 and/or MCF-7 cancer cell lines, lung ECs, and bone marrow MSCs, the localization of ECs is associated with breast cancer cell types and close interaction with MSCs in tumoroids. Cellular organization was more prominent in tumoroids containing MDA-MB-231 rather than MCF-7 cells. This effect would be related to the supportive role of MSCs in the stabilization of vascular units via direct juxtacrine interaction with ECs or pericytes [90, 91]. It is also mentioned that ECM-producing cells like fibroblasts can improve the angiogenic behavior of ECs within the tumoroid/spheroid structures [92]. Inside the body, fibroblasts, especially cancer-associated fibroblasts (CAFs), tend to localize in the core zone of tumors while proliferating cancer cells occupy the outer margins of solid tumors [93]. Of note, the exact location of fibroblasts within the tumoroid system determines morphological adaptation. For instance, CAFs and fibroblasts located near the core zone are round-shaped while these cells are elongated and flattened in the outer layer. This effect would be closely associated with topological net charge in which the deep layer exhibits positive values and these features are negative in the periphery of tumor masses [94]. It was shown that fibroblasts and especially CAFs can support tumor angiogenesis via the production of type I collagen, different pro-angiogenesis factors such as fibroblast growth factor (FGF), VEGF, etc., and stimulation of EMT phenomenon (Fig. 4) [95, 96]. In contrast to the cancer cells, E-cadherin and other adhesion molecules are normally distributed at the surface of normal cells, increasing the juxtacrine cell-to-cell interaction over time [97, 98]. Morphological changes and close interaction of cells within the spheroid/tumoroid system along with the hypoxic core are essential for angiogenesis potential [62, 99]. Emerging data have revealed that there is a close relationship between spheroid/tumoroid vascularization potential and activation of resistance mechanisms [100]. Ahn and co-workers developed unicellular HepG2 cells, and hybrid HepG2 plus HUVEC spheroids using fibrinogen matrix [100]. Compared to unicellular HepG2 spheroids, the expression of genes related to angiogenesis and vascular unit function (CD31, vWF, and FLT1), and metastasis (H19, VIM, LAMB3, ITGA5) were prominent in HepG2-HUVEC spheroids. These data confirmed that heterogeneous cell microstructures (such as tumoroids) can efficiently provide geometric clues for in vivo-like cell behavior and phenotype, especially cancer angiogenesis status [100]. Inside solid tumors, the existence of differentiation capacity from the epithelial-to-mesenchymal lineage, especially ECs, and other cell types can enhance the formation of intraparenchymal vessels, resulting in invasive tumor cell behavior and metastasis toward remote sites [101]. HepG2-HUVEC fibrin spheroids indicated eminent expression of EMT factors (LUG↑, MMP-9↑, and α-SMA↑), leading to the increase of intra-spheroid vascularization [100]. It has been indicated that ECs possess plasticity to acquire mesenchymal phenotype (EndMT) within the cancer parenchyma. Upon the activation of EndMT, ECs reduce protein levels of certain factors such as CD31, VE-cadherin, Tie-2, and vWF, and the expression of mesenchymal cell lineages such as α-SMA, type I/III collagen, and fibroblast-specific protein-1 is induced [102]. Inside the tumor niche, resistant cancer cells and CSCs prefer to locate at sites near the hypoxic zone to maintain their stemness features, angiogenesis potential (VEGF), and resistance mechanisms [103]. In contrast, the increase of O2 levels inside TME leads to CSC-to-normal cancer cell maturation, and loss of stemness feature loss [104]. It should be noted that the development of vascular units within the tumoroid/spheroid system indicates activated endothelial differentiation of CSCs, and proliferation of ECs in response to hypoxic conditions. In the hypoxic conditions, glioma CSCs are prone to acquire EC phenotype by the activation of A3 adenosine receptor [105]. It was shown that the exposure of spheroids to specific culture conditions such as cyclic fluid shear stress can improve the angiogenesis potential and endothelial differentiation of adipose MSCs (vWF↑, and CD144↑) [106]. The application of specific ECM, and/or ECM-like substrates with different cell lineages (multicellular spheroids) can yield a suitable platform for the analysis of tumor angiogenesis. However, it should be kept in mind that providing a hypoxic niche inside spheroids/tumoroids using certain techniques is an essential factor in an efficient angiogenesis analysis. It is suggested that the mean diameter of avascular tumoroids/spheroids should exceed 400 µm for obtaining a hypoxic core [107].
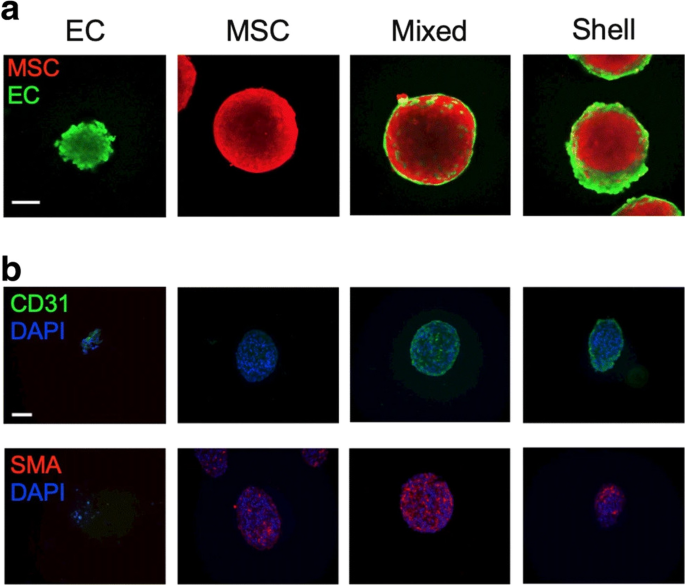
Copyright 2020 [228]. Journal of Molecular Medicine (Springer Nature Publishing Group)
The localization of human cord blood EC and bone marrow MSCs within the spheroids generated using agarose molds (Day 0 a–b). ECs and MSCs were stained with CellTrace™ Oregon Green® 488, and CellTrace™ Far Red Cell, respectively to be tracked within the spheroid structure. Immunofluorescence images indicate the localization of ECs in the periphery of spheroids (a, and b). Data showed that green CD31+ cells were located at the periphery of spheroids while red-colored α-SMA.+ MSCs distributed within the spheroid parenchyma. The nuclei were stained using DAPI. These data show that cell alignment and localization depend on types and functions within the spheroids in in vitro conditions.
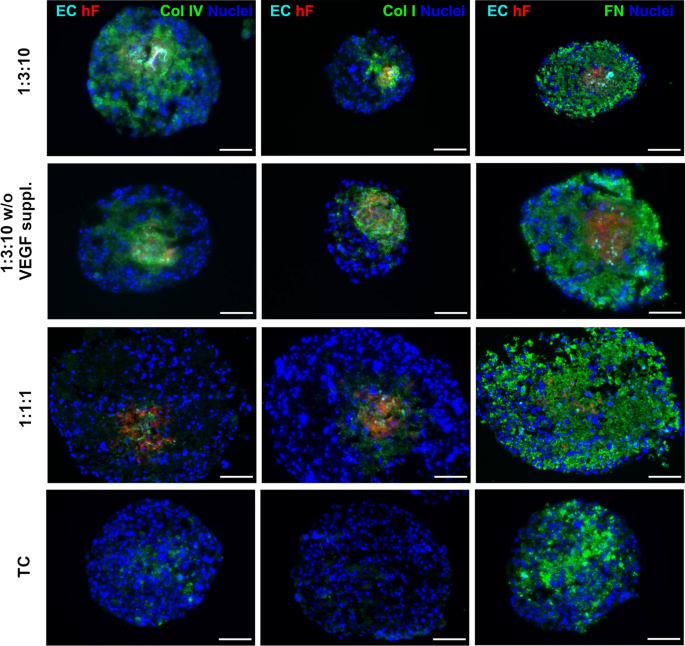
Copyright 2021. [236]. Frontiers in Bioengineering and Biotechnology (Frontiers Publishing Group)
Detection of ECM protein components [collagen IV (Col IV), collagen I (Col I), and fibronectin (FN)] in multicellular spheroids composed of breast HCC1954 cells (TC), PKH26-labeled dermal fibroblasts (hF), and Cell Tracker Deep Red-labeled HUVECs (ECs) with different ratios (TC 1: hF 1: EC 1, and TC 1: hF 3: EC 10) using immunofluorescence images. Spheroids were generated using ultra-low 96-well plates. Spheroids were incubated with VEGF [VEGF suppl. (0.5 ng/m)] or VEGF-deprived conditions (VEGF w/o) for 30 days, and exposed 2-day static culture system followed by agitation-based culture setting (rotation speed: 100 rpm). Data showed that fibronectin was produced in monotypic and heterotypic spheroid systems and is mainly at the center zone of spheroids, indicating the cancer cell origin. In contrast, the production of type I and IV collagen was promoted in the heterotypic spheroid system compared to the monotypic spheroids. The presence of VEGF can intensify the production of collagen by fibroblasts within the heterotypic spheroid system. These data highlight the role of cells in the production of certain components of ECM via the production of certain growth factors within the spheroid systems. The analyses were done in triplicate. Nuclei were stained with DAPI Scale bar, 100 μm.
During past decades, several techniques and assays have been used to assess the angiogenesis potential of cancer cell spheroids and tumoroids based on the EC function and activity. Among several techniques and approaches, spheroid-based sprouting angiogenesis is a simple, fast, and valid assay to measure the formation of new blood vessels and the activity of tip cells embedded in supporting dense matrices [67]. Sprouting parameters such as the number of sprouting and migrating ECs, mean envelope area, mean total outgrowth cell area, and the distance of ECs from the spheroid center, and border can be calculated in unicellular spheroids or multicellular spheroids composed of endothelial lineage and other cell types [108,109,110]. In an interesting study, Park and colleagues monitored the angiogenesis behavior of tumor spheroids composed of human fibroblasts with different cancer cell lines such as HepG2, U87MG, A549, and/or plasmacytoid dendritic cells in all-in-one-IMPACT system using the microfluidic platform and fibrin gel [111]. The formation of vascular sprouts within the different concentrations of fibrin hydrogel was assessed [111]. Several molecular analyses such as proteomic [i.e. immunofluorescence [112, 113], immunohistochemistry staining [114], western blotting [79], etc.], and genomic assays have been also to measure the angiogenesis status in several spheroid systems [62, 79, 115] following the exposure to different modalities [116,117,118].
Along with different in vitro spheroid-based angiogenesis assays, the transplantation of developed spheroids/tumoroids into animal models has been extensively used during the last decades [119]. Spheroid-plug model is one of the most applicable methods for the analysis of spheroid-derived angiogenesis in in vivo conditions. In this approach, unicellular and/or multicellular spheroids are embedded inside the supporting substrates, like Matrigel, etc., and directly injected into the subcutaneous area or transplanted into the target tissues [120, 121]. At distinct time points, the angiogenesis potential of transplanted spheroids/tumoroids was assessed using imaging techniques, i.e. ultrasonographic, proteomic, and genomic analyses [122]. Recently, Choi and co-workers investigated the formation of angiogenesis in a mouse xenograft model of oral mucosa after the injection of CAFs and head and neck squamous cell carcinoma (SCC) grown in a 2D monolayer and/or 3D spheroid culture systems [123]. Histological analyses revealed that the number of CD31+ was less in mice that received disassociated cells while the injection of spheroids resulted in the formation of a vascular bed with numerous CD31+ cells [123]. Interestingly, they found that the levels of exosomes (Exos) also increased in spheroids composed of CAFs, and SCC cells with the increase of angiogenesis potential (pdgf↑, vegf↑, vegfr-2↑, pdgfra↑). Data indicated that the presence of CAFs can increase the angiogenic potential of FaDu squamous cell carcinoma [123]. It has been shown that Exos are valid theranostics for monitoring molecular signatures in the parent cells and loading certain cargo for therapeutic purposes [124]. Exos are produced directly by the activity of the endosomal system inside the cytosol via the invagination of the endosome membrane. During this process, several signaling factors and pro-angiogenesis factors are sequestrated into the Exo lumen [125].
Due to their nano-sized scales, Exos can easily penetrate the spheroid structure for the analysis of angiogenesis potential. In most studies, supporting ECM is composed of collagen, gelatin, hyaluronic acid, polymeric carbohydrates such as alginate, and other substrates which can yield fibrous networks or hydrogel with nano- to micron-sized pores in the structure of spheroids/tumoroids [126,127,128]. To this end, Capik and co-workers incubated human EC spheroids with hypoxic oral SCC cell Exos for monitoring the angiogenesis potential [129]. Data indicated that exosomal miR-1825 led to the increase of sprouts in EC spheroids via the modulation of the TSC2/mTOR axis [129]. These data confirmed that Exos can efficiently harbor both genetics and proteomics in different cell layers within the spheroid structure. Whether the majority of Exos can be uptaken by cells located at outer or deep cell layers needs more elucidation. In an interesting study, paclitaxel/gemcitabine monophosphate-loaded Exos efficiently entered pancreatic ductal adenocarcinoma spheroids with about an average diameter of 300–350 µm. Immunofluorescence images revealed significant homing capability of fluorochrome-stained Exos in the deep layer of pancreatic ductal adenocarcinoma spheroids and orthotopic model in mice [130]. Hao and co-workers indicated the prominent penetrating properties of doxorubicin-loaded Exos in human adenocarcinoma A549 spheroids after 4 h in in vitro conditions [131]. These data show that Exos are valid diagnostics for the evaluation of pro-angiogenesis cargo. Thus, Exos can be used for the delivery of specific therapeutics into deep layers of tumoroids/spheroids using some sophisticated loading techniques.
Microfluidic devices and 3D bioprinting for fabrication of vascularized tumor spheroids
In recent years, 3D bioprinting technology along with microfluidic devices have been used for precise and spatial arrangement of cells and supporting materials to generate micro-sized aggregates and simulate in vivo tumor-like conditions [132]. Along with different bioprinting modalities, droplet-based bioprinting (such as inkjet, acoustic, and microvalve-based approaches) exhibits reproducibility, accuracy, adaptability to different substrates, etc. for the fabrication of tumor spheroids/organoids [133]. Using droplet-based bioprinting, both cell number and type can be controlled in the structure of final tumor spheroids [133]. Previously, Utama et al. used drop-on-demand bioprinter and alginate-based substrate for the fabrication of multicellular tumor spheroids (neuroblastoma, and lung cancer cell lines) with the potential to control parameters such as size, and dimensions of colonies [134]. Data indicated a rapid spheroid formation rate with high encapsulated cell numbers within certain dimensions. The current approach was eligible to preserve the stemness of neuroblastoma cells (CD133+ cells↑) within the tumor spheroids. Besides, the cell alignment and compactness were increased in 3D-bioprinted spheroids as compared with manually prepared spheroid counterparts. The presence of HIF-1α+ cells indicates typical hypoxic conditions in the inner layers of 3D-bioprinted spheroids which can be used for monitoring angiogenesis and drug screening [134]. In a similar work conducted by Hong and co-workers, they used a 3D bioprinting system for the evaluation of drug resistance in breast cancer spheroids containing MCF-7 CSCs and alginate-gelatin hydrogel [135]. The developed tumor spheroids preserve the stemness feature (CD44+/CD24−/ALDH+ cells↑) with simultaneous up-regulation of resistance markers such as GRP78 chaperon and ABCG2 [135].
In recent years microfluidic bioreactors have been increasingly used for the evaluation of angiogenesis response in tumor spheroids [100]. It has been shown that the integration of the tumor-on-a-chip technique with 3D bioprinting platforms can help us provide a heterogeneous TME and monitor angiogenesis as well [136]. Meng and co-workers fabricated 3D bioprinted modules consisting of photo-responsive microcapsules [GelMA core with EGF, and Au-functionalized PLGA film], A549 tumor cell droplets, endothelialized (HUVECs) microchannels, and fibroblast-loaded natural hydrogel. This platform can successfully mimic the TME and be eligible to monitor cancer cell migration in association with growth factor gradient and interaction with the stromal cells [137]. By combining 3D bioprinting technology and microfluidic devices, unicellular or multicellular tumor spheroids can be exposed directly to an EC-lined surface with predetermined flow rates and certain pro-, and anti-angiogenesis factors [138]. The angiogenesis rate is monitored in terms of vascularization area, and penetration of vascular cells to spheroid structure (Figs. 5, and 6) [100]. Han and co-workers studied the angiogenesis of U78 glioblastoma tumoroids plated on a bioprinted vascular niche composed of pulmonary fibroblasts and human ECs embedded in gelatin/alginate/fibrinogen composite [139]. Data confirmed the invagination of vascular ECs into tumoroid structure and migration of U78 cancer cells toward vessels and vice versa. On day 7, the expression of CD31 and α-actin is up-regulated coinciding with the formation of vascular network within the hydrogel. In the presence of anti-cancer compounds (temozolomide, and sunitinib), the intensity of vascular units was reduced in the tumoroid backbone compared to the cells treated with temozolomide alone. These features indicate the feasibility of the designed system for monitoring the efficiency of several anti-cancer drugs on certain tumor types in vitro [139].
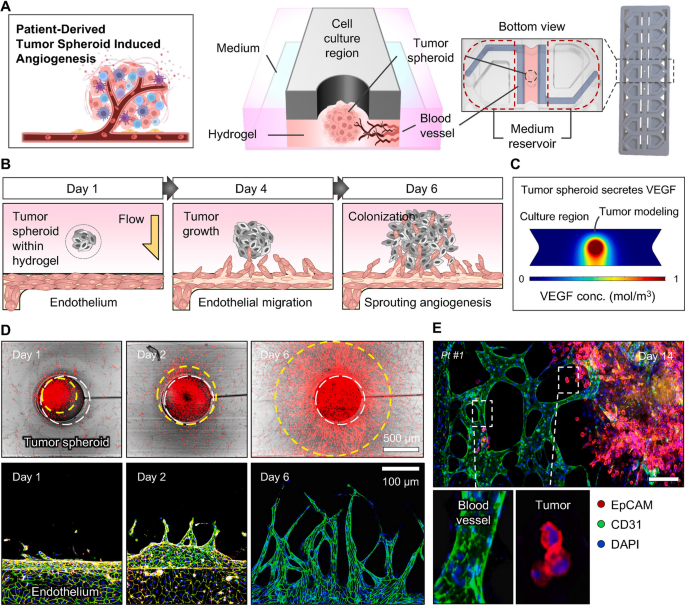
Copyright 2024. [138]. Biomaterials (Elsevier Publishing Group)
Application of microfluidic 3D culture system for monitoring the patient tumor (gastric adenocarcinoma)-derived spheroid angiogenesis response (A–E). The patient-derived tumor spheroids were generated using fibrin hydrogel. The microfluidic 3D culture system provided a valuable platform to measure the angiogenesis properties of spheroids after being exposed to HUVECs in the EC channel (A). The reciprocal interaction between the EC layer and hydrogel-embedded spheroids within the microfluidic 3D culture system (B). The existence of hydrostatic pressure stimulated tumor spheroids to release angiogenesis-related factors such as VEGF toward the EC channel (C). Tumor cell and EC growth were monitored using confocal microscopy for 6 days (D). After 14 days, the fluorescein-labeled Ulex Europaeus agglutinin I+ EC layer advanced toward Alexa Fluor 594-EpCAM.+ tumor spheroid to generate vascularized tumor mass. Scala bar: 10 µm.
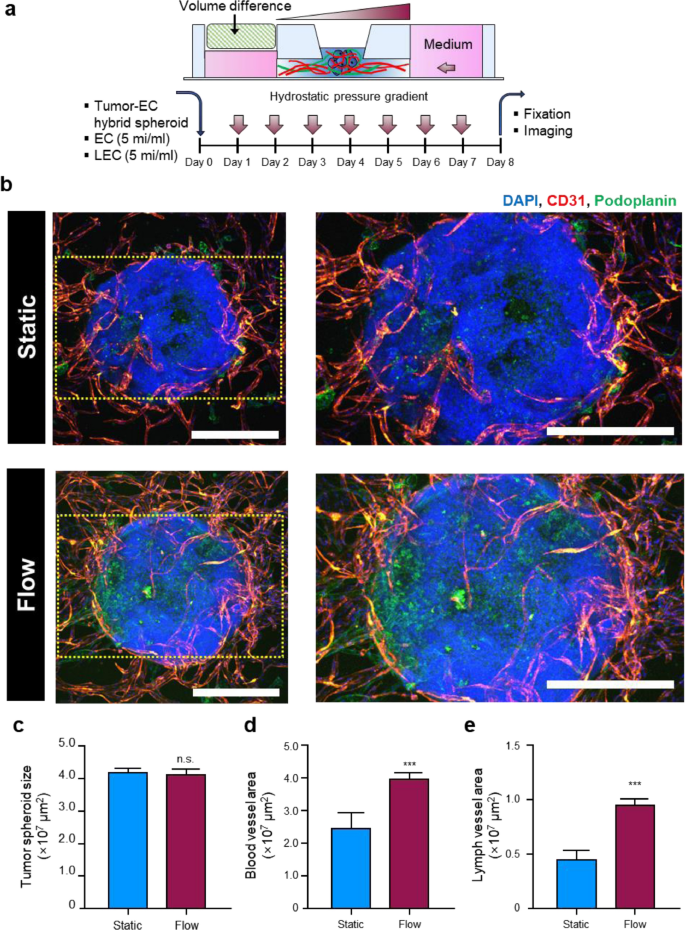
Copyright 2023. [100]. Acta Biomaterialia (Elsevier Publishing Group)
Comparison of lymphangiogenesis efficiency in tumor spheroids inside microfluidic chip device exposed to static and dynamic culture condition (a-e). Schematic illustration of interstitial flow effect on lymphangiogenesis and angiogenesis properties of tumor spheroids composed of fibrin-embedded HepG2 cells and HUVECs. Tumor spheroids (HepG2 cells + HUVECs) were injected and fixed in the central channel using fibrin clots containing vascular ECs and lymphatic ECs (a). Confocal images of angiogenesis (red-colored Alexa Fluor® 594 CD31+ cells) and lymphangiogenesis (green-colored Alexa Fluor® 488 podoplanin.+ cells) under static and dynamic culture conditions (b) Scale bars: 400 μm). Spheroid size (c), vascularization area (d), and lymphatic vessel area (e) were measured in conditions with static and dynamic culture systems (n = 4). Data indicated that both lymphangiogenesis and angiogenesis were stimulated in tumor spheroid systems after being exposed to a dynamic culture system. Non-paired student's t-test. ***p < 0.001. n.s: non-significant.
Under physiological conditions, the existence of shear stress with frictional forces is an essential element for the regulation of EC function and activity. Thus, the application of a perfusable culture system with simultaneous shear stress forces can yield reliable data compared to static culture platforms. In microfluidic systems, microfabrication approaches can be used for the fabrication of microchannels using suitable substrates for monitoring cell attachment, morphogenesis, and function in the dynamic aqueous phase [140]. In an interesting work, Miller and co-workers developed engineered vascular tissue consisting filament network composed of carbohydrate glass using 3D bioprinting [141]. The luminal surface was furnished with ECs and exposed to pulsative blood flow. Based on the complexity of TME, this approach can be applied to control several geometries such as vascular network dimension, EC function, impact of intervascular niche, and tumor cell behavior. The application of different cell lineages within certain substrates is also possible for achieving reliable in vivo-like data [141]. Using a tumor microfluidic model, immune cell function can be also monitored in response to tumoroids with different cell types [142]. In this regard, fibroblasts were co-incubated with different tumor cells (H69M lung cancer cells, OV90 ovarian cancer cells. SN12C kidney cancer cells) within the tumor-on-a-chips composed of PDMS for assessing angiogenesis potential [142]. The tumoroids were overlaid on HUVEC- and fibroblast-incorporated fibrin gel and vascularized tumoroids were evident after 7 days [142]. Application of continuous flow for 4 days showed that CAR T cells successfully penetrate the tumoroid microenvironment and interact with the apical surface vascular ECs. Along with the recruitment of CAR T cells into the tumor niche, the local IFN-γ levels were also increased, indicating the activation of CAR T cells after being exposed to tumor cells [142].
In most previously conducted works, ECs have been used as the main vascular cell component for monitoring angiogenesis in tumor model analyses. The inclusion of other vascular cells such as pericytes and perivascular niches can reflect more reliable in vitro data about the dynamic tumor cell growth comparably to in vivo conditions [143]. In an experiment conducted by Ngo et al., brain glioblastoma tumoroids were fabricated using brain microvascular ECs, pericytes, and astrocytes to monitor the possible role of perivascular cells in angiogenesis potential of tumoroids within the GelMA substrate [143]. Data indicated that mono, di, and triculture of ECs with pericytes, and astrocytes led to the formation of a primitive stable vascular network (CD31↑, ZO↑) within the tumor unicellular or multicellular aggregates. The presence of pericytes can increase the expression of tight junction molecules such as ZO, and CLDN5. Along with these changes, the expression of ECM components such as LAMA4 was also induced. The culture of GBM6 cell spheroids within the GelMA hydrogel containing ECs, pericytes, and astrocytes indicated numerous peripheral invading tumor cells after 7 days. On the other hand, ECs were also recruited to the proximity of tumor spheroids to generate vascular networks. Taken together, the existence of perivascular cells (pericytes) can stimulate the migration and metastasis of glioblastoma cells with enhanced vascular density [143].
Current bottlenecks and challenges
Despite the superiority of unicellular and multicellular spheroids over 2D culture systems in terms of cancer research, this platform faces some critical limitations that need further consideration. Compared to cultured cells in 2D systems, bright-field imaging and obtaining high-resolution scanning are usually difficult and laborious due to intricate geometries that affect light scattering, penetration, and absorption within the spheroid/tumoroid systems [144, 145]. These features make it difficult to make transparent images and study the central zone of multicellular complexes. Prolonged light exposure can also increase the possibly of phototoxicity and photobleaching effects that per se necessitate the application of more expensive techniques such as electron microscopy, and layer-by-layer fluorescence imaging [146, 147]. In most circumstances, the lack of an appropriate penetration rate or homogenous distribution of fluorochrome compounds reduces the possibility of certain biomarkers and positive cells within the deep layers of spheroid/tumoroid systems [7, 144, 148]. To fabricate interpretable 3D datasets, certain imaging software, and platforms with motorized and controllable microscope frames are mandatory [144]. It should not be forgotten that the mean diameter size of multicellular units is not completely similar which can affect the access of nutrients and other essential factors into central zones. In this regard, obtaining almost uniform data is problematic and makes the results difficult [149]. In contrast to in vivo conditions, the distribution of O2 and various nutrients in the 3D culture system is restricted in the static culture systems. Of note, dynamic culture platforms can in part circumvent these pitfalls but it is not completely similar to biological systems [150]. In terms of anatomical microstructure, the tumor masses are enclosed by solid tissues while in in vitro spheroid/tumoroid cultures are surrounded by aqueous phases. These features allow the cells located at the external surface to proliferate more actively because of unlimited access to growth factors and cell culture elements [7].
Notwithstanding, each tumor has specific supporting ECM components and the development of spheroid/tumoroid systems with native ECM compositions needs standard protocols and sophisticated synthesis protocols. It is believed that ECM composition and variation in supporting substrates can affect the genetic traits, cellular behavior within the spheroid/tumoroid complex, and response to therapeutics and drug screening protocols [144, 151]. Compared to the real tumor mass, the in vitro multicellular systems possess a relatively simple microstructure with a limited number of ECM components. The entity of ECM can change during the culture periods and selection of cell ratio in tumoroid systems. Therefore, the selection of appropriate cells with relatively similar tumor mass ratios is mandatory to establish more realistic models and compare the in vitro and in vivo data [150]. In most spheroid/tumoroid models, the lack of microstructure interconnectivity, ECM alignment, and specific cell types such as cancer-associated fibroblasts (CAFs), tumor-associated macrophages (TAMs), and other vascular components such as pericytes can affect the dynamic growth of cancer cells and tumor mass behavior [144, 152]. Unlike in vivo conditions, spheroids/tumoroids are devoid of neuroimmunoendocrine interactions which allows cancer cells to proliferate more actively compared to other cell types [150]. It is believed that M2 TAMs can exert immunosuppressive properties with the potential to change tumor cell migration, drug sensitivity and ECM components [153]. Whether and how co-cultured fibroblasts can acquire in vivo-like stable phenotypes (CAFs) is associated with the production of several cytokines, and physical interaction with the other cells [150]. CAFs are actively present in the tumor matrix and likely produce a large fraction of ECM components (fibronectin, collagen, etc.) along with certain growth factors. It has been thought the number of CAFs and ECM production correlate with stable and continuous hypoxic conditions and low pH values [153]. The co-culture of ECs with other cells within the multicellular system cannot fully mimic tumor parenchyma vascular networks while constant shear stress and perfusion should be provided [154]. Direct evidence for low-content intercellular junction factors, i.e., E-cadherin, leads to formation of less compact spheroid/tumoroid system. In most circumstances, the up-regulation of N-cadherin instead of E-cadherin loosen the cell-to-cell interaction [146]. Of course, certain ECM components such as collagen can facilitate the compactness of spheroids/tumoroids. Unfortunately, creating a multicellular system with a dense collagen matrix is somehow impossible and needs intricate synthesis protocols that may damage the cells [145, 146]. These features closely affect the penetration and tumoricidal properties of immune cells [150, 155].
Conclusions
Tumoroids/spheroids provide a valid cost-effective, fast, precise, and reproducible in vitro platform to recapitulate in vivo-like conditions for the analysis of angiogenesis status in response to drugs and other factors [156]. Besides, both multicellular and unicellular tumor spheroids enable us to study the paracrine and juxtacrine interaction between the homotypic and heterotypic cells. In most previously conducted experiments, several parameters such as tumor cell dynamic, metastasis, drug screening, etc. have been analyzed more than the angiogenesis potential. Thus, the current review article aims to scrutinize the importance of angiogenesis/vascularization in the dynamic growth of the tumoroid/spheroid system. Based on several studies, endothelial lineage, and/or stem cell sources are used to mimic blood vessel structure within the multicellular and unicellular tumor spheroids, and thus type, intensity, and duration of angiogenesis responses can differ based on the cell components and supporting matrices. Like tumoroids composed of different cell lines, patient-derived tumoroids are also valid analytic tools to find appropriate medications and screen novel therapies targeting the angiogenesis potential. It should not be forgotten that tumoroids/spheroids have their limitations despite several advantages compared to conventional 2D culture systems. The entity, structure, and vascular cells involved in the development of vascular niches are not completely similar to in vivo conditions. Thus, additional efforts are needed to recapitulate different aspects of the tumor microenvironment, such as the inclusion of immune cells recapitulation of appropriate vessel morphologies, or appropriate basement membrane or ECM composition. Finding the exact in vivo-like cell-to-cell ratio is another challenge that restricts the applicability of 3D culture system-based assays for therapeutic purposes. Commensurate with these facts, future studies are suggested to address these issues for the development of powerful culture system tools comparable to in vivo tumor conditions.
Availability of data and materials
Data sharing is not possible as no new data were created or analyzed in this study.
Abbreviations
- CSCs:
-
Cancer stem cells
- CAFs:
-
Cancer-associated fibroblasts
- ECs:
-
Endothelial cells
- EPCs:
-
Endothelial progenitor cells
- EndMT:
-
Endothelial-mesenchymal transition
- EMT:
-
Epithelial-mesenchymal transition
- Exos:
-
Exosomes
- ECM:
-
Extracellular matrix
- FGF:
-
Fibroblast growth factor
- HIF-1α:
-
Hypoxia-inducible factor 1 alpha
- Mscs:
-
Mesenchymal stem cells
- PLGA:
-
Poly (lactic-co-glycolic acid)
- PEG:
-
Polyethylene glycol
- PU:
-
Polyurethane
- SMCs:
-
Smooth muscle cells
- SCC:
-
Squamous cell carcinoma
- 3D:
-
Three dimension
- TGF-β:
-
Transforming growth factor-β
- TME:
-
Tumor microenvironment
- TAMs:
-
Tumor-associated macrophages
- 2D:
-
Two dimension
- HUVECs:
-
Umbilical cord vein ECs
- VEGF:
-
Vascular endothelial growth factor
References
Zhao Z, et al. Organoids. Nat Rev Methods Primers. 2022;2(1):94.
Cacciamali A, Villa R, Dotti S. 3D cell cultures: evolution of an ancient tool for new applications. Front Physiol. 2022;13: 836480.
Yin S, et al. Composite microfluidic petri dish-chip (MPD-Chip) without protein coating for 2D cell culture. Langmuir. 2023;39(44):15643–52.
Lerman MJ, et al. The evolution of polystyrene as a cell culture material. Tissue Eng Part B Rev. 2018;24(5):359–72.
Gao W, et al. Development of a novel and economical agar-based non-adherent three-dimensional culture method for enrichment of cancer stem-like cells. Stem Cell Res Ther. 2018;9(1):243.
Shah DD, et al. Harnessing three-dimensional (3D) cell culture models for pulmonary infections: state of the art and future directions. Naunyn Schmiedebergs Arch Pharmacol. 2023;396(11):2861–80.
Jubelin C, et al. Three-dimensional in vitro culture models in oncology research. Cell Biosci. 2022;12(1):155.
Law AMK, et al. Advancements in 3D cell culture systems for personalizing anti-cancer therapies. Front Oncol. 2021;11:782766.
Wang L, et al. Spatial topology of organelle is a new breast cancer cell classifier. iScience. 2023;26(7):107229.
Venkatesan M, et al. Spatial subcellular organelle networks in single cells. Sci Rep. 2023;13(1):5374.
Russell S, et al. Metabolic profiling of healthy and cancerous tissues in 2D and 3D. Sci Rep. 2017;7(1):15285.
Duval K, et al. Modeling physiological events in 2D vs. 3D cell culture. Physiology (Bethesda). 2017;32(4):266–77.
Dravid A, et al. A macroscopic diffusion-based gradient generator to establish concentration gradients of soluble molecules within hydrogel scaffolds for cell culture. Front Chem. 2019;7:638.
Montagner M, Dupont S. Mechanical forces as determinants of disseminated metastatic cell fate. Cells. 2020. https://doi.org/10.3390/cells9010250.
Baker BM, Chen CS. Deconstructing the third dimension–how 3D culture microenvironments alter cellular cues. J Cell Sci. 2012;125(13):3015–24.
Saraswathibhatla A, Indana D, Chaudhuri O. Cell–extracellular matrix mechanotransduction in 3D. Nat Rev Molecular Cell Biol. 2023;24:1–22.
Jensen C, Teng Y. Is it time to start transitioning from 2D to 3D cell culture? Front Mol Biosci. 2020;7:33.
Habanjar O, et al. 3D cell culture systems: tumor application, advantages, and disadvantages. Int J Mol Sci. 2021;22(22):12200.
Petersen OW, et al. Interaction with basement membrane serves to rapidly distinguish growth and differentiation pattern of normal and malignant human breast epithelial cells. Proc Natl Acad Sci. 1992;89(19):9064–8.
Sun M, et al. 3D cell culture—can it be as popular as 2d cell culture? Adv Nanobiomed Res. 2021;1(5):2000066.
Redmond J, et al. Advances in biofabrication techniques for collagen-based 3D in vitro culture models for breast cancer research. Mater Sci Eng C. 2021;122: 111944.
Nyga A, Cheema U, Loizidou M. 3D tumour models: novel in vitro approaches to cancer studies. J Cell Commun Signaling. 2011;5:239–48.
Moscona A, Moscona H. The dissociation and aggregation of cells from organ rudiments of the early chick embryo. J Anat. 1952;86(Pt 3):287.
Peng K, et al. HIF-1α promotes kidney organoid vascularization and applications in disease modeling. Stem Cell Res Ther. 2023;14(1):336.
Li X, et al. Assay establishment and validation of a high-throughput organoid-based drug screening platform. Stem Cell Res Ther. 2022;13(1):219.
Cacciamali A, Villa R, Dotti S. 3D cell cultures: evolution of an ancient tool for new applications. Front Physiol. 2022;13:836480.
Akhtar A. The flaws and human harms of animal experimentation. Camb Q Healthc Ethics. 2015;24(4):407–19.
Van Norman GA. Limitations of animal studies for predicting toxicity in clinical trials: is it time to rethink our current approach? JACC Basic Trans Sci. 2019;4(7):845–54.
Florian S, et al. A human organoid system that self-organizes to recapitulate growth and differentiation of a benign mammary tumor. Proc Natl Acad Sci USA. 2019;116(23):11444–53.
Lee S-Y, et al. In Vitro three-dimensional (3D) cell culture tools for spheroid and organoid models. SLAS Discovery. 2023;28(4):119–37.
Xu H, et al. Tumor organoids: applications in cancer modeling and potentials in precision medicine. J Hematol Oncol. 2022;15(1):58.
Tatla AS, et al. A vascularized tumoroid model for human glioblastoma angiogenesis. Sci Rep. 2021;11(1):19550.
Gunti S, et al. Organoid and spheroid tumor models: techniques and applications. Cancers (Basel). 2021;13(4):874.
Zhou Z, et al. Evaluation of the tumoricidal efficacy of adoptive cell transfer using hepatocellular carcinoma-derived organoids. J Gastrointest Oncol. 2022;13(2):732–43.
Makouei F, et al. 3D ultrasound versus computed tomography for tumor volume measurement compared to gross pathology-a pilot study on an animal model. J Imaging. 2022;8(12):329.
Pasini A, et al. Perfusion flow enhances viability and migratory phenotype in 3D-cultured breast cancer cells. Ann Biomed Eng. 2021;49(9):2103–13.
Atat OE, et al. 3D modeling in cancer studies. Hum Cell. 2022;35(1):23–36.
Li W, et al. 3D Biomimetic Models to Reconstitute Tumor Microenvironment In Vitro: spheroids, Organoids, and Tumor-on-a-Chip. Adv Healthcare Mater. 2023;12(18):2202609.
Kang SM, et al. Engineered microsystems for spheroid and organoid studies. Adv Healthc Mater. 2021;10(2):2001284.
Wang Y, Sun Y. Engineered organoids in oral and maxillofacial regeneration. iScience. 2023;26(1):105757.
Wang D, et al. Hyaluronic acid methacrylate/pancreatic extracellular matrix as a potential 3D printing bioink for constructing islet organoids. Acta Biomater. 2023;165:86–101.
Heo JH, et al. Engineering the extracellular matrix for organoid culture. Int J Stem Cells. 2022;15(1):60–9.
Liu H, et al. Advances in hydrogels in organoids and organs-on-a-chip. Adv Mater. 2019;31(50):1902042.
Cheng Y, et al. Sustained hedgehog signaling in medulloblastoma tumoroids is attributed to stromal astrocytes and astrocyte-derived extracellular matrix. Lab Invest. 2020;100(9):1208–22.
Cesarz Z, Tamama K. Spheroid culture of mesenchymal stem cells. Stem Cells Int. 2016;2016(1):9176357.
Lefort CT, Wojciechowski K, Hocking DC. N-cadherin cell-cell adhesion complexes are regulated by fibronectin matrix assembly*. J Biol Chem. 2011;286(4):3149–60.
Maître JL, Heisenberg CP. Three functions of cadherins in cell adhesion. Curr Biol. 2013;23(14):R626–33.
Dash S, et al. Heterophilic recognition between E-cadherin and N-cadherin relies on same canonical binding interface as required for E-cadherin homodimerization. Arch Biochem Biophys. 2022;727: 109329.
Wu SK, Yap AS. Patterns in space: coordinating adhesion and actomyosin contractility at E-cadherin junctions. Cell Commun Adhes. 2013;20(6):201–12.
Powan P, et al. Detachment-induced E-cadherin expression promotes 3D tumor spheroid formation but inhibits tumor formation and metastasis of lung cancer cells. Am J Physiol Cell Physiol. 2017;313(5):C556–66.
Zisis T, et al. Disentangling cadherin-mediated cell-cell interactions in collective cancer cell migration. Biophys J. 2022;121(1):44–60.
Derycke LD, Bracke ME. N-cadherin in the spotlight of cell-cell adhesion, differentiation, embryogenesis, invasion and signalling. Int J Dev Biol. 2004;48(5–6):463–76.
Ardizzone A, et al. Role of basic fibroblast growth factor in cancer: biological activity, targeted therapies, and prognostic value. Cells. 2023;12(7):1002.
Jia T, et al. FGF-2 promotes angiogenesis through a SRSF1/SRSF3/SRPK1-dependent axis that controls VEGFR1 splicing in endothelial cells. BMC Biol. 2021;19:1–26.
Yuan Y, et al. Role of the tumor microenvironment in tumor progression and the clinical applications (Review). Oncol Rep. 2016;35(5):2499–515.
Xu S, et al. The role of collagen in cancer: from bench to bedside. J Transl Med. 2019;17:1–22.
Borst R, Meyaard L, Pascoal Ramos MI. Understanding the matrix: collagen modifications in tumors and their implications for immunotherapy. J Trans Med. 2024;22(1):382.
Nazari SS. Generation of 3D tumor spheroids with encapsulating basement membranes for invasion studies. Curr Protoc Cell Biol. 2020;87(1): e105.
Namjoo AR, et al. Tissue engineering modalities in skeletal muscles: focus on angiogenesis and immunomodulation properties. Stem Cell Res Ther. 2023;14(1):90.
Chen K, et al. Comprehensive insight into endothelial progenitor cell-derived extracellular vesicles as a promising candidate for disease treatment. Stem Cell Res Ther. 2022;13(1):238.
Narmi MT, et al. Melatonin blunted the angiogenic activity in 3D colon cancer tumoroids by the reduction of endocan. Cancer Cell Int. 2023;23(1):118.
Beloglazova I, et al. New insight on 2D in vitro angiogenesis models: all that stretches is not a tube. Cells. 2022. https://doi.org/10.3390/cells11203278.
Kapałczyńska M, et al. 2D and 3D cell cultures—a comparison of different types of cancer cell cultures. Arch Med Sci. 2018;14(4):910–9.
Liu X, Bouman Chen Z. How their environment influences endothelial cells. Elife. 2023;12:e88248.
Heiss M, et al. Endothelial cell spheroids as a versatile tool to study angiogenesis in vitro. FASEB J. 2015;29(7):3076–84.
Liu Z-L, et al. Angiogenic signaling pathways and anti-angiogenic therapy for cancer. Signal Transduct Target Ther. 2023;8(1):198.
Kannan P, Schain M, Lane DP. An automated quantification tool for angiogenic sprouting from endothelial spheroids. Front Pharmacol. 2022;13:883083.
Guan Y, et al. Effects of hypoxia on cerebral microvascular angiogenesis: benefits or damages? Aging Dis. 2023;14(2):370.
Raghavan S, et al. Comparative analysis of tumor spheroid generation techniques for differential in vitro drug toxicity. Oncotarget. 2016;7(13):16948–61.
Suarez-Martinez E, et al. 3D and organoid culture in research: physiology, hereditary genetic diseases and cancer. Cell Biosci. 2022;12(1):39.
Wicks EE, Semenza GL. Hypoxia-inducible factors: cancer progression and clinical translation. J Clin Invest. 2022. https://doi.org/10.1172/JCI159839.
Yuan X, et al. Targeting hypoxia-inducible factors: therapeutic opportunities and challenges. Nat Rev Drug Discovery. 2024;23(3):175–200.
Magar AG, et al. A molecular perspective on HIF-1α and angiogenic stimulator networks and their role in solid tumors: an update. Int J Mol Sci. 2024;25(6):3313.
Pang X, et al. Weak acids produced during anaerobic respiration suppress both photosynthesis and aerobic respiration. Nat Commun. 2023;14(1):4207.
Pinto B, et al. Three-dimensional spheroids as in vitro preclinical models for cancer research. Pharmaceutics. 2020;12(12):1186.
Zhu Y, et al. 3D tumor spheroid and organoid to model tumor microenvironment for cancer immunotherapy. Organoids. 2022;1(2):149–67.
Kreitzer MA, et al. ATP-mediated increase in H+ efflux from retinal Müller cells of the axolotl. J Neurophysiol. 2024;131(1):124–36.
Faes S, et al. Acidic pH reduces VEGF-mediated endothelial cell responses by downregulation of VEGFR-2 relevance for anti-angiogenic therapies. Oncotarget. 2016;7(52):86026.
Mena HA, et al. Acidic preconditioning of endothelial colony-forming cells (ECFC) promote vasculogenesis under proinflammatory and high glucose conditions in vitro and in vivo. Stem Cell Res Ther. 2018;9(1):120.
Schmitz C, et al. Hypoxia onset in mesenchymal stem cell spheroids: monitoring with hypoxia reporter cells. Front Bioeng Biotechnol. 2021;9: 611837.
Schmitz C, et al. Live reporting for hypoxia: hypoxia sensor–modified mesenchymal stem cells as in vitro reporters. Biotechnol Bioeng. 2020;117(11):3265–76.
Knapp JP, et al. Tumor temperature: friend or foe of virus-based cancer immunotherapy. Biomedicines. 2022;10(8):2024.
Kumar A, et al. Synthesis and characterization of a fluorescent polymeric nano-thermometer: dynamic monitoring of 3D temperature distribution in co-cultured tumor spheroids. Analyst. 2023;148(9):2045–57.
Wang Y, et al. Microenvironment of a tumor-organoid system enhances hepatocellular carcinoma malignancy-related hallmarks. Organogenesis. 2017;13(3):83–94.
Schumacher A, et al. Enhanced microvasculature formation and patterning in iPSC–derived kidney organoids cultured in physiological hypoxia. Front Bioeng Biotechnol. 2022;10: 860138.
Podkalicka P, et al. Hypoxia as a driving force of pluripotent stem cell reprogramming and differentiation to endothelial cells. Biomolecules. 2020;10(12):1614.
Vorwald CE, Joshee S, Leach JK. Spatial localization of endothelial cells in heterotypic spheroids influences Notch signaling. J Mol Med (Berl). 2020;98(3):425–35.
Walser R, et al. Generation of co-culture spheroids as vascularisation units for bone tissue engineering. Eur Cell Mater. 2013;26:222–33.
Zuo X, et al. Spheroids of endothelial cells and vascular smooth muscle cells promote cell migration in hyaluronic acid and fibrinogen composite hydrogels. Research. 2020. https://doi.org/10.34133/2020/8970480.
Rajan AM, et al. Dual function of perivascular fibroblasts in vascular stabilization in zebrafish. PLoS Genet. 2020;16(10): e1008800.
Nassiri SM, Rahbarghazi R. Interactions of mesenchymal stem cells with endothelial cells. Stem Cells Dev. 2013;23(4):319–32.
Lamichhane SP, et al. Recapitulating epithelial tumor microenvironment in vitro using three dimensional tri-culture of human epithelial, endothelial, and mesenchymal cells. BMC Cancer. 2016;16(1):1–12.
Sarkar M, et al. Cancer-associated fibroblasts: the chief architect in the tumor microenvironment. Front Cell Dev Biol. 2023;11:1089068.
Turiv T, et al. Topology control of human fibroblast cells monolayer by liquid crystal elastomer. Sci Adv. 2020;6(20):eaaz6485.
Hughes CC. Endothelial-stromal interactions in angiogenesis. Curr Opin Hematol. 2008;15(3):204.
Huang B, Huang M, Li Q. Cancer-associated fibroblasts promote angiogenesis of hepatocellular carcinoma by VEGF-mediated EZH2/VASH1 pathway. Technol Cancer Res Treat. 2019;18:1533033819879905.
Lin RZ, et al. Dynamic analysis of hepatoma spheroid formation: roles of E-cadherin and beta1-integrin. Cell Tissue Res. 2006;324(3):411–22.
Steinberg MS. Differential adhesion in morphogenesis: a modern view. Curr Opin Genet Dev. 2007;17(4):281–6.
Zhou Y, et al. 3D culture increases pluripotent gene expression in mesenchymal stem cells through relaxation of cytoskeleton tension. J Cell Mol Med. 2017;21(6):1073–84.
Ahn J, et al. 3D microengineered vascularized tumor spheroids for drug delivery and efficacy testing. Acta Biomater. 2023;165:153–67.
Yoshimatsu Y, Watabe T. Emerging roles of inflammation-mediated endothelial–mesenchymal transition in health and disease. Inflammation Regeneration. 2022;42(1):9.
Choi KJ, et al. Endothelial-to-mesenchymal transition in anticancer therapy and normal tissue damage. Exp Mol Med. 2020;52(5):781–92.
Emami Nejad A, et al. The role of hypoxia in the tumor microenvironment and development of cancer stem cell: a novel approach to developing treatment. Cancer Cell Int. 2021;21(1):62.
Kim H, et al. The hypoxic tumor microenvironment in vivo selects the cancer stem cell fate of breast cancer cells. Breast Cancer Res. 2018;20:1–15.
Rocha R, et al. The adenosine A3 receptor regulates differentiation of glioblastoma stem-like cells to endothelial cells under hypoxia. Int J Mol Sci. 2018;19(4):1228.
Ueda Y, Sakamoto N. A combined application of cyclic stretching and spheroid culture promotes endothelial differentiation of mesenchymal stem cells. J Biorheol. 2023;37(2):88–95.
Riffle S, Hegde RS. Modeling tumor cell adaptations to hypoxia in multicellular tumor spheroids. J Exp Clin Cancer Res. 2017;36(1):102.
Blacher S, et al. Cell invasion in the spheroid sprouting assay: a spatial organisation analysis adaptable to cell behaviour. PLoS ONE. 2014;9(5): e97019.
Shah S, Kang K-T. Two-cell spheroid angiogenesis assay system using both endothelial colony forming cells and mesenchymal stem cells. Biomolecules Therapeutics. 2018;26(5):474.
Balcioglu HE, van de Water B, Danen EHJ. Tumor-induced remote ECM network orientation steers angiogenesis. Sci Rep. 2016;6(1):22580.
Park S, et al. One-step achievement of tumor spheroid-induced angiogenesis in a high-throughput microfluidic platform: one-step tumor angiogenesis platform. Organoid. 2023. https://doi.org/10.51335/organoid.2023.3.e3.
Chaddad H, et al. Combining 2D angiogenesis and 3D osteosarcoma microtissues to improve vascularization. Exp Cell Res. 2017;360(2):138–45.
He YJ, et al. Immobilized RGD concentration and proteolytic degradation synergistically enhance vascular sprouting within hydrogel scaffolds of varying modulus. J Biomater Sci Polym Ed. 2020;31(3):324–49.
Vakhrushev IV, et al. Heterotypic multicellular spheroids as experimental and preclinical models of sprouting angiogenesis. Biology (Basel). 2021;11(1):18.
Choi J, et al. FGF2-primed 3D spheroids producing IL-8 promote therapeutic angiogenesis in murine hindlimb ischemia. npj Regenerative Med. 2021;6(1):48.
Brüningk SC, et al. A cellular automaton model for spheroid response to radiation and hyperthermia treatments. Sci Rep. 2019;9(1):17674.
de Oliveira Silva N, et al. Cellular and molecular antiproliferative effects in 2D monolayer and 3D-cultivated HT-29 cells treated with zerumbone. Naunyn-Schmiedeberg’s Arch Pharmacol. 2023;397(3):1561–73.
Brüningk SC, et al. 3D tumour spheroids for the prediction of the effects of radiation and hyperthermia treatments. Sci Rep. 2020;10(1):1653.
Szade K, et al. Spheroid-plug model as a tool to study tumor development, angiogenesis, and heterogeneity in vivo. Tumor Biology. 2016;37(2):2481–96.
Durymanov M, et al. Subcutaneous inoculation of 3D pancreatic cancer spheroids results in development of reproducible stroma-rich tumors. Transl Oncol. 2019;12(1):180–9.
Tachibana T, et al. Establishment of an in vivo xenograft mouse model of a subcutaneous submillimeter HT-29 tumor formed from a single spheroid transplanted using radiation-crosslinked gelatin hydrogel microwell. Appl Sci. 2021. https://doi.org/10.3390/app11157031.
Wang T, et al. Antioxidants stimulate BACH1-dependent tumor angiogenesis. J Clin Invest. 2023. https://doi.org/10.1172/JCI169671.
Choi SY, et al. Differential angiogenic potential of 3-dimension spheroid of HNSCC cells in mouse xenograft. Int J Mol Sci. 2021;22(15):8245.
Izadpanah M, et al. Exosomes as theranostic agents in reproduction system. Adv Biol. 2023;8(2):2300258.
Ateeq M, et al. Extracellular vesicles&rsquo role in angiogenesis and altering angiogenic signaling. Med Sci. 2024. https://doi.org/10.3390/medsci12010004.
Pereira RC, et al. Elucidating the role of matrix porosity and rigidity in glioblastoma type IV progression. Appl Sci. 2020. https://doi.org/10.3390/app10249076.
Johnson PA, et al. A rapid high throughput bioprinted colorectal cancer spheroid platform for in vitro drug- and radiation-response. Biofabrication. 2023;15(1): 014103.
Fiore PF, et al. Different effects of NK cells and NK-derived soluble factors on cell lines derived from primary or metastatic pancreatic cancers. Cancer Immunol Immunother. 2023;72(6):1417–28.
Capik O, Gumus R, Karatas OF. Hypoxia-induced tumor exosomes promote angiogenesis through miR-1825/TSC2/mTOR axis in oral squamous cell carcinoma. Head Neck. 2023;45(9):2259–73.
Zhou Y, et al. Bone marrow mesenchymal stem cells-derived exosomes for penetrating and targeted chemotherapy of pancreatic cancer. Acta Pharm Sin B. 2020;10(8):1563–75.
Hao R, et al. A high-throughput nanofluidic device for exosome nanoporation to develop cargo delivery vehicles. Small. 2021;17(35):2102150.
Anthon SG, Valente KP. Vascularization strategies in 3D cell culture models: from scaffold-free models to 3D bioprinting. Int J Mol Sci. 2022;23(23):14582.
Liu C, et al. Droplet-based bioprinting for fabrication of tumor spheroids. Int J Bioprinting. 2024;10(1):1214.
Utama RH, et al. A 3D bioprinter specifically designed for the high-throughput production of matrix-embedded multicellular spheroids. Iscience. 2020. https://doi.org/10.1016/j.isci.2020.101621.
Hong S, Song JM. 3D bioprinted drug-resistant breast cancer spheroids for quantitative in situ evaluation of drug resistance. Acta Biomater. 2022;138:228–39.
Wang X, et al. Converging bioprinting and organoids to better recapitulate the tumor microenvironment. Trends Biotechnol. 2023;42:648.
Meng F, et al. 3D bioprinted in vitro metastatic models via reconstruction of tumor microenvironments. Adv Mater. 2019;31(10):1806899.
Ko J, et al. Patient-derived tumor spheroid-induced angiogenesis preclinical platform for exploring therapeutic vulnerabilities in cancer. Biomaterials. 2024;306:122504.
Han S, et al. 3D bioprinted vascularized tumour for drug testing. Int J Mol Sci. 2020;21(8):2993.
Li X, et al. Microfluidic 3D cell culture: potential application for tissue-based bioassays. Bioanalysis. 2012;4(12):1509–25.
Miller JS, et al. Rapid casting of patterned vascular networks for perfusable engineered three-dimensional tissues. Nat Mater. 2012;11(9):768–74.
Wan Z, et al. New strategy for promoting vascularization in tumor spheroids in a microfluidic assay. Adv Healthcare Mater. 2023;12(14):2201784.
Ngo MT, Sarkaria JN, Harley BAC. Perivascular stromal cells instruct glioblastoma invasion, proliferation, and therapeutic response within an engineered brain perivascular niche model. Adv Sci. 2022;9(31):2201888.
Mitrakas AG, et al. Applications and advances of multicellular tumor spheroids: challenges in their development and analysis. Int J Molecular Sci. 2023;24(8):6949.
Langhans SA. Three-dimensional in vitro cell culture models in drug discovery and drug repositioning. Front Pharmacol. 2018;23(9):6.
Han SJ, Kwon S, Kim KS. Challenges of applying multicellular tumor spheroids in preclinical phase. Cancer Cell Int. 2021;21:1–9.
Achilli TM, Meyer J, Morgan JR. Advances in the formation, use and understanding of multi-cellular spheroids. Expert Opin Biol Therapy. 2012;12(10):1347–60.
Serra D, et al. Self-organization and symmetry breaking in intestinal organoid development. Nature. 2019;569(7754):66–72.
Kim S, et al. Uniform sized cancer spheroids production using hydrogel-based droplet microfluidics: a review. Biomed Microdevices. 2024;26(2):1–9.
Franchi-Mendes T, et al. 3D cancer models: depicting cellular crosstalk within the tumour microenvironment. Cancers. 2021;13(18):4610.
Qu J, et al. Tumor organoids: synergistic applications, current challenges, and future prospects in cancer therapy. Cancer Commun. 2021;41(12):1331–53.
Marques dos Reis E, Vieira Berti F. Vasculogenic mimicry—an overview. In: Marques dos Reis E, Berti F, editors. Vasculogenic mimicry. Methods in molecular biology, vol. 2514. New York: Humana; 2022.
Bidan N, et al. Multicellular tumor spheroid model to study the multifaceted role of tumor-associated macrophages in PDAC. Drug Deliv Transl Res. 2024;14(8):2085–99.
Nashimoto Y, et al. Vascularized cancer on a chip: the effect of perfusion on growth and drug delivery of tumor spheroid. Biomaterials. 2020;229: 119547.
Nii T, Makino K, Tabata YJC. Three-dimensional culture system of cancer cells combined with biomaterials for drug screening. Cancers. 2020;12(10):2754.
Yao L, et al. Application of tumoroids derived from advanced colorectal cancer patients to predict individual response to chemotherapy. J Chemother. 2023;35(2):104–16.
Powley IR, et al. Patient-derived explants (PDEs) as a powerful preclinical platform for anti-cancer drug and biomarker discovery. Br J Cancer. 2020;122(6):735–44.
Quarta A, et al. Investigation on the composition of agarose-collagen I blended hydrogels as matrices for the growth of spheroids from breast cancer cell lines. Pharmaceutics. 2021;13(7):963.
Kim W, et al. Therapeutic strategies of three-dimensional stem cell spheroids and organoids for tissue repair and regeneration. Bioactive Mater. 2023;19:50–74.
Chai Q, Jiao Y, Yu X. Hydrogels for biomedical applications: their characteristics and the mechanisms behind them. Gels. 2017;3(1):6.
Sepantafar M, et al. Engineered hydrogels in cancer therapy and diagnosis. Trends Biotechnol. 2017;35(11):1074–87.
Spicer CD. Hydrogel scaffolds for tissue engineering: the importance of polymer choice. Polym Chem. 2020;11(2):184–219.
Emmermacher J, et al. Engineering considerations on extrusion-based bioprinting: interactions of material behavior, mechanical forces and cells in the printing needle. Biofabrication. 2020;12(2): 025022.
Leiva MC, et al. Breast cancer patient-derived scaffolds as a tool to monitor chemotherapy responses in human tumor microenvironments. J Cell Physiol. 2021;236(6):4709–24.
Parkinson GT, et al. Patient-derived scaffolds as a model of colorectal cancer. Cancer Med. 2021;10(3):867–82.
Permlid AM, et al. Unique animal friendly 3D culturing of human cancer and normal cells. Toxicol In Vitro. 2019;60:51–60.
Dondajewska E, et al. Heterotypic breast cancer model based on a silk fibroin scaffold to study the tumor microenvironment. Oncotarget. 2018;9(4):4935.
Xue J, et al. States U. Electrospinning and electrospun nano fibers: methods, materials, and applications. Chem Rev. 2019;119:5298.
Kuriakose AE, et al. Scaffold-based lung tumor culture on porous PLGA microparticle substrates. PLoS ONE. 2019;14(5): e0217640.
Bosnakovski D, et al. Chondrogenic differentiation of bovine bone marrow mesenchymal stem cells in pellet cultural system. Exp Hematol. 2004;32(5):502–9.
Zhang L, et al. Chondrogenic differentiation of human mesenchymal stem cells: a comparison between micromass and pellet culture systems. Biotech Lett. 2010;32:1339–46.
Caron MM, et al. Redifferentiation of dedifferentiated human articular chondrocytes: comparison of 2D and 3D cultures. Osteoarthritis Cartilage. 2012;20(10):1170–8.
Francioli SE, et al. Effect of three-dimensional expansion and cell seeding density on the cartilage-forming capacity of human articular chondrocytes in type II collagen sponges. J Biomed Mater Res Part A. 2010;95(3):924–31.
Loeser RF, et al. Osteoarthritis: a disease of the joint as an organ. Arthritis Rheum. 2012;64(6):1697.
Schulze-Tanzil G, et al. Redifferentiation of dedifferentiated human chondrocytes in high-density cultures. Cell Tissue Res. 2002;308:371–9.
Zhang L, et al. Chondrogenic differentiation of human mesenchymal stem cells: a comparison between micromass and pellet culture systems. Biotechnol Lett. 2010;32(9):1339–46.
Rai MF, et al. Molecular and phenotypic modulations of primary and immortalized canine chondrocytes in different culture systems. Res Vet Sci. 2009;87(3):399–407.
Fickert S, et al. One-year clinical and radiological results of a prospective, investigator-initiated trial examining a novel, purely autologous 3-dimensional autologous chondrocyte transplantation product in the knee. Cartilage. 2012;3(1):27–42.
Raffo-Romero A, et al. Establishment and characterization of canine mammary tumoroids for translational research. BMC Biol. 2023;21(1):23.
Zhu X, et al. Cancer evolution: a means by which tumors evade treatment. Biomed Pharmacother. 2021;133: 111016.
Timmins NE, Nielsen LK. Generation of multicellular tumor spheroids by the hanging-drop method. In: Hauser H, Fussenegger M, editors. Tissue engineering, vol. 140. New York: Humana Press; 2007.
Costa EC, et al. Spheroids formation on non-adhesive surfaces by liquid overlay technique: considerations and practical approaches. Biotechnol J. 2018;13(1):1700417.
Bhatia SN, Ingber DE. Microfluidic organs-on-chips. Nat Biotechnol. 2014;32(8):760–72.
Huh D. A human breathing lung-on-a-chip. Ann Am Thorac Soc. 2015;12(Supplement 1):S42–4.
Shrestha J, et al. Lung-on-a-chip: the future of respiratory disease models and pharmacological studies. Crit Rev Biotechnol. 2020;40(2):213–30.
Jang K-J, et al. Reproducing human and cross-species drug toxicities using a Liver-Chip. Sci Transl Med. 2019;11(517):eaax5516.
Dickson I. Multispecies liver-on-a-chip for improved drug toxicity testing. Nat Rev Gastroenterol Hepatol. 2020;17(1):4–4.
Wilmer MJ, et al. Kidney-on-a-chip technology for drug-induced nephrotoxicity screening. Trends Biotechnol. 2016;34(2):156–70.
Abulaiti M, et al. Establishment of a heart-on-a-chip microdevice based on human iPS cells for the evaluation of human heart tissue function. Sci Rep. 2020;10(1):19201.
Sirenko O et al. Evaluating Drug Response in 3D Triple Negative Breast Cancer Tumoroids with High Content Imaging and Analysis. 2022.
Melissaridou S, et al. The effect of 2D and 3D cell cultures on treatment response, EMT profile and stem cell features in head and neck cancer. Cancer Cell Int. 2019;19:16.
El Feky SE, et al. Cytotoxic, chemosensitizing and radiosensitizing effects of curcumin based on thioredoxin system inhibition in breast cancer cells: 2D vs. 3D cell culture system. Exp Ther Med. 2021;21(5):506.
Chen Z, et al. 3D hanging spheroid plate for high-throughput CAR T cell cytotoxicity assay. J Nanobiotechnol. 2022;20(1):30.
Zhao L, et al. A 3D printed hanging drop dripper for tumor spheroids analysis without recovery. Sci Rep. 2019;9(1):19717.
Kroupová J, Hanuš J, Štěpánek F. Surprising efficacy twist of two established cytostatics revealed by a-la-carte 3D cell spheroid preparation protocol. Eur J Pharm Biopharm. 2022;180:224–37.
Sokolova V, et al. Calcium phosphate nanoparticle-mediated transfection in 2D and 3D mono- and co-culture cell models. Acta Biomater. 2019;84:391–401.
Sarkar S, Peng CC, Tung YC. Comparison of VEGF-A secretion from tumor cells under cellular stresses in conventional monolayer culture and microfluidic three-dimensional spheroid models. PLoS ONE. 2020;15(11): e0240833.
Stryker ZI, et al. Evaluation of angiogenesis assays. Biomedicines. 2019;7(2):37.
Katt ME, et al. In vitro tumor models: advantages, disadvantages, variables, and selecting the right platform. Front Bioeng Biotechnol. 2016;4:12.
Carpentier G, et al. Angiogenesis analyzer for imageJ—a comparative morphometric analysis of “endothelial tube formation assay” and “fibrin bead assay.” Sci Rep. 2020;10(1):11568.
Xie D, et al. Effects of dulaglutide on endothelial progenitor cells and arterial elasticity in patients with type 2 diabetes mellitus. Cardiovasc Diabetol. 2022;21(1):200.
Khoo CP, Micklem K, Watt SM. A comparison of methods for quantifying angiogenesis in the Matrigel assay in vitro. Tissue Eng Part C Methods. 2011;17(9):895–906.
Nowak-Sliwinska P, et al. Consensus guidelines for the use and interpretation of angiogenesis assays. Angiogenesis. 2018;21(3):425–532.
Mason J, Öhlund D. Key aspects for conception and construction of co-culture models of tumor-stroma interactions. Front Bioeng Biotechnol. 2023;11:1150764.
Truelsen SLB, et al. The cancer angiogenesis co-culture assay: In vitro quantification of the angiogenic potential of tumoroids. PLoS ONE. 2021;16(7): e0253258.
Li S, et al. Identification of angiogenesis inhibitors using a co-culture cell model in a high-content and high-throughput screening platform. SLAS Technol. 2018;23(3):217–25.
Lin S, He X, He Y. Co-culture of ASCs/EPCs and dermal extracellular matrix hydrogel enhances the repair of full-thickness skin wound by promoting angiogenesis. Stem Cell Res Ther. 2021;12(1):129.
Perry L, et al. Co-culture systems for vasculogenesis. In: Holnthoner W, et al., editors. Vascularization for tissue engineering and regenerative medicine. Cham: Springer; 2021. p. 385–413.
Simons M, et al. State-of-the-art methods for evaluation of angiogenesis and tissue vascularization: a scientific statement from the american heart association. Circ Res. 2015;116(11):e99-132.
Chu P-Y, et al. Applications of the chick chorioallantoic membrane as an alternative model for cancer studies. Cells Tissues Organs. 2021;211(2):222–37.
Janser FA, et al. The Chick Chorioallantoic Membrane (CAM) Assay as a Three-dimensional Model to Study Autophagy in Cancer Cells. Bio Protoc. 2019;9(13): e3290.
Stoletov K, et al. Discovery of metastatic regulators using a rapid and quantitative intravital chick chorioallantoic membrane model. J Vis Exp. 2021;168: e62077.
Ribatti D. The CAM assay in the study of the metastatic process. Exp Cell Res. 2021;400(2): 112510.
Merckx G, et al. Chorioallantoic membrane assay as model for angiogenesis in tissue engineering: focus on stem cells. Tissue Eng Part B Rev. 2020;26(6):519–39.
Birsner AE, Benny O, D’Amato RJ. The corneal micropocket assay: a model of angiogenesis in the mouse eye. J Vis Exp. 2014;90:e51375.
Morbidelli L, Ciccone V, Ziche M. Studying angiogenesis in the rabbit corneal pocket assay. In: Ribatti D, editor. Vascular morphogenesis: methods and protocols. New York: Springer; 2021. p. 89–101.
Mir B. Laboratory study on the effect of plastic waste additive on shear strength of marginal soil. In: Sustainable Civil Engineering Practices: Select Proceedings of ICSCEP 2019. 2020. Springer.
Aref Z, Quax PHA. In vivo matrigel plug assay as a potent method to investigate specific individual contribution of angiogenesis to blood flow recovery in mice. Int J Mol Sci. 2021;22(16):8909.
Cabezas-Sáinz P, et al. Modeling cancer using zebrafish xenografts: drawbacks for mimicking the human microenvironment. Cells. 2020;9(9):1978.
Eberlein J, et al. Molecular and cellular mechanisms of vascular development in Zebrafish. Life (Basel). 2021;11(10):1088.
Makwana S, Mandal CC. Animal models for angiogenesis on cancer research. In: Pathak S, Banerjee A, Bisgin A, editors. Handbook of animal models and its uses in cancer research. Singapore: Springer; 2023. p. 397–419.
Kapoor A, Chen CG, Iozzo RV. A simplified aortic ring assay: a useful ex vivo method to assess biochemical and functional parameters of angiogenesis. Matrix Biol Plus. 2020;6–7: 100025.
Iqbal F, et al. Angiogenic potency evaluation of cell therapy candidates by a novel application of the in vitro aortic ring assay. Stem Cell Res Ther. 2017;8(1):184.
Kim SJ, et al. Molecular, cellular, and functional heterogeneity of retinal and choroidal endothelial cells. Invest Ophthalmol Vis Sci. 2023;64(10):35–35.
Tomita Y, et al. An ex vivo choroid sprouting assay of ocular microvascular angiogenesis. J Vis Exp. 2020;162:e61677.
Tetzlaff F, Fischer A. Human endothelial cell spheroid-based sprouting angiogenesis assay in collagen. Bio Protoc. 2018;8(17): e2995.
Augustin H, Korff T. SP0090 3D spheroids for the study of endothelial cell differentiation and angiogenesis. London: BMJ Publishing Group Ltd.; 2001.
Vorwald CE, Joshee S, Leach JK. Spatial localization of endothelial cells in heterotypic spheroids influences Notch signaling. J Mol Med. 2020;98(3):425–35.
Bauman E, et al. Xeno-free pre-vascularized spheroids for therapeutic applications. Sci Rep. 2018;8(1):230.
Eckermann CW, et al. Characterization and modulation of fibroblast/endothelial cell co-cultures for the in vitro preformation of three-dimensional tubular networks. Cell Biol Int. 2011;35(11):1097–110.
Dey M, et al. Studying tumor angiogenesis and cancer invasion in a three-dimensional vascularized breast cancer micro-environment. Adv Biol (Weinh). 2021;5(7): e2100090.
Park S, et al. One-step achievement of tumor spheroid-induced angiogenesis in a high-throughput microfluidic platform: one-step tumor angiogenesis platform. Organoid. 2023;3: e3.
Wartenberg M, et al. Tumor-induced angiogenesis studied in confrontation cultures of multicellular tumor spheroids and embryoid bodies grown from pluripotent embryonic stem cells. FASEB J. 2001;15(6):995–1005.
Chaddad H. Development of vascularized tumor spheroids mimicking the tumor environment: angiogenesis and hypoxia. 2019, Strasbourg.
Timmins N, Dietmair S, Nielsen L. Hanging-drop multicellular spheroids as a model of tumour angiogenesis. Angiogenesis. 2004;7:97–103.
Franchi-Mendes T, Lopes N, Brito C. Heterotypic tumor spheroids in agitation-based cultures: a scaffold-free cell model that sustains long-term survival of endothelial cells. Front Bioeng Biotechnol. 2021;9:649949.
Ko J, et al. Tumor spheroid-on-a-chip: a standardized microfluidic culture platform for investigating tumor angiogenesis. Lab Chip. 2019;19(17):2822–33.
Tae Joon K and Esak L, <em>In vitro</em> modeling of tumor spheroid interactions to perfused blood vessels. bioRxiv, 2020; 2020.08.03.234633.
Filho IPT, Hartley-Asp B, Borgström P. Quantitative angiogenesis in a syngeneic tumor spheroid model. Microvasc Res. 1995;49(2):212–26.
Alajati A, et al. Spheroid-based engineering of a human vasculature in mice. Nat Methods. 2008;5(5):439–45.
Bingle L, et al. Macrophages promote angiogenesis in human breast tumour spheroids in vivo. Br J Cancer. 2006;94(1):101–7.
Acknowledgements
Authors wish to thank the personnel of the Stem Cell Research Center for their help and guidance.
Funding
This study was supported by a grant (no: 72983) from Tabriz University of Medical Sciences under the ethical code of IR.TBZMED.VCR.REC.1402.216.
Author information
Authors and Affiliations
Contributions
Z. A-M., P.K., M.T.N., N.M., N.D.K., A.H., A.R., F.S., S.R., S.A.C., and G. R., collected data and wrote the draft. R.R. acquired financial support and supervised the study.
Corresponding author
Ethics declarations
Ethics approval and consent to participate
Not applicable.
Consent for publication
Not applicable.
Competing interests
The authors declare that they have no conflict of interest.
Additional information
Publisher's Note
Springer Nature remains neutral with regard to jurisdictional claims in published maps and institutional affiliations.
Rights and permissions
Open Access This article is licensed under a Creative Commons Attribution-NonCommercial-NoDerivatives 4.0 International License, which permits any non-commercial use, sharing, distribution and reproduction in any medium or format, as long as you give appropriate credit to the original author(s) and the source, provide a link to the Creative Commons licence, and indicate if you modified the licensed material. You do not have permission under this licence to share adapted material derived from this article or parts of it. The images or other third party material in this article are included in the article’s Creative Commons licence, unless indicated otherwise in a credit line to the material. If material is not included in the article’s Creative Commons licence and your intended use is not permitted by statutory regulation or exceeds the permitted use, you will need to obtain permission directly from the copyright holder. To view a copy of this licence, visit http://creativecommons.org/licenses/by-nc-nd/4.0/.
About this article
Cite this article
Abbasi-Malati, Z., Khanicheragh, P., Narmi, M.T. et al. Tumoroids, a valid preclinical screening platform for monitoring cancer angiogenesis. Stem Cell Res Ther 15, 267 (2024). https://doi.org/10.1186/s13287-024-03880-4
Received:
Accepted:
Published:
DOI: https://doi.org/10.1186/s13287-024-03880-4