Abstract
Background
By controlling hypercapnia, respiratory acidosis, and associated consequences, extracorporeal CO2 removal (ECCO2R) has the potential to facilitate ultra-protective lung ventilation (UPLV) strategies and to decrease injury from mechanical ventilation. We convened a meeting of European intensivists and nephrologists and used a modified Delphi process to provide updated insights into the role of ECCO2R in acute respiratory distress syndrome (ARDS) and to identify recommendations for a future randomized controlled trial.
Results
The group agreed that lung protective ventilation and UPLV should have distinct definitions, with UPLV primarily defined by a tidal volume (VT) of 4–6 mL/kg predicted body weight with a driving pressure (ΔP) ≤ 14–15 cmH2O. Fourteen (93%) participants agreed that ECCO2R would be needed in the majority of patients to implement UPLV. Furthermore, 10 participants (majority, 63%) would select patients with PaO2:FiO2 > 100 mmHg (> 13.3 kPa) and 14 (consensus, 88%) would select patients with a ventilatory ratio of > 2.5–3. A minimum CO2 removal rate of 80 mL/min delivered by continuous renal support machines was suggested (11/14 participants, 79%) for this objective, using a short, double-lumen catheter inserted into the right internal jugular vein as the preferred vascular access. Of the participants, 14/15 (93%, consensus) stated that a new randomized trial of ECCO2R is needed in patients with ARDS. A ΔP of ≥ 14–15 cmH2O was suggested by 12/14 participants (86%) as the primary inclusion criterion.
Conclusions
ECCO2R may facilitate UPLV with lower volume and pressures provided by the ventilator, while controlling respiratory acidosis. Since recent European Society of Intensive Care Medicine guidelines on ARDS recommended against the use of ECCO2R for the treatment of ARDS outside of randomized controlled trials, new trials of ECCO2R are urgently needed, with a ΔP of ≥ 14–15 cmH2O suggested as the primary inclusion criterion.
Similar content being viewed by others
Background
Clinical data suggest that mechanical ventilation (MV) can contribute to the negative outcomes in patients with acute respiratory distress syndrome (ARDS) through ventilator-induced lung injury (VILI) [1,2,3]. The ARDSNet investigators demonstrated that limiting tidal volume (VT) to 6 mL/kg of predicted body weight (PBW) and plateau pressure (PPlat) to < 30 cm H2O improved survival. However, this approach may not be fully protective as ~ 30% of patients exhibit tidal hyperinflation along with an increase in proinflammatory mediators in bronchoalveolar lavage fluid, a typical signal for VILI [4, 5]. Reducing VT even further to 4 mL/kg and PPlat to < 25 cmH2O, a strategy termed “ultra-protective ventilation”, has been proposed to reduce VILI effects [6,7,8]. Furthermore, other variables of risk reduction for VILI have been discussed: Amato et al. [9] suggested reduction of driving pressure (ΔP), reduction of respiratory rate [10, 11] and/or mechanical power [12,13,14,15] are other variables to be discussed in this context. However, this strategy entails the risks associated with hypercapnia and severe respiratory acidosis [16,17,18].
As an adjunct to MV, extracorporeal CO2 removal (ECCO2R) aims to clear CO2, enabling ultra-protective lung ventilation while limiting hypercapnia and respiratory acidosis [19,20,21,22]. In 2019, a European ECCO2R user group meeting identified factors influencing patient selection and clinical decision-making, as well as how to implement ECCO2R in the intensive care unit (ICU) [17]. The group considered ARDS to be the primary indication for ECCO2R, with the treatment goal being to facilitate ultra-protective lung ventilation by decreasing VT, PPlat, ΔP, and RR [17].
Since this framework was proposed, experience of ECCO2R and ultra-protective lung ventilation has increased. The COVID-19 pandemic provided experience of delivering ECCO2R to different patient groups [23,24,25]. While the REST study (NCT02654327) reported no difference in 90-day mortality in patients receiving ultra-low VT ventilation (ULTV) with ECCO2R compared with those receiving low VT ventilation (LTV) without ECCO2R [26], a secondary analysis suggested that the use of ECCO2R may improve survival in patients with a ventilatory ratio (VR, ≥ 3; a simple bedside index of impaired efficiency of ventilation, which correlates well with physiological dead space fraction) [27, 28]. However, uncertainty remains around the use of ECCO2R in ARDS, and the recent European Society of Intensive Care Medicine (ESICM) guidelines recommended that the use of ECCO2R for the treatment of ARDS should be limited to randomized controlled trials [29]. We therefore convened the second European ECCO2R Expert Roundtable Meeting to update the framework for ECCO2R and to outline further research in this area.
Methods
Participants
The ECCO2R Expert Roundtable Meeting was held in Brussels on the 5 October 2022 and was attended by 16 clinicians (1 chair [AC] plus 15 respondents) who regularly provide ECCO2R in clinical centers across Europe. Each participant was a senior clinician or an intensivist with direct clinical experience of ECCO2R, with several of the participants being principal investigators in recently completed or ongoing clinical trials. JK and KH are employees of Baxter who were engaged in the development of the questionnaire. They did not participate in the roundtable discussion but, like all other authors, they participated in drafting the manuscript and critically revising it for important intellectual content. There was no modification of the intellectual content by Baxter employees other than the listed authors. All authors take responsibility for the final content of the manuscript. Conflict of interest declarations for the attendees can be found at the end of the manuscript.
Objectives
The objectives of the Expert Roundtable Meeting were to understand current clinical practice for ECCO2R in patients with acute hypoxemic respiratory failure, including the clinical rationale for the use of ECCO2R, the criteria used for initiation, maintenance, and discontinuation in patients with mild-to-moderate ARDS, and practical considerations, including anticoagulation and vascular access strategies. The meeting also aimed to assess the impact of recent publications investigating the use of ECCO2R to support ultra-protective lung ventilation for acute respiratory failure [26], as well as the impact of the COVID-19 pandemic on current and future standards of practice.
Data collection and analysis
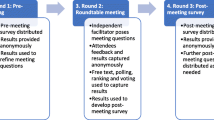
A modified Delphi-based methodology was used to assess the clinicians’ views on ECCO2R over four rounds of iterative questioning, including an anonymous pre-meeting survey, a live survey during the meeting, and two anonymous post-meeting surveys (Table 1). The meeting questions, as well as the pre-meeting and post-meeting surveys, were developed by AC in collaboration with JK, with independent medical writing support funded by Baxter. The questions are available in the supplementary appendix. JK and KH did not participate in answering the surveys.
The Round 1 pre-meeting survey consisted of a PDF questionnaire that was circulated to each participant individually in advance of the meeting, with results analyzed anonymously. Results from the Round 1 survey were presented to the group and used to inform the questions asked in the Round 2 meeting, which was moderated by an independent facilitator. In Round 2, participants were divided into four subgroups and questions were presented to the group by an independent facilitator. For closed questions, participants provided their responses anonymously through a web-based voting system. For open questions, responses from each group were collected after a period of discussion to facilitate interaction between participants. JK and KH were present as Baxter employees during the meeting but were not permitted to provide answers or responses. To further explore questions and topics raised during the meeting, a first post-meeting survey (Round 3) was shared with the authors. Based on the Round 3 survey and literature published following the meeting, including the secondary analysis of the REST trial, the ESICM guidelines and the VT4COVID trial [28,29,30], a second post-meeting survey (Round 4) was shared with the authors to understand their definition of ultra-protective ventilation and the role of ECCO2R. Both Round 3 and 4 surveys consisted of PDF questionnaires that were shared with each participant individually and the results were analyzed anonymously.
Responses to the survey questions at each round were evaluated to determine the level of agreement between participants. A threshold of ≥ 80% of participants in agreement was defined as a “consensus.” A threshold of ≥ 50% of participants in agreement was defined as a “majority,” while < 50% was defined as “no agreement.” These thresholds are consistent with the analysis conducted in 2019 [17].
To facilitate the analysis of responses for questions regarding respiratory parameters used for the implementation of lung protective ventilation and ECCO2R, a ranked scoring system was employed. Participants were asked to score respiratory parameters in order of perceived importance, giving them a score (e.g. from 1 to 5, depending on the number of variables). Scores were then combined to give a total score for each parameter, with higher scores indicating a higher perceived importance.
Results
Participant experience
The participants at the meeting were experienced clinicians who regularly provide ECCO2R in clinical centers across Europe; the average number of patients treated with ECCO2R therapy in their ICU/unit per year was 8 (range 1–18). Regarding patients with mild-to-moderate ARDS (defined by the 2012 Berlin definition), the average number of admissions per year to their ICU/unit was 112 (range 40–250). Participants used all available devices for ECCO2R present in the EU at that time, including devices from ALung, B-Braun and Fresenius. The majority of participants (93%) had experience with Baxter’s product PrismaLung +.
Definitions of lung protective ventilation and ultra-protective lung ventilation
The majority of participants (57%) agreed that lung protective ventilation and ultra-protective lung ventilation should have distinct definitions, with lower targets for VT, ΔP, PPlat, and RR identified as the parameters that define ultra-protective lung ventilation vs. more conventional lung protective ventilation. Participants ranked VT, ΔP, PPlat, and RR as the four most important respiratory parameters to monitor when implementing a protective ventilation strategy. In subsequent rounds, participants agreed that a protective ventilation strategy for patients with mild-to-moderate ARDS should have a target VT of 6 mL/kg PBW, maximum ΔP of 15 cmH2O, and maximum PPlat of 29–30 cmH2O (majority, Fig. 1A–C). No agreement was reached on maximum RR, with 10 participants selecting values of 21–30 breaths per minute (BPM) (Fig. 1D). Based on the ECCO2R data published after the meeting, the majority of participants (63%) indicated that ultra-protective lung ventilation should have a maximum VT ≤ 6 mL/kg PBW, with 6, 2, 7, and 1 participants defining VT as ≤ 6, ≤ 5, ≤ 4, and ≤ 3 mL/kg PBW, respectively (Fig. 2A). The majority of participants consider a VT < 4 mL/kg as the definition for ULTV.
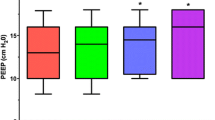
Acceptable threshold values for respiratory parameters when implementing protective ventilation to minimize or avoid VILI for a patient with mild-to-moderate ARDS. 14/15 participants answered this question. ≥ 12 responses indicate consensus, ≥ 7 responses indicate a majority, and < 7 responses indicate no agreement
Ventilatory objectives for ultra-protective lung ventilation. Target VT (A) and driving pressure (B) thresholds for ultra-protective lung ventilation as defined by the participants. (C) Rate of participants stating that ECCO2R would be required to implement ultra-protective lung ventilation. All participants answered this question. ≥ 12 responses indicate a consensus, ≥ 8 responses indicate a majority, and ≤ 7 responses indicate no agreement
Use of ECCO2R to facilitate protective ventilation in patients with ARDS
All participants indicated that ultra-protective lung ventilation facilitated by ECCO2R would require a maximum ΔP (100%, consensus), with the majority (56%) selecting 14–15 cmH2O as their preference; 12–13 cmH2O and 16–17 cmH2O were selected by three participants each (19%, Fig. 2B). Furthermore, 10 participants (majority, 63%) would select patients using a minimum PaO2:FiO2 of > 100 mmHg (> 13.3 kPa) and 14 (consensus, 88%) would select a minimum VR of > 2.5–3. Fourteen (93%) participants agreed that ECCO2R would be needed in the majority of patients to implement ultra-protective lung ventilation (consensus) (Fig. 2C).
Initiation and discontinuation of ECCO2R
Partial pressure of CO2 (PaCO2), pH, ΔP, and RR were ranked as the four most important respiratory parameters to consider when deciding whether to initiate ECCO2R in a patient who is sedated and ventilated with mild-to-moderate ARDS. In subsequent rounds, the majority agreed they would initiate ECCO2R once PaCO2 reached > 60 mmHg (> 8 kPa) and pH < 7.25 (both majority) (Table 2). No agreement was reached on ΔP threshold; however, 10 participants selected either > 14 or > 15 cmH2O. No agreement was reached on a threshold for RR.
When discontinuing ECCO2R, pH, ΔP, RR, and Pplat were indicated as being the four most important respiratory parameters to consider (evaluated with sweep gas off on the ECCO2R device). In subsequent rounds, most participants would discontinue ECCO2R once pH reached > 7.3 (majority) (Table 2). No agreement was reached on thresholds for ΔP, RR, or Pplat.
Anticoagulation strategy for ECCO2R
Fourteen out of 15 participants (93%) would use unfractionated heparin for anticoagulation when implementing ECCO2R (consensus), with one participant stating they would use regional citrate-based anticoagulation (although this treatment is not recommended with flow higher than 150 mL/min) (Table 3). Among participants who preferred heparin, the majority agreed they would target an activated partial thromboplastin time ratio of 1.5–2.0 × control (majority). Half of participants (7/14) reported using anti-Xa testing, with all of them agreeing on a target range of 0.3–0.5 IU/mL (consensus). No agreement was reached on bolus or infusion dosage for unfractionated heparin.
Optimal blood flow rate for ECCO2R and vascular access
Most participants selected either a range of 251–350 mL/min or 351–450 mL/min as the minimum blood flow rate they believed was required for effective use of ECCO2R (Fig. 3A). A majority of participants (73%) believed that a minimum CO2 removal rate of 80 mL/min delivered by technology based on peristaltic (roller) pumps as in renal support devices was required for ECCO2R to be effective (Fig. 3B). The right internal jugular vein was the preferred vascular access point for the majority of participants (Table 4); however, femoral access was discussed as suitable, especially in conscious patients. All participants preferred using a double-lumen catheter (consensus), with most participants specifying a length of 16–17 cm (for the right internal jugular access) and a diameter of 14 French (both majority). All considered the use of vascular ultrasound necessary to safely guide venous catheter insertion using the Seldinger technique (consensus) (Table 5).
Minimum requirements for ECCO2R therapy. A Minimum blood flow rate required for effective use of ECCO2R. All 15 participants answered this question. ≥ 12 responses indicate consensus, ≥ 8 responses indicate a majority, and ≤ 7 responses indicate no agreement. B Minimum CO2 removal rate for an ECCO2R device. 14/15 participants answered this question. ≥ 12 responses indicate consensus, ≥ 7 responses indicate a majority, and < 7 responses indicate no agreement
Use of neuromuscular blocking agents and prone positioning during ECCO2R
Most participants (9/14, 64%, majority) use neuromuscular blockade in patients who are sedated with ventilator asynchrony receiving ECCO2R. They (8/13, 62%, majority) reported routinely using prone positioning for sedated and ventilated patients with ARDS who are receiving ECCO2R.
Need for and design of another randomized trial of ECCO2R for patients with acute hypoxemic respiratory failure
During the post-meeting survey, 14/15 (93%, consensus) participants stated that a new randomized trial of ECCO2R is needed in patients with ARDS. A ΔP of ≥ 14–15 cmH2O was suggested by 12/14 participants (86%) as the primary inclusion criterion. No agreement existed for the primary endpoint, although mortality and the duration of MV were mentioned as suitable outcome parameters. Major bleeding (including central nervous system hemorrhage) was the most frequently indicated safety endpoint (8/12, 67%).
Discussion
This consensus provides fresh insights into the use of ECCO2R for mitigating VILI in patients with ARDS. The group agreed that lung protective ventilation and ultra-protective lung ventilation should have distinct definitions, with ultra-protective lung ventilation primarily defined by a VT 4–6 mL/kg PBW with a ΔP ≤ 14–15 cmH2O. ECCO2R may have a significant role in patients with ARDS by controlling hypercapnia and respiratory acidosis induced by these low VT levels. While this provides a broad framework to help guide implementation of a protective or ultra-protective lung ventilation strategy supported by ECCO2R, it should be noted that the use of ECCO2R outside of randomized clinical trials is not recommended in the latest ESICM guidelines [29]. To this end, the group provided recommendations to guide the development of a trial to help overcome the uncertainties around the use of ECCO2R.
The previous ECCO2R Roundtable Meeting was held in July 2019. Since then, further insights into lung protective and ultra-protective lung ventilation and the use of ECCO2R have emerged. Firstly, the REST trial, the first large-scale randomized controlled trial of patients receiving MV facilitated by ECCO2R, was halted due to futility [26]. No significant benefit of ECCO2R on 90-day mortality vs. standard care was observed (41.5% vs. 39.5%, respectively; p = 0.68) [26]. In addition, serious adverse events were reported more commonly in the ECCO2R group, the majority related to bleeding complications, including intracranial hemorrhage. Bleeding complications associated with ECCO2R were indeed identified by our panel as one of the major endpoints to evaluate in a future trial of ECCO2R in ARDS. They also suggested unfractionated heparin should remain the first-line anticoagulant for ECCO2R, although new drugs with more favorable efficacy/safety profiles are currently under development and evaluation [31]. The REST trial had other major limitations. At randomization, ARDS was present in only 59% of the patients, ΔP was < 15 cm H2O in 50% of patients and, despite marked hypoxemia (median PaO2:FiO2, 118 mmHg), the median positive end-expiratory pressure (10 cm H2O) was lower than in other ARDS trials with similar patients and only 11% of the patients had been proned. On Day 2 post-randomization, the decreases in VT (6.3–4.5 mL/kg) and in ΔP (15–12 cm H2O) from baseline were modest, while increases in the RR (from 24 to 27 BPM) and in PaCO2 (from 54 to 61 mmHg) were observed. These data suggest that the device used in this study may have provided insufficient CO2 removal to reach ultra-protective ventilation while controlling respiratory acidosis. Furthermore, as noted by the trial authors, most of the trial’s study sites were naive to the intervention, and inexperience may have negatively affected outcomes [26]. Interestingly, a secondary analysis of the REST trial has suggested a benefit of low VT ventilation facilitated by ECCO2R on 90-day survival in a subset of the patients who had a high VR (> 3) and in patients with PaO2:FiO2 110 mmHg or higher [28].
Secondly, the VT4COVID study conducted across 10 ICUs in France compared LTV (6 mL/kg PBW) or ULTV (4 mL/kg PBW) during the COVID-19 pandemic. There was no significant difference in the primary composite outcome of all-cause mortality at Day 90 and ventilator-free days at Day 60. Forty-six (44%) of 105 patients in the ULTV group and 43 (39%) of 109 in the LTV group died by Day 90 (absolute difference 4% [− 9 to 18]; p = 0.52) [30]. Severe respiratory acidosis in the first 28 days was higher in the ULTV group than in the LTV group (33% vs. 13%; absolute difference 20% [95% confidence interval 9–31]; p = 0.0004), suggesting the potential need for ECCO2R to facilitate ultra-protective lung ventilation. A major limitation of this trial is that the median ΔP was only 11 cm H2O at inclusion (with a modest reduction in ΔP of only 2 mm H2O in the ULTV group), with the benefit of further lowering VT being very unlikely to outweigh the possible risks of heavy sedation, neuromuscular blockade, and diaphragm deconditioning. In a further development, the authors of the VT4COVID trial have since indicated that they are analyzing their trial database to identify a threshold of ΔP above which ULTV would be beneficial [30]. Another limitation of the VT4COVID trial is that the possible benefit of the reduction in TV in the ULTV group may have been masked by the increase of respiratory rates, with such increases in respiratory rates potentially a consequence of respiratory acidosis and hypercapnia in this population. Although not necessarily supporting the concept of ULTV, these observations may suggest that its application without sufficient extracorporeal reduction of CO2 load may limit its beneficial effects for patients.
Thirdly, the ESICM guidelines on ARDS recommended against the use of ECCO2R for the treatment of ARDS outside of randomized controlled trials [29]. This is based on a meta-analysis of the primary analysis of the REST trial and the smaller Xtravent trial, which suggests the use of ECCO2R did not reduce mortality as well as the side effects experienced in the REST trial in patients receiving ECCO2R. However, the ESICM experts do acknowledge the need for further research to clarify the current uncertainty around ECCO2R; specifically, understanding device-specific safety and efficacy profiles, the identification of long-term multidimensional outcomes, and defining a population of patients with ARDS who may respond to ECCO2R [29]. Indeed, during our meeting, there was a recurring discussion on the importance of a patient-centric approach to ECCO2R, highlighting that the parameters necessary for initiation and discontinuation of protective ventilation and/or ECCO2R must be adapted to the patient’s disease severity, comorbidities, and ventilatory parameters associated with lung injury. Specifically, the participants emphasized that parameters reflecting alterations in lung mechanics, such as an increase in ΔP, might serve as better inclusion criteria for ECCO2R in patients with acute hypoxemic respiratory failure than the degree of hypoxemia. The group also believed that a minimum CO2 removal rate of 80 mL/min delivered by continuous renal support machines was required for ECCO2R to be effective, with a short, double-lumen catheter inserted into the right internal jugular vein as the preferred vascular access. Furthermore, we note the type of device use to deliver ECCO2R may have some import. That is, at the flow rates typically used for ECCO2R (~ 300 to ~ 1500 mL/min), centrifugal devices (used in the REST trial) [26] have an associated risk of hemolysis and destruction of platelets [32] that may not occur with peristaltic pumps. Our recommendations provided here on the use of ECCO2R and on clinical trial design should aid the development of a trial that could help answer the questions posed by the ESICM guidelines on ARDS.
This work does have some limitations. Firstly, the findings relate to the experiences of a relatively small number of physicians from centers across Europe and do not replace the need for a randomized controlled trial to determine the optimal use of ECCO2R to facilitate LTV strategies. Secondly, the group focused on the use of ECCO2R to facilitate ventilation in patients with ARDS—these experiences may not translate to other rarer indications. Thirdly, we did not include discussions related to the amount of CO2 removal required depending on ideal/predicted body weight or other external factors that could influence a patient’s CO2 production. The amount of CO2 that is produced by the patient is dependent on multiple factors (such as muscle activity, inflammatory reactions and nutrition). As a result, it cannot be defined easily and there is no method available that can be used easily at the bedside to determine this production rate reliably. This is another reason why more physiological and interventional studies are needed to explore the ability of the ECCO2R device to control hypercapnia while providing ultra-protective lung ventilation. Although the authors took every opportunity to ensure all relevant major articles were cited when constructing surveys, a comprehensive systematic literature analysis was considered out of scope of this project. Readers are reminded that the discussions outlined here are the authors’ personal experiences and are not a replacement for formal guidelines. Practicing clinicians should continue to prioritize their patients’ individual needs and consult guidelines.
Conclusions
The authors consider that ECCO2R may facilitate UPLV with lower volume and pressures by the ventilator while controlling respiratory acidosis. ECCO2R may be delivered using blood flows currently delivered by continuous renal support machines, providing that a minimum CO2 removal rate of 80 mL/min can be obtained. Since recent ESICM guidelines on ARDS recommended against the use of ECCO2R for the treatment of ARDS outside of randomized controlled trials, a new trial of ECCO2R is now urgently needed (with ΔP of ≥ 14–15 cmH2O suggested as the primary inclusion criterion).
Availability of data and materials
All data generated or analyzed during this study are included in this published article [and its supplementary information files].
Abbreviations
- ∆P:
-
Driving pressure
- ARDS:
-
Acute respiratory distress syndrome
- BPM:
-
Breaths per minute
- ECCO2R:
-
Extracorporeal CO2 removal
- ESICM:
-
European Society of Intensive Care Medicine
- FiO2 :
-
Fraction of inspired oxygen
- ICU:
-
Intensive care unit
- LTV:
-
Low VT ventilation
- MV:
-
Mechanical ventilation
- PaCO2 :
-
Partial pressure of CO2
- PaO2 :
-
Partial pressure of arterial oxygen
- PPlat :
-
Plateau pressure
- RR:
-
Respiratory rate
- ULTV:
-
Ultra-low VT ventilation
- UPLV:
-
Ultra-protective lung ventilation
- VILI:
-
Ventilator-induced lung injury
- VR:
-
Ventilatory ratio
- VT :
-
Tidal volume
References
Gorman EA, O’Kane CM, McAuley DF. Acute respiratory distress syndrome in adults: diagnosis, outcomes, long-term sequelae, and management. Lancet. 2022;400(10358):1157–70.
Saha R, Pham T, Sinha P, Maddali MV, Bellani G, Fan E, et al. Estimating the attributable fraction of mortality from acute respiratory distress syndrome to inform enrichment in future randomised clinical trials. Thorax. 2023;78(10):990–1003.
Acute Respiratory Distress Syndrome N, Brower RG, Matthay MA, Morris A, Schoenfeld D, Thompson BT, Wheeler A. Ventilation with lower tidal volumes as compared with traditional tidal volumes for acute lung injury and the acute respiratory distress syndrome. N Engl J Med. 2000;342(18):1301–8.
Terragni PP, Rosboch G, Tealdi A, Corno E, Menaldo E, Davini O, et al. Tidal hyperinflation during low tidal volume ventilation in acute respiratory distress syndrome. Am J Respir Crit Care Med. 2007;175(2):160–6.
Bellani G, Guerra L, Musch G, Zanella A, Patroniti N, Mauri T, et al. Lung regional metabolic activity and gas volume changes induced by tidal ventilation in patients with acute lung injury. Am J Respir Crit Care Med. 2011;183(9):1193–9.
Terragni PP, Del Sorbo L, Mascia L, Urbino R, Martin EL, Birocco A, et al. Tidal volume lower than 6 ml/kg enhances lung protection: role of extracorporeal carbon dioxide removal. Anesthesiology. 2009;111(4):826–35.
Pelosi P, Ball L, Barbas CSV, Bellomo R, Burns KEA, Einav S, et al. Personalized mechanical ventilation in acute respiratory distress syndrome. Crit Care. 2021;25(1):250.
Fanelli V, Ranieri MV, Mancebo J, Moerer O, Quintel M, Morley S, et al. Feasibility and safety of low-flow extracorporeal carbon dioxide removal to facilitate ultra-protective ventilation in patients with moderate acute respiratory distress syndrome. Crit Care. 2016;20:36.
Amato MB, Meade MO, Slutsky AS, Brochard L, Costa EL, Schoenfeld DA, et al. Driving pressure and survival in the acute respiratory distress syndrome. N Engl J Med. 2015;372(8):747–55.
Grasso S, Stripoli T, Mazzone P, Pezzuto M, Lacitignola L, Centonze P, et al. Low respiratory rate plus minimally invasive extracorporeal Co2 removal decreases systemic and pulmonary inflammatory mediators in experimental Acute Respiratory Distress Syndrome. Crit Care Med. 2014;42(6):e451–60.
Laffey JG, Bellani G, Pham T, Fan E, Madotto F, Bajwa EK, et al. Potentially modifiable factors contributing to outcome from acute respiratory distress syndrome: the LUNG SAFE study. Intensive Care Med. 2016;42(12):1865–76.
Haudebourg AF, Tuffet S, Perier F, Razazi K, de Prost N, Mekontso Dessap A, Carteaux G. Driving pressure-guided ventilation decreases the mechanical power compared to predicted body weight-guided ventilation in the Acute Respiratory Distress Syndrome. Crit Care. 2022;26(1):185.
Costa ELV, Slutsky AS, Brochard LJ, Brower R, Serpa-Neto A, Cavalcanti AB, et al. Ventilatory variables and mechanical power in patients with acute respiratory distress syndrome. Am J Respir Crit Care Med. 2021;204(3):303–11.
Gattinoni L, Collino F, Camporota L. Mechanical power: meaning, uses and limitations. Intensive Care Med. 2023;49(4):465–7.
Gattinoni L, Collino F, Camporota L. Ventilator induced lung injury: a case for a larger umbrella? Intensive Care Med. 2024;50(2):275–8.
Combes A, Brodie D, Aissaoui N, Bein T, Capellier G, Dalton HJ, et al. Extracorporeal carbon dioxide removal for acute respiratory failure: a review of potential indications, clinical practice and open research questions. Intensive Care Med. 2022;48(10):1308–21.
Combes A, Auzinger G, Capellier G, du Cheyron D, Clement I, Consales G, et al. ECCO2R therapy in the ICU: consensus of a European round table meeting. Crit Care. 2020;24(1):490.
Goligher EC, Combes A, Brodie D, Ferguson ND, Pesenti AM, Ranieri VM, et al. Determinants of the effect of extracorporeal carbon dioxide removal in the SUPERNOVA trial: implications for trial design. Intensive Care Med. 2019;45(9):1219–30.
Chiumello D, Pozzi T, Mereto E, Fratti I, Chiodaroli E, Gattinoni L, Coppola S. Long term feasibility of ultraprotective lung ventilation with low-flow extracorporeal carbon dioxide removal in ARDS patients. J Crit Care. 2022;71: 154092.
Stommel AM, Herkner H, Kienbacher CL, Wildner B, Hermann A, Staudinger T. Effects of extracorporeal CO(2) removal on gas exchange and ventilator settings: a systematic review and meta-analysis. Crit Care. 2024;28(1):146.
Pequignot B, Combes A, Lescroart M, Levy B, Koszutski M. Contribution of electrical impedance tomography to personalize positive end-expiratory pressure under ECCO(2)R. Crit Care. 2024;28(1):124.
Molina Lobo R, Lobo-Valbuena B, Gordo F. Management of severe ARDS due to SARS-CoV-2 pneumonia using low-flow extracorporeal CO(2) extraction. Crit Care. 2021;25(1):362.
Cambria G, Spelde AE, Olia SE, Biscotti M, Mackay E, Ibrahim M, et al. Extracorporeal carbon dioxide removal to de-escalate venovenous extracorporeal membrane oxygenation in severe COVID-19 acute respiratory distress syndrome. J Cardiothorac Vasc Anesth. 2024;38(3):717–23.
Milewski RC, Chatterjee S, Merritt-Genore H, Hayanga JWA, Grant MC, Roy N, et al. ECMO during COVID-19: a society of thoracic surgeons/extracorporeal life support organization survey. Ann Thorac Surg Short Rep. 2023;1(1):168–73.
Ding X, Chen H, Zhao H, Zhang H, He H, Cheng W, et al. ECCO(2)R in 12 COVID-19 ARDS patients with extremely low compliance and refractory hypercapnia. Front Med (Lausanne). 2021;8: 654658.
McNamee JJ, Gillies MA, Barrett NA, Perkins GD, Tunnicliffe W, Young D, et al. Effect of lower tidal volume ventilation facilitated by extracorporeal carbon dioxide removal vs standard care ventilation on 90-day mortality in patients with acute hypoxemic respiratory failure: the REST randomized clinical trial. JAMA. 2021;326(11):1013–23.
Sinha P, Calfee CS, Beitler JR, Soni N, Ho K, Matthay MA, Kallet RH. Physiologic analysis and clinical performance of the ventilatory ratio in acute respiratory distress syndrome. Am J Respir Crit Care Med. 2019;199(3):333–41.
Dianti J, McNamee James J, Slutsky Arthur S, Fan E, Ferguson Niall D, McAuley Daniel F, Goligher Ewan C. Determinants of effect of extracorporeal CO2 removal in hypoxemic respiratory failure. NEJM Evid. 2023;2(5):EVIDoa2200295.
Grasselli G, Calfee CS, Camporota L, Poole D, Amato MBP, Antonelli M, et al. ESICM guidelines on acute respiratory distress syndrome: definition, phenotyping and respiratory support strategies. Intensive Care Med. 2023;49(7):727–59.
Richard JC, Terzi N, Yonis H, Chorfa F, Wallet F, Dupuis C, et al. Ultra-low tidal volume ventilation for COVID-19-related ARDS in France (VT4COVID): a multicentre, open-label, parallel-group, randomised trial. Lancet Respir Med. 2023;11(11):991–1002.
Van Edom CJ, Gorog DA, Vandenbriele C. Anticoagulation in the ICU: a future for contact pathway inhibition? Intensive Care Med. 2023;49(11):1388–91.
Gross-Hardt S, Hesselmann F, Arens J, Steinseifer U, Vercaemst L, Windisch W, et al. Low-flow assessment of current ECMO/ECCO(2)R rotary blood pumps and the potential effect on hemocompatibility. Crit Care. 2019;23(1):348.
Acknowledgements
Medical writing support for the development of this manuscript was provided by Anna Woroniuk, PhD, and Daniel Johnson, PhD, of Nucleus Global, funded by Baxter.
Funding
Funding for this work, including medical writing support for the manuscript, was provided by Baxter International Inc., Deerfield, Illinois.
Author information
Authors and Affiliations
Contributions
AC developed the questions, and analyzed and interpreted the responses. As part of the Delphi process, AC, GA, LC, GCo, GCa, AGC, WD, RD, OD, CF, JF, MPH, DP, NR, RT, FT participated in the surveys before, during and after the consensus meeting. FT discussed the previous and the current draft of the study. All authors read and approved the final manuscript and approved it for submission.
Corresponding author
Ethics declarations
Ethics approval and consent to participate
Not applicable.
Consent for publication
Not applicable.
Competing interests
AC reports grants from Getinge, and personal fees from Getinge, Baxter and Xenios. LC has no financial interests to declare. GCa has received honoraria from Fresenius and Baxter and is a consultant for ASTEN Santé (France). AGC has received lectures honoraria from Baxter International Inc., Deerfield, Illinois. WD has no financial interests to declare. RD has received honoraria and has received laboratory material for an in vitro study from Baxter International Inc., Deerfield, Illinois. CFG has no financial interests to declare. JF has conducted advisory work and received honoraria from Baxter. MPH is the principal investigator of a clinical trial (NCT05316532) for which partial funding has been provided by the Baxter Foundation, Deerfield, United States. DP has received honoraria from Baxter International Inc. and Braun. NR has received lecture honoraria from Baxter Hellas. RT has received honoraria from Baxter International Inc., Deerfield, Illinois. FT received a Grant for a clinical study on Oxiris in AKI—Septic shock from Baxter International Inc., Deerfield, Illinois. JK and KH are full time employees of Baxter International with ownership interests.
Additional information
Publisher's Note
Springer Nature remains neutral with regard to jurisdictional claims in published maps and institutional affiliations.
Supplementary Information
Rights and permissions
Open Access This article is licensed under a Creative Commons Attribution 4.0 International License, which permits use, sharing, adaptation, distribution and reproduction in any medium or format, as long as you give appropriate credit to the original author(s) and the source, provide a link to the Creative Commons licence, and indicate if changes were made. The images or other third party material in this article are included in the article's Creative Commons licence, unless indicated otherwise in a credit line to the material. If material is not included in the article's Creative Commons licence and your intended use is not permitted by statutory regulation or exceeds the permitted use, you will need to obtain permission directly from the copyright holder. To view a copy of this licence, visit http://creativecommons.org/licenses/by/4.0/.
About this article
Cite this article
Combes, A., Auzinger, G., Camporota, L. et al. Expert perspectives on ECCO2R for acute hypoxemic respiratory failure: consensus of a 2022 European roundtable meeting. Ann. Intensive Care 14, 132 (2024). https://doi.org/10.1186/s13613-024-01353-8
Received:
Accepted:
Published:
DOI: https://doi.org/10.1186/s13613-024-01353-8