Abstract
Background
Algorithmic decision-making (ADM) utilises algorithms to collect and process data and develop models to make or support decisions. Advances in artificial intelligence (AI) have led to the development of support systems that can be superior to medical professionals without AI support in certain tasks. However, whether patients can benefit from this remains unclear. The aim of this systematic review is to assess the current evidence on patient-relevant benefits and harms, such as improved survival rates and reduced treatment-related complications, when healthcare professionals use ADM systems (developed using or working with AI) compared to healthcare professionals without AI-related ADM (standard care)—regardless of the clinical issues.
Methods
Following the PRISMA statement, MEDLINE and PubMed (via PubMed), Embase (via Elsevier) and IEEE Xplore will be searched using English free text terms in title/abstract, Medical Subject Headings (MeSH) terms and Embase Subject Headings (Emtree fields). Additional studies will be identified by contacting authors of included studies and through reference lists of included studies. Grey literature searches will be conducted in Google Scholar. Risk of bias will be assessed by using Cochrane’s RoB 2 for randomised trials and ROBINS-I for non-randomised trials. Transparent reporting of the included studies will be assessed using the CONSORT-AI extension statement. Two researchers will screen, assess and extract from the studies independently, with a third in case of conflicts that cannot be resolved by discussion.
Discussion
It is expected that there will be a substantial shortage of suitable studies that compare healthcare professionals with and without ADM systems concerning patient-relevant endpoints. This can be attributed to the prioritisation of technical quality criteria and, in some cases, clinical parameters over patient-relevant endpoints in the development of study designs. Furthermore, it is anticipated that a significant portion of the identified studies will exhibit relatively poor methodological quality and provide only limited generalisable results.
Systematic review registration
This study is registered within PROSPERO (CRD42023412156).
Similar content being viewed by others

Explore related subjects
Discover the latest articles, news and stories from top researchers in related subjects.Background
Artificial intelligence (AI) is a broad term referring to the field of computer science that develops algorithms mimicking human cognitive functions such as learning, perception, problem-solving and decision-making. AI encompasses various approaches, including machine learning (ML) and deep learning. It comprises a range of technologies and techniques, including algorithmic decision-making (ADM) ([9]: 1). ADM refers to the process of using these algorithms to gather, process, model and use input data to make or support decisions. Feedback from these decisions can then be used for improving the system ([2]: 612). An ADM can take various forms depending on how it is framed and presented to the user or decision subject. It can be a simple algorithm that has been known and used for decades, such as classification trees [37], or a more complex system like a recommender or AI that can provide recommendations to human decision-makers, nudge its users in a certain direction or perform fully automated decision-making processes without human involvement ([2]: 613). We specify AI-related algorithmic decision-making systems (AI-related ADM) as decision support systems that either apply AI (relying on ML models) or have been developed with the help of AI.
Recent advances in AI have resulted in the development of more complex and sophisticated systems that can outperform humans in certain tasks. For example, in the field of computer vision, systems like DeepMind’s AlphaFold have revolutionised protein structure prediction, solving a decades-old challenge in biology by accurately predicting 3D protein structures [18]. Additionally, AI innovations have transformed financial services, with machine learning models now being used to predict market trends, optimise trading strategies and enhance fraud detection [12]. Furthermore, generative AI has demonstrated remarkable capabilities in generating human-like text and performing a wide range of language-related tasks with unprecedented accuracy [13]. Recently, ChatGPT was evaluated for its clinical reasoning ability by testing its performance on questions from the United States Medical Licensing Examination, where it scored at or near the passing threshold on all three exams without any special training or reinforcement [21].
These advances in AI seem to have enormous potential to transform many different fields and industries, which begs the question: will AI do so in healthcare?
In clinical trials, AI systems have already shown potential to help clinicians make better diagnoses [3, 22], help personalise medicine and monitor patient care [6, 16] and contribute to drug development [7]. However, successful application in practice is limited ([30]: 77) and potential issues that may be responsible for this gap between research and practice should be revealed by our work.
By searching PubMed for the term ‘artificial intelligence’, we found over 2000 systematic reviews and meta-analyses published in the last 10 years, with a yearly increasing trend. These include several reviews conducted in the area of AI in healthcare that provide an overview of the current state of AI technologies in specific clinical areas, including AI systems for breast cancer diagnosis in screening programmes [8], ovarian cancer [38], early detection of skin cancer [17], COVID-19 and other pneumonia [15], prediction of preterm birth [1] or diabetes management [19]. Other reviews have focused on comparing clinicians and AI systems in terms of their performance to show their capabilities in a clinical setting [24, 27, 34].
Although these reviews are crucial to the further development of AI systems, they offer little insight into whether patients actually benefit from their use by medical professionals. Indeed, these studies focus on the analytical performance of these systems, rather than on healthcare-related metrics. In most of the studies mentioned here, the underlying algorithms have been evaluated using a variety of parameters, such as the F1 score for error classification, balanced accuracy, false positive rate and area under the receiver operating characteristic curve (AUROC). However, measures of a system’s accuracy often provide non-replicable results ([25]: 4), do not necessarily indicate clinical efficiency ([20]: 1), AUROC does not necessarily indicate clinical applicability ([10]: 935) and in fact, none of these measures reflects beneficial change in patient care ([4]: 1727, [33]: 1).
To summarise, as with any other new technology introduced into healthcare, the clinical effectiveness and safety of AI compared to the standard of care must be evaluated through properly designed studies to ensure patient safety and maximise benefits while minimising any unintended harm ([31]: 328). Therefore, a critical analysis of patient-relevant outcomes is needed, especially the benefits and harms of decisions informed by or made by AI systems.
To this end, this review goes beyond previous studies in several ways. First, we study clinical AI systems that enable algorithmic decision-making (AI-related ADM) in general and therefore do not limit ourselves to selected clinical problems. In particular, we focus on machine learning systems that infer rules from observations. Although we omit rule-based systems, we apply the term AI throughout our work because it is often incorrectly and redundantly used for ML and deep learning in the literature we study. Second, we focus on studies that report patient-relevant outcomes that, according to German Institute for Quality and Efficiency in Healthcare ([14]: 44), describe how patients feel, how they can perform their functions and activities or if they survive. These may include, for example, mortality, morbidity (with regard to complaints and complications), length of hospital stay, readmission, time to intervention and health-related quality of life. Third, we focus only on studies that compare medical professionals supported by AI-related ADM systems with medical professionals without AI-related ADM systems (standard care). By doing so, this review provides an overview of the current literature on clinical AI-related ADM systems, summarises the empirical evidence on their benefits and harms for patients and highlights research gaps that need to be addressed in future studies.
Objectives
The aim of this review is to systematically assess the current evidence on patient-relevant benefits and harms of ADM systems which are developed or used with AI (AI-related ADM) to support medical professionals compared to medical professionals without this support (standard care).
-
1.
Are there studies that compare patient-relevant effectiveness of AI-related ADM for medical professionals compared to medical professionals without AI-related ADM?
-
2.
Do these studies show adequate methodological quality and are their findings generalisable?
-
3.
Can AI-related ADM systems help medical professionals to make better decisions in terms of benefits and harms for patients?
Methods/design
In accordance with the Preferred Reporting Items for Systematic Review and Meta-Analysis Protocols (PRISMA-P) statement [26], the study protocol for this systematic is registered on the International Prospective Register of Systematic Reviews (PROSPERO) database (CRD42023412156). If necessary, post-registration changes to the protocol will be detailed under the PROSPERO record with an accompanying rationale.
We will follow the Preferred Reporting Items for Systematic Reviews and Meta-Analyses (PRISMA) statement [29] and the Methodological Expectations of Cochrane Intervention Reviews (MECIR) standards [11].
Searches
We will search systematically using English free text terms in title/abstract, Medical Subject Headings (MeSH) terms and Embase Subject Headings (Emtree) fields for various forms of keywords related to ‘artificial intelligence’ and relevant subcategories of computer generated and processed decision-making algorithms, ‘medical professionals’ and keywords describing effectiveness parameters and outcomes as well as preferred study types. Based on the block building approach, keywords and terms are combined using the Boolean operators AND and OR and progressively checked for relevant hits.
Databases to be used for searches
MEDLINE and PubMed (via PubMed), Embase (via Elsevier) and Institute of Electrical and Electronics Engineers (IEEE) Xplore will be searched for peer-reviewed articles as well as ClinicalTrials.gov and ICTRP (via CENTRAL) for ongoing trials and protocols.
To reduce potential publication bias, additional studies will be identified by contacting authors of included studies, contacting experts in the field and through reference lists of relevant studies. Grey literature searches will be conducted in Google Scholar. For this purpose, the keywords used in the systematic search will be used in different combinations, as well as their German equivalents. Google Scholar will be searched up to the 10th hit page. The detailed search strategy for each database will be reported under the PROSPERO record once the searches have been conducted.
Search strategy
We developed our search strategy using the PICOS scheme (Table 1).
While doing preliminary searches for basic literature in MEDLINE and PubMed (via PubMed), we noticed that study conductors from different scientific fields (e.g. computer scientists) used different terms for the intervention outcomes we were looking for. In addition, some studies were not indexed appropriately in PubMed, which complicated our initial search strategy. To carry out the search strategy, we have created and tested the blocks consecutively to gather the best results from each block, expanding and narrowing the search strategy. To assess the right direction of the search strategy, we have used fundamental literature, such as Choudhury and Asan [5], Park et al. [31] and Nagendran et al. [27] as test sets, making sure the results of our search had common ground with these studies.
The resulting search string for MEDLINE and PubMed in the individual blocks can be found in Table 2 and describes the basis for other databases.
Types of studies to be included
For the systematic search, peer-reviewed interventional and observational studies published in German or English 10 years retrospectively from the date of the search will be considered. For the search of grey literature, scientific reports published in German or English 10 years retrospectively from the date of the search will be considered. To extract potentially relevant studies from (systematic) reviews and meta-analyses, secondary studies will be gathered and screened. However, secondary studies will not be included in the synthesis.
In contrast to studies of effectiveness and safety, pure efficacy studies (e.g. focusing on algorithms accuracy) will be excluded as these outcomes are not directly relevant for patients. Patient-relevant outcomes will be defined according to the IQEHC method paper [14]. In addition, studies that used AI systems beyond our scope, such as robotics (systems that support the implementation of decisions), will be excluded. Editorials, commentaries, letters and other informal publication types will be excluded as well.
We will provide a list of all references screened in full text including exclusion reasons in the appendix of the final study.
Participants
Our study is focusing on human patients without restriction of age or sex. Therefore, the input data for the algorithms must include real human data gathered either during routine care and saved for use in research or generated specifically for the individual study.
Intervention
Out study is focusing on medical professionals utilising an AI-related ADM system to address a clinical problem.
In our working definition, a medical professional is a qualified individual who has the authority to perform necessary medical procedures within their professional scope of practice. Their goal is to improve, maintain or restore the health of individuals by examining, diagnosing, prognosticating and/or treating clinical problems. This may include medical doctors, registered nurses and other medical professionals. Clinical problems can encompass illnesses, injuries and physical or mental disorders, among other conditions.
In our working definition, an AI-related ADM system is a clinical decision support system that either applies AI in the sense of machine learning (ML, excluding rule-based systems) or has been developed with the help of ML. Clinical decision support models without any involvement of AI will be excluded.
Control
Medical professionals, as described in the working definition, are addressing a clinical problem without the support of an AI-related ADM system (standard care).
Outcomes
Patient-relevant benefits and harms, according to the IQEHC method paper [14], are gathered. These may include, for example, mortality, morbidity (with regard to complaints and complications), length of hospital stay, readmission, time to intervention and health-related quality of life.
Study types
We will collect both interventional and observational studies, which may encompass randomised controlled trials, cohort studies, case–control studies, randomised surveys, retrospective and prospective studies and phase studies, as well as non-inferiority or diagnostic studies.
Data extraction
Records arising from the literature search will be stored in the citation manager Citavi 6 (c) by Swiss Academic Software. After removing duplicates, two reviewers will independently review all titles and abstracts via the browser application Rayyan [28]. Studies potentially meeting the inclusion criteria will then be screened in full text independently by two reviewers using Citavi 6 (c). Disagreements over eligibility of studies will be discussed and, if necessary, resolved by a third reviewer. Authors of the included studies will be contacted if clarification of their data or study methods is required. The PRISMA 2020 flow diagram [29] will be used to keep the study selection process transparent.
Using a standardised data collection form, two reviewers will extract data independently from the included studies and will compare them for discrepancies. Missing data will be requested from study authors. Extracted data will include country of conduction, setting, study design, observational period, patient-relevant outcomes, intervention, comparator, characteristics of patient and medical professional populations and characteristics of the used algorithm. Additionally, studies will be classified by type of system, medical specialty or clinical area, prediction or classification goal of the AI-related ADM, supported decision, investigated benefits and harms, private or public study funding, applicable regulation (e.g. FDA, MDR), medical device classification (based on the risk and nature of the product) and whether the product is commercially available in its respective class (Table 3).
Risk of bias and quality assessment
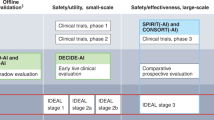
Risk of bias will be assessed by using the revised Cochrane risk-of-bias tool for randomised trials (RoB 2) [36] and the risk-of-bias in non-randomised studies for interventions (ROBINS-I) tool [35]. Disagreements between the authors over the risk of bias in the included studies will be resolved by discussion or with involvement of a third author if necessary. Transparent reporting of the included studies will be assessed trough the Consolidated Standards of Reporting Trials interventions involving Artificial Intelligence (CONSORT-AI) extension by Liu et al. [23]. The CONSORT-AI extension includes 14 new items that were considered sufficiently important for AI interventions to be routinely reported in addition to the core CONSORT items by Schulz et al. [32]. CONSORT-AI aims to improve the transparency and completeness in reporting clinical trials for AI interventions. It will assist to understand, interpret and critically appraise the quality of clinical trial design and risk of bias in the reported outcomes. We will assess studies conducted prior to the introduction of the CONSORT-AI guidelines in 2020 against these standards where possible. Although these studies may not fully meet the new criteria, application of the guidelines may still identify potential reporting gaps and ensure a consistent assessment framework across studies. We will discuss limitations related to this retrospective requirement to ensure a balanced and comprehensive analysis.
Data synthesis
Given the expected likelihood of heterogeneity between studies in the different medical specialties in terms of outcome measures, study designs and interventions, we do not know if performing a meta-analysis will be possible. However, a systematic narrative synthesis will be provided of the results with an overview of the relevant effects for the outcomes, with information presented in the text and tables to summarise and explain the characteristics and findings of the included studies. We will analyse the geographic distribution, study settings and medical specialties of the included studies. Additionally, we will examine funding sources and conduct a detailed risk of bias assessment. Compliance with reporting standards, such as CONSORT-AI and TRIPOD-AI, will be evaluated. We also plan to analyse patient demographics, including age, sex and race/ethnicity, as well as the involvement and training of medical professionals. ADM systems will be categorised into applicable regulation (e.g. FDA, MDR), medical device classification (based on the risk and nature of the product) and whether the product is commercially available in its respective class. Outcome analyses will focus on assessing both benefits and harms. Furthermore, we will analyse the validation of algorithms, considering both internal and external validation, and review the data availability statements to evaluate the accessibility of data used for algorithm development. Studies with an unclear or high risk of bias are not excluded to avoid potential selection bias and to ensure that valuable findings, particularly in emerging areas, are not lost. By including them, but clearly acknowledging and discussing their limitations, we aim to provide a more comprehensive overview of the available evidence. For this reason, our narrative synthesis emphasises the qualitative aspects of the data and focuses on identifying and describing trends, patterns and inconsistencies in the studies, rather than attempting to quantify effect sizes. This is consistent with the approach of recent reviews examining the methodological quality of machine learning systems in clinical settings (e.g. [27]).
Discussion
It is to be expected that there is a significant lack of suitable studies comparing healthcare professionals with and without AI-related ADM systems regarding patient-relevant outcomes. It is assumed that this is due to, first, the lack of approval regulations for AI systems, second, the prioritisation of technical and clinical parameters over patient-relevant outcomes in the development of study designs and, third, the prioritisation of AI for supporting clinical processes (e.g. administration). In addition, it is to be expected that a large proportion of the studies to be identified are of rather poor methodological quality and provide results that are rather difficult to generalise. Although reporting guidelines such as the Consolidated Standards of Reporting Trials (CONSORT) statement [32] are well-known and widely used in medical and public health research, they do not necessarily correspond to the novel protocol and study designs that are relevant for the assessment of the research questions relevant here. The extension of the Reporting Guidelines for Clinical Study Reports of Interventions Using Artificial Intelligence (CONSORT-AI) [23] may fill the gap but this guideline is relatively new and not necessarily always applied.
Availability of data and materials
Not applicable.
Abbreviations
- ADM:
-
Algorithmic decision-making
- AI:
-
Artificial intelligence
- AUROC:
-
Area under the receiver operating characteristic curve
- CENTRAL:
-
Cochrane Central Register of Controlled Trials
- CNN:
-
Convolutional neural network
- CONSORT:
-
Consolidated Standards of Reporting Trials
- CONSORT-AI:
-
Consolidated Standards of Reporting Trials for Artificial Intelligence
- CRD:
-
Centre for Reviews and Dissemination
- Emtree:
-
Embase Subject Headings
- HR:
-
Heart rate
- ICU:
-
Intensive care unit
- IEEE:
-
Institute of Electrical and Electronics Engineers
- IQEHC:
-
German Institute for Quality and Efficiency in Healthcare
- MECIR:
-
Methodological Expectations of Cochrane Intervention Reviews
- MeSH:
-
Medical Subject Headings
- ML:
-
Machine learning
- nRCT:
-
Non-randomised controlled trial
- PICO:
-
Participants, Intervention, Control, Outcome
- PRISMA:
-
Preferred Reporting Items for Systematic Review and Meta-Analysis
- PRISMA-P:
-
Preferred Reporting Items for Systematic Review and Meta-Analysis Protocols
- PROSPERO:
-
International Prospective Register of Systematic Reviews
- RCT:
-
Randomised controlled trial
- ResNet-18:
-
A convolutional neural network that is 18 layers deep
- RoB 2:
-
Revised Cochrane risk-of-bias tool for randomised trials
- ROBINS-I:
-
Risk-of-bias in non-randomised studies for interventions
- RR:
-
Respiratory rate
- SD:
-
Standard deviation
- SpO2:
-
Oxygen saturation
- SRQR:
-
Standards for Reporting Qualitative Research
References
Akazawa M, Hashimoto K. Prediction of preterm birth using artificial intelligence: a systematic review. J Obstet Gynaecol. 2022;42(6):1662–8. https://doi.org/10.1080/01443615.2022.2056828.
Araujo T, Helberger N, Kruikemeier S, de Vreese CH. In AI we trust? Perceptions about automated decision-making by artificial intelligence. AI & Soc. 2020;35:611–23. https://doi.org/10.1007/s00146-019-00931-w.
Bahl M, Barzilay R, Yedidia AB, Locascio NJ, Yu L, Lehman CD. High-risk breast lesions: a machine learning model to predict pathologic upgrade and reduce unnecessary surgical excision. Radiology. 2018;286(3):810–8. https://doi.org/10.1148/radiol.2017170549.
Brocklehurst P, Field D, Greene K, Juszczak E, Keith R, Kenyon S, et al. Computerised interpretation of fetal heart rate during labour (INFANT): a randomised controlled trial. Lancet. 2017;389:1719–29. https://doi.org/10.1016/s0140-6736(17)30568-8.
Choudhury A, Asan O. Role of artificial intelligence in patient safety outcomes: systematic literature review. JMIR Med Inform. 2020;8(7):e18599. https://doi.org/10.2196/18599.
Ciervo J, Shen SC, Stallcup K, Thomas A, Farnum MA, Lobanov VS, Agrafiotis DK. A new risk and issue management system to improve productivity, quality, and compliance in clinical trials. JAMIA Open. 2019;2(2):216–21. https://doi.org/10.1093/jamiaopen/ooz006.
Ekins S, Puhl AC, Zorn KM, Lane TR, Russo DP, Klein JJ, Hickey AJ, Clark AM. Exploiting machine learning for end-to-end drug discovery and development. Nat Mater. 2019;18(5):435–41. https://doi.org/10.1038/s41563-019-0338-z.
Freeman K, Geppert J, Stinton C, Todkill D, Johnson S, Clarke A, Taylor-Phillips S. Use of artificial intelligence for image analysis in breast cancer screening programmes: systematic review of test accuracy. BMJ. 2021;1(374):n1872. https://doi.org/10.1136/bmj.n1872.
Graili P, Ieraci L, Hosseinkhah N, Argent-Katwala M. Artificial intelligence in outcomes research: a systematic scoping review. Expert Rev Pharmacoecon Outcomes Res. 2021;21(4):601–23. https://doi.org/10.1080/14737167.2021.1886083.
Halligan S, Altman DG, Mallett S. Disadvantages of using the area under the receiver operating characteristic curve to assess imaging tests: a discussion and proposal for an alternative approach. Eur Radiol. 2015;25(4):932–9. https://doi.org/10.1007/s00330-014-3487-0.
Higgins J, Lasserson T, Chandler J, Tovey D, Thomas J, Flemyng E, Churchill R. Methodological Expectations of Cochrane Intervention Reviews (MECIR). Standards for the conduct and reporting of new Cochrane Intervention Reviews, reporting of protocols and the planning, conduct and reporting of updates. 2021. https://community.cochrane.org/sites/default/files/uploads/MECIR-February-2021.pdf. Accessed 12 Nov 2023.
Hilpisch Y. Artificial intelligence in finance: a Python-based guide. Sebastopol: O’Reilly Media; 2020.
Hughes A. ChatGPT: Everything you need to know about OpenAI’s GPT-3 tool. BBC Science Focus Magazine. https://www.sciencefocus.com/future-technology/gpt-3/. 2023. Accessed 12 Nov 2023.
Institute for Quality and Efficiency in Healthcare (IQEHC). Allgemeine Methoden. Version 6.1, from 24th of January 2022. https://www.iqwig.de/methoden/allgemeine-methoden-v6-1.pdf. 2022. Accessed 12 Nov 2023.
Jia LL, Zhao JX, Pan NN, Shi LY, Zhao LP, Tian JH, Huang G. Artificial intelligence model on chest imaging to diagnose COVID-19 and other pneumonias: a systematic review and meta-analysis. Eur J Radiol Open. 2022;9:100438. https://doi.org/10.1016/j.ejro.2022.100438.
Jiang F, Jiang Y, Zhi H, Dong Y, Li H, Ma S, Wang Y, Dong Q, Shen H, Wang Y. Artificial intelligence in healthcare: past, present and future. Stroke Vasc Neurol. 2017;2(4):230–43. https://doi.org/10.1136/svn-2017-000101.
Jones OT, Matin RN, van der Schaar M, Prathivadi Bhayankaram K, Ranmuthu CKI, Islam MS, Behiyat D, Boscott R, Calanzani N, Emery J, Williams HC, Walter FM. Artificial intelligence and machine learning algorithms for early detection of skin cancer in community and primary care settings: a systematic review. Lancet Digit Health. 2022;4(6):e466–76. https://doi.org/10.1016/S2589-7500(22)00023-1.
Jumper J, Evans R, Pritzel A, Green T, Figurnov M, Ronneberger O, Tunyasuvunakool K, Bates R, Žídek A, Potapenko A, Bridgland A, Meyer C, Kohl SA, Ballard AJ, Cowie A, Romera-Paredes B, Nikolov S, Jain R, Adler J, Back T, Petersen S, Reiman D, Clancy E, Zielinski M, Steinegger M, Pacholska M, Berghammer T, Bodenstein S, Silver D, Vinyals O, Senior A, Kavukcuoglu K, Kohli P, Hassabis D. Highly accurate protein structure prediction with AlphaFold. Nature. 2021;596(7873):583–9. https://doi.org/10.1038/s41586-021-03819-2.
Kamel Rahimi A, Canfell OJ, Chan W, Sly B, Pole JD, Sullivan C, Shrapnel S. Machine learning models for diabetes management in acute care using electronic medical records: a systematic review. Int J Med Inform. 2022;2(162):104758. https://doi.org/10.1016/j.ijmedinf.2022.104758.
Keane PA, Topol EJ. With an eye to AI and autonomous diagnosis. NPJ Digit Med. 2018;28(1):40. https://doi.org/10.1038/s41746-018-0048-y.
Kung TH, Cheatham M, Medenilla A, Sillos C, De Leon L, Elepaño C, Madriaga M, Aggabao R, Diaz-Candido G, Maningo J, Tseng V. Performance of ChatGPT on USMLE: potential for AI-assisted medical education using large language models. PLOS Digit Health. 2023;2(2):e0000198. https://doi.org/10.1371/journal.pdig.0000198.
Li Q, Zhao K, Bustamante CD, Ma X, Wong WH. Xrare: a machine learning method jointly modeling phenotypes and genetic evidence for rare disease diagnosis. Genet Med. 2019;21(9):2126–34. https://doi.org/10.1038/s41436-019-0439-8.
Liu X, Cruz Rivera S, Moher D, Calvert MJ, Denniston AK, SPIRIT-AI and CONSORT-AI Working Group. Reporting guidelines for clinical trial reports for interventions involving artificial intelligence the CONSORT-AI extension. Nat Med. 2020;26(9):1364–74. https://doi.org/10.1038/s41591-020-1034-x.
Liu X, Faes L, Kale AU, Wagner SK, Fu DJ, Bruynseels A, Mahendiran T, Moraes G, Shamdas M, Kern C, Ledsam JR, Schmid MK, Balaskas K, Topol EJ, Bachmann LM, Keane PA, Denniston AK. A comparison of deep learning performance against health-care professionals in detecting diseases from medical imaging: a systematic review and meta-analysis. Lancet Digit Health. 2019;1(6):e271–97. https://doi.org/10.1016/S2589-7500(19)30123-2.
McDermott MBA, Wang S, Marinsek N, Ranganath R, Foschini L, Ghassemi M. Reproducibility in machine learning for health research: Still a ways to go. Sci Transl Med. 2021 Apr;13(586). doi: 10.1126/scitranslmed.abb1655.
Moher D, Shamseer L, Clarke M, Ghersi D, Liberati A, Petticrew M, Shekelle P, Stewart LA. Preferred Reporting Items for Systematic Review and Meta-Analysis Protocols (PRISMA-P) 2015 statement. Syst Rev. 2015;4(1):1. https://doi.org/10.1609/aimag.v27i4.1904.
Nagendran M, Chen Y, Lovejoy CA, Gordon AC, Komorowski M, Harvey H, Topol EJ, Ioannidis JPA, Collins GS, Maruthappu M. Artificial intelligence versus clinicians: systematic review of design, reporting standards, and claims of deep learning studies. BMJ. 2020;25(368):m689. https://doi.org/10.1136/bmj.m689.
Ouzzani M, Hammady H, Fedorowicz Z, Elmagarmid A. Rayyan - a web and mobile app for systematic reviews. Syst Rev. 2016;5:210. https://doi.org/10.1186/s13643-016-0384-4.
Page MJ, McKenzie JE, Bossuyt PM, Boutron I, Hoffmann TC, Mulrow CD, Shamseer L, Tetzlaff JM, Akl EA, Brennan SE, Chou R, Glanville J, Grimshaw JM, Hróbjartsson A, Lalu MM, Li T, Loder EW, Mayo-Wilson E, McDonald S, McGuinness LA, Stewart LA, Thomas J, Tricco AC, Welch VA, Whiting P, Moher D. The PRISMA 2020 statement: an updated guideline for reporting systematic reviews. BMJ. 2021;29(372):n71. https://doi.org/10.1136/bmj.n71.
Panch T, Mattie H, Celi LA. The “inconvenient truth” about AI in healthcare. NPJ Digit Med. 2019;16(2):77. https://doi.org/10.1038/s41746-019-0155-4.
Park Y, Jackson GP, Foreman MA, Gruen D, Hu J, Das AK. Evaluating artificial intelligence in medicine: phases of clinical research. JAMIA Open. 2020;3(3):326–31. https://doi.org/10.1093/jamiaopen/ooaa033.
Schulz KF, Altman DG, Moher D; CONSORT Group. CONSORT 2010 statement: updated guidelines for reporting parallel group randomised trials. BMJ. 2010 340:c332. https://doi.org/10.1136/bmj.c332.
Shah NH, Milstein A, Bagley PhD SC. Making machine learning models clinically useful. JAMA. 2019;322(14):1351–2. https://doi.org/10.1001/jama.2019.10306.
Shen J, Zhang CJP, Jiang B, Chen J, Song J, Liu Z, He Z, Wong SY, Fang PH, Ming WK. Artificial intelligence versus clinicians in disease diagnosis: systematic review. JMIR Med Inform. 2019;7(3):e10010. https://doi.org/10.2196/10010.
Sterne JAC, Hernán MA, Reeves BC, Savović J, Berkman ND, Viswanathan M, Henry D, Altman DG, Ansari MT, Boutron I, Carpenter JR, Chan AW, Churchill R, Deeks JJ, Hróbjartsson A, Kirkham J, Jüni P, Loke YK, Pigott TD, Ramsay CR, Regidor D, Rothstein HR, Sandhu L, Santaguida PL, Schünemann HJ, Shea B, Shrier I, Tugwell P, Turner L, Valentine JC, Waddington H, Waters E, Wells GA, Whiting PF, Higgins JP. ROBINS-I: a tool for assessing risk of bias in non-randomised studies of interventions. BMJ. 2016;12(355):i4919. https://doi.org/10.1136/bmj.i4919.
Sterne JAC, Savović J, Page MJ, Elbers RG, Blencowe NS, Boutron I, Cates CJ, Cheng HY, Corbett MS, Eldridge SM, Emberson JR, Hernán MA, Hopewell S, Hróbjartsson A, Junqueira DR, Jüni P, Kirkham JJ, Lasserson T, Li T, McAleenan A, Reeves BC, Shepperd S, Shrier I, Stewart LA, Tilling K, White IR, Whiting PF, Higgins JPT. RoB 2: a revised tool for assessing risk of bias in randomised trials. BMJ. 2019;28(366):l4898. https://doi.org/10.1136/bmj.l4898.
Winterfeldt DV, Edwards W. Decision analysis and behavioral research. Cambridge University Press; 1986.
Xu HL, Gong TT, Liu FH, Chen HY, Xiao Q, Hou Y, Huang Y, Sun HZ, Shi Y, Gao S, Lou Y, Chang Q, Zhao YH, Gao QL, Wu QJ. Artificial intelligence performance in image-based ovarian cancer identification: a systematic review and meta-analysis. EClinicalMedicine. 2022;17(53):101662. https://doi.org/10.1016/j.eclinm.2022.101662.
Acknowledgements
Not applicable.
Funding
Open Access funding enabled and organized by Projekt DEAL. This research does not receive any funding.
Author information
Authors and Affiliations
Contributions
Christoph Wilhelm conceptualised, developed the methodology and wrote this protocol. He is the guarantor of the manuscript. Prof. Dr Anke Steckelberg validated the methodology of this protocol. Dr Felix G. Rebitschek conceptualised, reviewed, edited and supervised this protocol.
Corresponding author
Ethics declarations
Ethics approval and consent to participate
Not applicable.
Consent for publication
Not applicable.
Competing interests
The authors declare that they have no competing interests.
Additional information
Publisher’s Note
Springer Nature remains neutral with regard to jurisdictional claims in published maps and institutional affiliations.
Rights and permissions
Open Access This article is licensed under a Creative Commons Attribution 4.0 International License, which permits use, sharing, adaptation, distribution and reproduction in any medium or format, as long as you give appropriate credit to the original author(s) and the source, provide a link to the Creative Commons licence, and indicate if changes were made. The images or other third party material in this article are included in the article's Creative Commons licence, unless indicated otherwise in a credit line to the material. If material is not included in the article's Creative Commons licence and your intended use is not permitted by statutory regulation or exceeds the permitted use, you will need to obtain permission directly from the copyright holder. To view a copy of this licence, visit http://creativecommons.org/licenses/by/4.0/.
About this article
Cite this article
Wilhelm, C., Steckelberg, A. & Rebitschek, F.G. Is artificial intelligence for medical professionals serving the patients? . Syst Rev 13, 228 (2024). https://doi.org/10.1186/s13643-024-02646-6
Received:
Accepted:
Published:
DOI: https://doi.org/10.1186/s13643-024-02646-6