Abstract
Purposes
The influence of gender on the epidemiology of and outcome from SA-AKI in ICU has not been fully clarified. Our aim is to elucidate these differences.
Methods
This study included adult patients with sepsis in MIMIC IV (V 2.2), and propensity matching analysis, cox regression and logistic regression were used to analyze gender differences in incidence, mortality and organ support rate.
Results
Of the 24,467 patients included in the cohort, 18,128 were retained after propensity score matching. In the matched cohort, the incidence of SA-AKI in males is higher than that in females (58.6% vs. 56.2%; P = 0.001).males were associated with a higher risk of SA-AKI (OR:1.07(1.01–1.14), P = 0.026;adjusted OR:1.07(1.01–1.14), P < 0.033).In SA-AKI patients, males were associated with a lower risk of ICU mortality(HR:0.803(0.721–0.893), P < 0.001;adjusted HR:0.836(0.746–0.937), P = 0.002) and in-hospital mortality(HR: 0.820(0.748–0.899), P < 0.001;adjusted HR:0.853(0.775–0.938), P = 0.003).there were no statistically significant differences between male and female patients in 1-year all-cause mortality (36.9% vs. 35.8%, P = 0.12), kidney replacement therapy rate (7.8% vs.7.4%, P = 0.547), mechanical ventilation rate 64.8% vs.63.9%, P = 0.369), and usage of vasoactive drugs (55.4% vs. 54.6%, P = 0.418).
Conclusions
Gender may affect the incidence and outcomes of SA-AKI, further research is needed to fully understand the impact of gender on SA-AKI patients.
Similar content being viewed by others
Introduction
Sepsis is a condition caused by the immune response dysfunction of the host due to infection, leading to multiple organ dysfunction [1]. There are over 18 million cases of sepsis worldwide each year, and it is a dangerous condition with high mortality rates [2]. The kidneys are vulnerable organs in sepsis, and statistics show that 60% of sepsis patients will develop Acute Kidney Injury (AKI), with a 50% mortality rate among AKI patients. Even for survivors, there is an increased risk of developing chronic kidney disease, often requiring long-term dialysis treatment [3]. Sepsis-associated acute kidney injury (SA-AKI) is independently associated with poor prognosis for patients, imposing significant burdens on both individuals and society [4]. Currently, apart from kidney transplantation, there are no effective treatment options available. This is due to the complex and yet unclear pathophysiological mechanisms underlying SA-AKI. SA-AKI presents as a complex clinical syndrome that can result in various clinical phenotypes and subtypes based on interactions between genotypes and exposures. It is precisely this heterogeneity that complicates the treatment and assessment of therapeutic efficacy for SA-AKI [5]. Identifying specific genotypic profiles and clinical phenotypes in patients has important implications for precision medicine treatments as well as evaluating treatment effectiveness.
Gender, as a genetic modifier in biology and disease [6, 7], leads to differences in the prevalence, prognosis, and treatment of many diseases between males and females [8,9,10,11,12]. However, the role of gender in SA-AKI is still unclear. On one hand, excessive inflammatory response and immune suppression during sepsis are closely related to SA-AKI [13]. Sex steroids have binary effects on immunity; estrogen may have a protective effect in an infectious environment while testosterone may be harmful due to its immunosuppressive properties [14]. This may reduce the susceptibility of females to SA-AKI. On the other hand, endothelial dysfunction activation during sepsis is closely associated with SA-AKI. Compared to males, females exhibit significant activation of endothelial cell function during sepsis [15], which may increase their susceptibility to SA-AKI. In conclusion, the differences in incidence rate, organ support rate, and prognosis of SA-AKI based on gender have not been fully elucidated and further research is needed.
Our research primarily aims to analyze SA-AKI patients in international large-scale databases. Firstly, we aim to evaluate the impact of gender on the incidence rate of SA-AKI in critically ill sepsis patients. Secondly, we aim to assess the influence of gender on organ support rates among SA-AKI patients. Lastly, we aim to evaluate the effect of gender on both short-term and long-term survival rates for these patients.
Method
Data source
This is a retrospective cohort study using the MIMIC-IV (version 2.2) database to analyze different populations. The MIMIC-IV database is a publicly available multi-parameter intensive care database provided by the Massachusetts Institute of Technology (MIT) [16]. It includes critically ill patients admitted to the ICU at Beth Israel Deaconess Medical Center in Boston, Massachusetts, from 2008 to 2019. Since this study is based on analysis of a third-party anonymous public database and has obtained institutional review board approval in advance, ethical review is not required. To access this database, we have completed the online training course and Protecting Human Research Participants exam offered by the National Institutes of Health (No. 54780440).
Study population
This study selected adult septic patients who were admitted to the ICU from the MIMIC-IV database from 2008 to 2019. Patients with severe chronic kidney disease (CKD), defined as CKD stage ≥ 4 or estimated glomerular filtration rate (eGFR) < 30 mL/min/1.73m2, and patients undergoing long-term dialysis treatment were excluded.
Identification of sepsis
According to the definition of SEPSIS-3, we identified patients with confirmed or suspected infection and a Sequential Organ Failure Assessment (SOFA) score increase of two or more [1]. We determined the clinician's recognition of suspected infection through two simultaneous events in the electronic health records: (1) prescription of antibiotics, and (2) ordering specific fluid cultures. These two events need to occur within a specific time frame and have the following options: In option 1, fluid culture is performed first, and antibiotic use must be initiated within 72 h. In option 2, antibiotic dosing is administered first, and fluid culture must be completed within 24 h. We excluded all antibiotics given as a single dose in the operating room. We also excluded antibiotics that were not accompanied by fluid cultures. We included fluid cultures from multiple sites: abdomen, bronchoalveolar lavage, blood, bone marrow, cerebrospinal fluid, catheter/device tips, pleural effusion, skin/tissue samples, stool, and urinary tract. Cultures types included bacterial, fungal, viral. We assumed a SOFA score of zero prior to ICU admission. If individual components of SOFA were missing, no contribution was made to the total score [17]. The daily total SOFA scores were calculated, and an increase of two points within 24 h was considered abnormal [18]. Considering difficulties in interpreting neurological SOFA when sedation therapy is being concurrently administered, it was not included in the overall scoring category [19].
Identification of acute kidney injury
The diagnostic criteria for AKI follow the standards of Kidney Disease: Improving Global Outcomes (KDIGO): an increase in serum creatinine (Scr) exceeding 26.5 μmol/L (0.3 mg/dl) within 48 h; an increase in serum creatinine by more than 50% from baseline, lasting for 7 days; urine output less than 0.5 ml/(kg·h), lasting for more than 6 h [20]. The minimum Scr value available within the first 7 days prior to admission is used as the baseline Scr [21, 22]. When pre-admission Scr is not available, the first measured Scr upon admission is used as the baseline Scr [23]. Either urine-based or creatinine-based criteria, or a combination of both, are used to determine if a patient meets KDIGO AKI criteria.
Identification of sepsis-associated acute kidney injury
After determining sepsis and acute kidney injury separately, we applied the definition of SA-AKI from the ADQI 28 working group. We compared the diagnosis day of sepsis with the diagnosis day of AKI. If AKI occurs within 1–7 days after the diagnosis of sepsis, patients are classified as SA-AKI according to ADQI criteria [5]. If AKI occurs before sepsis, patients do not meet the definition of SA-AKI.
Outcomes
The main outcome was the incidence of SA-AKI. Secondary outcomes included ICU mortality, in-hospital mortality, 1-year all-cause mortality, mechanical ventilation rate, renal replacement therapy rate, and use of vasoactive drugs.
Data extraction and preprocessing
The following variables were extracted from the database, including patient demographics, vital signs, medical history, laboratory tests, and scoring data. Organ support data included the use of vasoactive drugs, mechanical ventilation, and renal replacement therapy. Outcome variables included the occurrence of SA-AKI within 7 days after ICU admission, ICU length of stay, ICU mortality rate, hospital length of stay, hospital mortality rate, and 1-year all-cause mortality rate. Considering that some laboratory data may be measured multiple times within 24 h, this study extracted the first value of the day for those variables. For missing experimental data that accounted for less than 15% of the total population size, multiple imputation was used for handling [24, 25].
Statistical methods
All analyses in this study were conducted in two cohorts: unmatched and propensity score-matched. Baseline patient characteristics were stratified by gender. Normally distributed continuous data are presented as mean ± standard deviation (X ± s), while non-normally distributed continuous data are presented as median (interquartile range) [Median (IQR)]. Group comparisons were performed using t-tests or rank-sum tests. Categorical data are presented as frequency (N) and percentage (%), with group comparisons analyzed using chi-square tests. Propensity scores were calculated for gender and matched 1:1, The variables for propensity matching include age, BMI, race, admission type, microorganisms, infection sources, comorbidities, interventions [33]. Multivariable Cox regression or logistic regression was used to assess the association between gender and the incidence of SA-AKI, organ support rate, ICU mortality rate, hospital mortality rate, and 1-year all-cause mortality rate. All analyses were performed using R software version 4.62.
Result
Baseline characteristics of the population
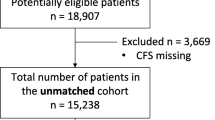
As shown in Fig. 1, a total of 33,177 sepsis patients were included in this study. Patients with multiple repeated ICU admissions and those requiring kidney dialysis treatment for CKD stage 4 or above were excluded.24467 patients with sepsis were ultimately included in the analysis, and 14,090 patients developed AKI within 7 days after the onset of sepsis, including 8221 males and 5869 females. The baseline demographic and clinical biochemical characteristics are presented in Table 1. In the unmatched cohort, there was no significant difference between males and females in terms of BMI. Females had a higher age than males (70.2 [58.0–81.3] vs. 66.5 [55.6–77.1], P < 0.001). Male patients had a higher proportion of emergency department admissions compared to female patients (16.3% vs. 13.1%, P < 0.001). The prevalence rates of coronary heart disease (35.4% vs. 22.4%, P < 0.001), peripheral vascular disease (12.7% vs. 10.6%, P < 0.001), chronic kidney disease (19.5% vs. 16.6%, P < 0.001), chronic liver disease (11.1% vs. 8.23%, P < 0.001), and cancer (15.3% vs. 13.0%, P < 0.001) were higher among male patients. Female patients had a higher prevalence rate of hypertension (44.8% vs. 43.5%, P = 0.041), chronic heart failure (29.3% vs. 27.1%, P < 0.001), cardiovascular diseases (15.6% vs. 13.5%, P = 0.001), and chronic lung diseases (30.4% vs. 23.0%, P < 0.001). In terms of infection sources, female patients had a higher prevalence rate of pulmonary infections (42.9% vs. 39.5%, P < 0.001), gastrointestinal infections (16.4% vs. 13.6%, P < 0.001), and urinary tract infections (27.5% vs.13.3%, P < 0.001) compared to male patients. Male patients had a higher prevalence rate of catheter-related infections compared to female patients (8.48% vs. 7.14%, P < 0.001). In the matched cohort, female patients were older than male patients, while other baseline demographic characteristics were generally consistent (Table 1).
Association of sex with risk of sepsis associated acute kidney injury
In the unmatched cohort, a total of 14,090 cases (57.1%) of sepsis-associated acute kidney injury (SA-AKI) occurred in ICU patients. The incidence rate of SA-AKI was higher in males than females (58.4% vs. 56.5%, P = 0.005), with the majority of patients in both groups classified as AKI stage 1–2 (males: 79.9%, females: 75.8%). In Table 2, we conducted logistic regression analysis on the occurrence of SA-AKI, and found that males had a higher risk associated with SA-AKI in the unmatched cohort (OR: 1.08; 95% CI 1.02–1.13; P < 0.001) (Model 1). After adjusting for age, BMI, race, infection source, admission type, comorbidities, treatment and laboratory variables, the association between males and SA-AKI remained significant (OR: 1.07; 95% CI 1.01–1.13; P = 0 0.028) (Model6). This association was also observed in propensity-matched cohort analysis indicating that male patients still had a higher risk of developing SA-AKI (Table 2). Subgroup analyses were further performed separately in matched and unmatched cohorts by stratifying age into ≥ 55 years old and < 55 years old categories which showed that male patients had a higher incidence rate of SA-AKI compared to female patients across different age strata (Fig. 2).
Association of sex with mortality after sepsis associated acute kidney injury
In the unmatched SA-AKI patient cohort, the ICU mortality (13.5% vs. 11.6%, P = 0.001), in-hospital mortality (19.0% vs. 16.3%, P < 0.001), and 1-year all-cause mortality (37.6% vs. 33.3%, P < 0.001) were higher in female patients compared to male patients (Table 3). Kaplan–Meier analysis showed that male patients had significantly higher 30-day ICU survival rate (log rank P < 0.001), 90-day hospital survival rate (log rank P < 0.001), and 1-year survival rate (log rank P < 0.001) than female patients (Fig. 3). Cox regression analysis demonstrated that male patients had a lower risk of ICU death, hospital death, and 1-year all-cause death compared to female patients, with hazard ratios of 0.851[95% CI (0.774–0.935), P < 0.001], 0.845[95% CI (0.780–0.915)], P < 0.001], and 0.858[95% CI (0.811–0.907)], P < 0.001] respectively. After adjusting for age, BMI, race, infection source, admission type, comorbidities, interventions, severity scale and laboratory variables, male patients had a lower risk of ICU mortality, in-hospital mortality, and all-cause mortality within 1 year, the hazard ratios were 0.861[95% CI (0.778–0.952), P = 0.003], 0.875[95%CI (0.803–0.953), P = 0.002], and 0.985 [95% CI (0.827–1.05)], P = 0.61] respectively (Table 4). In the matched cohort population, male patients still had independent associations with reduced risks of ICU death and hospital death. However, the 1-year all-cause mortality in female patients, although higher than that in male patients, was not statistically significant (36.9% vs. 35.8%, P = 0.12) (Tables 3, 4, Fig. 3).
Association of sex with organ supports after sepsis associated acute kidney injury
Figure 4 shows a comparison of organ support rates between male and female patients with SA-AKI. In the unmatched cohort, the rate of mechanical ventilation support and use of vasoactive drugs was higher in male patients than in female patients (P < 0.001). However, there was no statistically significant difference in the rate of kidney replacement therapy support between male and female patients, although it remained higher in males (P = 0.174) even after adjusting for variables. After adjustment, male patients still had higher rates of mechanical ventilation support (OR, 1.15; 95% CI 1.04–1.29; P < 0.001) and vasoactive drug use (OR, 1.10; 95% CI 1.01–1.20; P = 0.03) compared to female patients (Table 5). However, in the matched cohort, there were no statistically significant differences between male and female patients in terms of kidney replacement therapy, mechanical ventilation treatment or vasoactive drug use (Fig. 4).
Discussion
In this propensity-matched cohort study based on the MIMIC-IV database, the incidence of female SA-AKI during ICU hospitalization was lower than that of males. Females were associated with higher ICU and in-hospital mortality, but there was no statistically significant difference in 1-year all-cause mortality between the two groups. Among SA-AKI patients, there were no significant differences between males and females in terms of renal replacement therapy rate, mechanical ventilation support rate, and vasopressor use rate.
In our study, univariate analysis revealed that the incidence of SA-AKI in female patients with sepsis in the ICU was lower than that in male patients. Even after adjusting for age, BMI, race, infection source, admission type, comorbidities, treatment and laboratory variables, we still found that female patients with sepsis had a lower risk of developing SA-AKI compared to males. Furthermore, even after propensity score matching analysis on the population, we obtained the same results. However, our study design does not allow us to provide detailed explanations for the possible pathophysiological reasons behind this finding. SA-AKI is a complex clinical syndrome with intricate mechanisms of onset. Immune dysfunction combined with high levels of circulating endotoxins and cytokines accelerates the progression of SA-AKI [26]. Previous studies have also found gender differences in the incidence of sepsis. Adrie et al. discovered a lower incidence rate of severe sepsis among females [27]. Wichmann et al. found a significantly lower occurrence rate of severe sepsis/septic shock in female ICU patients aged 60–79 compared to male patients [28]. Additionally, Sperry et al. also observed a significant decrease in multiple organ failure and hospital infection rates among females [29]. This may be attributed to hormonal levels and other characteristics affecting immune function and inflammation levels in female patients; elevated estrogen levels may enhance immune function [30,31,32], while an advantage in anti-inflammatory mediators provides protection against critical sepsis for females [30]. These factors may contribute to reducing susceptibility to SA-AKI among females.
There are many factors in clinical practice that are independently associated with poor prognosis of SA-AKI [33,34,35,36]. In this article, we also reported the gender differences in the outcomes of SA-AKI. In both unmatched and matched cohorts, we found that females had an increased independent risk of ICU mortality and in-hospital mortality. There have been fewer reports on gender-related outcomes in SA-AKI, but there has been sufficient attention given to the differences in outcomes based on gender in sepsis, although conflicting results have been obtained. Schröder et al. found that female patients with surgical sepsis had a higher survival rate than males [37]. However, this study was limited to surgical sepsis patients and included a small number of participants. Adrie et al., in a large case–control study including 1692 critically ill septic patients, also reported similar results [38]. Some basic experimental studies have also found that female animals with sepsis have better survival rates compared to males [39]. The increase in pro-inflammatory cytokine levels is believed to be the cause of this phenomenon, and sex steroids can regulate inflammatory responses and may subsequently affect post-sepsis outcomes [40].
Our research results are contrary to some previous studies [30, 37]. We found that women have a higher risk of ICU mortality and in-hospital mortality independently associated with SA-AKI patients. Our findings are consistent with those of Shapati et al., who demonstrated that women are an independent predictor for increased mortality in critically ill patients with infection [41]. Recently, Combes et al. analyzed gender-related outcomes in a mixed population of patients with hospital-acquired infections in the ICU and reported an increased risk of ICU mortality among women [42]. Previous studies have reported differences in care between male and female patients during hospitalization, with male patients potentially receiving better care [43]. In addition, our study found that female patients had higher severity scores upon admission to the ICU. Female APS III (50.0 [37.0–69.0] vs. 48.0[35.0–68.0], P < 0.001), SAPS II (38.0 [30.0–48.0] vs. 37.0 [29.0–47.0], P < 0001), OASIS (35.0[29.0–42.0] vs. 34.0 [27.0–40.0], P < 0.001) were all higher than males, which may contribute to the increased mortality rate among females during their stay in the ICU as well as throughout their hospitalization period.
In this study, we explored the issue of gender differences in organ support among SA-AKI patients. In the unmatched cohort, female patients had a lower likelihood of receiving mechanical ventilation and vasopressor therapy. However, in the matched cohort, there was no statistically significant difference between males and females. Furthermore, there were no gender-related statistical differences in kidney replacement therapy observed in both matched and unmatched cohorts. Previous studies have found gender differences in organ support among critically ill patients in the ICU [44, 45]. A recent meta-analysis showed that female patients had a lower proportion of receiving organ support (including kidney replacement therapy and mechanical ventilation) during ICU treatment compared to males [46]. However, this study had substantial heterogeneity and potential bias within its sample population. In contrast, our study focused specifically on SA-AKI patients and concluded that there were no gender differences associated with organ support within this specific population. Additionally, our findings are consistent with another meta-analysis regarding organ support during hospitalization for sepsis patients [47]. However, it is worth noting that we did not obtain information such as pre-admission care, discussions or changes during ICU period or decision-makers for substitution therapies nor other social factors that may influence decisions regarding provision of organ support within our cohort. Therefore, further research is needed to better understand the impact of gender differences on rates of organ support in the ICU setting.
Our study has many advantages. It is an observational study based on a large database, using propensity score matching and non-matching cohort analysis to evaluate gender differences in the incidence of SA-AKI, ICU mortality, in-hospital mortality, and organ support rate among the included population. Propensity scores matching analysis retained enough patients and significantly reduced bias and standardized differences in important demographic and clinical characteristics between male and female patients. Additionally, we used the latest definition of SA-AKI [5] to comprehensively describe for the first time the gender differences in SA-AKI in the ICU setting. However, our study also has some limitations. Firstly, it is an observational retrospective design based on a database that only considers traditional parameters without including certain gender-specific variables such as hormone levels which may help explain potential mechanisms underlying our findings. Secondly, decisions regarding initiation and withdrawal of life-sustaining treatments in the ICU are multifactorial. While we attempted to balance clinical factors that could influence this decision-making process, we lack information on social factors such as gender roles or socioeconomic status that could confound this relationship. Although we were able to significantly reduce standardized differences for many important confounding factors by using propensity score matching, there still remains some variability between male and female patients that cannot be balanced within our model and must be excluded from propensity scores. Therefore, there may be residual confounding factors beyond our control present in our analysis. Furthermore, when preadmission creatinine was missing, the first creatinine on admission was used. As we did not use the lowest creatinine value during the entire ICU stay, this may have led to an underestimation of AKI, especially in emergency cases. Lastly, our study is limited to septic patients in ICUs; therefore, caution should be exercised when attempting to generalize our findings to the entire population.
Conclusions
In the ICU setting, male patients have a higher incidence of SA-AKI, while female SA-AKI patients face a higher risk of ICU mortality and in-hospital mortality. Gender may affect the incidence rate and clinical outcomes of SA-AKI, further research is needed to replicate our research and fully understand the impact of gender on SA-AKI patients.
Availability of data and materials
Data are available upon reasonable request.
References
Singer M, Deutschman CS, Seymour CW, et al. The third international consensus definitions for sepsis and septic shock (Sepsis-3). JAMA. 2016;315(8):801–10.
Rudd KE, Johnson SC, Agesa KM, et al. Global, regional, and national sepsis incidence and mortality, 1990–2017: analysis for the Global Burden of Disease Study. Lancet. 2020;395(10219):200–11.
Peerapornratana S, Manrique-Caballero CL, GóMEZ H, et al. Acute kidney injury from sepsis: current concepts, epidemiology, pathophysiology, prevention and treatment. Kidney Int. 2019;96(5):1083–99.
Barbar SD, Clere-Jehl R, Bourredjem A, et al. Timing of renal-replacement therapy in patients with acute kidney injury and sepsis. N Engl J Med. 2018;379(15):1431–42.
Zarbock A, Nadim MK, Pickkers P, et al. Sepsis-associated acute kidney injury: consensus report of the 28th Acute Disease Quality Initiative workgroup. Nat Rev Nephrol. 2023;19(6):401–17.
Klein SL, Jedlicka A, Pekosz A. The Xs and Y of immune responses to viral vaccines. Lancet Infect Dis. 2010;10(5):338–49.
Oliva M, Muñoz-Aguirre M, Kim-Hellmuth S, et al. The impact of sex on gene expression across human tissues. Science. 2020;369(6509):eaba3066.
Mauvais-Jarvis F, Merz NB, Barnes PJ, et al. Sex and gender: modifiers of health, disease, and medicine. Lancet. 2020;396(10250):565–82.
Beltrame A, Salguero P, Rossi E, et al. Association between sex hormone levels and clinical outcomes in patients with COVID-19 admitted to hospital: an observational, retrospective, cohort study. Front Immunol. 2022;13:834851.
Chichareon P, Modolo R, Kerkmeijer L, et al. Association of sex with outcomes in patients undergoing percutaneous coronary intervention: a subgroup analysis of the GLOBAL LEADERS randomized clinical trial. JAMA Cardiol. 2020;5(1):21–9.
Kondo Y, Miyazato A, Okamoto K, et al. Impact of sex differences on mortality in patients with sepsis after trauma: a nationwide cohort study. Front Immunol. 2021;12:678156.
Manwani B, Fall P, Zhu L, et al. Increased P450 aromatase levels in post-menopausal women after acute ischemic stroke. Biol Sex Differ. 2021;12(1):8.
Liang M, Ren X, Huang D, et al. The association between lactate dehydrogenase to serum albumin ratio and the 28-day mortality in patients with sepsis-associated acute kidney injury in intensive care: a retrospective cohort study. Ren Fail. 2023;45(1):2212080.
Zellweger R, Wichmann MW, Ayala A, et al. Females in proestrus state maintain splenic immune functions and tolerate sepsis better than males. Crit Care Med. 1997;25(1):106–10.
van Vught LA, Scicluna BP, Wiewel MA, et al. Association of gender with outcome and host response in critically ill sepsis patients. Crit Care Med. 2017;45(11):1854–62.
Johnson AEW, Bulgarelli L, Shen L, et al. MIMIC-IV, a freely accessible electronic health record dataset. Sci Data. 2023;10(1):1.
Raith EP, Udy AA, Bailey M, et al. Prognostic accuracy of the SOFA score, SIRS criteria, and qSOFA score for in-hospital mortality among adults with suspected infection admitted to the intensive care unit. JAMA. 2017;317(3):290–300.
Lambden S, Laterre PF, Levy MM, et al. The SOFA score-development, utility and challenges of accurate assessment in clinical trials. Crit Care. 2019;23(1):374.
Seymour CW, Liu VX, Iwashyna TJ, et al. Assessment of clinical criteria for sepsis: for the third international consensus definitions for sepsis and septic shock (Sepsis-3). JAMA. 2016;315(8):762–74.
Kellum JA, Lameire N. Diagnosis, evaluation, and management of acute kidney injury: a KDIGO summary (Part 1). Crit Care. 2013;17(1):204.
Siew ED, Ikizler TA, Matheny ME, et al. Estimating baseline kidney function in hospitalized patients with impaired kidney function. Clin J Am Soc Nephrol. 2012;7(5):712–9.
Huber M, Ozrazgat-Baslanti T, Thottakkara P, et al. Cardiovascular-specific mortality and kidney disease in patients undergoing vascular surgery. JAMA Surg. 2016;151(5):441–50.
Angeli P, Ginès P, Wong F, et al. Diagnosis and management of acute kidney injury in patients with cirrhosis: revised consensus recommendations of the International Club of Ascites. J Hepatol. 2015;62(4):968–74.
White IR, Royston P, Wood AM. Multiple imputation using chained equations: issues and guidance for practice. Stat Med. 2011;30(4):377–99.
Moons KG, Donders RA, Stijnen T, et al. Using the outcome for imputation of missing predictor values was preferred. J Clin Epidemiol. 2006;59(10):1092–101.
Ronco C, Bellomo R, Kellum JA. Acute kidney injury. Lancet. 2019;394(10212):1949–64.
Adrie C, Alberti C, Chaix-Couturier C, et al. Epidemiology and economic evaluation of severe sepsis in France: age, severity, infection site, and place of acquisition (community, hospital, or intensive care unit) as determinants of workload and cost. J Crit Care. 2005;20(1):46–58.
Wichmann MW, Inthorn D, Andress HJ, et al. Incidence and mortality of severe sepsis in surgical intensive care patients: the influence of patient gender on disease process and outcome. Intensive Care Med. 2000;26(2):167–72.
Sperry JL, Nathens AB, Frankel HL, et al. Characterization of the gender dimorphism after injury and hemorrhagic shock: are hormonal differences responsible? Crit Care Med. 2008;36(6):1838–45.
Schröder J, Kahlke V, Staubach KH, et al. Gender differences in human sepsis. Arch Surg. 1998;133(11):1200–5.
Knöferl MW, Diodato MD, Angele MK, et al. Do female sex steroids adversely or beneficially affect the depressed immune responses in males after trauma-hemorrhage? Arch Surg. 2000;135(4):425–33.
Knöferl MW, Angele MK, Diodato MD, et al. Female sex hormones regulate macrophage function after trauma-hemorrhage and prevent increased death rate from subsequent sepsis. Ann Surg. 2002;235(1):105–12.
Liu J, Xie H, Ye Z, et al. Rates, predictors, and mortality of sepsis-associated acute kidney injury: a systematic review and meta-analysis. BMC Nephrol. 2020;21(1):318.
Chen JJ, Kuo G, Fan PC, et al. Neutrophil-to-lymphocyte ratio is a marker for acute kidney injury progression and mortality in critically ill populations: a population-based, multi-institutional study. J Nephrol. 2022;35(3):911–20.
Peerapornratana S, Manrique-Caballero CL, Gómez H, et al. Acute kidney injury from sepsis: current concepts, epidemiology, pathophysiology, prevention and treatment. Kidney Int. 2019;96(5):1083–99.
Jiang W, Zhang C, Yu J, et al. Development and validation of a nomogram for predicting in-hospital mortality of elderly patients with persistent sepsis-associated acute kidney injury in intensive care units: a retrospective cohort study using the MIMIC-IV database. BMJ Open. 2023;13(3):e069824.
Schröder J, Kahlke V, Book M, et al. Gender differences in sepsis: genetically determined? Shock. 2000;14(3):307–10.
Adrie C, Azoulay E, Francais A, et al. Influence of gender on the outcome of severe sepsis: a reappraisal. Chest. 2007;132(6):1786–93.
Diodato MD, Knöferl MW, Schwacha MG, et al. Gender differences in the inflammatory response and survival following haemorrhage and subsequent sepsis. Cytokine. 2001;14(3):162–9.
Trentzsch H, Stewart D, de Maio A. Genetic background conditions the effect of sex steroids on the inflammatory response during endotoxic shock. Crit Care Med. 2003;31(1):232–6.
Eachempati SR, Hydo L, Barie PS. Gender-based differences in outcome in patients with sepsis. Arch Surg. 1999;134(12):1342–7.
Combes A, Luyt CE, Trouillet JL, et al. Gender impact on the outcomes of critically ill patients with nosocomial infections. Crit Care Med. 2009;37(9):2506–11.
Todorov A, Kaufmann F, Arslani K, et al. Gender differences in the provision of intensive care: a Bayesian approach. Intensive Care Med. 2021;47(5):577–87.
Fowler RA, Sabur N, Li P, et al. Sex-and age-based differences in the delivery and outcomes of critical care. CMAJ. 2007;177(12):1513–9.
Hessey E, Montgomery C, Zuege DJ, et al. Sex-specific prevalence and outcomes of frailty in critically ill patients. J Intensive Care. 2020;8:75.
Modra LJ, Higgins AM, Abeygunawardana VS, et al. Sex differences in treatment of adult intensive care patients: a systematic review and meta-analysis. Crit Care Med. 2022;50(6):913–23.
Failla KR, Connelly CD. Systematic review of gender differences in sepsis management and outcomes. J Nurs Scholarsh. 2017;49(3):312–24.
Funding
This work was supported by Yangzhou Social Development Project(YZ2023105);Jiangsu Provincial Medical Key Discipline Cultivation Unit (JSDW20221);National key clinical specialty, Financial Appropriations of National No.176[2022].
Author information
Authors and Affiliations
Contributions
All the authors participated in literature retrieval and viewpoint discussion in this article. WJ and LS are the main contributor to article ideas, writing, and data analysis. YZ, JB, JY and XL are the main contributors to data extraction, data analysis. RZ, JY, and JS revised this article. All authors have read and approved the final manuscript. RZ is responsible for all the study work as the guarantor.
Corresponding authors
Ethics declarations
Ethics approval and consent to participate
Given that this study was based on an analysis of a third-party anonymous public database and prior approval was obtained from the institutional review committee, an ethical review was therefore not required.
Consent for publication
My co-authors have all contributed to approve of this submission.
Competing interests
The authors have no competing interest to declare.
Additional information
Publisher's Note
Springer Nature remains neutral with regard to jurisdictional claims in published maps and institutional affiliations.
Rights and permissions
Open Access This article is licensed under a Creative Commons Attribution 4.0 International License, which permits use, sharing, adaptation, distribution and reproduction in any medium or format, as long as you give appropriate credit to the original author(s) and the source, provide a link to the Creative Commons licence, and indicate if changes were made. The images or other third party material in this article are included in the article's Creative Commons licence, unless indicated otherwise in a credit line to the material. If material is not included in the article's Creative Commons licence and your intended use is not permitted by statutory regulation or exceeds the permitted use, you will need to obtain permission directly from the copyright holder. To view a copy of this licence, visit http://creativecommons.org/licenses/by/4.0/. The Creative Commons Public Domain Dedication waiver (http://creativecommons.org/publicdomain/zero/1.0/) applies to the data made available in this article, unless otherwise stated in a credit line to the data.
About this article
Cite this article
Jiang, W., Song, L., Zhang, Y. et al. The influence of gender on the epidemiology of and outcome from sepsis associated acute kidney injury in ICU: a retrospective propensity-matched cohort study. Eur J Med Res 29, 56 (2024). https://doi.org/10.1186/s40001-024-01651-8
Received:
Accepted:
Published:
DOI: https://doi.org/10.1186/s40001-024-01651-8