Abstract
Background
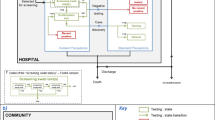
Hospitals in any given region can be considered as part of a network, where facilities are connected to one another – and hospital pathogens potentially spread – through the movement of patients between them. We sought to describe the hospital admission patterns of patients known to be colonised with carbapenemase-producing Enterobacterales (CPE), and compare them with CPE-negative patient cohorts, matched on comorbidity information.
Methods
We performed a linkage study in Victoria, Australia, including datasets with notifiable diseases (CPE notifications) and hospital admissions (admission dates and diagnostic codes) for the period 2011 to 2020. Where the CPE notification date occurred during a hospital admission for the same patient, we identified this as the ‘index admission’. We determined the number of distinct health services each patient was admitted to, and time to first admission to a different health service. We compared CPE-positive patients with four cohorts of CPE-negative patients, sampled based on different matching criteria.
Results
Of 528 unique patients who had CPE detected during a hospital admission, 222 (42%) were subsequently admitted to a different health service during the study period. Among these patients, CPE diagnosis tended to occur during admission to a metropolitan public hospital (86%, 190/222), whereas there was a greater number of metropolitan private (23%, 52/222) and rural public (18%, 39/222) hospitals for the subsequent admission. Median time to next admission was 4 days (IQR, 0–75 days). Admission patterns for CPE-positive patients was similar to the cohort of CPE-negative patients matched on index admission, time period, and age-adjusted Charlson comorbidity index.
Conclusions
Movement of CPE-positive patients between health services is not a rare event. While the most common movement is from one public metropolitan health service to another, there is also a trend for movement from metropolitan public hospitals into private and rural hospitals. After accounting for clinical comorbidities, CPE colonisation status does not appear to impact on hospital admission frequency or timing. These findings support the potential utility of a centralised notification and outbreak management system for CPE positive patients.
Similar content being viewed by others
Introduction
Carbapenemase-producing Enterbacterales (CPE) are a significant public health concern [1]. In Victoria, Australia, the estimated carriage rate is low compared to international prevalence, at just 1.42 per 100,000 individuals [2]. However, CPE cases have been steadily increasing in recent years despite a centralised ‘search and contain’ policy [2], with acquisition primarily associated with international travel and healthcare exposure [2,3,4]. This trend poses a serious threat to patient outcomes, as CPE infections are associated with increased morbidity, mortality, and healthcare costs [5].
Hospitals in any given administrative or geographical region can be considered as part of a network, where facilities are connected to one another through the movement of patients between them [6, 7]. Patients may either be directly transferred from one hospital to another, or admitted to a given hospital at some time after discharge from another hospital. In this way, patients colonised by a multidrug resistant bacteria, such as CPE, in one hospital can transmit it to patients in another [8,9,10] with models suggesting a region-wide approach could be necessary to achieve control of infection [11,12,13].
This inter-hospital transmission risk is acknowledged in the Victorian Department of Health’s CPE guidelines [14]. Victoria has no centralised laboratory information or alert system to share information about CPE colonisation; therefore, patients colonised by CPE, and their primary healthcare providers, are instructed that in case of future hospital admission, they should inform the facility about their CPE colonisation status to facilitate isolation with appropriate contact precautions. There is currently no data about the frequency with which patients colonised by CPE present to other healthcare facilities. Such data would help quantify the transmission risk posed by patients colonised with CPE, particularly in the context of reliance on self-reported CPE-colonisation status.
Our primary aim was to describe the hospital admission patterns of patients known to be colonised with CPE, using linked patient data, including the type of health service and timing of admissions. Our secondary aim was to test the hypothesis that patients colonised with CPE have similar admission patterns to other healthcare system patients.
Methods
Setting and population
Victoria, Australia, has a population of 6.5 million people, with a median age of 38 years [15]. The hospital system consists of more than 300 hospital campuses, which vary greatly in size, case mix and services. A map of these services is shown in Appendix 1.
Hospitals in Victoria are classified as “public” or “private” services. Public health services are freely available to the entire population, with funding provided by the state and federal government. Private health services service a subset of patients, generally those with private health insurance.
Public health services usually govern several hospital campuses, with 149 public hospital campuses (52 of which are in metropolitan areas) divided into 80 unique health services. Private hospitals have a much more complex and varied governance structure, with dynamic ownership structures. There are 146 private hospitals in metropolitan areas, and 23 in rural areas. The hospital system in Victoria operates using a ‘devolved governance’ structure [16], with each health service having its own board that dictates it’s policies.
The Victorian Guideline on Carbapenemase-producing Enterobacteriaceae for Health Services was released in December 2015, and involved a centralised genomic and epidemiological surveillance and response program [2]. CPE became a notifiable condition in Victoria in December 2018.
The study period was 1 January 2011 and 30 November 2020. All Victorian hospital admissions during the study period are included. Patients were identified as CPE-positive based on the presence of a notification in the Public Health Events Surveillance System (PHESS) database between May 2013 (the first notified case) and 30 November 2020 (N = 628). Patients without a recorded notification in this database were considered CPE-negative.
Data sources
The PHESS database contains patient demographics and epidemiologic data relevant to each case. For our analysis, we focused on the ‘calculated onset date’, determined by the Department of Health as follows: if the patient’s presumed acquisition date (that is, the date of transmission from another case based on epidemiologic and genomic investigation) was determined, it was used as the calculated onset date; if the acquisition date was unavailable, the specimen collection date was used instead; and if no other information was available, the calculated onset date was defined as the date when the notification was received by the health department. For the purposes of this analysis, calculated onset date is considered to be the best estimate of the date at which a patient became CPE positive.
The hospital admissions are sourced from the Victorian Admitted Episode Dataset (VAED) [17]. Linkage between the PHESS and VAED datasets was performed by the Centre for Victorian Data Linkage, as part of the Victorian Linkage Map and Integrated Data Resource. The linkage allowed build a sequence of admission and CPE status for each patient, and analyse how their admission patterns may have changed post-CPE positivity, and to compare population groups post-hoc.
Analysis
Our hypothesis that CPE positive patients have similar hospital admission risk to CPE negative patients is tested by analysing differences in two key metrics between CPE positive and CPE negative patients: the number of health services visited after diagnosis, and the time until the next admission after diagnosis.
To test this hypothesis, we use survival analysis to analyse the differences in estimated times of re-admission. The survival function, denoted \(\:\widehat{S}\left(t\right)\), represents the probability that the event of interest (here re-admission) has not yet occurred by time \(\:t\). Specifically, we calculate Kaplan-Meier survival curves, calculated as
where \(\:{d}_{i}\) is the number of events that have occurred up to time \(\:{t}_{i}\) and \(\:{n}_{i}\) is the number of individuals who have not yet experienced the event or been censored up to time \(\:{t}_{i}\).
The admission associated with CPE identification (index admission) is chosen to be the admission which encloses the calculated onset date from the patient in PHESS. Index hospital admissions could be identified for 528 patients. Patients who were not admitted at the CPE onset date were removed from further analysis (N = 100). Patients who died during the index admission were removed from further analysis (N = 81).
To determine the time of risk and the time of readmission event for conducting the survival analysis, the period of risk (time 0) was defined as the end date of the index admission. If the patient was transferred directly to another hospital campus within the same health service, then time 0 was the date of discharge from the health service. The event time was set as the beginning of the subsequent admission to a different health service. Any admissions occurring after time 0 to the same health service where the patient was initially diagnosed were excluded from consideration. Patients who do not appear in an admission to a different health service to that of the index admission are assumed to be censored at the end of the data period (30 November, 2020).
Four CPE-negative patient cohorts were created by sampling from the whole population using different matching criteria. These matching criteria were chosen to produce cohorts with increasing levels of similarity to the CPE-positive cohort:
-
Cohort (1) Random subset: patients are randomly sampled from the population.
-
Cohort (2) Campus and time: Patients are matched to CPE-positive patients based on the hospital campus and the quarter-year (i.e. 3-month period) of admission.
-
Cohort (3) Campus, time and age: As per Cohort 2, plus the inclusion of five-year age band.
-
Cohort (4) Campus, time and comorbidities: As per Cohort 2, plus the inclusion of age-adjusted Charlson Comorbidity Index category.
ICD-10-AM codes were used to calculate the age-adjusted Charlson Comorbidity Index [18]. Age adjustment was applied by increasing the Charlson score by 1 for every 10 years of patient age beyond 40 [19]. The resulting age-adjusted scores were categorized into four groups: None (0 points), Mild (1–2 points), Moderate (3–4 points), or Severe ( > = 5 points). To ensure robustness, the sampling process was repeated 100 times with replacement. Each CPE-positive patient had at least 10 potential matching CPE-negative patients identified for the analysis.
Kaplan-Meier survival curves were calculated separately for each patient cohort, using R version 4.1.0 and the survival package.
Results
The study population includes 528 unique CPE positive patients. Figure 1 shows the number of notifications for confirmed cases, by month, over the data period. We note that CPE was made notifiable in Victoria in December of 2018, with an increase in notifications from that time. Patients can have more than one notification for CPE i.e. if they are colonised by more than one CPE, with distinct species of carbapenemase gene. All notifications are shown in Fig. 1, but only the earliest onset is considered for further analysis.
Number of CPE notifications, by onset date, grouped by month notified to the Victorian Department of Health. Grey shaded region indicates that CPE infection had become notifiable, although surveillance had been established in December 2015. Coloured bars represent whether the patient’s calculated onset date falls in a hospital admission. The proportion of onsets within and outside of a hospital admission remains approximately constant over time
Patient demographic summaries are contained in Table 1. Of the 528 CPE positive patients included in the study, almost two thirds (63%) were male. The age distribution of positive patients tended toward older age groups, with 59% of patients being over 60 years of age.
Of the 528 patients in our cohort, 222 (42%) were admitted to more than one health service within the study period. Among these patients who moved between health services, most (90.6%, 201/222) notifications occurred at times when patients were admitted into a public hospital (Fig. 2). Of those diagnosed while in a public hospital, 84% (169/201) had their subsequent admission in a public hospital, with the remaining 16% being admitted to a private hospital. For those diagnosed in private health services (n = 21), two thirds were re-admitted to a different health service. We note that a very large percentage of patients (306 [58%]) were never admitted to a different health service within the study period. These patients are not included in the description of diagnosis-readmission pathways.
The number of unique health services visited after the beginning of risk period, as well as the index hospitalisation, is presented for CPE-positive patients and the four matched cohorts (Fig. 3). While most CPE-positive patients are only admitted to one health service, 155 (29%), 44 (8.3%) and 20 (3.7%) patients are admitted to two, three, and four or more distinct health services, respectively. Certain factors, such as index hospital campus and time, were found to impact on the number of unique health services visited. However, it appears that severity of illness is the main driver for this measure of behaviour, rather than CPE colonisation status. There is no significant difference between Cohort 4 (Campus, time and comorbidities) and the CPE + cohort (C-test, p < 0.9079), while there is a significant difference (C-test, p < 0.001) between Cohorts 1, 2, and 3 and the CPE + cohort respectively.
Number of unique health services visited for patients diagnosed with CPE, and the four matched cohorts. The service of admission was determined using the admission date, or the notification date for patients. Significance indicators are at the p = 0.05 level according to the C-test for differences in Poisson means
Time from the end of the index admission until next admission to a different health service is shown in shown for CPE positive patients, and the four matched cohorts (Fig. 4). At 90-days, 1-year, and 2 years, 40%, 50% and 57% of CPE-positive patients had been admitted to a different health service. There is no difference in the median survival times (i.e. time until next admission) between CPE-positive patients and the cohort matched on Charlson Comorbidity Index bracket (log-rank test, p > 0.05 / 400, Bonferroni correction), which means that patients who are CPE-positive are hospitalised no more frequently than those who have not tested positive. There is a large proportion of censored patients in this dataset, both in the CPE-positive and matched cohorts, which is reflective of patients appearing in hospital once, and never returning.
Survival curves for patients who have tested positive for CPE, against those who have not tested positive. Each line shows a survival curve for a particular sampled population. As matching criteria becomes stricter, the difference between CPE positive patients and the matched populations decreases. There is no significant difference between any of the populations in Cohort 4 compared with the CPE + group (log-rank test)
Of note, 71 (15.8%) patients experience a transfer to another health service immediately after discharge (i.e., have a time of event of 0 days). This is approximately double that observed in the most closely matched cohort of CPE negative patients (cohort 4). Of those who are directly transferred, three are censored (as they are discharged on the last day of the dataset). There are 48 patients who travel between public health services, and 13 who are diagnosed in a public service, then re-appear in a private service.
Conclusions
Patients known to be colonised with CPE are frequently re-admitted to different health services to the one they are diagnosed in. However, using survival analysis, we have shown that this frequency of readmission is not significantly higher than other healthcare system patients, after controlling for comorbidities.
We have also shown that majority of CPE colonisation identification and re-admission occurs in public, metropolitan hospitals in Victoria. This is perhaps correlated with hospital capability and centrality.
One unexpected trend observed in this data is the bias toward male patients. This trend hasn’t previously been reported, and further investigation as to whether this is a health service coding issue or an actual trend is warranted to further the epidemiologic understanding.
This work uses administrative data on patient admissions and separations across Victoria, which includes a large amount of quality assurance testing and input restrictions. By combining with notifiable disease information, which is legally required to be reported upon detection, this analysis uses very high quality input data. By using statistical linkage, we were able to follow patient journeys through the healthcare system, which is highly valuable when considering patient hospital admission risk.
Testing for CPE infection is based on highly varied screening procedures, with increased screening being performed generally in intensive care units, and after an outbreak is detected [20]. As a result, it is unlikely that detection of CPE infection is perfect. This means that potentially individuals who are CPE colonised (and therefore a potential source of transmission) may not be included in this analysis, and these patients may have different hospital admission risk.
Most studies of CPE colonisation are prospective studies [21, 22] that are focussed in one facility or ward. By using routinely reported data, we are able to get a greater understanding of the breadth of patients who may be admitted while colonised with CPE, and also understand their future health seeking behaviour. However, a key limitation of retrospective studies is the inability to change sampling strategies, or investigate trends further than what is present in the data.
The linked nature of the data used in this study is another key strength. The impact of inter-facility patient transfer has been studied previously for CPE [13] and other hospital associated infections [21]. These studies have only been able to draw associations between hospital incidence and patient admissions, while this data allowed us to look explicitly at the patterns of those colonised with CPE.
We focussed on CPE cases (infection or colonisation detection) that had a date of onset while hospitalised. While nosocomial transmission is likely to be the primary source of infection, community and household based transmission has been quantified previously [24]. We note that almost 16% of the patients in the notifiable disease database do not have an associated hospital admission. Many of these are likely returning travellers, but it is possible that community transmission could be a contributor to overall disease burden.
There is a potential impact of censoring of admissions of patients in the CPE positive cohort. As the data covers a fixed period (independent of date of CPE notification), those diagnosed late in the data period may not have had a chance to re-present to hospital. While there is a delay between notification of a new CPE case and their calculated onset date, this delay is small (median 5 days, IQR 1–5 days), and so the downward trend in CPE notifications at the end of the data period is likely to reflect the local epidemiology at the time.
Among the patients who are not readmitted within the study period, we assumed that the reasons are similar between the CPE-positive and CPE-negative cohorts, i.e. the combination of hospitalisation not required, moving out of Victoria, or death. It is possible that the reasons for this lack of presentation differ between population groups. Investigating these potential differences in cohorts requires linkage with an additional dataset (the Victorian Death Index), and is a direction of future work.
During this analysis, we excluded subsequent admissions to the health service where the index admission occurred. This is because we assumed that the health service where CPE was detected would have a process for recording the CPE-positive status of patients and, as a result, would take proactive measures to prevent onward transmission. It is not possible to verify this assumption with this dataset, as information about what type of room and use of transmission-based precautions was not available. Given the focus on detection and management of CPE cases in this region, the assumption is likely to be reasonable in this cohort.
Our description of where CPE cases are detected (i.e. predominantly metropolitan, public health services) may be subject to detection bias, as we can’t exclude the possibility that these health services are undertaking more active surveillance than other services. Nevertheless, we suggest that there is benefit to describing the current pattern of patient flow among known CPE cases.
While we have shown that patients colonised with CPE are frequently transferred around the hospital system, we have not evaluated the impact this may have on transmission. Dynamic models of disease and its control are required for this purpose, and are a direction of future work. We have also not been able to identify the frequency with which patients are appropriately placed in contact precautions after CPE diagnosis when admitted to a different health service.
Patients colonised with CPE are not fundamentally different in their hospital admission risk than those who are similarly ill. From an infection prevention perspective, this means that there is still significant infection risk from the transfer of patients who are known to be positive. This risk results in ample opportunity for between-facility transmission shortly after detection. This supports the potential utility of a centralised alert system to identify CPE positive patients on hospital presentation in Australia.
Data availability
Data required to reproduce the figures is available from the online supplement and online here: https://doi.org/10.5281/zenodo.10467483. Raw data cannot be provided due to ethics considerations, but can be made available upon reasonable request and ethical approval processes. Please contact the corresponding author for this purpose.
References
Murray CJL, et al. Global burden of bacterial antimicrobial resistance in 2019: a systematic analysis. Lancet. Feb. 2022;399(10325):629–55. https://doi.org/10.1016/S0140-6736(21)02724-0
Lane CR et al. Dec., Search and Contain: Impact of an Integrated Genomic and Epidemiological Surveillance and Response Program for Control of Carbapenemase-producing Enterobacterales, Clin. Infect. Dis, vol. 73, no. 11, pp. e3912–e3920, 2021, https://doi.org/10.1093/cid/ciaa972
Sidjabat HE et al. Jun., Dominance of IMP-4-Producing Enterobacter cloacae among Carbapenemase-Producing Enterobacteriaceae in Australia, Antimicrob. Agents Chemother, vol. 59, no. 7, pp. 4059–4066, 2015, https://doi.org/10.1128/aac.04378-14
Peleg AY, Franklin C, Bell JM, Spelman DW. Dissemination of the Metallo-β-Lactamase Gene blaIMP-4 among Gram-Negative Pathogens in a Clinical Setting in Australia, Clin. Infect. Dis, vol. 41, no. 11, pp. 1549–1556, Dec. 2005, https://doi.org/10.1086/497831
Budhram DR, Mac S, Bielecki JM, Patel SN, Sander B. Health outcomes attributable to carbapenemase-producing Enterobacteriaceae infections: a systematic review and meta-analysis. Infect Control Hosp Epidemiol. Jan. 2020;41(1):37–43. https://doi.org/10.1017/ice.2019.282
Donker T, Wallinga J, Grundmann H. Patient referral patterns and the spread of hospital-acquired infections through National Health Care Networks. PLOS Comput Biol. Mar. 2010;6(3):e1000715. https://doi.org/10.1371/journal.pcbi.1000715
Huang SS et al. Nov., Quantifying Interhospital Patient Sharing as a Mechanism for Infectious Disease Spread, Infect. Control Hosp. Epidemiol. Off. J. Soc. Hosp. Epidemiol. Am, vol. 31, no. 11, pp. 1160–1169, 2010, https://doi.org/10.1086/656747
Lee BY et al. Mar., The Potential Trajectory of Carbapenem-Resistant Enterobacteriaceae, an Emerging Threat to Health-Care Facilities, and the Impact of the Centers for Disease Control and Prevention Toolkit, Am. J. Epidemiol, vol. 183, no. 5, pp. 471–479, 2016, https://doi.org/10.1093/aje/kwv299
Ray MJ, et al. Regional Spread of an outbreak of Carbapenem-Resistant Enterobacteriaceae through an Ego Network of Healthcare Facilities. Clin Infect Dis. Jul. 2018;67(3):407–10. https://doi.org/10.1093/cid/ciy084
Ray MJ, Lin MY, Weinstein RA, Trick WE. Spread of Carbapenem-Resistant Enterobacteriaceae Among Illinois Healthcare Facilities: The Role of Patient Sharing, Clin. Infect. Dis, vol. 63, no. 7, pp. 889–893, Oct. 2016, https://doi.org/10.1093/cid/ciw461
Donker T, Wallinga J, Slack R, Grundmann H. Hospital Networks and the dispersal of hospital-acquired pathogens by patient transfer. PLoS ONE. Apr. 2012;7(4):e35002. https://doi.org/10.1371/journal.pone.0035002
Ciccolini M, et al. Infection prevention in a connected world: the case for a regional approach. Int J Med Microbiol. Aug. 2013;303(6):380–7. https://doi.org/10.1016/j.ijmm.2013.02.003
Nekkab N, Crépey P, Astagneau P, Opatowski L, Temime L. Assessing the role of inter-facility patient transfer in the spread of carbapenemase-producing Enterobacteriaceae: the case of France between 2012 and 2015, Sci. Rep, vol. 10, no. 1, Art. no. 1, Sep. 2020, https://doi.org/10.1038/s41598-020-71212-6
A. Department of Health. Victoria, Victorian guideline on CPO for health services. Accessed: Oct. 04, 2023. [Online]. Available: https://www.health.vic.gov.au/infectious-diseases/victorian-guideline-on-cpo-for-health-services
Australian Bureau of Statistics. Snapshot of Victoria. Accessed: Sep. 01, 2023. [Online]. Available: https://www.abs.gov.au/articles/snapshot-vic-2021
The King’s Fund. Managing health services through devolved governance: A perspective from Victoria, Australia.
A. Department of Health. Victoria, Victorian Admitted Episodes Dataset. Accessed: Oct. 04, 2023. [Online]. Available: https://www.health.vic.gov.au/data-reporting/victorian-admitted-episodes-dataset
Quan H, et al. Coding algorithms for defining comorbidities in ICD-9-CM and ICD-10 administrative data. Med Care. 2005;43(11):1130–9.
Charlson M, Szatrowski TP, Peterson J, Gold J. Validation of a combined comorbidity index. J Clin Epidemiol. Nov. 1994;47(11):1245–51. https://doi.org/10.1016/0895-4356(94)90129-5
Spilsbury S, et al. Screening for carbapenemase-producing enterobacteriaceae following transmission: experience at a large victorian healthcare facility. Infect Dis Health. Nov. 2018;23:S. https://doi.org/10.1016/j.idh.2018.09.044
Yan L, Sun J, Xu X, Huang S. Epidemiology and risk factors of rectal colonization of carbapenemase-producing Enterobacteriaceae among high-risk patients from ICU and HSCT wards in a university hospital, Antimicrob. Resist. Infect. Control, vol. 9, no. 1, p. 155, Sep. 2020, https://doi.org/10.1186/s13756-020-00816-4
Legeay C, Thépot-Seegers V, Pailhoriès H, Hilliquin D, Zahar J-R. Is cohorting the only solution to control carbapenemase-producing Enterobacteriaceae outbreaks? A single-centre experience. J Hosp Infect. Aug. 2018;99(4):390–5. https://doi.org/10.1016/j.jhin.2018.02.003
Pi L, Expert P, Clarke JM, Jauneikaite E, Costelloe CE. Electronic health record enabled track and trace in an urban hospital network: implications for infection prevention and control, medRxiv, p. 2021.03.15.21253584, Mar. 2021, https://doi.org/10.1101/2021.03.15.21253584
Jamal AJ et al. Dec., Household Transmission of Carbapenemase-producing Enterobacterales in Ontario, Canada, Clin. Infect. Dis, vol. 73, no. 11, pp. e4607–e4615, 2021, https://doi.org/10.1093/cid/ciaa1295
Acknowledgements
We would like to acknowledge the Victorian Department of Health as the source of VAED and PHESS datasets for this study, and the Centre for Victorian Data Linkage (Victorian Department of Health) for the provision of the data linkage.
The opinions expressed are those of the authors do not represent the views, nor the policy directions, of the Victorian Department of Health.
Funding
This research was funded by the Australian National Health and Medical Research Council (GNT1156742). A.J.S. was supported by an Early Career Fellowship from the National Health and Medical Research Council (GNT1141398).
Author information
Authors and Affiliations
Contributions
MJL wrote the main manuscript text, prepared figures, analysed data and performed statistical analysis. TD, DW provided analysis and interpretation of data and statistical results; CG, AT, BPH provided interpretation of data; ME, DH, AW provided input on design of the study, provision and interpretation of data and results; AYP, BSC, AJS conceived the study and provided input on the results. All others reviewed the manuscript.
Corresponding author
Ethics declarations
Ethics approval and consent to participate
Ethics permission was obtained from the Alfred Health ethics board, submission number 445/21.
Consent for publication
Not applicable.
Competing interests
Andrew Stewardson is an Editor-in-chief for Antimicrobial Resistance and Infection Control.
Additional information
Publisher’s Note
Springer Nature remains neutral with regard to jurisdictional claims in published maps and institutional affiliations.
Appendix
Appendix
Rights and permissions
Open Access This article is licensed under a Creative Commons Attribution 4.0 International License, which permits use, sharing, adaptation, distribution and reproduction in any medium or format, as long as you give appropriate credit to the original author(s) and the source, provide a link to the Creative Commons licence, and indicate if changes were made. The images or other third party material in this article are included in the article’s Creative Commons licence, unless indicated otherwise in a credit line to the material. If material is not included in the article’s Creative Commons licence and your intended use is not permitted by statutory regulation or exceeds the permitted use, you will need to obtain permission directly from the copyright holder. To view a copy of this licence, visit http://creativecommons.org/licenses/by/4.0/. The Creative Commons Public Domain Dedication waiver (http://creativecommons.org/publicdomain/zero/1.0/) applies to the data made available in this article, unless otherwise stated in a credit line to the data.
About this article
Cite this article
Lydeamore, M.J., Donker, T., Wu, D. et al. Carbapenemase-producing enterobacterales colonisation status does not lead to more frequent admissions: a linked patient study. Antimicrob Resist Infect Control 13, 82 (2024). https://doi.org/10.1186/s13756-024-01437-x
Received:
Accepted:
Published:
DOI: https://doi.org/10.1186/s13756-024-01437-x