Abstract
Background
Sodium-glucose co-transporter 2 (SGLT2) inhibitors have been shown to lower the risk of re-hospitalization and cardiovascular mortality among heart failure (HF) patients. Nevertheless, the impact of these agents on ventricular arrhythmias (VAs) has not been thoroughly investigated. To assess the beneficial impact of SGLT2 inhibitors on VAs in patients at various stages of HF, a systematic review and meta-analysis of randomized controlled trials involving SGLT2 inhibitors in this patient population was performed.
Methods
A comprehensive search of the PubMed, Embase, Ovid, ProQuest, Scopus, and Cochrane databases was performed for clinical trials published up to November 21, 2024. The primary outcomes of interest were incidences of VAs and sudden cardiac death (SCD) between the groups receiving SGLT2 inhibitors and the control drugs. For the outcomes observed in the populations of the included trials and in specific subgroups, hazard ratios (HRs) and 95% confidence intervals (CIs) were pooled and meta-analysed across the analyses.
Results
A total of 23 randomized trials (22 placebo-controlled trials and 1 active-controlled trial) involving 74,380 patients (37,372 receiving SGLT2 inhibitors and 37,008 in the control group) were included. The analysed SGLT2 inhibitors included canagliflozin, dapagliflozin, empagliflozin, bexagliflozin, sotagliflozin, and ertugliflozin. The participants were non-advanced HF patients, including at-risk for HF, pre-HF, and symptomatic HF, with follow-up duration ranging from 12 to 296 weeks. Compared with the control, treatment with SGLT2 inhibitors was associated with significantly reduced risk of VAs (risk ratio (RR) 0.85, 95% confidence interval (CI) 0.74–0.98; P = 0.02) and SCD (RR 0.79, 95% CI 0.64–0.98; P = 0.03). Subgroup analyses indicated that longer follow-up (≥ 1 year) taking SGLT2 inhibitors can still reduce the risk of VAs (RR 0.79, 95% CI 0.65–0.96; P = 0.02) and SCD (RR 0.80, 95% CI 0.65–0.99; P = 0.04).
Conclusion
SGLT2 inhibitors have beneficial effects on lowering risks of VAs and SCD in patients with type 2 diabetes, cardiovascular diseases, heart failure with reduced ejection fraction (HFrEF), heart failure with preserved ejection fraction (HFpEF), and heart failure with mildly reduced ejection fraction (HFmrEF), with longer follow-up duration reinforcing these findings. However, future prospective trials are needed to verify the effects of SGLT2 inhibitors on VAs and SCD.
Systematic review registration
PROSPERO (CRD42024601914)
Highlights
1. Redefine outcomes of ventricular arrhythmias (VAs) and sudden cardiac death (SCD) for SGLT2 inhibitor medication in heart failure patients.
2. SGLT2 inhibitors lower the risks of VAs and SCD in patients at various stages of HF, excluding advanced HF.
3. Longer therapeutic effects were associated with a significant reduction in the incidence of VAs and SCD.
Similar content being viewed by others
Introduction
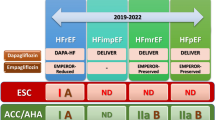
Heart failure (HF) is a growing health and economic burden worldwide, with its prevalence increasing to 1–3% in the general adult population, with high mortality and substantial annual healthcare costs per patient [1]. According to the AHA/ACC/HFSA Guideline for the Management of Heart Failure, HF is categorized into four distinct stages: at-risk for HF (Stage A), pre-HF (Stage B), symptomatic HF (Stage C), and advanced HF (Stage D) [2]. In the guideline, sodium‐glucose co-transporter 2 (SGLT2) inhibitors were recommended at different stages of HF, including Stage A patients with type 2 diabetes (T2DM) and cardiovascular disease (CVD) or high risks for CVD and Stage C patients of heart failure with reduced ejection fraction (HFrEF), heart failure with preserved ejection fraction (HFpEF), or heart failure with mildly reduced ejection fraction (HFmrEF) [2].
SGLT2 inhibitors are a class of anti-hyperglycemic drugs that include empagliflozin, dapagliflozin, canagliflozin, sotagliflozin, and ertugliflozin, all of which are currently available in the clinic. SGLT2 is located in segment 1 of the proximal renal tubules and contributes to the re-absorption of approximately 90% of the glucose filtered by the kidney [3]. Inhibition of this transporter leads to a reduction in glucose re-absorption by the kidney and an increase in glucose excretion in the urine [4]. Although these agents are initially designed to lower plasma glucose levels, several studies have demonstrated their protective effects on the prognosis of HF patients, regardless of diabetes status, indicating that their benefits extend beyond mere blood glucose control [5,6,7].
It is widely known that ventricular arrhythmias (VAs) are associated with high mortality in HF patients and HF can increase the occurrence of VAs [8, 9]. In addition to VAs, sudden cardiac death (SCD) is also a major cause of death in HF patients, with the majority of SCD cases attributed to VAs [8, 10]. However, conclusions regarding the effects of SGLT2 inhibitors on VAs/SCD remain contentious due to variations in inclusion criteria across studies. Several meta-analyses have shown a reduction in ventricular tachycardia following SGLT2 inhibitor treatment, although these findings did not reach statistical significance [11,12,13,14]. Recent studies have shown that SGLT2 inhibitors are effective in addressing HF-induced VAs, necessitating a systematic evaluation of their effects [15, 16]. This meta-analysis aims to evaluate whether SGLT2 inhibitor medication can lead to a reduction in VAs among individuals at various stages of non-advanced HF, including T2DM and CVD or high risks for CVD, HFrEF, HFpEF, and HFmrEF.
Methods
Protocol and registration
The protocol for this meta-analysis was registered in advance with the International Prospective Register of Systematic Reviews (CRD42024601914).
Search strategy
This meta-analysis was performed in accordance with the Preferred Reporting Items for Systematic Reviews and Meta-Analyses (PRISMA) guidelines. A systematic literature search of relevant randomized clinical trials was conducted across multiple databases, including PubMed, Embase, Cochrane, Web of Science, ProQuest, Scopus, Ovid, and ClinicalTrials.gov, with a cut-off date of November 21, 2024. PubMed is frequently used in biomedicine, offering extensive literature in biomedical and life sciences. Embase, a biomedical and pharmacology database by Elsevier, focuses on drug information and research. Cochrane Library offers high-quality healthcare info, including systematic reviews, clinical trials, and decision support, suitable for evidence-based medicine. Web of Science, a comprehensive citation database, provides impact and trend insights into research literature. ProQuest offers academic resources like journal articles, dissertations, and conference papers. Scopus, a large citation and abstract database by Elsevier, covers science, technology, and medicine. Ovid is a comprehensive online information and literature search platform integrating multiple databases, including MEDLINE and EMBASE. ClinicalTrials.gov provides clinical trial registration and results, crucial for trial transparency and integrity. Searching these databases enhances meta-analysis quality and reliability, ensuring scientific and objective research outcomes.
Both published and unpublished randomized controlled trials (RCTs) were reviewed via the search strategy outlined in eTable 3 in the Supplement. No language restrictions were applied. Additionally, the references listed in the identified studies were carefully checked to ensure that no additional studies were omitted.
Inclusion and exclusion criteria
Eligible RCTs were required to meet the following criteria: (i) patients aged 18 years or older with various stages of HF; (ii) a comparative analysis between SGLT2 inhibitors and either a placebo or an active control; and (iii) the reporting of relevant outcomes of interest. RCTs focused on type 1 diabetes mellitus (T1DM), or advanced HF were excluded. Trial eligibility was confirmed by two independent reviewers (M.L. and L.Z.), and discrepancies between reviewers were resolved by consensus or, if necessary, by a third investigator (X.F.).
Outcomes of interest
The outcomes of interest were reported as serious adverse events according to the Medical Dictionary for Drug Regulatory Activities (MedDRA) to reduce potential bias. The specific outcomes examined included the incidence of various forms of VAs and SCD. In this meta-analysis, VAs included ventricular tachycardia, ventricular fibrillation, ventricular flutter, ventricular extrasystole, ventricular asystole, Brugada syndrome, and Torsades de Pointes, and the detailed definition of SCD is presented in eTable 1. Additionally, subgroup analyses were conducted to compare the effects of SGLT2 inhibitor treatment across different drug types and lengths of follow-up.
Data extraction and quality assessment
Two reviewers (M.L. and L.Z.) independently extracted the data via a standard form that included the following items: (i) characteristics of the studies, sample size, study design, and follow-up duration; (ii) baseline characteristics of the study population; and (iii) interventions, comparisons, and outcomes of interest. Study quality was evaluated via the Cochrane Risk of Bias Tool, a process carried out by both M.L. and L.Z. Any discrepancies during this evaluation were resolved through consensus or adjudication by a third author (X. F.).
Statistical analysis
We used pooled relative risks (RRs) with corresponding 95% confidence intervals (CIs) to present the incidence of SCD or VAs. The assessment of heterogeneity was assessed via the Cochran Q test statistic and Higgins and Thompson I. For the Q test, a P value of 50% indicated at least moderate heterogeneity. If significant heterogeneity was detected, we used the random-effects (RE) model; otherwise, the fixed-effects (FE) model was applied when heterogeneity was not significant. The analysis was conducted with the Review Manager (RevMan, version 5.3; Copenhagen: The Nordic Cochrane Centre, The Cochrane Collaboration, 2014).
Results
Characteristics of eligible studies
A total of 11,795 records were identified, of which 23 RCTs [17,18,19,20,21,22,23,24,25,26,27,28,29,30,31,32,33,34,35,36,37,38,39] met inclusion criteria in this meta-analysis, comprising a cumulative sample size of 74,380 participants (37,008 patients treated with SGLT2 inhibitors and 37,372 control subjects) (Fig. 1). The baseline characteristics of the included studies and participants were summarized in Table 1. Among the 23 published trials reviewed, 22 were placebo-controlled, while the left one was active-controlled (with glimepiride). These trials were categorized by specific types of SGLT2 inhibitors, with seven trials focusing on empagliflozin, five trials on canagliflozin, six trials on dapagliflozin, two on ertugliflozin, one on bexagliflozin, and two on sotagliflozin. Fourteen RCTs reported SCD, and 22 reported VAs. A summary of the specific events of interest reported in the included trials can be found in eTable 1. The mean age of the participants ranged from 63 to 71.9 years old, and the percentage of males ranged from 43.2 to 92.7%, with a median follow-up period spanning from 12 to 296 weeks.
The quality assessment results are presented in eTable 2. Most RCTs were considered high methodological quality. One trial had an open-label design, given the well-defined diagnostic criteria, clear protocol, and well-executed methodology, we considered it to have a low risk of performance bias [28]. Two trials reported minor imbalances in baseline characteristics between the empagliflozin and placebo groups [30, 37]. Since these imbalances were minor, we also deemed these two trials to be at low risk of performance bias. In another study, the percentage of participants who discontinued treatment due to serious events exceeded 20%, indicating a high risk of attrition bias [22]. Nonetheless, the included patients who discontinued follow-up were limited and the percentage of patients who lost follow-up was less than 5%, the characteristics of the included two groups’ patients are balanced. Consequently, we assessed this trial as having a low risk of performance bias as well.
Incidence of ventricular arrhythmias
VAs events were reported in 22 RCTs, 21 of which involved comparisons between SGLT2 inhibitors and placebo [17,18,19,20,21,22,23,24,25,26,27, 29,30,31,32,33,34,35,36, 38, 39], whereas one trial compared SGLT2 inhibitors with an active control [28]. 277 patients receiving SGLT2 inhibitors experienced VAs (n = 37,274), whereas 320 individuals in the control group experienced VAs (n = 36,689). The aggregated results indicated that SGLT2 inhibitor therapy was associated with a lower risk of VAs than the control (RR 0.85, 95% CI 0.74–0.98; P = 0.02) (Fig. 2A).
In the subgroup analysis by specific SGLT2 inhibitor type, canagliflozin (RR 0.63, 95% CI 0.34–1.20; P = 0.16), sotagliflozin (RR 0.78, 95% CI 0.39–1.57; P = 0.49), dapagliflozin (RR 0.91, 95% CI 0.71–1.15; P = 0.39), ertugliflozin (RR 0.89, 95% CI 0.44–1.81; P = 0.75) and empagliflozin (RR 0.75, 95% CI 0.50–1.11; P = 0.15) showed no significant reduction in risk of VAs compared with the control group (Fig. 3A). To evaluate the effect of compliance of SGLT2 inhibitor treatment on VAs incidence, subgroup analysis for follow-up duration was conducted and results showed that longer follow-up durations (≥ 1 year; RR 0.79, 95% CI 0.65–0.96; P = 0.02) significantly reduced the incidence of VAs compared to shorter durations (< 1 year) (Fig. 3B). These findings suggested that a longer therapeutic effect was associated with a significant reduction in the incidence of VAs. All analyses performed above revealed low heterogeneity. The results of the funnel plot with Begg’s and Egger’s tests showed no significant publication bias (eFig. 1).
Subgroup analysis of the effects of various drugs and durations on the efficacy of SGLT-2 inhibitors in reducing the incidence of ventricular arrhythmias. A Effects of different sodium‒glucose cotransporter-2 inhibitors on the incidence of ventricular arrhythmias. B The duration of pharmacotherapy influences the effectiveness of SGLT2 inhibitors in reducing the incidence of ventricular arrhythmias
Incidence of sudden cardiac death
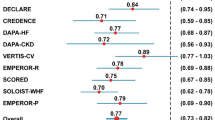
Fourteen RCTs reported SCD events, with one trial taking glimepiride as an active placebo in the control arm [28] and the remaining studies using placebo in the control arm [20,21,22, 25, 26, 29,30,31,32, 34, 35, 37, 39]. Among the total 360 SCD events, 159 occurred in the SGLT2 inhibitor group (n = 36,172), and 201 occurred in the placebo group (n = 35,814). After pooling the data from these 14 trials, a significantly reduced SCD risk was identified for SGLT2 inhibitor therapy compared to placebo, with RR of 0.79 and 95% CI of 0.64–0.98 (P = 0.03) (Fig. 2B). However, the subgroup analysis based on drug type revealed inconsistent results relative to the overall effect: empagliflozin (RR 0.72, 95% CI 0.47–1.12; P = 0.15), canagliflozin (RR 0.78, 95% CI 0.52–1.16; P = 0.22), sotagliflozin (RR 1.24, 95% CI 0.65–2.36; P = 0.51), and dapagliflozin (RR 0.72, 95% CI 0.52–1.02; P = 0.06) (Fig. 4A).
Subgroup analysis of various drugs and treatment durations on the efficacy of SGLT2 inhibitors in mitigating the occurrence of sudden cardiac death. A Differential effects of various SGLT2 inhibitors on the rates of sudden cardiac death. B The duration of pharmacotherapy influences the effectiveness of SGLT2 inhibitors in reducing the incidence of sudden cardiac death
In terms of the impact of the follow-up period on SCD incidence, a shorter duration (< 1 year, RR 0.67, 95% CI 0.27–1.64; P = 0.38) did not significantly affect SCD incidence. Conversely, a longer duration (≥ 1 year, RR 0.80, 95% CI 0.65–0.99; P = 0.04) was associated with a lower SCD incidence (Fig. 4B). All the pooled results above presented low heterogeneity across the studies. Additionally, a visual inspection of the funnel plot with Begg’s and Egger’s tests showed no significant publication bias (eFig. 2).
Discussion
In this meta-analysis of 23 trials involving 74,380 patients at different stages of HF, we observed that overall, therapy with SGLT2 inhibitors was associated with a lower risk of SCD and VAs in patients with non-advanced HF, differing from the findings of several previous studies demonstrating no relativity [11,12,13,14]. Notably, within the subgroup for a longer follow-up duration (> 1 year) of SGLT2 inhibitor therapy, a significant reduction in the incidence of reported VAs and SCD events remains.
The anti-arrhythmic effects of SGLT2 inhibitors have also gained attention. In the EMBODY trial, heart rate variability and turbulence indicators of cardiac sympathetic and parasympathetic nerve activities were improved in the empagliflozin group compared with the placebo group. Such improvements in autonomic nerve activity can lead to a decreased incidence of VAs [40]. Additionally, dapagliflozin has been shown to benefit patients with T2DM and HFrEF by reducing the burden of ventricular ectopic beats, decreasing heart rate variability, and reducing the heterogeneity of ventricular repolarization [41, 42]. Furthermore, another study reported that SGLT2 inhibitor treatment could significantly attenuate the burden of inflammation and reduce composite cardiac outcomes, VAs included, in patients with T2DM [43].
Studies on the direct association between SGLT2 inhibitor treatment and VAs have also been explored as in the EMPA-ICD and the ERASE trials investigated the effects of empagliflozin on VAs in T2DM and HF patients, respectively. These two trials revealed that empagliflozin significantly reduced VAs occurrence in T2DM or/and HF patients with an ICD/CRT-D [17, 18]. With the device implantation, these trials directly showed that SGLT2 inhibitors reduced the occurrence of VAs from the recordings by ICD/CRT-D. However, these trials have limited enrolled patients. Further large clinical trials need to be conducted to investigate the direct effects of SGLT2 inhibitors on VAs and SCD in HF patients. On the other side, meta-analysis provides an alternative way to study the association between SGLT2 inhibitor treatment and arrhythmia in a real world and large population way. One meta-analysis reported that SGLT2 inhibitors were associated with a lower rate of ventricular tachycardia in HF, T2DM, and chronic kidney disease (CKD) patient populations. However, it was limited to only seven studies [44]. Another meta-analysis of randomized trials in HF, T2DM, and chronic CKD patients suggested that SGLT2 inhibitor treatment was not associated with the incidence of VAs [11,12,13,14]. With the update of clinical guidelines, we included patients at Stages A, B, and C of HF, and excluded studies involving patients with advanced HF, defined by severe and persistent symptoms of HF (NYHA class IV) [2]. By extending the inclusion of participants ranging across different stages of HF, we found that SGLT2 inhibitor treatment was associated with a 19% risk reduction in VAs, agreeing with other clinical studies suggested that SGLT2 inhibitor treatment may significantly attenuate VAs, though encompassed as part of composite cardiac outcomes [45, 46].
VA is a major cause of SCD [47]. Investigating the association between SGLT2 inhibitors and SCD can also help explore the anti-arrhythmic properties of these pharmacological agents. A meta-analysis indicated that SGLT2 inhibitor therapy was associated with a significantly lower risk of SCD in patients with HF receiving conventional treatments [48]. Additionally, another meta-analysis revealed that SGLT2 inhibitors are linked to a significant decrease in the incidence of SCD in patients with T2DM [11]. Although some meta-analyses suggested that the relationship between SGLT2 inhibitors and SCD is not significant [12, 49], our results supported the SGLT2 inhibitor treatment significantly reduced the risk of SCD to various stages of HF patients, which in accordance with the EMPA-REG OUTCOME study revealed that empagliflozin significantly reduced cardiovascular death, including SCD [50].
Currently, there is a lack of extensive clinical trials specifically examining the anti-arrhythmic effects of SGLT2 inhibitors via a comparative analysis of arrhythmic incidence prior to and following SGLT2 inhibitor treatment. As SGLT2 inhibitor drugs have gained increasing attention for their cardio-protective properties, some ongoing clinical trials are investigating the impact of SGLT2 inhibitors on VAs and SCD. Some studies have designated VAs/SCD as one of their endpoints; however, these studies are currently in various stages of participant recruitment or are not yet recruiting. The ClinicalTrials.gov identifiers of these trials include NCT03271879, NCT06124937, NCT04117763, NCT05550441, and NCT03977116. With these trials complete, the association between SGLT2 inhibitor treatment and the occurrence of VAs/SCD will be clearer.
As previously noted, the discrepancies observed in various meta-analyses regarding the effect of SGLT2 inhibitors on VAs or SCD could be due to varied inclusion criteria. Our meta-analysis specifically paid more attention to patients at high risk of HF, defined by a history of T2DM lasting 10 years or more and a systolic blood pressure > 140 mmHg (average of 3 readings) recorded during the screening visits. These high-risk factors for HF correspond to Stage A: at-risk for HF. The Stages B and C of HF patients were also included in this meta-analysis. We excluded patients with advanced HF. Consequently, these novel eligibility criteria employed in our study resulted in different patient characteristics compared with those of previous studies, leading to our findings showing that SGLT2 inhibitor treatment could effectively reduce the incidence of VAs and SCD in non-advanced HF patients. Furthermore, the low event recordings of VAs and SCD may lead to an inaccurate estimation of the real incidence, as VAs/SCD were not designated primary or secondary outcomes in the studies reviewed. All included clinical trials reported VAs as appendages in adverse events and by regular 12-lead electrocardiogram measurements. This method of monitoring contributes to an underestimation of VAs events. Thus, the frequency and duration of VAs events among different patients remain largely unknown. Future clinical trials should consider employing alternative rhythm monitoring techniques, such as cardiac implantable devices, and should prioritize the assessment of VAs as either a primary or secondary outcome to elucidate the interaction between SGLT2 inhibitor treatment and VAs.
In addition to these clinical studies and meta-analyses, preclinical studies have investigated the underlying mechanisms of the anti-arrhythmic effects of SGLT2 inhibitors. Firstly, SGLT2 inhibitor treatment can attenuate prolonged action potential duration, which is achieved by modulating various ion currents involved in each phase of the action potential under pathological conditions. Specifically, SGLT2 inhibitor treatment can mitigate the increased late sodium current (INa,L) by modifying the states of cardiac sodium channels in both diabetic and HF models [51,52,53]. Additionally, these inhibitors can reduce the increased activity of Na+/H+ exchangers (NHEs) in the cardiomyocytes of failing hearts [52, 54], leading to alleviation of intracellular Na+ accumulation and subsequent cytoplasmic Ca2+ overload, as well as mitochondrial Ca2+ depletion. Furthermore, SGLT2 inhibitors can modulate the activity/phosphorylation of calcium/calmodulin-dependent protein kinase II (CaMKII) to regulate RyR2 and sarcoplasmic reticulum calcium ATPase (SERCA)/PLN, resulting in increased Ca2+ reuptake and decreased Ca2+ leakage from the sarcoplasmic reticulum (SR), thereby reducing VAs [51, 55]. Moreover, SGLT2 inhibitors have been shown to restore mitochondrial calcium homeostasis in HF, avoiding adversely affecting the regeneration of nicotinamide‐adenine dinucleotide, compromising the antioxidant system and subsequently reducing the incidence of VAs [56, 57]. Secondly, SGLT2 inhibitors such as dapagliflozin can attenuate the increased sympathetic nerve activities observed in HF model [58] . In failing hearts, the activation of neural hormone pathways can lead to abnormal electrical automaticity and exacerbate HF. Additionally, SGLT2 inhibitors such as dapagliflozin reduce inflammation in models of HF and diabetes [59, 60]. Finally, the promotion of osmotic diuresis and natriuresis to alleviate cardiac load and improve left ventricular function alterations in hemodynamic and metabolic parameters (such as ketone bodies) to protect against myocardial remodelling, reduction in epicardial adipose tissue may also count [61, 62].
Limitations
This meta-analysis has several limitations. First, accurately estimating the incidence of SCD and VAs is challenging due to diverse definitions, and some qualified studies were excluded because SCD or VA events were unidentified from the comprehensive safety events. Additionally, subgroup analysis has insignificant results due to the limited clinical publications. Second, the types of VA included in the analysis varied, potentially resulting in the omission of certain specific VA types. The lack of ECG monitoring, such as the use of implantable cardioverter defibrillators (ICDs) and cardiac resynchronization therapy (CRT), may under-report the number of SCD and VA events. Third, the distribution of patients across different stages of HF was not uniform, with a few studies possibly including patients with advanced HF. Finally, some trials investigating VAs and SCD as primary or secondary outcomes are currently ongoing.
Conclusions
SGLT2 inhibitor therapy was associated with an overall lower risk of VAs and SCD in patients with non-advanced HF, including T2DM, CVD, HFrEF, HFpEF, and HFmrEF. Besides, a long treatment duration (> 1 year) has also exhibited a consistent reduction of VAs and SCD occurrence. However, further prospective studies with large populations, including advanced HF and longer follow-up periods, are needed before definitive conclusions can be reached.
Data availability
The data used in this review were extracted from published studies, and the original data could be obtained by searching databases. Other data supporting the results of this review are available from the corresponding author upon reasonable request.
References
Savarese G, Becher PM, Lund LH, et al. Global burden of heart failure: a comprehensive and updated review of epidemiology. Cardiovasc Res. 2023;118(17):3272–87. https://doi.org/10.1093/cvr/cvac013.
Heidenreich PA, Bozkurt B, Aguilar D, et al. 2022 AHA/ACC/HFSA guideline for the management of heart failure: a report of the American College of Cardiology/American Heart Association Joint Committee on Clinical Practice Guidelines. Circulation. 2022;145(18):e895–1032. https://doi.org/10.1161/CIR.0000000000001063.
Wright EM, Loo DDF, Hirayama BA. Biology of human sodium glucose transporters. Physiol Rev. 2011;91(2):733–94. https://doi.org/10.1152/physrev.00055.2009.
Zelniker TA, Braunwald E. Mechanisms of cardiorenal effects of sodium-glucose cotransporter 2 inhibitors: JACC state-of-the-art review. J Am Coll Cardiol. 2020;75(4):422–34. https://doi.org/10.1016/j.jacc.2019.11.031.
Inzucchi SE, Kosiborod M, Fitchett D, et al. Improvement in cardiovascular outcomes with empagliflozin is independent of glycemic control. Circulation. 2018;138(17):1904–7. https://doi.org/10.1161/circulationaha.118.035759.
McMurray JJV, Solomon SD, Inzucchi SE, et al. Dapagliflozin in patients with heart failure and reduced ejection fraction. New Engl J Med. 2019;381(21):1995–2008. https://doi.org/10.1056/NEJMoa1911303.
Packer M, Anker SD, Butler J, et al. Cardiovascular and renal outcomes with empagliflozin in heart failure. Ne Engl J Med. 2020;383(15):1413–24. https://doi.org/10.1056/NEJMoa2022190.
Lip GY, Heinzel FR, Gaita F, et al. European Heart Rhythm Association/Heart Failure Association joint consensus document on arrhythmias in heart failure, endorsed by the Heart Rhythm Society and the Asia Pacific Heart Rhythm Society. Europace. 2016;18(1):12–36. https://doi.org/10.1093/europace/euv191.
Podrid PJ, Fogel RI, Fuchs TT. Ventricular arrhythmia in congestive heart failure. American journal of cardiology. 1992;69(18):82G-95G; discussion 95G-96G. https://doi.org/10.1016/0002-9149(92)91257-5.
Watanabe E, Tanabe T, Osaka M, et al. Sudden cardiac arrest recorded during Holter monitoring: prevalence, antecedent electrical events, and outcomes. Heart Rhythm. 2014;11(8):1418–25. https://doi.org/10.1016/j.hrthm.2014.04.036.
Fernandes GC, Fernandes A, Cardoso R, et al. Association of SGLT2 inhibitors with arrhythmias and sudden cardiac death in patients with type 2 diabetes or heart failure: a meta-analysis of 34 randomized controlled trials. Heart Rhythm. 2021;18(7):1098–105. https://doi.org/10.1016/j.hrthm.2021.03.028.
Sfairopoulos D, Zhang N, Wang Y, et al. Association between sodium-glucose cotransporter-2 inhibitors and risk of sudden cardiac death or ventricular arrhythmias: a meta-analysis of randomized controlled trials. Europace. 2022;24(1):20–30. https://doi.org/10.1093/europace/euab177.
Yin Z, Zheng H, Guo Z. Effect of sodium-glucose co-transporter protein 2 inhibitors on arrhythmia in heart failure patients with or without type 2 diabetes: a meta-analysis of randomized controlled trials. Front Cardiovasc Med. 2022;9: 902923. https://doi.org/10.3389/fcvm.2022.902923.
Scheen AJ. Glucose-lowering agents and risk of ventricular arrhythmias and sudden cardiac death: a comprehensive review ranging from sulphonylureas to SGLT2 inhibitors. Diabetes Metab. 2022;48(6): 101405. https://doi.org/10.1016/j.diabet.2022.101405.
Fawzy AM, Rivera-Caravaca JM, Underhill P, Fauchier L, Lip GYH. Incident heart failure, arrhythmias and cardiovascular outcomes with sodium-glucose cotransporter 2 (SGLT2) inhibitor use in patients with diabetes: insights from a global federated electronic medical record database. Diabetes Obes Metab. 2023;25(2):602–10. https://doi.org/10.1111/dom.14854.
Curtain JP, Docherty KF, Jhund PS, et al. Effect of dapagliflozin on ventricular arrhythmias, resuscitated cardiac arrest, or sudden death in DAPA-HF. Euro Heart J. 2021;42(36):3727–38. https://doi.org/10.1093/eurheartj/ehab560.
Fujiki S, Iijima K, Nakagawa Y, et al. Effect of empagliflozin on ventricular arrhythmias in patients with type 2 diabetes treated with an implantable cardioverter-defibrillator: the EMPA-ICD trial. Cardiovasc Diabetol. 2024;23(1):224. https://doi.org/10.1186/s12933-024-02309-9.
Benedikt M, Oulhaj A, Rohrer U, et al. Ertugliflozin to reduce arrhythmic burden in patients with ICDs/CRT-Ds. NEJM evidence. 2024;3(10):EVIDoa2400147. https://doi.org/10.1056/EVIDoa2400147.
McMurray JJV, Docherty KF, de Boer RA, et al. Effect of dapagliflozin versus placebo on symptoms and 6-minute walk distance in patients with heart failure: the DETERMINE randomized clinical trials. Circulation. 2024;149(11):825–38. https://doi.org/10.1161/circulationaha.123.065061.
Solomon SD, McMurray JJV, Claggett B, et al. Dapagliflozin in heart failure with mildly reduced or preserved ejection fraction. N Engl J Med. 2022;387(12):1089–98. https://doi.org/10.1056/NEJMoa2206286.
Spertus JA, Birmingham MC, Nassif M, et al. The SGLT2 inhibitor canagliflozin in heart failure: the CHIEF-HF remote, patient-centered randomized trial. Nat Med. 2022;28(4):809–13. https://doi.org/10.1038/s41591-022-01703-8.
Anker SD, Butler J, Filippatos G, et al. Empagliflozin in heart failure with a preserved ejection fraction. N Engl J Med. 2021;385(16):1451–61. https://doi.org/10.1056/NEJMoa2107038.
Nassif ME, Windsor SL, Borlaug BA, et al. The SGLT2 inhibitor dapagliflozin in heart failure with preserved ejection fraction: a multicenter randomized trial. Nat Med. 2021;27(11):1954–60. https://doi.org/10.1038/s41591-021-01536-x.
Nassif ME, Qintar M, Windsor SL, et al. Empagliflozin effects on pulmonary artery pressure in patients with heart failure: results from the EMBRACE-HF trial. Circulation. 2021;143(17):1673–86. https://doi.org/10.1161/circulationaha.120.052503.
Bhatt DL, Szarek M, Steg PG, et al. Sotagliflozin in patients with diabetes and recent worsening heart failure. N Engl J Med. 2021;384(2):117–28. https://doi.org/10.1056/NEJMoa2030183.
Bhatt DL, Szarek M, Pitt B, et al. Sotagliflozin in patients with diabetes and chronic kidney disease. N Engl J Med. 2021;384(2):129–39. https://doi.org/10.1056/NEJMoa2030186.
Santos-Gallego CG, Vargas-Delgado AP, Requena-Ibanez JA, et al. Randomized trial of empagliflozin in nondiabetic patients with heart failure and reduced ejection fraction. J Am Coll Cardiol. 2021;77(3):243–55. https://doi.org/10.1016/j.jacc.2020.11.008.
Tanaka A, Hisauchi I, Taguchi I, et al. Effects of canagliflozin in patients with type 2 diabetes and chronic heart failure: a randomized trial (CANDLE). ESC Heart Failure. 2020;7(4):1585–94. https://doi.org/10.1002/ehf2.12707.
Zelniker TA, Bonaca MP, Furtado RHM, et al. Effect of dapagliflozin on atrial fibrillation in patients with type 2 diabetes mellitus: insights from the DECLARE-TIMI 58 trial. Circulation. 2020;141(15):1227–34. https://doi.org/10.1161/circulationaha.119.044183.
McMurray JJV, Freeman MW, Massaro JO, et al. 32-OR: the Bexagliflozin Efficacy and Safety Trial (BEST): a randomized, double-blind, placebo-controlled, phase IIII, clinical trial. Diabetes. 2020;69(Supplement_1). https://doi.org/10.2337/db20-32-OR.
Cannon CP, Pratley R, Dagogo-Jack S, et al. Cardiovascular outcomes with ertugliflozin in type 2 diabetes. N Engl J Med. 2020;383(15):1425–35. https://doi.org/10.1056/NEJMoa2004967.
Perkovic V, Jardine MJ, Neal B, et al. Canagliflozin and renal outcomes in type 2 diabetes and nephropathy. N Engl J Med. 2019;380(24):2295–306. https://doi.org/10.1056/NEJMoa1811744.
Verma S, Mazer CD, Yan AT, et al. Effect of empagliflozin on left ventricular mass in patients with type 2 diabetes mellitus and coronary artery disease: the EMPA-HEART CardioLink-6 randomized clinical trial. Circulation. 2019;140(21):1693–702. https://doi.org/10.1161/circulationaha.119.042375.
Neal B, Perkovic V, Mahaffey KW, et al. Canagliflozin and cardiovascular and renal events in type 2 diabetes. N Engl J Med. 2017;377(7):644–57. https://doi.org/10.1056/NEJMoa1611925.
Zinman B, Wanner C, Lachin JM, et al. Empagliflozin, cardiovascular outcomes, and mortality in type 2 diabetes. N Engl J Med. 2015;373(22):2117–28. https://doi.org/10.1056/NEJMoa1504720.
Leiter LA, Cefalu WT, de Bruin TW, et al. Dapagliflozin added to usual care in individuals with type 2 diabetes mellitus with preexisting cardiovascular disease: a 24-week, multicenter, randomized, double-blind, placebo-controlled study with a 28-week extension. J Am Geriatr Soc. 2014;62(7):1252–62. https://doi.org/10.1111/jgs.12881.
Barnett AH, Mithal A, Manassie J, et al. Efficacy and safety of empagliflozin added to existing antidiabetes treatment in patients with type 2 diabetes and chronic kidney disease: a randomised, double-blind, placebo-controlled trial. Lancet Diabetes Endocrinol. 2014;2(5):369–84. https://doi.org/10.1016/s2213-8587(13)70208-0.
Yale JF, Bakris G, Cariou B, et al. Efficacy and safety of canagliflozin in subjects with type 2 diabetes and chronic kidney disease. Diabetes Obes Metab. 2013;15(5):463–73. https://doi.org/10.1111/dom.12090.
McMurray JJV, Solomon SD, Inzucchi SE, et al. Dapagliflozin in patients with heart failure and reduced ejection fraction. N Engl J Med. 2019;381(21):1995–2008. https://doi.org/10.1056/NEJMoa1911303.
Shimizu W, Kubota Y, Hoshika Y, et al. Effects of empagliflozin versus placebo on cardiac sympathetic activity in acute myocardial infarction patients with type 2 diabetes mellitus: the EMBODY trial. Cardiovasc Diabetol. 2020;19(1):148. https://doi.org/10.1186/s12933-020-01127-z.
Ilyas F, Jones L, Tee SL, et al. Acute pleiotropic effects of dapagliflozin in type 2 diabetic patients with heart failure with reduced ejection fraction: a crossover trial. ESC heart failure. 2021;8(5):4346–52. https://doi.org/10.1002/ehf2.13553.
Nakase M, Yahagi K, Horiuchi Y, et al. Effect of dapagliflozin on ventricular repolarization in patients with heart failure with reduced ejection fraction. Heart Vessels. 2023;38(12):1414–21. https://doi.org/10.1007/s00380-023-02298-x.
Sardu C, Massetti M, Testa N, et al. Effects of Sodium-Glucose Transporter 2 Inhibitors (SGLT2-I) in patients with Ischemic Heart Disease (IHD) treated by coronary artery bypass grafting via MiECC: inflammatory burden, and clinical outcomes at 5 years of follow-up. Front Pharmacol. 2021;12: 777083. https://doi.org/10.3389/fphar.2021.777083.
Li HL, Lip GYH, Feng Q, et al. Sodium-glucose cotransporter 2 inhibitors (SGLT2i) and cardiac arrhythmias: a systematic review and meta-analysis. Cardiovasc Diabetol. 2021;20(1):100. https://doi.org/10.1186/s12933-021-01293-8.
Chen HY, Huang JY, Siao WZ, Jong GP. The association between SGLT2 inhibitors and new-onset arrhythmias: a nationwide population-based longitudinal cohort study. Cardiovasc Diabetol. 2020;19(1):73. https://doi.org/10.1186/s12933-020-01048-x.
Jhuo SJ, Lin TH, Lin YH, et al. Clinical observation of SGLT2 inhibitor therapy for cardiac arrhythmia and related cardiovascular disease in diabetic patients with controlled hypertension. J Pers Med. 2022;12(2): 271. https://doi.org/10.3390/jpm12020271.
Zeppenfeld K, Tfelt-Hansen J, de Riva M, et al. 2022 ESC guidelines for the management of patients with ventricular arrhythmias and the prevention of sudden cardiac death. Euro Heart J. 2022;43(40):3997–4126. https://doi.org/10.1093/eurheartj/ehac262.
Oates CP, Santos-Gallego CG, Smith A, et al. SGLT2 inhibitors reduce sudden cardiac death risk in heart failure: Meta-analysis of randomized clinical trials. J Cardiovasc Electrophysiol. 2023;34(5):1277–85. https://doi.org/10.1111/jce.15894.
Liao J, Ebrahimi R, Ling Z, et al. Effect of SGLT-2 inhibitors on arrhythmia events: insight from an updated secondary analysis of > 80,000 patients (the SGLT2i-arrhythmias and sudden cardiac death). Cardiovasc Diabetol. 2024;23(1):78. https://doi.org/10.1186/s12933-024-02137-x.
Fischereder M, Schönermarck U. Empagliflozin, cardiovascular outcomes, and mortality in type 2 diabetes. New Engl J Med. 2016;374(11):1092–3. https://doi.org/10.1056/NEJMc1600827.
Wen Q, Zhang R, Ye K, et al. Empagliflozin rescues pro-arrhythmic and Ca(2+) homeostatic effects of transverse aortic constriction in intact murine hearts. Sci Rep. 2024;14(1):15683. https://doi.org/10.1038/s41598-024-66098-7.
Lee TI, Chen YC, Lin YK, et al. Empagliflozin attenuates myocardial sodium and calcium dysregulation and reverses cardiac remodeling in streptozotocin-induced diabetic rats. Int J Mol Sci. 2019;20(7). https://doi.org/10.3390/ijms20071680.
Philippaert K, Kalyaanamoorthy S, Fatehi M, et al. Cardiac late sodium channel current is a molecular target for the sodium/glucose cotransporter 2 inhibitor empagliflozin. Circulation. 2021;143(22):2188–204. https://doi.org/10.1161/circulationaha.121.053350.
Baartscheer A, Schumacher CA, Wüst RC, et al. Empagliflozin decreases myocardial cytoplasmic Na(+) through inhibition of the cardiac Na(+)/H(+) exchanger in rats and rabbits. Diabetologia. 2017;60(3):568–73. https://doi.org/10.1007/s00125-016-4134-x.
Mustroph J, Wagemann O, Lücht CM, et al. Empagliflozin reduces Ca/calmodulin-dependent kinase II activity in isolated ventricular cardiomyocytes. ESC heart failure. 2018;5(4):642–8. https://doi.org/10.1002/ehf2.12336.
Bode D, Semmler L, Wakula P, et al. Dual SGLT-1 and SGLT-2 inhibition improves left atrial dysfunction in HFpEF. Cardiovasc Diabetol. 2021;20(1):7. https://doi.org/10.1186/s12933-020-01208-z.
Kohlhaas M, Liu T, Knopp A, et al. Elevated cytosolic Na+ increases mitochondrial formation of reactive oxygen species in failing cardiac myocytes. Circulation. 2010;121(14):1606–13. https://doi.org/10.1161/circulationaha.109.914911.
Zhang N, Feng B, Ma X, et al. Dapagliflozin improves left ventricular remodeling and aorta sympathetic tone in a pig model of heart failure with preserved ejection fraction. Cardiovasc Diabetol. 2019;18(1):107. https://doi.org/10.1186/s12933-019-0914-1.
Arow M, Waldman M, Yadin D, et al. Sodium-glucose cotransporter 2 inhibitor dapagliflozin attenuates diabetic cardiomyopathy. Cardiovasc Diabetol. 2020;19(1):7. https://doi.org/10.1186/s12933-019-0980-4.
Byrne NJ, Matsumura N, Maayah ZH, et al. Empagliflozin blunts worsening cardiac dysfunction associated with reduced NLRP3 (nucleotide-binding domain-like receptor protein 3) inflammasome activation in heart failure. Circ Heart Fail. 2020;13(1): e006277. https://doi.org/10.1161/circheartfailure.119.006277.
Jhuo SJ, Liu IH, Tsai WC, et al. Effects of secretome from fat tissues on ion currents of cardiomyocyte modulated by sodium-glucose transporter 2 inhibitor. Molecules (Basel). 2020;25(16). https://doi.org/10.3390/molecules25163606.
Sato T, Aizawa Y, Yuasa S, et al. The effect of dapagliflozin treatment on epicardial adipose tissue volume. Cardiovasc Diabetol. 2018;17(1):6. https://doi.org/10.1186/s12933-017-0658-8.
Acknowledgements
We would like to gratefully acknowledge all the investigators who participated in this work.
Funding
This work was supported by the Sichuan Science and Technology Program (2024YFFK0180 to Dr. Ou, 2024JDHJ0051 to Dr. Tan), Sichuan Education Department Project (17ZA0434 to Dr. Ou), Luzhou Science and Technology Bureau (2023RCX174 and LY-63 to Dr. Ou), National Natural Science Foundation of China (81900300 to Dr. Wen), Collaborative Innovation Center for Prevention and Treatment of Cardiovascular Disease of Sichuan Province (xtcx-2016–10 to Dr. Fan), and Ph.D. Programs Foundation of the Affiliated Hospital of Southwest Medical University (2016-QB-25 to Dr. Fan).
Author information
Authors and Affiliations
Contributions
XO, XF and QW contributed to the study concept and design. ML, LZ and SZ conducted the meta-analysis, interpreted the data, and drafted the manuscript. ML, CY, YP, and YL were responsible for the literature search, data collection, and quality assessment. XO, XF, XT, and QW contributed to the verification and supervision. All the authors critically revised the manuscript for important intellectual content and approved the final version of the manuscript.
Corresponding authors
Ethics declarations
Ethics approval and consent to participate
Not applicable.
Consent for publication
All the authors provided consent for publication.
Competing interests
The authors declare that they have no competing interests.
Additional information
Publisher’s Note
Springer Nature remains neutral with regard to jurisdictional claims in published maps and institutional affiliations.
Supplementary Information
13643_2025_2766_MOESM1_ESM.docx
Supplementary Material 1. eTable 1. Number of patients with SCD or ventricular arrhythmias. eTable 2. Risk of bias assessment. eTable 3. Search strategy. eFigure 1. Funnel plot of meta-analysis for incidence of ventricular arrhythmias. eFigure 2. Funnel plot of meta-analysis for incidence of sudden death.
Rights and permissions
Open Access This article is licensed under a Creative Commons Attribution-NonCommercial-NoDerivatives 4.0 International License, which permits any non-commercial use, sharing, distribution and reproduction in any medium or format, as long as you give appropriate credit to the original author(s) and the source, provide a link to the Creative Commons licence, and indicate if you modified the licensed material. You do not have permission under this licence to share adapted material derived from this article or parts of it. The images or other third party material in this article are included in the article’s Creative Commons licence, unless indicated otherwise in a credit line to the material. If material is not included in the article’s Creative Commons licence and your intended use is not permitted by statutory regulation or exceeds the permitted use, you will need to obtain permission directly from the copyright holder. To view a copy of this licence, visit http://creativecommons.org/licenses/by-nc-nd/4.0/.
About this article
Cite this article
Lin, M., Zhang, S., Zhang, L. et al. Redefining outcomes of ventricular arrhythmia for SGLT2 inhibitor medication in heart failure patients: a meta-analysis of randomized controlled trials. Syst Rev 14, 31 (2025). https://doi.org/10.1186/s13643-025-02766-7
Received:
Accepted:
Published:
Version of record:
DOI: https://doi.org/10.1186/s13643-025-02766-7