Abstract
Background
This comprehensive systematic review and meta-analysis investigated the mid- to long-term efficacy and safety of stem cell therapy in patients with acute myocardial infarction (AMI).
Methods
The study encompassed 79 randomized controlled trials with 7103 patients, rendering it the most up-to-date and extensive analysis in this field. This study specifically focused on the impact of stem cell therapy on left ventricular ejection fraction (LVEF), major adverse cardiac events (MACE), and infarct size.
Results
Stem cell therapy significantly improved LVEF at 6, 12, 24, and 36 months post-transplantation compared to control values, indicating its potential for long-term cardiac function enhancement. A trend toward reduced MACE occurrence was observed in the intervention groups, suggesting the potential of stem cell therapy to lower the risk of cardiovascular death, reinfarction, and stroke. Significant LVEF improvements were associated with long cell culture durations exceeding 1 week, particularly when combined with high injected cell quantities (at least 108 cells). No significant reduction in infarct size was observed.
Conclusions
This review highlights the potential of stem cell therapy as a promising therapeutic approach for patients with AMI, offering sustained LVEF improvement and a potential reduction in MACE risk. However, further research is required to optimize cell culture techniques, determine the optimal timing and dosage, and investigate procedural variations to maximize the efficacy and safety of stem cell therapy in this context.
Similar content being viewed by others
Background
Despite significant prognostic advancements over the past decade, acute myocardial infarction (AMI) remains a significant contributor to global morbidity and mortality [1]. AMI continues to emerge as the primary driver of heart failure (HF), with a substantial impact on the patient’s quality of life and healthcare costs [2]. Thus, emphasizing heart function preservation in patients with AMI is crucial, considering its implications for patient survival and the economic burden associated with HF progression [3].
Existing conventional treatments effectively and temporarily control the disease, underscoring the need for innovative methods aimed at preventing and reversing heart dysfunction. Stem cell therapy has significant regenerative potential in addressing the short-term effects of cardiac damage following AMI [4]. Research on this treatment method is ongoing, and although short-term effects (6 months) on cardiac function have been reported [4, 5], long-term evaluations ranging from 18 months to 3 years have yielded inconsistent data on whether cell transplantation improves cardiac function because of the small number of patients recruited in individual studies [6, 7].
It has been reported that there is an effective improvement in cardiac function as the number of injected stem cells increases [8]. However, the administration of a substantial number of stem cells necessitates a significant harvest from either the patient's blood or bone marrow (BM), a task often fraught with difficulty owing to the challenge of securing an adequate quantity of stem cells. In autologous stem cell transplantation, concerted efforts have been made to increase the quantity of stem cells through in vitro cultivation and proliferation. The duration of isolation and culture, and the timing of subsequent administration are also considered to influence stem cell therapy outcomes in patients with AMI [5, 9]. However, there is a lack of clarity regarding the optimal number of cells. Moreover, the effects of therapy and the appropriate length of time for cell culture to enable the injection of a large number of cells have not yet been discussed.
Administering stem cell therapy before complete myocardial damage may be an effective alternative to current treatment methods [10]. However, injecting stem cells too early can increase the procedural risks. Therefore, questions have been raised regarding the optimal time required from primary percutaneous coronary intervention (PCI) to cell infusion to ensure safe and effective treatment.
Traditionally, the primary outcomes used to evaluate the effectiveness of stem cell infusion include left ventricular ejection fraction (LVEF), left ventricle end-diastolic volume, and infarct size. However, these indicators often involve subjective interpretations by evaluators, as is the case with echocardiography, which cannot be eliminated in most studies [11]. Therefore, increasing attention has been paid to major adverse cardiac events (MACEs) as the patient outcomes, with a focus on observable events. A MACE is a composite endpoint event that includes cardiovascular death, reinfarction, and stroke [12]. As a critical composite endpoint, MACE has frequently been used to evaluate the safety and efficacy of treatment strategies in patients with acute coronary syndrome [13]. MACE significantly contributes to the morbidity and mortality of patients with AMI [13].
Through this systematic review, we aim to evaluate the mid- to long-term effectiveness of stem cell therapy in patients with AMI. We also intend to determine the appropriate cell quantity and optimal transplantation time to maximize treatment efficacy while ensuring safety.
Methods
The protocol for this review has been prospectively registered in the PROSPERO systematic review database (CRD42023422818). The protocol was prepared according to the Preferred Reporting Items for Systematic Reviews and Meta-Analyses protocol checklist. The final report was prepared and submitted according to the Cochrane Handbook for Systematic Reviews of Interventions.
Search strategy
In this systematic review, searches were performed in the following databases: Ovid-MEDLINE, Ovid-EMBASE, Cochrane Library, KoreaMed, KMBASE, KISS, RISS, and DBpia, up to May 11th, 2023, to find relevant studies. The search terms included Medical Subject Headings in the titles and abstracts. We used the following keywords: “myocardial infarction,” “ST elevation myocardial infarction,” “non-ST elevated myocardial infarction,” “angina pectoris,” “myocardial ischemia,” coronary artery disease,” “coronary occlusion,” “coronary stenosis,” “acute coronary syndrome,” “STEMI,” “NSTEMI,” “stem cells,” “bone marrow cells,” “mesenchymal stem cells,” “mononuclear cells,” “mesenchymal stromal cells,” “pluripotent stromal cells,” “embryonic stromal cells,” and “cardiac progenitor cells.” We applied the “removes records about animal” and “RCT” filters and English language limitations. Only peer-reviewed studies were included in this analysis.
Study selection criteria
The intervention groups included patients with AMI who underwent PCI and received stem cell therapy by injection into the coronary arteries, myocardium, or veins. Patients with AMI who underwent PCI intervention but did not receive stem cell therapy comprised the control group.
We included the following study types: (1) randomized controlled trials (RCTs); (2) studies including patients diagnosed with AMI as per the International Classification of Diseases, Eleventh Edition, definition (myocardial infarction specified as acute or with a stated duration of 4 weeks [28 days] or fewer from onset) within the specified timeframe; (3) clinical trials in which allogenic or autologous stem cells were transplanted; and (4) studies in which more than one proper clinical outcome (LVEF, MACE, infarct size, etc.) was reported. Studies were excluded if they were: (1) non-human or pre-clinical studies; (2) non-original articles (systematic reviews, editorials, letters, comments, opinion pieces, reviews, guidelines, notes, news articles, etc.); (3) non-RCT trials; (4) continuous or duplicate studies; or (5) not available with the complete original text.
Study identification was performed by two independent reviewers (HSL and SHL). Any discrepancies and/or disagreements were resolved by discussion with a third reviewer (YJH). We eliminated duplicate studies and conducted a screening based on titles and abstracts. Subsequently, we identified potentially relevant studies and examined their full text. Finally, 79 RCTs were selected for inclusion in the systematic review. Sixty-nine RCTs were included in the meta-analysis.
Data extraction
Data were collected by two reviewers (HSL and YJH) using standardized forms. Data on publication characteristics (year of publication, journal, country, and corresponding author), study populations (eligibility criteria, age, and sex), intervention details (diagnosis, cell type, cell dose, culture period, injection route, time from the onset of myocardial infarction to the first intervention [cell injection], and number of injected cells), study designs (methods, sample size, and follow-up months), and clinical endpoints (efficacy and safety) were recorded.
Outcome measures
Primary outcomes
The primary outcomes of our study were post-treatment efficacy indices, such as LVEF, infarct size, and MACEs, which are defined as composite outcomes of cardiovascular death, and non-fatal myocardial infarction or stroke [12]. In several studies, cardiac function was measured using more than one modality; however, we included only one modality per study for the analysis of LVEF outcomes. If a single study reported LVEF using multiple modalities, we analyzed the data based on echocardiography, which is the commonly used method in most studies. In the absence of echocardiographic results, we analyzed results from magnetic resonance imaging (MRI) followed by single-photon emission computed tomography (SPECT). A subgroup meta-analysis was conducted to identify the differences in results based on the methods (echocardiography, MRI, SPECT, angiography) used to measure efficacy.
A subgroup analysis was conducted to analyze the differences in LVEF improvement based on various stem cell characteristics. The following subgroups were defined by baseline characteristics: (1) whether the cells were cultured, (2) length of time the cells had been cultured, and (3) measurement methods. The cut-off points for the length of time the cells were cultured [14] and the number of injected cells were based on the results of previous cell therapy studies [15, 16].
The secondary outcome was safety, assessed based on the occurrence of adverse events (AEs). We defined procedure-related AEs as complications that occurred during hospitalization in patients receiving stem cell injections. Whereas, non-procedure-related AEs were the events that developed during the follow-up period after hospital discharge for patients receiving stem cell injections. For procedure-related AEs, we analyzed events such as death, obstruction and/or thrombus of the related artery, coronary dissection, coronary spasm, and arrhythmia. We also investigated procedure-related complications associated with BM suppression or granulocyte colony-stimulating factor administration. Safety outcomes during the follow-up period included mortality, rehospitalization, stroke, cancer, and restenosis of the related artery.
Quality assessment
A single reviewer (HSL) assessed the selected studies for quality, and a second reviewer (YJH) confirmed the evaluation using the Cochrane Collaboration tool for assessing the risk of bias in randomized trials. The assessment criteria included random sequence generation, allocation concealment, blinding of the participants and personnel, blinding of the outcome assessments, incomplete outcome data, and selective reporting (Additional file 1).
Statistical analysis
We performed a meta-analysis using Review Manager version 5.4 from the Cochrane Library. Odds ratios (ORs) for dichotomous variables and mean differences and standardized mean differences for continuous variables were computed using a fixed-effects model. Statistical heterogeneity among the selected studies was evaluated using the chi-square test, with a significance level set at p < 0.10, and I2 statistics were used to quantify the degree of heterogeneity.
Results
Search and selection of stem cell studies
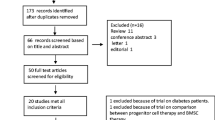
We identified 14,912 potentially relevant studies and screened them for eligibility, selecting 121 pertinent stem cell therapy studies for full-text review. Of the 121 studies, 42 were excluded because they did not describe AMI (n = 9), were not RCTs (n = 19), were duplicate reports (n = 3), were irrelevant interventions (n = 10), or had insufficient outcomes (n = 1). Finally, 79 RCTs with 7,103 patients were included in the review [11, 14, 17,18,19,20,21,22,23,24,25,26,27,28,29,30,31,32,33,34,35,36,37,38,39,40,41,42,43,44,45,46,47,48,49,50,51,52,53,54,55,56,57,58,59,60,61,62,63,64,65,66,67,68,69,70,71,72,73,74,75,76,77,78,79,80,81,82,83,84,85,86,87,88,89,90,91,92,93] and 69 RCTs were included in the meta-analysis (Fig. 1).
Study characteristics
Table 1 shows the characteristics of all the included studies. The selected studies were published between 2004 and 2022. The study size ranged from 15 to 375 patients, and the follow-up duration ranged from 1 to 60 months. Of the 79 studies, 49 were conducted in Europe, 9 in China, 7 in the USA, 5 in Korea, 3 in Iran, 2 in India, 2 in Brazil, 2 in Pan-Europe, and 1 in Russia. Among the 7103 patients, 4014 received stem cell therapy, and 3,120 were in the control group. Of the 79 studies, 75 used autologous stem cells and 4 used allogeneic stem cells [17,18,19,20].
Of the 75 studies using autologous stem cells, 66 used BM cells (BMCs), 3 used granulocyte colony-stimulating factor-mobilized peripheral blood stem cells (PBSCs) via leukapheresis [87,88,89], and 4 used both BMCs and PBSCs [90,91,92,93]. One study used umbilical cord-derived cells [19] and human cardiac stem cells [17]. Most studies used mononuclear cells (MNCs) (n = 63), and nine studies used mesenchymal stem cells (MSCs) cultured from BM aspirates. Two studies used BM-derived cluster of differentiation (CD)133 + cells [55, 56], one study used CD34 + and C-X-C chemokine receptor (CXCR)4 + cells [73], and one used both CD133 + and CD34 + cells [68]. Two studies used progenitor cells [53, 67]. Twenty-one studies have conducted cell culture, out of which, thirteen employed cell culture duration exceeding seven days.
Most cell injections (n = 75) were performed through intra-coronary infusion within 28 days of the primary PCI using the stopped-flow technique. Two studies infused cells intravenously after PCI [18, 20], and two other studies injected cells intramuscularly through the epicardium during coronary artery bypass graft operations [39, 40]. Except for one study [74] where stem cells were infused at 3–7 days and after 3 months, all studies infused a single injection of stem cells. The total numbers of injected cells are listed in Table 1. In 53 studies, cells were injected at quantities equal to or greater than 108. The comparisons included standard treatment (n = 40) and placebo (n = 30) groups. Seven studies did not report interventions received by the control group.
Left ventricular ejection fraction
Analyses based on a fixed-effects model for differences in LVEF, MACE, and infarct size are shown in Fig. 2. Stem cell therapy for patients with AMI improved LVEF at 6 months (2.91% increase; p < 0.001), 1 year (2.22% increase; p < 0.001), 2 years (2.61% increase; p < 0.001), and 3 years (2.50% increase; p = 0.005) compared with that in the control group (Fig. 2A–D). One study reporting a 5-year follow-up found no significant difference in LVEF between the intervention and control groups (Fig. 2E). In the subgroup analysis based on cell culture, studies with and without cell culture demonstrated a greater improvement in the intervention group than in the control group at 6 and 12 months of observation (Fig. 2F, G). After 24 months of observation, studies with cell culture showed a significant improvement in the intervention group compared to control values (5.11% increase; p < 0.001), whereas those without cell culture showed no significant difference (1.28% decrease; p = 0.23) (Fig. 2H).
Forest plots of left ventricular ejection fraction (LVEF) improvement. A–E Forest plots for LVEF at the A 6-, B 12-, C 24-, D 36-, and E 60-month follow-ups. F–H Subgroup comparisons of LVEF between the cultured cell therapy and non-cultured cell therapy groups at the F 6-, G 12-, and H 24-month follow-ups. I–K Subgroup analyses of LVEF based on the length of cell culture time at the I 6-, J 12-, and K 24-month follow-ups. L–N Subgroup comparisons of LVEF between patients treated with mononuclear cells (MNCs) and mesenchymal stem cells (MSCs) at the L 6-, M 12-, and N 24-month follow-ups
This analysis focused on cell culture studies, specifically examining the mean change in LVEF based on the duration of the cell culture. Among the studies with a cell culture period exceeding 1 week, the intervention group showed a significant improvement in LVEF at 6 months (4.32% increase; p < 0.001), 12 months (1.89% increase; p < 0.001), and 24 months (5.23% increase; p < 0.001) (Fig. 2I–K). However, in studies with a cell culture period of 1 week or less, there was a significant improvement only at 6 months (3.38% increase; p < 0.001), with no significant differences between the intervention and control groups at 12 months (1.40% increase; p = 0.46) or 24 months (1.96% increase; p = 0.66).
Cell type-based analyses showed a significant increase in LVEF in the intervention group compared with that of the control group at 6 and 12 months for treatment with MNCs and MSCs (Fig. 2L, M), which were the most commonly used cells. At 6 months, there was a 2.35% (p < 0.001) and a 4.47% (p < 0.001) increase in LVEF during MNC and MSC treatments, respectively. However, at 12 months, we observed a 1.87% (p < 0.001) and a 2.43% (p = 0.001) increase in LVEF during MNC and MSC treatments, respectively. At 24 months, there was no significant difference in LVEF for MNC treatment (p = 0.29), whereas a meta-analysis of two studies using MSCs (5.23% increase; p < 0.001) showed a significant improvement in LVEF in the intervention group compared to that in the control group (Fig. 2N).
Major cardiac adverse events
No significant difference in MACE occurrence was observed between the intervention and control groups at the 6-month observation point (OR 0.78; 95% confidence interval [CI]: 0.46, 1.31; p = 0.34; Fig. 3A). At the 12-month observation point (OR 0.62; 95% CI 0.38, 1.01; p = 0.05; Fig. 3B) and between 18 and 36 months (OR 0.63; 95% CI 0.39, 1.02; p = 0.06; Fig. 3C), the intervention group showed a tendency toward a lower risk of MACE than that shown by the control group. However, at the 60-month observation point (Fig. 3D), there was no significant difference in MACE occurrence between the intervention and control groups (OR 1.00; 95% CI 0.58, 1.72; p = 0.99).
Forest plots of major adverse cardiac events (MACEs). A–D Forest plots for MACE occurrence at the 6-, B 12-, C 18-, 24-, 36-, and D 60-month follow-ups. E–G Subgroup analyses of MACE occurrence between patients treated with mononuclear cells (MNCs) and mesenchymal stem cells (MSCs) at the (E) 6-, F 12-, and G 24-month follow-ups
Risk analysis of MACE, according to cell type, showed no significant difference between the intervention group and the control group at 6 months for either MNC (OR 0.58; 95% CI 0.30, 1.13; p = 0.11) or MSC (OR 1.23; 95% CI 0.52, 2.91; p = 0.63; Fig. 3E) treatment. At 12 months and between 18 and 36 months, the intervention group that received MNCs showed a significantly lower MACE risk than the control group (12 months OR 0.57; 95% CI 0.34, 0.97; p = 0.04) (18 and 36 months OR 0.61; 95% CI 0.37, 0.98; p = 0.04; Fig. 3F); whereas the group that received MSCs showed no significant difference in MACE risk from the control group (12 months OR 1.05; 95% CI 0.25, 4.36; p = 0.95) (18 and 36 months OR 3.15; 95% CI 0.12, 81.74; p = 0.49; Fig. 3G).
The occurrence of AEs related to stem cell injection resulted in one death each in three studies [19, 27, 82]; however, there were either no controls or no significant differences from the control group in AE occurrence. Additionally, cases of coronary artery restenosis, thrombosis, and coronary artery dissection were reported, but all were successfully treated.
Infarct size
Infarct size after stem cell therapy in patients with AMI showed no significant difference between the intervention and control groups at the 6-month (− 0.02; 95% CI − 0.14, 0.10; p = 0.75; Fig. 4A), 1-year (− 0.29; 95% CI − 0.29, 0.06; p = 0.19; Fig. 4B), 2-year (0.12; 95% CI, − 0.26, 0.50; p = 0.53; Fig. 4C), and 3–4-year observation points (0.01; 95% CI, − 0.44, 0.46; p = 0.95; Fig. 4D).
Discussion
This systematic review included 79 RCTs that investigated stem cell therapy in patients with AMI. Our work is the most recent and comprehensive systematic review. Additionally, this is the only study that has conducted an analysis based on the duration of cell culture and discusses the adequacy of infused cell counts and the appropriate timing of stem cell injection. The major finding of this study is the enhancement of LVEF in patients undergoing stem cell therapy, as compared to the control group, at 6 and 12 months, and 24 and 36 months durations. Additionally, the intervention groups undergoing stem cell transplantation had a lower MACE risk as compared to the control groups. Moreover, significant enhancements in LVEF were observed in studies employing cell culture, especially when the culture duration exceeded 1 week and the cell quantity of at least 108 was administered.
Mid- to long-term improvement in LVEF with stem cell therapy
We found that the intervention group showed modest improvements in LVEF at 6, 12, 24, and 36 months compared to the control group. Additionally, these improvements were more pronounced in patients receiving MSC injections. Previous systematic reviews have reported only the short-term (approximately 6 and 12 months) effectiveness of stem cell therapy. The studies evaluating patients from 18 months to 3 years are limited, resulting in inconsistent data on whether cell transplantation improves cardiac function [4, 6]. The current systematic review indicates that the effect of stem cell therapy on LVEF in patients with AMI may last up to 3 years. However, the effects at 5 years remain unclear as a limited number of studies report the follow-up results up to 5 years after stem cell injection.
In contrast to the improvement in LVEF, the reduction in infarct size showed no significant difference between the intervention and control groups in the observed 6- to 48-month period, indicating unclear recovery of the infarcted area in AMI with stem cell injection. The improvements in LVEF and lack of improvements in infarct size are consistent with the findings of previous systematic reviews [4]. The observed improvement in infarct-related regional wall motion abnormalities did not correspond to a significant change in infarct size, rendering this discrepancy difficult to explain. Infarct size measurement involves various modalities, such as MRI, echocardiography, and SPECT, leading to limitations owing to the lack of consistency in measurement techniques.
Potential role of stem cell therapy in reducing MACE risk
Our systematic review revealed a trend toward fewer MACEs in the intervention group than in the control group at 12 (p = 0.05) and 18–36 months (p = 0.06) after stem cell transplantation. In recent years, there has been significant emphasis on reporting MACEs as objective clinical outcomes in patients with heart disease. However, there is a notable scarcity of systematic reviews reporting MACEs as indicators of the efficacy or safety of stem cell transplantation for patients with AMI. Few studies have reported a reduction in MACE incidence after stem cell therapy. This could be attributed to the low incidence of MACEs in intervention groups when compared with that in the well-treated control groups receiving standard therapy that is highly effective. Another possibility is that patients with severe AMI might not have been recruited for stem cell therapy. Considering these points, although not statistically significant, the observed trends in cardiovascular death, non-fatal reinfarction, and non-fatal stroke are noteworthy given the difficulty in demonstrating improvement with cell therapy [7]. Thus, comprehensive analyses that integrate the results from additional studies are necessary to ascertain the true efficacy of cell-based treatments. When analyzed by cell type, the intervention group that received MNCs showed a significant reduction in MACE occurrence compared to the control group at 12 and 18–36 months. However, studies involving MSC injections did not show a significant difference in MACE risk between intervention and control groups. Due to the limited number of studies and MACE occurrences in MSC therapy research, it is difficult to conclude the incidence of MACEs in patients receiving MSC injections based on this meta-analysis. Further research is needed to validate these findings and provide more robust evidence for the effectiveness of stem cell transplantation in reducing MACE risk in patients with AMI.
Although reports of mortality and recurrent myocardial infarction within the hospitalization period exist for patients with AMI who received stem cell injections, no significant difference in frequency was found compared with that in the control group. Moreover, the reported rates of in-hospital mortality after PCI in previous studies ranged from 0.53 to 2.0% [94, 95], and the myocardial infarction recurrence rate was 0.7% [96], which was not significantly higher than control values, indicating relative safety. Most studies analyzed in this systematic review employed intra-coronary stem cell injection. In terms of complications, intra-coronary stem cell injection is generally considered safer than direct cell injection into the myocardium (trans-endocardial or trans-epicardial cell injection) [97].
Role of cell culture in increasing cell numbers and appropriate number of injected stem cells
In this study, a subgroup analysis was conducted by distinguishing studies that did and did not perform cell culture, demonstrating a significant improvement in LVEF during the mid-term period (12–24 months) in patients when cell culture was performed. Furthermore, the analysis of various studies with cell culture periods of less than 1 week and those exceeding 1 week revealed a significant improvement in LVEF during the mid-term period (12–24 months) in studies with a culture period exceeding 1 week. To our knowledge, few studies have analyzed the effects of cell therapy on cardiac function improvement considering whether cell culture was conducted and for how long. The results of this systematic review suggest that enhancing the purity of injected cells has a positive impact on the preservation or recovery of cardiac function. Therefore, we hypothesize that achieving a homogeneous cell population through culture may enhance therapeutic efficacy.
Ensuring an adequate number of selected cells is important to ensure the sufficient recovery of cardiac function. Therefore, recent research on cell therapy for myocardial infarction treatment has also focused on using selected cell products such as MSCs and CD34 + cells rather than BM-MNCs [7]. Although it is important to conduct cell processing and isolation effectively, increasing the number of cells may also be necessary. Therefore, in the context of autologous stem cell transplantation, efforts have been made to increase the number of stem cells selected through in vitro cultivation and proliferation.
A systematic review comprising 40 randomized controlled trials reported a significant increase in LVEF when the BMC dosage exceeded 108 cells [6]. Another systematic review analyzing 41 RCTs also concluded that the mortality risk was reduced in patients who received > 108 to ≤ 109 cells [8], similar to our study findings. Given the hostile environment of AMI, higher doses may be necessary to counteract the initial cell death caused by hypoxia in transplanted cells [98]. MSC doses lower or higher than 107 cells did not show differential improvements in LVEF, and using even higher cell doses (≥ 1010) did not significantly increase LVEF compared with that in the control group [6]. Administering a greater number of injections may pose a risk of myocardial damage, potentially diminishing the effectiveness of therapy and complicating the correlation between cell quantity and clinical benefits [98]. Considering the results of our study, injecting a cell quantity of at least 108 is preferable, whereas cell doses exceeding 1010 are unlikely to provide additional benefits. Moreover, when performing cell culture for cultivation and proliferation, ensuring a culture period of more than 1 week could be advantageous for increasing cell purity.
Additional research on methods to create purified cell populations is required and should include those employing processes such as cell culture that result in the selection of homogeneous cell populations. Moreover, further studies are required to improve the repair and regeneration functions of the stem cells. The methods for grafting the injected cells into damaged myocardial areas should also be investigated. Most studies included in this review involved autologous stem cells. However, the characteristics of each patient's stem cells were heterogeneous, and the results evaluated at the endpoint also exhibited a heterogeneous tendency. Subsequent studies should be conducted to inject sufficiently standardized allogeneic cells cultured from multiple patients and verify the outcomes.
Appropriate timing of stem cell injection for optimal effectiveness
In addition to cell dosage, the optimal timing of stem cell transplantation to achieve the greatest efficacy in improving cardiac function post-AMI has been investigated. Our study confirmed that ensuring a sufficient number of injected cells would help in the recovery of left ventricular function with an adequate culture period (more than 1 week). Similarly, some studies have suggested that the best transplantation time to secure an adequate culture period is between 7 and 14 days after PCI [14]. This strategy is advantageous because it allows time for the recovery of the damaged myocardium and coronary arteries.
However, contrary to our assertion, previous systematic reviews have suggested that the optimal timing for improving myocardial function is within 3–7 days post-AMI [15]. A systematic review of studies involving MSC transplantation post-AMI reported that, when performed during the first week [99], transplantation shows a higher efficacy in increasing LVEF, thereby improving the left ventricular end-systolic dimension and reducing the incidence of revascularization [100]. If stem cell transplantation is excessively delayed, its effectiveness may decrease due to myocardial cell loss and fibrosis [100]. However, this poses a risk of overlooking the inefficiency of excessively early stem cell transplantation and potential damage to the weakened heart. Immediately after AMI (1–2 days), an increased local apoptosis of transplanted stem cells is observed presumably due to significant myocardial ischemia and inflammation, post-reperfusion oxygen burst, and severe peroxidation injury. Therefore, the efficacy of stem cell therapy is poor [6]. The early injection of stem cells may be restricted owing to the risk of arrhythmias when cells are injected into a damaged heart with significant swelling, inflammation, and microvascular blockage, as well as potential coronary embolization and decreased blood flow. Additionally, the administration of heavy antiplatelet and anticoagulant medications can result in bleeding.
In our study, we have addressed crucial aspects of the previously reported optimal timing for stem cell transplantation, which is within 1 week of AMI. Further research is needed to explore the full extent of optimal timing for stem cell transplantation in patients post-AMI. Additionally, investigating potential strategies to mitigate the risks associated with early or delayed transplantation, such as minimizing AEs on coronary circulation, would be beneficial for enhancing the efficacy and safety of stem cell therapy.
In a study on repeated cell injection, when comparing 12 patients who received a single stem cell infusion at 3–7 days with 15 patients who received an initial infusion at 3–7 days followed by a second infusion at 3 months, the latter group showed more pronounced improvements in LVEF and reductions in infarct size, as assessed by MRI at 12 months, than the former group [74]. Further studies with larger patient populations are needed to draw definitive conclusions about the effectiveness of repeated stem cell infusions.
This study has some limitations. The analysis did not thoroughly scrutinize the procedural aspects of the stem cell therapy process, such as the cell collection technique or other preprocessing steps. Thus, further research is required to investigate whether variations in these processes lead to differences in efficacy. Moreover, the incidence of MACEs was too low in both the control and intervention groups to detect any statistically significant differences. Hence, further studies with larger sample sizes are necessary to elucidate the effects of stem cell transplantation on MACE occurrence. Long-term studies extending up to 5 years are warranted to provide a comprehensive understanding of the sustained effects of stem cell therapy on cardiac function post-AMI.
Conclusions
Our findings revealed the sustained enhancement of LVEF for up to 36 months post-transplantation and a trend toward decreased MACE risk in the intervention groups versus the control groups. Notably, significant LVEF improvements were observed with longer cell culture durations and higher injected cell quantities. Nevertheless, no significant reduction in infarct size was noted, which is consistent with previous reviews. Future research should explore the optimal timing and dosages while addressing procedural variations to enhance the efficacy and safety of stem cell therapy in patients with AMI.
Availability of data and materials
The data underlying this research will be shared on reasonable request to the corresponding author.
Abbreviations
- AMI:
-
Acute myocardial infarction
- LVEF:
-
Left ventricular ejection fraction
- MACE:
-
Major adverse cardiac event
- HF:
-
Heart failure
- PCI:
-
Percutaneous coronary intervention
- MRI:
-
Magnetic resonance imaging
- SPECT:
-
Single-photon emission computed tomography
- AE:
-
Adverse event
- OR:
-
Odds ratio
- RCT:
-
Randomized controlled trial
- BM:
-
Bone marrow
- BMC:
-
Bone marrow cell
- PBSC:
-
Peripheral blood stem cell
- MNC:
-
Mononuclear cell
- MSC:
-
Mesenchymal stem cell
- CD:
-
Cluster of differentiation
- CI:
-
Confidence interval
References
Reed GW, Rossi JE, Cannon CP. Acute myocardial infarction. Lancet. 2017;389(10065):197–210. https://doi.org/10.1016/S0140-6736(16)30677-8.
Groenewegen A, Rutten FH, Mosterd A, Hoes AW. Epidemiology of heart failure. Eur J Heart Fail. 2020;22(8):1342–56. https://doi.org/10.1002/ejhf.1858.
Salari N, Morddarvanjoghi F, Abdolmaleki A, Rasoulpoor S, Khaleghi AA, Hezarkhani LA, et al. The global prevalence of myocardial infarction: a systematic review and meta-analysis. BMC Cardiovasc Disord. 2023;23(1):206. https://doi.org/10.1186/s12872-023-03231-w.
Lee SH, Hong JH, Cho KH, Noh JW, Cho HJ. Discrepancy between short-term and long-term effects of bone marrow-derived cell therapy in acute myocardial infarction: a systematic review and meta-analysis. Stem Cell Res Ther. 2016;7(1):153. https://doi.org/10.1186/s13287-016-0415-z.
Botleroo RA, Bhandari R, Ahmed R, Kareem R, Gyawali M, Venkatesan N, et al. Stem cell therapy for the treatment of myocardial infarction: how far are we now? Cureus. 2021;13(8): e17022. https://doi.org/10.7759/cureus.17022.
Xu JY, Cai WY, Tian M, Liu D, Huang RC. Stem cell transplantation dose in patients with acute myocardial infarction: a meta-analysis. Chronic Dis Transl Med. 2016;2(2):92–101. https://doi.org/10.1016/j.cdtm.2016.09.006.
Bolli R. Cell therapy for acute myocardial infarction: requiescat in Pace. Eur Heart J. 2020;41(38):3711–4. https://doi.org/10.1093/eurheartj/ehaa802.
Otsuka T, Bär S, Losdat S, Kavaliauskaite R, Ueki Y, Zanchin C, et al. Effect of timing of staged percutaneous coronary intervention on clinical outcomes in patients with acute coronary syndromes. J Am Heart Assoc. 2021;10(23): e023129. https://doi.org/10.1161/JAHA.121.023129.
Yamada Y, Minatoguchi S, Kanamori H, Mikami A, Okura H, Dezawa M, et al. Stem cell therapy for acute myocardial infarction-focusing on the comparison between Muse cells and mesenchymal stem cells. J Cardiol. 2022;80(1):80–7. https://doi.org/10.1016/j.jjcc.2021.10.030.
Traverse JH, Henry TD, Ellis SG, Pepine CJ, Willerson JT, Zhao DX, et al. Effect of intracoronary delivery of autologous bone marrow mononuclear cells 2 to 3 weeks following acute myocardial infarction on left ventricular function: the late TIME randomized trial. JAMA. 2011;306(19):2110–9. https://doi.org/10.1001/jama/2011/1670.
Lee JW, Lee SH, Youn YJ, Ahn MS, Kim JY, Yoo BS, et al. A randomized, open-label, multicenter trial for the safety and efficacy of adult mesenchymal stem cells after acute myocardial infarction. J Korean Med Sci. 2014;29(1):23–31. https://doi.org/10.3346/jkms.2014.29.1.23.
Bosco E, Hsueh L, McConeghy KW, Gravenstein S, Saade E. Major adverse cardiovascular event definitions used in observational analysis of administrative databases: a systematic review. BMC Med Res Methodol. 2021;21(1):241. https://doi.org/10.1186/s12874-021-01440-5.
Duan H, Sun Z, Dong W, Huang Z. Utilizing dynamic treatment information for MACE prediction of acute coronary syndrome. BMC Med Inform Decis Mak. 2019;19(1):5. https://doi.org/10.1186/s12911-018-0730-7.
Zhang R, Yu J, Zhang N, Li W, Wang J, Cai G, et al. Bone marrow mesenchymal stem cells transfer in patients with ST-segment elevation myocardial infarction: single-blind, multicenter, randomized controlled trial. Stem Cell Res Ther. 2021;12(1):33. https://doi.org/10.1186/s13287-020-02096-6.
Delewi R, Hirsch A, Tijssen JG, Schächinger V, Wojakowski W, Roncalli J, et al. Impact of intracoronary bone marrow cell therapy on left ventricular function in the setting of ST-segment elevation myocardial infarction: a collaborative meta-analysis. Eur Heart J. 2014;35(15):989–98. https://doi.org/10.1093/eurheartj/eht372.
Shen T, Xia L, Dong W, Wang J, Su F, Niu S, et al. A systematic review and meta-analysis: safety and efficacy of mesenchymal stem cells therapy for heart failure. Curr Stem Cell Res Ther. 2021;16(3):354–65. https://doi.org/10.2174/1574888X15999200820171432.
Fernández-Avilés F, Sanz-Ruiz R, Bogaert J, Plasencia AC, Gilaberte I, Belmans A, et al. Safety and efficacy of intracoronary infusion of allogeneic human cardiac stem cells in patients with ST-segment elevation myocardial infarction and left ventricular dysfunction. Circ Res. 2018;123(5):579–89. https://doi.org/10.1161/CIRCRESAHA.118.312823.
Chullikana A, Majumdar AS, Gottipamula S, Krishnamurthy S, Kumar AS, Prakash VS, et al. Randomized, double-blind, phase I/II study of intravenous allogeneic mesenchymal stromal cells in acute myocardial infarction. Cytotherapy. 2015;17(3):250–61. https://doi.org/10.1016/j.jcyt.2014.10.009.
Gao LR, Chen Y, Zhang NK, Yang XL, Liu HL, Wang ZG, et al. Intracoronary infusion of Wharton’s jelly-derived mesenchymal stem cells in acute myocardial infarction: double-blind, randomized controlled trial. BMC Med. 2015;13:162. https://doi.org/10.1186/s12916-015-0399-z.
Hare JM, Traverse JH, Henry TD, Dib N, Strumpf RK, Schulman SP, et al. A randomized, double-blind, placebo-controlled, dose-escalation study of intravenous adult human mesenchymal stem cells (prochymal) after acute myocardial infarction. J Am Coll Cardiol. 2009;54(24):2277–86. https://doi.org/10.1016/j.jacc.2009.06.055.
Kim SH, Cho JH, Lee YH, Lee JH, Kim SS, Kim MY, et al. Improvement in left ventricular function with intracoronary mesenchymal stem cell therapy in a patient with anterior wall ST-Segment elevation myocardial infarction. Cardiovasc Drugs Ther. 2018;32(4):329–38. https://doi.org/10.1007/s10557-018-6804-z.
Lamirault G, de Bock E, Sébille V, Delasalle B, Roncalli J, Susen S, et al. Sustained quality of life improvement after intracoronary injection of autologous bone marrow cells in the setting of acute myocardial infarction: results from the BONAMI trial. Qual Life Res. 2017;26(1):121–5. https://doi.org/10.1007/s11136-016-1366-7.
Manrique A, Lemarchand P, Delasalle B, Lairez O, Sportouch-Duckan C, Lamirault G, et al. Predictors of ventricular remodelling in patients with reperfused acute myocardial infarction and left ventricular dysfunction candidates for bone marrow cell therapy: insights from the BONAMI trial. Eur J Nucl Med Mol Imaging. 2016;43(4):740–8. https://doi.org/10.1007/s00259-015-3279-z.
Wang X, Xi WC, Wang F. The beneficial effects of intracoronary autologous bone marrow stem cell transfer as an adjunct to percutaneous coronary intervention in patients with acute myocardial infarction. Biotechnol Lett. 2014;36(11):2163–8. https://doi.org/10.1007/s10529-014-1589-z.
Gao LR, Pei XT, Ding QA, Chen Y, Zhang NK, Chen HY, et al. A critical challenge: dosage-related efficacy and acute complication intracoronary injection of autologous bone marrow mesenchymal stem cells in acute myocardial infarction. Int J Cardiol. 2013;168(4):3191–9. https://doi.org/10.1016/j.ijcard.2013.04.112.
Jazi SM, Esfahani MH, Fesharaki M, Moulavi F, Gharipour M. Initial clinical outcomes of intracoronary infusion of autologous progenitor cells in patients with acute myocardial infarction. ARYA Atheroscler. 2012;7(4):162–7.
Quyyumi AA, Waller EK, Murrow J, Esteves F, Galt J, Oshinski J, et al. CD34(+) cell infusion after ST elevation myocardial infarction is associated with improved perfusion and is dose dependent. Am Heart J. 2011;161(1):98–105. https://doi.org/10.1016/j.ahj.2010.09.025.
Roncalli J, Mouquet F, Piot C, Trochu JN, Corvoisier PL, Neuder Y, et al. Intracoronary autologous mononucleated bone marrow cell infusion for acute myocardial infarction: results of the randomized multicenter BONAMI trial. Eur Heart J. 2011;32(14):1748–57. https://doi.org/10.1093/eurheartj/ehq455.
Kaminek M, Meluzin J, Panovský R, Metelkova I, Budikova M, Richter M. Long-term results of intracoronary bone marrow cell transplantation: the potential of gated sestamibi SPECT/FDG PET imaging to select patients with maximum benefit from cell therapy. Clin Nucl Med. 2010;35(10):780–7. https://doi.org/10.1097/RLU.0b013e3181e4d9c5.
Grajek S, Popiel M, Gil L, Breborowicz P, Lesiak M, Czepczyński R, et al. Influence of bone marrow stem cells on left ventricle perfusion and ejection fraction in patients with acute myocardial infarction of anterior wall: randomized clinical trial: impact of bone marrow stem cell intracoronary infusion on improvement of microcirculation. Eur Heart J. 2010;31(6):691–702. https://doi.org/10.1093/eurheartj/ehp536.
Meluzín J, Janousek S, Mayer J, Groch L, Hornácek I, Hlinomaz O, et al. Three-, 6-, and 12-month results of autologous transplantation of mononuclear bone marrow cells in patients with acute myocardial infarction. Int J Cardiol. 2008;128(2):185–92. https://doi.org/10.1016/j.ijcard.2007.04.098.
Kaminek M, Meluzin J, Panovsky R, Janousek S, Mayer J, Prasek J, et al. Individual differences in the effectiveness of intracoronary bone marrow cell transplantation assessed by gated sestamibi SPECT/FDG PET imaging. J Nucl Cardiol. 2008;15(3):392–9. https://doi.org/10.1016/j.nuclcard.2008.02.016.
Meluzín J, Mayer J, Groch L, Janousek S, Hornácek I, Hlinomaz O, et al. Autologous transplantation of mononuclear bone marrow cells in patients with acute myocardial infarction: the effect of the dose of transplanted cells on myocardial function. Am Heart J. 2006;152(5):975. https://doi.org/10.1016/j.ahj.2006.08.004.
Ge J, Li Y, Qian J, Shi J, Wang Q, Niu Y, et al. Efficacy of emergent transcatheter transplantation of stem cells for treatment of acute myocardial infarction (TCT-STAMI). Heart. 2006;92(12):1764–7. https://doi.org/10.1136/hrt.2005.085431.
Chen SL, Fang WW, Ye F, Liu YH, Qian J, Shan SJ, et al. Effect on left ventricular function of intracoronary transplantation of autologous bone marrow mesenchymal stem cell in patients with acute myocardial infarction. Am J Cardiol. 2004;94(1):92–5. https://doi.org/10.1016/j.amjcard.2004.03.034.
Mathur A, Sim DS, Choudry F, Veerapen J, Colicchia M, Tyrlejski T, et al. Five-year follow-up of intracoronary autologous cell therapy in acute myocardial infarction: the REGENERATE-AMI trial. ESC Heart Fail. 2022;9(2):1152–9. https://doi.org/10.1002/ehf2.13786.
Mathur A, Fernández-Avilés F, Bartunek J, Belmans A, Crea F, Dowlut S, et al. The effect of intracoronary infusion of bone marrow-derived mononuclear cells on all-cause mortality in acute myocardial infarction: the BAMI trial. Eur Heart J. 2020;41(38):3702–10. https://doi.org/10.1093/eurheartj/ehaa651.
Yang YJ, Qian HY, Song L, Geng YJ, Gao RL, Li N, et al. Strengthening effects of bone marrow mononuclear cells with intensive atorvastatin in acute myocardial infarction. Open Heart. 2020;7(1): e001139. https://doi.org/10.1136/openhrt-2019-001139.
Laguna G, DI Stefano S, Maroto L, Fulquet E, Echevarría JR, Revilla A, et al. Effect of direct intramyocardial autologous stem cell grafting in the sub-acute phase after myocardial infarction. J Cardiovasc Surg Torino. 2018;59(2):259–67. https://doi.org/10.23736/S0021-9509.17.10126-6.
Naseri MH, Madani H, Ahmadi Tafti SH, Farahani MM, Saleh DK, Hosseinnejad H, et al. COMPARE CPM-RMI trial: intramyocardial transplantation of autologous bone marrow-derived CD133+ cells and MNCs during CABG in patients with recent MI: a phase II/III, multicenter, placebo-controlled, randomized, double-blind clinical trial [published correction appears in Cell J. 2018 Oct;20(3):449]. Cell J. 2018;20(2):267–277. https://doi.org/10.22074/cellj.2018.5197.
Nicolau JC, Furtado RHM, Silva SA, Rochitte CE, Rassi A, Moraes JBMC, et al. Stem-cell therapy in ST-segment elevation myocardial infarction with reduced ejection fraction: a multicenter, double-blind randomized trial. Clin Cardiol. 2018;41(3):392–9. https://doi.org/10.1002/clc.22882.
Traverse JH, Henry TD, Pepine CJ, Willerson JT, Chugh A, Yang PC, et al. TIME trial: effect of timing of stem cell delivery following ST-elevation myocardial infarction on the recovery of global and regional left ventricular function: final 2-year analysis. Circ Res. 2018;122(3):479–88. https://doi.org/10.1161/CIRCRESAHA.117.311466.
Quyyumi AA, Vasquez A, Kereiakes DJ, Klapholz M, Schaer GL, Abdel-Latif A, et al. PreSERVE-AMI: a randomized, double-blind, placebo-controlled clinical trial of intracoronary administration of autologous CD34+ cells in patients with left ventricular dysfunction post STEMI. Circ Res. 2017;120(2):324–31. https://doi.org/10.1161/CIRCRESAHA.115.308165.
Choudry F, Hamshere S, Saunders N, Veerapen J, Bavnbek K, Knight C, et al. A randomized double-blind control study of early intra-coronary autologous bone marrow cell infusion in acute myocardial infarction: the REGENERATE-AMI clinical trial. Eur Heart J. 2016;37(3):256–63. https://doi.org/10.1093/eurheartj/ehv493.
Sürder D, Manka R, Moccetti T, Cicero VL, Emmert MY, Klersy C, et al. Effect of bone marrow-derived mononuclear cell treatment, early or late after acute myocardial infarction: twelve months CMR and long-term clinical results. Circ Res. 2016;119(3):481–90. https://doi.org/10.1161/CIRCRESAHA.116.308639.
Nair V, Madan H, Sofat S, Ganguli P, Jacob MJ, Datta R, et al. Efficacy of stem cell in improvement of left ventricular function in acute myocardial infarction–MI3 trial. Indian J Med Res. 2015;142(2):165–74. https://doi.org/10.4103/0971-5916.164245.
San Roman JA, Sánchez PL, Villa A, Sanz-Ruiz R, Fernandez-Santos ME, Gimeno F, et al. Comparison of different bone marrow-derived stem cell approaches in reperfused STEMI: a multicenter, prospective, randomized, open-labeled TECAM trial. J Am Coll Cardiol. 2015;65(22):2372–82. https://doi.org/10.1016/j.jacc.2015.03.563.
Assmus B, Leistner DM, Schächinger V, Erbs S, Elsässer A, Haberbosch W, et al. Long-term clinical outcome after intracoronary application of bone marrow-derived mononuclear cells for acute myocardial infarction: migratory capacity of administered cells determines event-free survival. Eur Heart J. 2014;35(19):1275–83. https://doi.org/10.1093/eurheartj/ehu062.
Sürder D, Manka R, Lo Cicero V, Moccetti T, Rufibach K, Soncin S, et al. Intracoronary injection of bone marrow-derived mononuclear cells early or late after acute myocardial infarction: effects on global left ventricular function. Circulation. 2013;127(19):1968–79. https://doi.org/10.1161/CIRCULATIONAHA.112.001035.
Wöhrle J, von Scheidt F, Schauwecker P, Wiesneth M, Markovic S, Schrezenmeier H, et al. Impact of cell number and microvascular obstruction in patients with bone-marrow derived cell therapy: final results from the randomized, double-blind, placebo controlled intracoronary Stem Cell therapy in patients with Acute Myocardial Infarction (SCAMI) trial. Clin Res Cardiol. 2013;102(10):765–70. https://doi.org/10.1007/s00392-013-0595-9.
Skalicka H, Horak J, Kobylka P, Palecek T, Linhart A, Aschermann M. Intracoronary injection of autologous bone marrow-derived mononuclear cells in patients with large anterior acute myocardial infarction and left ventricular dysfunction: a 24-month follow up study. Bratisl Lek Listy. 2012;113(4):220–7. https://doi.org/10.4149/bll_2012_051.
Traverse JH, Henry TD, Pepine CJ, Willerson JT, Zhao DXM, Ellis SG, et al. Effect of the use and timing of bone marrow mononuclear cell delivery on left ventricular function after acute myocardial infarction: the TIME randomized trial [published correction appears in JAMA. 2013 Jan 23;309(4):343] [published correction appears in JAMA. 2015 Jul 7;314(1):86]. JAMA. 2012;308(22):2380–2389. https://doi.org/10.1001/jama.2012.28726.
Turan RG, Bozdag-T I, Turan CH, Ortak J, Akin I, Kische S, et al. Enhanced mobilization of the bone marrow-derived circulating progenitor cells by intracoronary freshly isolated bone marrow cells transplantation in patients with acute myocardial infarction. J Cell Mol Med. 2012;16(4):852–64. https://doi.org/10.1111/j.1582-4934.2011.01358.x.
Beitnes JO, Gjesdal O, Lunde K, Solheim S, Edvardsen T, Arnesen H, et al. Left ventricular systolic and diastolic function improve after acute myocardial infarction treated with acute percutaneous coronary intervention, but are not influenced by intracoronary injection of autologous mononuclear bone marrow cells: a 3 year serial echocardiographic sub-study of the randomized-controlled ASTAMI study. Eur J Echocardiogr. 2011;12(2):98–106. https://doi.org/10.1093/ejechocard/jeq116.
Colombo A, Castellani M, Piccaluga E, Pusineri E, Palatresi S, Longari V, et al. Myocardial blood flow and infarct size after CD133+ cell injection in large myocardial infarction with good recanalization and poor reperfusion: results from a randomized controlled trial. J Cardiovasc Med (Hagerstown). 2011;12(4):239–48. https://doi.org/10.2459/JCM.0b013e328343d708.
Mansour S, Roy DC, Bouchard V, Stevens LM, Gobeil F, Rivard A, et al. One-year safety analysis of the COMPARE-AMI trial: comparison of intracoronary injection of CD133 bone marrow stem cells to placebo in patients after acute myocardial infarction and left ventricular dysfunction. Bone Marrow Res. 2011;2011: 385124. https://doi.org/10.1155/2011/385124.
Miettinen JA, Ylitalo K, Hedberg P, Kervinen K, Niemelä M, Säily M, et al. Effects of intracoronary injection of autologous bone marrow-derived stem cells on natriuretic peptides and inflammatory markers in patients with acute ST-elevation myocardial infarction. Clin Res Cardiol. 2011;100(4):317–25. https://doi.org/10.1007/s00392-010-0246-3.
Plewka M, Krzemińska-Pakuła M, Peruga JZ, Lipiec P, Kurpesa M, Wierzbowska-Drabik K, et al. The effects of intracoronary delivery of mononuclear bone marrow cells in patients with myocardial infarction: a two year follow-up results. Kardiol Pol. 2011;69(12):1234–40.
Traverse JH, Henry TD, Ellis SG, Pepine CJ, Willerson JT, Zhao DXM, et al. Effect of intracoronary delivery of autologous bone marrow mononuclear cells 2 to 3 weeks following acute myocardial infarction on left ventricular function: the lateTIME randomized trial. JAMA. 2011;306(19):2110–9.
Assmus B, Rolf A, Erbs S, Elsässer A, Haberbosch W, Hanbrecht R, et al. Clinical outcome 2 years after intracoronary administration of bone marrow-derived progenitor cells in acute myocardial infarction. Circ Heart Fail. 2010;3(1):89–96. https://doi.org/10.1161/CIRCHEARTFAILURE.108.843243.
Piepoli MF, Vallisa D, Arbasi M, Cavanna L, Cerri L, Mori M, et al. Bone marrow cell transplantation improves cardiac, autonomic, and functional indexes in acute anterior myocardial infarction patients (Cardiac Study). Eur J Heart Fail. 2010;12(2):172–80. https://doi.org/10.1093/eurjhf/hfp183.
Schaefer A, Zwadlo C, Fuchs M, Meyer GP, Lippolt P, Wollert KC, et al. Long-term effects of intracoronary bone marrow cell transfer on diastolic function in patients after acute myocardial infarction: 5-year results from the randomized-controlled BOOST trial—an echocardiographic study. Eur J Echocardiogr. 2010;11(2):165–71. https://doi.org/10.1093/ejechocard/jep191.
Wöhrle J, Merkle N, Mailänder V, Nusser T, Schauwecker P, von Scheidt F, et al. Results of intracoronary stem cell therapy after acute myocardial infarction. Am J Cardiol. 2010;105(6):804–12. https://doi.org/10.1016/j.amjcard.2009.10.060.
Beitnes JO, Hopp E, Lunde K, Solheim S, Arnesen H, Brinchmann JE, et al. Long-term results after intracoronary injection of autologous mononuclear bone marrow cells in acute myocardial infarction: the ASTAMI randomised, controlled study. Heart. 2009;95(24):1983–9. https://doi.org/10.1136/hrt.2009.178913.
Cao F, Sun D, Li C, Narsinh K, Zhao L, Li X, et al. Long-term myocardial functional improvement after autologous bone marrow mononuclear cells transplantation in patients with ST-segment elevation myocardial infarction: 4 years follow-up. Eur Heart J. 2009;30(16):1986–94. https://doi.org/10.1093/eurheartj/ehp220.
Dill T, Schächinger V, Rolf A, Möllmann S, Thiele H, Tillmanns H, et al. Intracoronary administration of bone marrow-derived progenitor cells improves left ventricular function in patients at risk for adverse remodeling after acute ST-segment elevation myocardial infarction: results of the Reinfusion of Enriched Progenitor cells And Infarct Remodeling in Acute Myocardial Infarction study (REPAIR-AMI) cardiac magnetic resonance imaging substudy. Am Heart J. 2009;157(3):541–7. https://doi.org/10.1016/j.ahj.2008.11.011.
Herbots L, D’hooge J, Eroglu E, Thijs D, Ganame J, Claus P, et al. Improved regional function after autologous bone marrow-derived stem cell transfer in patients with acute myocardial infarction: a randomized, double-blind strain rate imaging study. Eur Heart J. 2009;30(6):662–70. https://doi.org/10.1093/eurheartj/ehn532.
Lipiec P, Krzemińska-Pakuła M, Plewka M, Kuśmierek J, Płachcińska A, Szumiński R, et al. Impact of intracoronary injection of mononuclear bone marrow cells in acute myocardial infarction on left ventricular perfusion and function: a 6-month follow-up gated 99mTc-MIBI single-photon emission computed tomography study. Eur J Nucl Med Mol Imaging. 2009;36(4):587–93. https://doi.org/10.1007/s00259-008-0988-6.
Meyer GP, Wollert KC, Lotz J, Pirr J, Rager U, Lippolt P, et al. Intracoronary bone marrow cell transfer after myocardial infarction: 5-year follow-up from the randomized-controlled BOOST trial. Eur Heart J. 2009;30(24):2978–84. https://doi.org/10.1093/eurheartj/ehp374.
Nogueira FB, Silva SA, Haddad AF, Peixoto CM, de Carvalho RM, Tuche FA, et al. Systolic function of patients with myocardial infarction undergoing autologous bone marrow transplantation. Arq Bras Cardiol. 2009;93(4):374–372. https://doi.org/10.1590/s0066-782x2009001000010.
Plewka M, Krzemińska-Pakuła M, Lipiec P, Peruga JZ, Jezewski T, Kidawa M, et al. Effect of intracoronary injection of mononuclear bone marrow stem cells on left ventricular function in patients with acute myocardial infarction. Am J Cardiol. 2009;104(10):1336–42. https://doi.org/10.1016/j.amjcard.2009.06.057.
Schächinger V, Assmus B, Erbs S, Elsässer A, Haberbosch W, Hambrecht R, et al. Intracoronary infusion of bone marrow-derived mononuclear cells abrogates adverse left ventricular remodelling post-acute myocardial infarction: insights from the reinfusion of enriched progenitor cells and infarct remodelling in acute myocardial infarction (REPAIR-AMI) trial. Eur J Heart Fail. 2009;11(10):973–9. https://doi.org/10.1093/eurjhf/hfp113.
Tendera M, Wojakowski W, Ruzyłło W, Chojnowska L, Kepka C, Tracz W, et al. Intracoronary infusion of bone marrow-derived selected CD34+CXCR4+ cells and non-selected mononuclear cells in patients with acute STEMI and reduced left ventricular ejection fraction: results of randomized, multicentre Myocardial Regeneration by Intracoronary Infusion of Selected Population of Stem Cells in Acute Myocardial Infarction (REGENT) Trial. Eur Heart J. 2009;30(11):1313–21. https://doi.org/10.1093/eurheartj/ehp073.
Yao K, Huang R, Sun A, Qian J, Liu X, Ge L, et al. Repeated autologous bone marrow mononuclear cell therapy in patients with large myocardial infarction. Eur J Heart Fail. 2009;11(7):691–8. https://doi.org/10.1093/eurjhf/hfp062.
Huikuri HV, Kervinen K, Niemelä M, Ylitalo K, Säily M, Koistinen P, et al. Effects of intracoronary injection of mononuclear bone marrow cells on left ventricular function, arrhythmia risk profile, and restenosis after thrombolytic therapy of acute myocardial infarction. Eur Heart J. 2008;29(22):2723–32. https://doi.org/10.1093/eurheartj/ehn436.
Lunde K, Solheim S, Aakhus S, Arnesen H, Moum T, Abdelnoor M, et al. Exercise capacity and quality of life after intracoronary injection of autologous mononuclear bone marrow cells in acute myocardial infarction: results from the Autologous Stem cell Transplantation in Acute Myocardial Infarction (ASTAMI) randomized controlled trial. Am Heart J. 2007;154(4):710.e1-710.e7108. https://doi.org/10.1016/j.ahj.2007.07.003.
SuárezdeLezo J, Herrera C, Pan M, Romero M, Pavlovic D, Segura J, et al. Tratamiento regenerativo en pacientes con infarto agudo anterior revascularizado y función ventricular deprimida [Regenerative therapy in patients with a revascularized acute anterior myocardial infarction and depressed ventricular function]. Rev Esp Cardiol. 2007;60(4):357–65.
Janssens S, Dubois C, Bogaert J, Theunissen K, Deroose C, Desmet W, et al. Autologous bone marrow-derived stem-cell transfer in patients with ST-segment elevation myocardial infarction: double-blind, randomised controlled trial. Lancet. 2006;367(9505):113–21. https://doi.org/10.1016/S0140-6736(05)67861-0.
Lunde K, Solheim S, Aakhus S, Arnesen H, Abdelnoor M, Egeland T, et al. Intracoronary injection of mononuclear bone marrow cells in acute myocardial infarction. N Engl J Med. 2006;355(12):1199–209. https://doi.org/10.1056/NEJMoa055706.
Meyer GP, Wollert KC, Lotz J, Steffens J, Lippolt P, Fichtner S, et al. Intracoronary bone marrow cell transfer after myocardial infarction: eighteen months’ follow-up data from the randomized, controlled BOOST (BOne marrOw transfer to enhance ST-elevation infarct regeneration) trial. Circulation. 2006;113(10):1287–94. https://doi.org/10.1161/CIRCULATIONAHA.105.575118.
Schächinger V, Erbs S, Elsässer A, Haberbosch W, Hambrecht R, Hölschermann H, et al. Intracoronary bone marrow-derived progenitor cells in acute myocardial infarction. N Engl J Med. 2006;355(12):1210–21. https://doi.org/10.1056/NEJMoa060186.
Schächinger V, Erbs S, Elsässer A, Haberbosch W, Hambrecht R, Hölschermann H, et al. Improved clinical outcome after intracoronary administration of bone-marrow-derived progenitor cells in acute myocardial infarction: final 1-year results of the REPAIR-AMI trial. Eur Heart J. 2006;27(23):2775–83. https://doi.org/10.1093/eurheartj/ehl388.
Schaefer A, Meyer GP, Fuchs M, Klein G, Kaplan M, Wollert KC, et al. Impact of intracoronary bone marrow cell transfer on diastolic function in patients after acute myocardial infarction: results from the BOOST trial. Eur Heart J. 2006;27(8):929–35. https://doi.org/10.1093/eurheartj/ehi817.
Karpov RS, Popov SV, Markov VA, Suslova TE, Ryabov VV, Poponina YS, et al. Autologous mononuclear bone marrow cells during reparative regeneratrion after acute myocardial infarction. Bull Exp Biol Med. 2005;140(5):640–3. https://doi.org/10.1007/s10517-006-0043-1.
Ruan W, Pan CZ, Huang GQ, Li YL, Ge JB, Shu XH. Assessment of left ventricular segmental function after autologous bone marrow stem cells transplantation in patients with acute myocardial infarction by tissue tracking and strain imaging. Chin Med J (Engl). 2005;118(14):1175–81.
Wollert KC, Meyer GP, Lotz J, Ringes-Lichtenberg S, Lippolt P, Breidenbach C, et al. Intracoronary autologous bone-marrow cell transfer after myocardial infarction: the BOOST randomised controlled clinical trial. Lancet. 2004;364(9429):141–8. https://doi.org/10.1016/S0140-6736(04)16626-9.
Kang HJ, Kim MK, Lee HY, Park KW, Lee W, Cho YS, et al. Five-year results of intracoronary infusion of the mobilized peripheral blood stem cells by granulocyte colony-stimulating factor in patients with myocardial infarction. Eur Heart J. 2012;33(24):3062–9. https://doi.org/10.1093/eurheartj/ehs231.
Chang SA, Kim HK, Lee HY, Choi SY, Koo BK, Kim YJ, et al. Restoration of left ventricular synchronous contraction after acute myocardial infarction by stem cell therapy: new insights into the therapeutic implication of stem cell therapy for acute myocardial infarction. Heart. 2008;94(8):995–1001. https://doi.org/10.1136/hrt.2007.124701.
Kang HJ, Lee HY, Na SH, Chang SA, Park KW, Kim HK, et al. Differential effect of intracoronary infusion of mobilized peripheral blood stem cells by granulocyte colony-stimulating factor on left ventricular function and remodeling in patients with acute myocardial infarction versus old myocardial infarction: the MAGIC Cell-3-DES randomized, controlled trial. Circulation. 2006;114(1 Suppl):I145–51. https://doi.org/10.1161/CIRCULATIONAHA.105.001107.
Delewi R, van der Laan AM, Robbers LF, Hirsch A, Nijveldt R, van der Vleuten PA, et al. Long term outcome after mononuclear bone marrow or peripheral blood cells infusion after myocardial infarction. Heart. 2015;101(5):363–8. https://doi.org/10.1136/heartjnl-2014-305892.
Robbers LF, Nijveldt R, Beek AM, Hirsch A, van der Laan AM, Delewi R, et al. Cell therapy in reperfused acute myocardial infarction does not improve the recovery of perfusion in the infarcted myocardium: a cardiac MR imaging study. Radiology. 2014;272(1):113–22. https://doi.org/10.1148/radiol.14131121.
Hirsch A, Nijveldt R, van der Vleuten PA, Tijssen JG, van der Giessen WJ, Tio RA, et al. Intracoronary infusion of mononuclear cells from bone marrow or peripheral blood compared with standard therapy in patients after acute myocardial infarction treated by primary percutaneous coronary intervention: results of the randomized controlled HEBE trial. Eur Heart J. 2011;32(14):1736–47. https://doi.org/10.1093/eurheartj/ehq449.
van der Laan AM, Hirsch A, Haeck JD, Nijveldt R, Delewi R, Biemond BJ, et al. Recovery of microcirculation after intracoronary infusion of bone marrow mononuclear cells or peripheral blood mononuclear cells in patients treated by primary percutaneous coronary intervention the Doppler substudy of the Hebe trial. JACC Cardiovasc Interv. 2011;4(8):913–20. https://doi.org/10.1016/j.jcin.2011.05.005.
Al’Aref SJ, Singh G, van Rosendael AR, Kolli KK, Ma X, Maliakal G, et al. Determinants of in-hospital mortality after percutaneous coronary intervention: a machine learning approach. J Am Heart Assoc. 2019;8(5): e011160. https://doi.org/10.1161/JAHA.118.011160.
Roe MT, Messenger JC, Weintraub WS, Cannon CP, Fonarow GC, Dai D, et al. Treatments, trends, and outcomes of acute myocardial infarction and percutaneous coronary intervention. J Am Coll Cardiol. 2010;56(4):254–63. https://doi.org/10.1016/j.jacc.2010.05.008.
Singh M, Peterson ED, Roe MT, Ou FS, Spertus JA, Rumsfeld JS, et al. Trends in the association between age and in-hospital mortality after percutaneous coronary intervention: national cardiovascular data registry experience. Circ Cardiovasc Interv. 2009;2(1):20–6. https://doi.org/10.1161/CIRCINTERVENTIONS.108.826172.
Liu C, Han D, Liang P, Li Y, Cao F. The current dilemma and breakthrough of stem cell therapy in ischemic heart disease. Front cell dev biol. 2021;9: 636136. https://doi.org/10.3389/fcell.2021.636136.
Florea V, Rieger AC, DiFede DL, El-Khorazaty J, Natsumeda M, Banerjee MN, et al. Dose comparison study of allogeneic mesenchymal stem cells in patients with ischemic cardiomyopathy (The TRIDENT Study). Circ Res. 2017;121(11):1279–90. https://doi.org/10.1161/CIRCRESAHA.117.311827.
Zhang S, Sun A, Xu D, Yao K, Huang Z, Jin H, et al. Impact of timing on efficacy and safetyof intracoronary autologous bone marrow stem cells transplantation in acute myocardial infarction: a pooled subgroup analysis of randomized controlled trials. Clin Cardiol. 2009;32(8):458–66. https://doi.org/10.1002/clc.20575.
Attar A, Bahmanzadegan Jahromi F, Kavousi S, Monabati A, Kazemi A. Mesenchymal stem cell transplantation after acute myocardial infarction: a meta-analysis of clinical trials. Stem Cell Res Ther. 2021;12(1):600. https://doi.org/10.1186/s13287-021-02667-1.
Acknowledgements
The authors would like to thank the Division of Regenerative Medicine Policy for their support.
Funding
This study was funded by the Innovative Regenerative Medicine Clinical Research Support Program from the Ministry of Health and Welfare, Republic of Korea.
Author information
Authors and Affiliations
Contributions
SHL contributed to the concept and design of the study. HL and YH assisted in statistical analysis and administrative support. The first draft of the manuscript was written by HL, YH, and SHL. HL, HC, and SHL contributed to manuscript writing and preparing the final version of the manuscript. All authors read and approved the final manuscript.
Corresponding author
Ethics declarations
Ethics approval and consent to participate
The protocol of this systematic review and meta-analysis was previously registered in PROSPERO (CRD42023422818). Since this study did not involve human participants, consent to participate was not required.
Consent for publication
Not applicable.
Competing interests
The authors declare no conflict of interests.
Additional information
Publisher's Note
Springer Nature remains neutral with regard to jurisdictional claims in published maps and institutional affiliations.
Supplementary Information
Additional file 1
. Summary of the risk of bias in the included studies. Figures 1 and 2.
Rights and permissions
Open Access This article is licensed under a Creative Commons Attribution-NonCommercial-NoDerivatives 4.0 International License, which permits any non-commercial use, sharing, distribution and reproduction in any medium or format, as long as you give appropriate credit to the original author(s) and the source, provide a link to the Creative Commons licence, and indicate if you modified the licensed material. You do not have permission under this licence to share adapted material derived from this article or parts of it. The images or other third party material in this article are included in the article’s Creative Commons licence, unless indicated otherwise in a credit line to the material. If material is not included in the article’s Creative Commons licence and your intended use is not permitted by statutory regulation or exceeds the permitted use, you will need to obtain permission directly from the copyright holder. To view a copy of this licence, visit http://creativecommons.org/licenses/by-nc-nd/4.0/.
About this article
Cite this article
Lee, H., Cho, HJ., Han, Y. et al. Mid- to long-term efficacy and safety of stem cell therapy for acute myocardial infarction: a systematic review and meta-analysis. Stem Cell Res Ther 15, 290 (2024). https://doi.org/10.1186/s13287-024-03891-1
Received:
Accepted:
Published:
DOI: https://doi.org/10.1186/s13287-024-03891-1