Abstract
Objective
In this study, we evaluated the efficacy and safety of 1 μg/kg dexmedetomidine as an adjuvant treatment to ropivacaine in children undergoing upper limb surgeries under ultrasound-guided axillary brachial plexus blocks and general anesthesia.
Methods
We enrolled 90 children (aged 1–8 years; ASA I-II) undergoing closed reduction and internal fixation for upper extremity fractures at the Xiamen Children's Hospital and randomly assigned them to one of two groups: L (injection with 0.25% ropivacaine) or D (injection with 0.25% ropivacaine containing 1 μg/kg dexmedetomidine) using the random number table method. The main outcome indicators recorded were the facial expression, leg activity, position, crying, and Face, Legs, Activity, Cry, and Consolability (FLACC) scale scores of children after surgery and the duration of block and analgesia maintenance. The secondary outcome indicators were vital sign data at the time of ultrasound probe placement (T1), at the time of block completion (T2), prior to the beginning of surgery (T3), 5 min after the beginning of surgery (T4), and at the end of surgery (T5), as well as the time of postoperative recovery, the number of cases of remedial analgesia, and complications.
Results
There was no statistical difference between the two groups in terms of general data, block completion time, postoperative recovery time, and complications (P > 0.05). Compared to the L group, the D group had significantly lower FLACC scores at 6 h after surgery, as well as significantly lower systolic blood pressure, diastolic blood pressure, and heart rate values at T4 and T5, and significantly longer duration of postoperative analgesia maintenance (all P < 0.05).
Conclusion
Dexmedetomidine (1 μg/kg) as a local anesthetic adjuvant to ropivacaine can alleviate pain at 6 h postoperatively, prolong analgesia maintenance, and reduce intraoperative blood pressure and heart rate in pediatric patients undergoing closed reduction and internal fixation for upper extremity fractures, with no obvious complications or delayed recovery.
Clinical registry number
Registration website: www.chictr.org.cn, Registration number: ChiCTR2200065163, Registration date: October, 30, 2022.
Similar content being viewed by others
Introduction
Pediatric patients are more sensitive to pain than adults and have a lower pain domain. In children, the onset of pain is frequently accompanied by relatively strong physiological and biochemical changes, including increased respiration, heart rate, blood pressure, and intracranial pressure, accelerated metabolism, elevated oxygen consumption, and changes in plasma and cerebrospinal fluid endorphin levels [1]. In addition, children lack the ability to verbalize pain and frequently resort to exaggerated behavior in order to gain parental attention and sympathy. Unfortunately, these factors have a significant negative impact on the postoperative care and speedy recovery of children. Consequently, it is more important to address the problem of pediatric perioperative pain, particularly acute postoperative pain in trauma [2].
Axillary brachial plexus block is the most used regional anesthesia technique in upper extremity surgery, due to its numerous advantages, such as ease of operation, clear vascular markings, favorable analgesic effect, fewer complications, faster functional recovery, and greater patient satisfaction [3]. However, the analgesic duration (8 ~ 14 h) of long-acting local anesthetics such as ropivacaine for nerve block is not enough to meet the requirements of postoperative analgesia, so adjuvants are needed to prolong the duration of local anesthetics [4]. Dexmedetomidine, a new adjuvant, is among the many adjuvants that have garnered considerable attention. In addition, numerous clinical studies and systematic reviews of dexmedetomidine in adults are available [5, 6]. Dexmedetomidine is widely known to increase the block effect and prolong the maintenance of analgesia [7, 8]. Current research focuses on the safety and efficacy of pre-neural administration of dexmedetomidine.
Reportedly, ropivacaine can be used safely and effectively for axillary brachial plexus block in pediatric patients [9]. However, there are few clinical studies of dexmedetomidine as a brachial plexus block adjuvant in pediatric patients. In this prospective, randomized, double-blind, controlled trial, we investigated the efficacy and safety of dexmedetomidine as an adjuvant for axillary brachial plexus block for postoperative pain in closed reduction and internal fixation for pediatric upper extremity fractures, with the aim of reducing postoperative pain and extending the duration of analgesia in children.
Materials and methods
This study included off-label technique,which was approved by the ethics committee of the Xiamen Children's Hospital (Ethics Review No. 34 of [2021]). Before the surgery, the parents/guardians of all the children signed the informed consent form. The study was recorded in the Chinese Clinical Trials Registry (registration website: www.chictr.org.cn, registration number: ChiCTR2200065163, registration date: October 30, 2022).
Participants and groups
From November1, 2022 to April 1, 2023, we enrolled 90 children with upper extremity fractures who underwent closed reduction and internal fixation at the Xiamen Children's Hospital. The types of humerus fractures were ulnar, radial, and distal.
Children (both genders; 1–8 years; American Society of Anesthesiologists [ASA] I-II) who were able to lay supine and abduct the affected extremity met the inclusion criteria for this study. Exclusion criteria for participants included children with abnormal coagulation, morbid obesity, anatomical abnormalities of the upper arm, puncture site infections, neurological disorders, a history of local anesthetic allergy, and cardiovascular, respiratory, hepatic, and renal diseases.
Using the random number table method, the children were divided into two groups: ropivacaine + normal saline (L) and ropivacaine + dexmedetomidine (D).
Sample size estimation
This study is a prospective, randomized, double-blind, and controlled clinical trial in which the analgesic effect of nerve block and the duration of analgesia maintenance in the participants were observed as the primary outcome indicators. According to the classical literature and the results of the pre-test [8], the mean duration of analgesia maintenance in the L group was 12.58 ± 10.59 h, whereas in the D group, this duration could be increased by 5.21 h. With bilateral a = 0.05 and a certainty of 80%, PASS 11 software was used to calculate the sample size: N1 = 37 cases for the D group and N2 = 37 cases for the L group. Considering nerve block failure, loss to follow-up, and refusal at 20%, each of the two groups required at least 45 participants, with a total of at least 90 participants.
Perioperative management and axillary brachial plexus block
Without preoperative medication, all children fasted for six hours and did not consume any liquids for two hours prior to surgery. Children were transported to the operation room on a flatbed, and their electrocardiograms, noninvasive blood pressure, and finger pulse oximetry oxygen saturation readings were all monitored throughout the procedure. Children were injected intravenously with 0.02 mg/kg atropine (Hubei Xinghua Pharmaceutical Co., Ltd.; H42020590) and 2–2.5 mg/kg propofol (Guangdong Jiabo Pharmaceutical Co., Ltd.; H20133248) successively by anesthesiologists for general anesthesia induction. After the child fell asleep, 6% sevoflurane (Jiangsu Hengrui Pharmaceuticals Co., Ltd.; H20040772) was inhaled through the face mask. After the jaw of the child had relaxed and the eyelash reflex had vanished, an appropriate laryngeal mask was placed. After connection to the anesthesia machine, spontaneous respiratory ventilation was maintained, and anesthesia was maintained by inhalation of one minimum effective alveolar concentration (MAC) of sevoflurane ( the MAC values of sevoflurane in children aged 1–3 years and 3–8 years were 2.8% and 2.5%, respectively). After draping and sterilization, the affected upper extremity was abducted in the salute position, and the probe was placed in the axillary plane perpendicular to the long axis of the humerus. The axillary artery, brachial plexus nerve, and surrounding structures (Supplement Fig. 1A) were identified by a trained attending physician using the high-frequency ultrasound probe of a color Doppler ultrasound machine (Philips Investments Ltd., model CX30). The needle was inserted in the plane of the ultrasound beam, and a small amount of normal saline was used to minimize damage to the vessels and nerves during hydrodissection needle insertion. The local anesthetic was injected until it wrapped the axillary artery (Supplement Fig. 1B) [10]. Children were injected with 0.5 mL/kg of 0.25% ropivacaine (Reyoung Pharmaceutical Co., Ltd.; H20183152) or 0.25% ropivacaine containing 1 μg/kg dexmedetomidine (Yangtze River Pharmaceutical Group Co., Ltd.; H20183220) after the needle reached the axillary artery and no blood or gas was pumped back [9, 11]. Using normal saline as the solvent, two groups of local anesthetics were configured and labeled with serial numbers by specialized personnel, which contained 1:200000 adrenaline (it is helpful for the early detection of local anesthetics mistakenly injected into blood vessels). The operating doctor was not aware of the grouping information. Fifteen minutes after giving block, the surgery began. After surgery, the laryngeal mask was removed at a 1 MAC end-expiratory sevoflurane concentration. When the vital signs of the children were stable and their breathing was regular, they were sent to the post-anesthesia recovery room for resuscitation.
Acquisition and recording of experimental data
As pain descriptions of children are usually inexact [12], we used the Face, Legs, Activity, Cry, and Consolability (FLACC) scale, which consists of five items: facial expression, leg activity, position, crying, and consolability (each item was scored 0, 1, or 2, with a total score of 10; 0 = relaxed and comfortable; 1–3 = mild discomfort; 4–6 = moderate pain; 7–10 = severe pain, discomfort or both). If the total score was > 3, paracetamol suppositories (30 mg/kg) were administered per the children's and parents' preferences. Imperfect nerve block was not included in the study group. The main outcome indicators observed were FLACC scores at 6 h, 12 h, 18 h and 24 h after surgery and analgesia maintenance time. The secondary outcome indicators were systolic blood pressure, diastolic blood pressure, heart rate, finger pulse oxygen saturation and respiratory rate when placing the ultrasound probe (T1), when the block was completed (T2), before the start of the operation (T3), 5 min after the start of the operation (T4) and at the end of the operation (T5), as well as nerve block completion time, postoperative recovery time, complications (including local anesthetic poisoning, hematoma, bradycardia, hypotension, etc.). The test grouping information is confidential to the test data recording and evaluators.
The completion time of the nerve block was calculated between the initial placement of the ultrasound probe and the removal of the puncture needle. The duration of analgesia maintenance was the time between the removal of the needle and the first postoperative pain report by children. The postoperative recovery period was defined as the time between the removal of the laryngeal mask during surgery and the spontaneous opening of children's eyes. Imperfect nerve block was defined as the presence of motor responses after surgical skin incision, accompanied by a 10% and 20% increase in heart rate and respiratory rate compared to pre-surgery levels. When the imperfect nerve block occurred, a total dose of fentanyl ≤ 2 μg/kg was injected intravenously. Failure of the nerve block occurred if the surgery could not be completed due to motor response despite intravenous fentanyl administration [13]. When bradycardia (heart rate decreased by 30% or more than baseline), hypotension (mean arterial pressure decreased by 30% or more than baseline) or hypoxemia (SpO2 < 90%) occurred, atropine, vasopressor or oxygen mask were used respectively [8].
Statistical analysis
The SPSS 19.0 statistical software was used to analyze the data. The measurement data are expressed as the mean ± standard deviation (x ± s) and compared within groups utilizing repeated measures analysis of variance and between groups with the two-samples t test. Count data are summarized as cases (n, %) and analyzed using the chi-squared test or Fisher's exact probability method. Differences were considered statistically significant at P < 0.05.
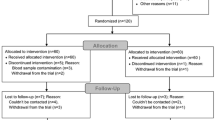
Consort diagram
Study design is shown in Fig. 1.
Results
-
2.1.
A total of 90 patients were included in this study, and 3 patients (2 in L group and 1 in D group) who were converted to open surgery were excluded. Finally, 43 patients in L group and 44 patients in D group were included. There was no statistical difference between the two groups as to the number of cases, gender, age, body weight, ASA classification, or duration of surgery (P > 0.05) (Table 1).
-
2.2.
Six hours after surgery, there were statistically significant differences in FLACC scores between the D and L groups (P < 0.05). In particular, the number of patients with scores of 0–3 (comfort and mild discomfort) at six hours after surgery was greater in the D group (41 patients) than in the L group (33 patients) (P < 0.05). The number of children in the D group with scores of 4–10 (moderate to severe pain) at 6 h postoperatively was significantly lower than in the L group (10 cases) (P < 0.05). The differences in FLACC scores at 12, 18, and 24 h after surgery between the two groups were not statistically significant (P > 0.05). (Fig. 2).
-
2.3.
Nerve blocks were performed successfully on all children, with no children experiencing imperfect blocks or failures. The duration of analgesia maintenance was significantly longer in the D group 17.6 ± 7.0 h than in the L group (12.3 ± 8.1 h) (P < 0.05). Oxygen saturation and respiratory rate were compared between the two groups at each time during operation. The duration of anesthesia and the duration of postoperative recovery were not statistically different between the two groups (P > 0.05) (Fig. 3).
-
2.4.
The systolic and diastolic blood pressure of D group at T4 and T5 and heart rate at T3–T5 were significantly lower than those of the L group (P < 0.05), as determined by intergroup comparisons. In addition, finger pulse oximetry oxygen saturation and respiratory rate at T1–T5 were not statistically significantly different between the two groups (P > 0.05). Comparisons of data at various time points within groups revealed that the systolic and diastolic blood pressure and heart rate of both groups were lower at T3–T5 than at T1 (P < 0.05). The oxygen saturation and respiratory rate values measured by a finger pulse oximeter at T1–T5 within each group did not differ significantly (P > 0.05) (Fig. 4).
-
2.5.
Eight patients in the D group required first remedial analgesia (analgesic drugs were used for the first time in children with postoperative pain) within 24 h, which was not significantly lower than the 10 cases in the L group (P > 0.05). Children in the L group did not develop complications, whereas one child in the D group developed a hematoma at the puncture site (P > 0.05), which resolved following compression. The difference between the two groups in terms of complications was not statistically significant (P > 0.05) (Table 2).
FLACC pain assessment scale score at 24 h after operation. * represents P < 0.05; when compared with group L, the number of patients with scores of 0–3 points in group D was significantly higher than that in group L at 6 h after operation. # represents P < 0.05; when compared with group L, the number of children with scores of 4–10 points in group D was significantly less than that in group L at 6 h after operation
a Comparison of analgesia maintenance time between the two groups. *represents P < 0.05; when compared with group L, the duration of analgesia in group D was significantly longer than that in group L. b Comparison of block completion time and postoperative recovery time between the two groups. There was no significant difference between the two groups (P > 0.05)
a Systolic blood pressure, diastolic blood pressure, and heart rate were compared between the two groups at each time during operation. # represents P < 0.05; when compared with group L, systolic blood pressure at T4 and T5 in group D was lower than that in group L. & represents P < 0.05; when compared with group L, diastolic blood pressure at T4 and T5 in group D was lower than that in group L * represents P < 0.05; when compared with group L, the heart rate of group D was lower than that of group L at T3–T5. T1: When placing the ultrasonic probe, T2: When block is completed, T3: Before surgery begins, T4: 5 min after the surgery started, T5: At the end of surgery. b Oxygen saturation and respiratory rate were compared between the two groups at each time during the operation. There was no significant difference between the two groups (P > 0.05)
Discussion
For pediatric patients with upper extremity fractures, minimally invasive reduction can reduce trauma and hasten recovery. However, postoperative pain still requires consideration. In this study, we examined postoperative pain in pediatric patients who underwent closed reduction and internal fixation for upper extremity fractures using ultrasound-guided axillary brachial plexus block as perioperative analgesia. In our study, the intercostobrachial and musculocutaneous nerves were not blocked due to the small space and loose tissue structure in the axilla of pediatric patients, the ease of local anesthetic distribution, and the absence of tourniquet use [14]. Nonetheless, the duration of analgesia of the long-acting local anesthetic ropivacaine is insufficient to meet clinical needs for postoperative analgesia [15, 16]. An earlier study analyzed the application of dexmedetomidine in combination with ropivacaine in axillary brachial plexus blocks in children and explored the effect on inflammatory factors, providing a basis for the use of dexmedetomidine in the pediatric population [8]. Another study revealed that the combination of dexmedetomidine and ropivacaine increased the duration of nerve block maintenance while decreasing the levels of inflammatory factors such as TNF-α, IL-1β, and IL-6, which exerted significant anti-inflammatory effects and decreased the risk of possible neurological damage caused by local anesthetics while exhibiting neuroprotective effects [17, 18]. In our study, dexmedetomidine was added to ropivacaine as an adjuvant to increase the duration of postoperative analgesia and decrease postoperative pain.
The analgesic mechanism of dexmedetomidine may be to reduce the release of substance P by stimulating the α2 receptor of nociceptive neurons in the spinal dorsal horn, and to inhibit the release of norepinephrine by acting on the a2 adrenergic receptor and G protein in the central nervous system, resulting in sedative, hypnotic and analgesic effects, thereby reducing the degree of postoperative pain. [19, 20] Dexmedetomidine as an adjuvant to ropivacaine reduced pain levels in children at 6 h postoperatively but had no significant effect at 12, 18, or 24 h according to our data. This result is not entirely consistent with literature, wherein studies have reported that the addition of dexmedetomidine to ropivacaine decreased pain intensity 24 h after surgery [21, 22]. This difference is the result of three factors. First, the mean duration of analgesia maintenance was 17.6 h in the D group and 12.2 h in the L group. Therefore, the administration of remedial analgesic paracetamol suppositories was likely initiated 12 h after surgery. No statistical differences in the FLACC scores of children at 12, 18, and 24 h after surgery can be attributed to remedial analgesic interventions. Second, the duration of analgesia maintenance is subjectively reported by the child, and the FLACC scale is an objective score obtained by a specially followed-up medical staff based on the performance of the child: the two have different perspectives on pain assessment and are not necessarily completely consistent. Third, the majority of relevant research was conducted on adults. Therefore, their findings may not necessarily apply to pediatric patients [23]. This distinction highlights the exclusivity of the pediatric population, which is not a subset of the adult population.
Dexmedetomidine inhibits the action potential by inhibiting the expression of TNF-α, IL-1β and IL-6, promoting the hyperpolarization of neuronal cation currents, and contracting local blood vessels to slow down the systemic absorption of local anesthetics, thereby prolonging the maintenance of analgesia. [18, 24, 25] In addition, 1 µg/kg dexmedetomidine as an adjuvant to ropivacaine exerts sedative and analgesic effects without causing delayed recovery. In the current study, dexmedetomidine had no noticeable effect on postoperative recovery time, but prolonged the duration of analgesia maintenance in children. This conclusion is not entirely consistent with literature—in adults, dexmedetomidine is effective as an adjuvant for prolonging the duration of nerve block, resulting in varying degrees of delayed recovery [26]. We speculate that the dexmedetomidine dosage and the timing of administration may determine the degree of recovery delay: in this study, the use of dexmedetomidine before the start of surgery significantly reduced the risk of postoperative drowsiness and delayed recovery in children. In addition, the effects of adjuvant application may vary by age group, particularly among children with faster metabolism [27]. Therefore, additional high-quality clinical trials are required to validate the precise role and efficacy of dexmedetomidine as a local anesthetic adjuvant in the pediatric population.
In terms of hemodynamics, our study revealed that dexmedetomidine decreases the heart rate during surgery and the systolic and diastolic blood pressure after the beginning of surgery. This result may be attributable to the fact that dexmedetomidine is a highly selective α2-adrenergic receptor agonist, which can exert sedative effects and decrease blood pressure and heart rate by inhibiting sympathetic nerve excitability and decreasing norepinephrine release from α2 subtype receptors. This result is generally consistent with the result of the description in literature [18]. Extensive research indicates that the addition of dexmedetomidine to ropivacaine significantly lengthens the duration of brachial plexus block while increasing the risk of bradycardia and hypotension. [19, 28] No bradycardia and hypotension occurred because of the application of atropine to excite sympathetic nerve and adrenaline to contract local blood vessels for slowing down the systemic absorption of dexmedetomidine in this experiment, it is still crucial to be on the alert for and manage such events promptly [29, 30].
In our study, there was no statistically significant difference between the two groups in terms of the number of patients requiring analgesia within 24 h of surgery or the incidence of complications, indicating that dexmedetomidine as a local anesthetic adjuvant, exerted perioperative analgesic effects without causing significant complications. However, most of the research on adults demonstrated that dexmedetomidine decreased the need for postoperative supplementary analgesics [19]. This discrepancy may be attributable to the unique characteristics of the pediatric population: the use of postoperative remedial analgesics in children is influenced by a variety of variables, including cultural values, religion, parental beliefs, and anxiety level. Additionally, non-pharmacological methods such as position modification, parental comfort, and listening to music can alleviate postoperative pain in children. Consequently, medication is not the only method for reducing postoperative pain in children.
In conclusion, the addition of dexmedetomidine to ropivacaine alleviated postoperative pain, prolonged analgesia maintenance, and decreased intraoperative blood pressure and heart rate without causing recovery delays or obvious complications. First, 1 µg/kg dexmedetomidine as an adjuvant reduced FLACC scores at 6 h after surgery in children, but had no effect on FLACC scores at 12, 18, and 24 h. Second, 1 µg/kg dexmedetomidine increased the duration of axillary brachial plexus block analgesia but had no significant effect on supplementary analgesia or postoperative recovery time. Third, 1 µg/kg dexmedetomidine as an adjuvant may cause a slight decrease in the intraoperative heart rate and blood pressure of children, which must be monitored and treated promptly to ensure patient safety. To ensure safety, heart rate and blood pressure must be monitored more closely, and low heart rate and blood pressure must be treated promptly. Finally, 1 μg/kg dexmedetomidine as an adjuvant is safe and has no serious complications. This trial is a prospective double-blind randomized controlled trial, which provides new ideas and methods for perioperative analgesia of closed reduction and internal fixation of upper limb fractures in children, and provides new evidence for the application of dexmedetomidine in axillary brachial plexus block in children. In a small number of pediatric-related studies [31], this trial supported the establishment of evidence-based recommendations for dexmedetomidine as an adjuvant for pediatric nerve block, and laid a new foundation for dose exploration.
Shortcomings and limitations
There are shortcomings in this research. In numerous adult studies, the axillary brachial plexus block exerts excellent analgesic effects during forearm surgery but is less effective during upper arm surgery. In our study, only the distal upper arm was operated on, which may have influenced the block effect. Second, although the difference in the number of first remedial analgesia between the two groups was not statistically significant, the time from the removal of the puncture needle to the first use of remedial analgesic drugs due to pain was not recorded, which may have varied between the two groups. Third, there was no comparison of the efficacy and safety of different doses of dexmedetomidine as an adjuvant to local anesthetics. Fourth, the postoperative sedative effect of dexmedetomidine was not evaluated; Finally, children who used non-pharmacological methods to relieve postoperative pain were not excluded.
Future research should investigate the optimal dose of dexmedetomidine for maximizing the therapeutic effect while minimizing the risk of side effects. In addition, future studies examining the neurotoxicity and delayed neurological effects of dexmedetomidine should extend the duration of the follow-up period.
Conclusion
Collectively, 1 µg/kg dexmedetomidine as an adjuvant to 0.25% ropivacaine in ultrasound-guided perivascular axillary brachial plexus block relieved the pain 6 h after surgery, prolonged the duration of analgesia maintenance, slightly reduced intraoperative blood pressure and heart rate, did not cause delayed recovery or significant complications, and had high safety in pediatric patients aged 1–8 years undergoing closed reduction and internal fixation for upper extremity fractures.
Availability of data and materials
Date will be made available on request.
References
Jin L, Saroj R, Ruikang L, et al. One additional shot of brachial plexus block equates to less postoperative pain for younger children with elbow surgeries. J Orthop Surg Res. 2020;15(1):246. https://doi.org/10.1186/s13018-020-01778-4.
Kathryn D, Megan F, Wilson Lauren A, et al. The utilization of regional anesthesia among pediatric patients: a retrospective study. HSS J. 2020. https://doi.org/10.1007/s11420-020-09805-0.
Casas-Arroyave FD, Ramírez-Mendoza E, Ocampo-Agudelo AF. Complications associated with three brachial plexus blocking techniques: systematic review and meta-analysis. Rev Esp Anestesiol Reanim. 2021;68(7):392–407. https://doi.org/10.1016/j.redare.2020.10.
Tommy G, Guillaume B, Marc P, et al. Minimum effective concentration of ropivacaine for 90% ultrasound-guided axillary brachial plexus block, with or without intravenous dexamethasone. J Clin Anesth. 2021;75:110468. https://doi.org/10.1016/j.jclinane.2021.110468.
Nazanin H, Hesameddin M, Esmail M, et al. Effects of adding dexmedetomidine, fentanyl, and verapamil to 0.5% ropivacaine on onset and duration of sensory and motor block in forearm surgeries: a randomized controlled trial. Med Gas Res. 2021;11(2):47–52. https://doi.org/10.4103/2045-9912.311488.
Nidhi S, Shikha G, Suneet K. Dexmedetomidine vs dexamethasone as an adjuvant to 0.5% ropivacaine in ultrasound-guided supraclavicular brachial plexus block. J Anaesthesiol Clin Pharmacol. 2020;36(2):238–43. https://doi.org/10.4103/joacp.JOACP_176_19.
Wu Yu, Yuling K, Yanli Li, et al. Impact of ultrasound-guided deep serratus anterior plane block combined with dexmedetomidine as an adjuvant to ropivacaine inpatient quality of recovery scores undergoing modified radical mastectomy: a randomized controlled trial. Front Oncol. 2022. https://doi.org/10.3389/fonc.2022.858030.
Haikou Y, Wei F, Yong Y, et al. Application of dexmedetomidine combined with ropivacaine in axillary brachial plexus block in children and its effect on inflammatory factors. Cell Mol Biol. 2020;66(5):73–9.
Liang C, Yang S, Shuangmei L, et al. Minimum effective volume of 0.2% ropivacaine for ultrasound-guided axillary brachial plexus block in preschool-age children. Sci Rep. 2021;11(1):17002. https://doi.org/10.1038/s41598-021-96582-3.
Liandi Li, Yanjing Z, Ling G, et al. Ultrasound guidance enhances the efficiency of brachial plexus block and ameliorates the vascular injury compared with nerve stimulator guidance in hand surgery patients. J Investigative Surgery. 2020;33(6):530–5. https://doi.org/10.1080/08941939.2018.1539792.
Jing Y, Cui Y, Rong C, et al. Dexmedetomidine as an adjunct to peripheral nerve blocks in pediatric patients. World J Pediatr. 2022;18(4):251–62. https://doi.org/10.1007/s12519-021-00507-z.
Robert A, Barbara R, Zsofia K, et al. Onset times and duration of analgesic effect of various concentrations of local anesthetic solutions in standardized volume used for brachial plexus blocks. Heliyon. 2020;6(9): e04718. https://doi.org/10.1016/j.heliyon.2020.e04718.
Murat YA, Sevim C, Figen O, et al. Comparison of the lateral sagittal and costoclavicular approaches for ultrasound-guided infraclavicular block in pediatric patients: a prospective randomized study. Brazilian J Anesthesiol. 2021. https://doi.org/10.1016/j.bjane.2021.05.005.
Krithika K, Umesh KS, Purnachandra T. Incidence and factors influencing tourniquet pain. Chinese J Traumatol. 2021;24(5):291–4. https://doi.org/10.1016/j.cjtee.2021.05.002.
Shrestha Niti Wu, Liang WX, et al. Preemptive infiltration with betamethasone and ropivacaine for postoperative pain in laminoplasty or laminectomy (PRE-EASE): study protocol for a randomized controlled trial. Trials. 2020;21(1):381. https://doi.org/10.1186/s13063-020-04308-z.
Hui L, Liang N. Application of hydromorphone and ropivacaine in ultrasound-guided brachial plexus block of children. J Perianesth Nurs. 2022;37(5):662–8. https://doi.org/10.1016/j.jopan.2021.11.013.
Yuandong Q, Zhiwei T. Dexmedetomidine attenuates LPS-induced acute lung injury in rats by activating the Nrf2/ARE pathway. J Healthcare Eng. 2022;2022:4185195. https://doi.org/10.1155/2022/4185195.
Ivan U, Guadalupe VC, Hamed A, et al. A comprehensive review and update of the use of dexmedetomidine for regional blocks. Psychopharmacol Bull. 2020;50:121–41.
Srikantha R, Niraja. R. Dexmedetomidine as an adjunct for regional anesthetic nerve blocks. Curr Pain Headache R. 2021;25(2):8. https://doi.org/10.1007/s11916-020-00926-z.
Ye Weidi Hu, Yuntao YW, et al. Retrobulbar dexmedetomidine in pediatric vitreoretinal surgery eliminates the need for intraoperative fentanyl and postoperative analgesia: a randomized controlled study. Indian J Ophthalmol. 2019;67(6):922–7. https://doi.org/10.4103/ijo.IJO_1905_18.
Wei L, Jingwen G, Jun Z, et al. Low-dose dexmedetomidine as a perineural adjuvant for postoperative analgesia: a randomized controlled trial. BMC anesthesiol. 2022;22(1):249. https://doi.org/10.1186/s12871-022-01791-6.
Kashish G, Neerja B, Sandhya Y, et al. Efficacy of dexmedetomidine as an adjunct to ropivacaine in transversus abdominis plane block for paediatric laparoscopic surgeries: a double-blinded randomised trial. Indian J Anaesth. 2021;65(Suppl 1):S27–33. https://doi.org/10.4103/ija.IJA_1207_20.
Daisy K, Swastika S, Ranjan MP, et al. Effect of dexmedetomidine as an adjuvant to ropivacaine in ilioinguinal-iliohypogastric nerve blocks for inguinal hernia repair in pediatric patients: a randomized, double-blind Control Trial. Anesth Essays Res. 2018;12(4):924–9. https://doi.org/10.4103/aer.AER_169_18.
Yongmei P, Qigang Y, Wenwei W, et al. Dexmedetomidine as an adjuvant to local anesthetics in brachial plexus blocks: a meta-analysis of randomized controlled trials. Medicine. 2017. https://doi.org/10.1097/MD.0000000000005846.
Kariem E-B, Richard B, Herman S, et al. Perineural dexmedetomidine is more effective than clonidine when added to local anesthetic for supraclavicular brachial plexus block: a systematic review and meta-analysis. Anesth Analg. 2017;124(6):2008–20. https://doi.org/10.1213/ANE.0000000000002014.
Krishan GK, Prasad PB, Gurpreet S, et al. Evaluation of dexmedetomidine as an adjuvant to ropivacaine in transversus abdominis plane block for postoperative analgesia in unilateral infraumbilical surgeries-a randomized prospective trial. Asian J Anesthesiol. 2022;60(1):19–25. https://doi.org/10.6859/aja.202203_60(1).0003.
Mostafa Mohamed F, Aal Fatma Abdel A, Ibrahim Hassan A, et al. Dexmedetomidine during suprazygomatic maxillary nerve block for pediatric cleft palate repair, randomized double-blind controlled study. Korean J Pain. 2020;33(1):81–9. https://doi.org/10.3344/kjp.2020.33.1.81.
Shi-Hui Y, Wei-Guo S, Yong-le Li, et al. Effects of different doses of dexmedetomidine combined with ropivacaine for brachial plexus nerve block in children undergoing polydactyly surgery. Nan Fang Yi Ke Da Xue Xue Bao. 2017;37(6):833–6.
Shahryar S, Shahram S, Parang G, et al. The effect of dexmedetomidine in combination with bupivacaine on sensory and motor block time and pain score in supraclavicular block. Pain Res Manage. 2021;2021:8858312. https://doi.org/10.1155/2021/8858312.
Mostafa Mohamed F, Esam H, Amin Ahmed H, et al. Dexmedetomidine versus clonidine adjuvants to levobupivacaine for ultrasound-guided transversus abdominis plane block in paediatric laparoscopic orchiopexy: randomized, double-blind study. Eur J Pain. 2020;25(2):497–507. https://doi.org/10.1002/ejp.1689.
Markus Z, Philipp O, Peter M, et al. Brachial plexus block with ultrasound guidance for upper-limb trauma surgery in children: a retrospective cohort study of 565 cases. British J Anaesthesia. 2020;125(1):104–9. https://doi.org/10.1016/j.bja.2020.03.012.
Acknowledgements
Not applicable.
Funding
This study was supported by a grant from Guided project of Fujian Provincial Science and Technology Department (No.2022Y0028).
Author information
Authors and Affiliations
Contributions
Conception and design of the research:Jian-Bin Chen,Zhi-Yuan Chen ,Xue-Shan Li Acquisition of data:Xiao Qi Zhang,Li-Ming Su,Xue-Shan Li Analysis and interpretation of the data:Jian-Bin Chen,Ying Liu ,Zhi-Yuan Chen,Li-Ming Su Statistical analysis:Ying Liu,Xiao-qi Zhang,Li-Ming Su Writing of the manuscript:Jian-Bin Chen Critical revision of the manuscript for intellectual content:Jian-Bin Chen,Zhi-Yuan Chen All authors read and approved the final draft.
Corresponding authors
Ethics declarations
Ethics approval and consent to participate
This study was conducted in accordance with the declaration of Helsinki. This study was conducted with approval from the Ethics Committee of Xiamen Children’s Hospital (〔2021〕34). A written informed consent was obtained from legal guardians of all participants.
Consent for publication
Consent for publication was obtained from every individual (legal guardians) whose data are included in this manuscript.
Competing interests
The authors declare no competing interests.
Additional information
Publisher's Note
Springer Nature remains neutral with regard to jurisdictional claims in published maps and institutional affiliations.
Supplementary Information
Rights and permissions
Open Access This article is licensed under a Creative Commons Attribution-NonCommercial-NoDerivatives 4.0 International License, which permits any non-commercial use, sharing, distribution and reproduction in any medium or format, as long as you give appropriate credit to the original author(s) and the source, provide a link to the Creative Commons licence, and indicate if you modified the licensed material. You do not have permission under this licence to share adapted material derived from this article or parts of it. The images or other third party material in this article are included in the article’s Creative Commons licence, unless indicated otherwise in a credit line to the material. If material is not included in the article’s Creative Commons licence and your intended use is not permitted by statutory regulation or exceeds the permitted use, you will need to obtain permission directly from the copyright holder. To view a copy of this licence, visit http://creativecommons.org/licenses/by-nc-nd/4.0/.
About this article
Cite this article
Chen, JB., Su, LM., Zhang, XQ. et al. Ropivacaine combined with dexmedetomidine in ultrasound-guided axillary brachial plexus block in children, a randomized controlled trial. Eur J Med Res 29, 416 (2024). https://doi.org/10.1186/s40001-024-01997-z
Received:
Accepted:
Published:
DOI: https://doi.org/10.1186/s40001-024-01997-z