Abstract
Background
MicroRNAs (miRNAs) are presented in the uterine lumen of many mammals, and in vitro experiments have determined that several miRNAs are important for the regulation of endometrial and trophoblast functions. Our aim was to identify and contrast the miRNAs present in extracellular vesicles (EVs) in the uterine lumen fluid (ULF) at the onset of attachment in cattle pregnancies (gestation d 18) initiated by artificial insemination (AI) or by the transfer of an in vitro-produced blastocyst (IVP-ET). A third group had no conceptus after the transfer of an IVP embryo.
Results
The abundance of 263 annotated miRNAs was quantified in the EVs collected from ULF. There was an increase in the transcript abundance of 20 miRNAs in the ULF EVs from the AI pregnant group, while 4 miRNAs had a lower abundance relative to the group not containing a conceptus. Additionally, 4 miRNAs were more abundant in ULF EVs in the AI pregnant group relative to IVP-ET group (bta-mir-17, bta-mir-7-3, MIR7-1, MIR18A). Specific miRNAs in the ULF EVs were co-expressed with messenger RNAs expressed in extra-embryonic tissues and endometrium, including genes that are known to be their targets.
Conclusions
The results provide biological insights into the participation of miRNAs in the regulation of trophoblast proliferation and differentiation, as well as in endometrium receptivity. The knowledge that in vitro cultured embryos can contribute to the altered abundance of specific miRNAs in the uterine lumen can lead to the development of corrective approaches to reduce conceptus losses during the first month of pregnancy in cattle.
Similar content being viewed by others
Background
In cattle, the embryo enters the uterus by gestation d 4–5 and hatches from the zona pellucida by d 8 post-fertilization [1, 2]. At this time, the outer monolayer of trophectoderm cells and the uterine luminal epithelium (LE) have direct contact. The hatched blastocyst begins to produce interferon tau (IFNT) [3], which is the major pregnancy recognition signal that inhibits the development of the endometrial luteolytic mechanism [4, 5]. On gestation d 12–14, the blastocyst is ovoid in shape (~ 2–5 mm in length) and transitions into a tubular shape by d 14–15, at which time it can be termed a conceptus [6]. In cattle, elongation is also coincident with a greater release of IFNT [7]. The conceptus elongates via the proliferation of the trophectoderm and parietal endoderm cells [8] and reaches 20 cm or more in length by d 19–20 [8, 9]. Then, the trophectoderm begins to attach to the endometrial lining [8, 10] thereby initiating cell-to-cell communication mediated by adhesion [10]. By gestation d 25, a small proportion of trophectoderm cells have differentiated into binuclear cells [11], and the formation of the chorion marks the onset of the epitheliochorial placentation [1, 9, 12]. Ruminants have an epitheliochorial nature of placentation [1, 13, 14] with an extended time of non-invasive implantation followed by a limited invasion of the endometrium [10, 13].
After the primary signals or hormonal controllers exert their roles to progress endometrial and conceptus functionality, a myriad of cellular signals, mediated by bioactive molecules named embryotrophins [15], is triggered. The embryo can modulate the regulation of gene expression in the endometrium as early as on d 7 of gestation [16], when it can also promote alterations in the metabolite composition of the uterine lumen fluid (ULF) [17]. By gestation d 15–16, global alterations in endometrial gene expression occur in response to the elongating conceptus [18,19,20]. Thereafter, a synchrony of gene regulation between the extra-embryonic tissue (EET) and the endometrium is established [21] and contributes to a carefully orchestrated cell-to-cell communication between the conceptus and endometrium.
As the trophoblast and endometrium lining have direct contact, cell-to-cell interactions [10, 22, 23] can be established through ligand-receptor mediated signaling [20, 24, 25]. The transfer of RNAs from cell to cell is another concept [26], supporting the possible exchange of signals between trophoblast and endometrium [27]. These RNAs can be transported between cells in extracellular vesicles (EVs), which may also contain proteins and other macromolecules such as lipids [26, 28, 29]. EVs present in the ULF carrying microRNAs (miRNAs) have gained much attention in the past decade due to their importance in modulating trophoblast function and health [30] and endometrial remodeling [31]. Alterations in the miRNA content of EVs in the ULF have been associated with embryo implantation failure in women (reviewed in [32, 33]).
Cattle blastocysts [34,35,36], d 16 conceptuses [37] and endometrial cells [38, 39] produce EVs containing miRNAs. Alterations in miRNA profiles of EVs present in the ULF have been detected as early as pregnancy d 7 of gestation in cattle [40]. Also on d 7, blastocysts produced in vitro may produce and export miRNAs in EVs that are different from their in vivo generated counterparts [41]. EVs are present in the ULF during the attachment period [42,43,44,45] containing miRNAs among other molecules in their cargo [42, 43]. In vitro experiments have determined that the cargo in EVs present in the uterine lumen of pregnant cows can modulate gene transcription in cattle endometrial [42, 43, 45] and trophoblast [46] cell lines. However, the extent to which miRNAs present in the ULF contribute to the conceptus-maternal communication in cattle remains unclear. In the present study, our aims were to determine differences in the miRNA profiles in the ULF EVs of d 18 pregnancies harboring an in vivo derived conceptus versus ULF EVs when there is not conceptus present, and also to determine differences in the miRNA profiles in the ULF EVs of d 18 pregnancies harboring an in vivo or in vitro derived conceptus. Herein we tested two hypotheses: (a) that the miRNA profile in the ULF of d 18 pregnant uteri harboring in vivo conceptus is different than those of uteri harboring in vitro derived conceptus, and (b) that miRNAs can form co-expression regulatory networks with genes expressed in the conceptus and endometrium.
Methods
All animal procedures for live handling were approved by the Institutional Animal Care and Use Committee, Auburn University, under protocol 2016-2874.
In vitro production of embryos and cryopreservation
All chemicals were obtained from Sigma-Aldrich (St. Louis, MO, USA) or Fisher (Pittsburgh, PA, USA), unless otherwise stated. Embryo production procedures utilized in this study to produce embryos were consistent with procedures detailed previously [47,48,49]. Cumulus-oocyte complexes were aspirated from follicles (3–8 mm in diameter) of abattoir-derived ovaries. The cumulus-oocyte complexes were washed in Tissue Culture Medium-199 with Hanks salts supplemented with 25 mmol/L HEPES followed by in vitro maturation in Tissue Culture Medium-199 with Earle salts (Gibco, Grand Island, NY, USA) supplemented with 10% fetal bovine serum, 100 IU/mL penicillin, 100 μg/mL streptomycin, 0.2 mmol/L sodium pyruvate, 2 mmol/L L-glutamine, 50 ng/mL recombinant human epidermal growth factor (Invitrogen, Waltham, MA, USA), and 5 μg/mL of follicle-stimulating hormone (Bioniche Animal Health, Athens, GA, USA). In vitro maturation was carried out for 22–24 h at 38.5 °C in a humidified atmosphere containing 5% CO2.
In vitro matured cumulus-oocyte complexes were washed three times with HEPES-TALP medium and placed in IVF-TALP medium for in vitro fertilization. Sperm from a single sire was prepared by density gradient centrifugation utilizing the ISolate sperm separation kit (Irvine Scientific, Santa Ana, CA, USA) and washed twice by centrifugation in SP-TALP. Sperm was added to the fertilization dish at the concentration of 1 × 106/mL, followed by the addition of penicillamine-hypotaurine-epinephrine solution. In vitro fertilization was carried out for 17–19 h at 38.5 °C in a humidified atmosphere containing 5% CO2. Cumulus cells were removed from putative zygotes by vortexing in 400 μL/of HEPES-TALP. Putative zygotes were then cultured in groups of up to 50 in 500 μL of SOF-BE2, covered with 300 μL of light mineral oil. In vitro culture was carried out at 38.5 °C in a humidified atmosphere containing 5% CO2, 5% O2 and 90% N2. Seven d after in vitro fertilization, grade one [50] blastocysts were cryopreserved using the slow-freezing procedure in ethylene-glycol solution [51].
Estrous synchronization, artificial insemination, and embryo transfer
Nulliparous heifers of Angus-cross genetic background (15–19 months of age, weighing > 296 kg) were utilized for this experiment. Animals were randomized into one of the two experimental groups, based on whether they would be artificially inseminated or serve as recipients for embryo transfer.
Estrous synchronization [52] was initiated by inserting a controlled internal drug release (CIDR, 1.38 g progesterone), which was removed after 14 d. Sixteen d post removal of CIDR, 25 mg of prostaglandin F2 alpha (Lutalyse®, Zoetis, Parsippany-Troy Hills, NJ, USA) was administered along the application of an estrus detection patch (Estrotect™; Rockway Inc., Spring Valley, WI, USA) mid-way between the hip and tail head. All animals were observed for estrus by two investigators, and heifers that showed clear signs of standing estrus [53] and ≥ 50% of color change on the estrus detection patch continued the protocol. Alternatively, when there was no sign of estrus, the heifer was re-enrolled in estrous synchronization.
If the heifer was assigned to be artificially inseminated, AI was conducted 12–16 h after the onset of standing estrus. All heifers were inseminated with semen from one sire, which was the same sire used for in vitro embryo production.
For embryo transfer, 7 d post-estrus, the presence of a corpus luteum was evaluated by transrectal ultrasonography. If a corpus luteum was present, one embryo was deposited in the uterine horn ipsilateral to the corpus luteum. If the heifer did not present a corpus luteum, she was re-enrolled in estrous synchronization.
Collection of the uterine lumen contents
Heifers were euthanized with captive bolt on d 18 of pregnancy (herein considered 18 d post fertilization). The reproductive tract was removed from each heifer within 15 min of euthanasia and immediately prepared for flushing. To prepare the reproductive tract for flushing, first, mesometrium was removed from the uterine horns. Next, the cervix was removed by cutting the base of the uterine body. Then, an 18-g needle coupled to a syringe was inserted into the distal portion of the ipsilateral horn and 20 mL of nuclease-free phosphate-buffered saline solution were flushed towards the base of the uterine body. The fluid was flushed into a cell strainer placed in a 50-mL conical tube, which retained a conceptus, when present, and clumps of cells. The flushed solution was centrifuged (3,000 × g for 15 min at 4 °C) for the palletization of cells and other debits. The supernatant was filtered using a polyvinylidene difluoride 0.45 μm membrane filters for the removal of potential remaining cells. The flushed material was stored at –80 °C until extraction of miRNAs.
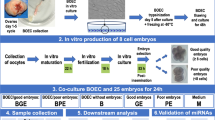
Samples were categorized as obtained from pregnancies initiated by artificial insemination (AI, n = 7), or in vitro produced embryo followed by embryo transfer (IVP-ET, n = 7), or no conceptus present (NCP, n = 5) after embryo transfer (Fig. 1A).
Overview of the experiment and study carried out. A Experimental groups included in the experiment. B Schematics of the procedures and data produced, as well as the results obtained. C Depiction of the data obtained from the public data base. Created with BioRender.com
Processing of the uterine luminal flush and high throughput sequencing of small RNAs
Starting with 10 mL of ULF, EVs were captured and isolated using the ExoQuick-TC™ Tissue Culture Media Exosome Precipitation Solution (System Biosciences, Palo Alto, CA, USA) following the manufacturer’s protocol (Fig. 1B). The kit is based on the purification with polyethylene glycol [54] along with centrifugation, which is an acceptable method for the collection of EVs [55] and has been validated to precipitate EVs that are positive for the tetraspanins CD9 [56] or CD63 [57, 58] including in cattle follicular fluid [57]. Both markers are characteristic of EVs [55].
Particle size analysis was conducted in two representative samples (three technical replicates per sample) as a service provided by System Biosciences, Palo Alto, CA, USA. The size of EVs was assessed using a NanoSight NS300 instrument (NanoSight Ltd., Amesbury, UK) and a sCMOS camera, under shutter setting 1,000 and gain 400. The assays were conducted in parallel with standard beads (100 nm latex bead – standardized by the National Institute of Standards and Technology), which reported a 5% coefficient of variation in the measurements of the standard beads.
To further validate the enrichment of EVs, we separated two samples for precipitation of EVs as indicated above. We assayed EVs markers using the Exo-Check Exosome Antibody Arrays (System Biosciences, Palo Alto, CA, USA) according to the manufacturer’s manual. This slot blot has 12 pre-printed spots and out of which antibodies for exosome markers (CD63, CD81, ALIX, FLOT1, ICAM1, EpCam, ANXA5 and TSG101), and GM130, a cis-Golgi marker to identify cellular contamination. The signal was developed by chemiluminescence using WesternBright™ Sirius™ HRP substrate (Advansta, San Jose, CA, USA). The array was imaged on a iBright™ CL750 Imaging System (ThermoFisher Scientific).
After obtaining the pellet with EVs, small RNAs extracted using TRIzol Reagent [59] following procedures optimized for small sample size [60]. We accessed the small RNAs using the Agilent Small RNA kit (Agilent, Waldbronn, Germany) in a 2100 Bioanalyzer Instrument (Agilent) (Fig. 1B) and submitted the samples for sequencing at the VANTAGE (Vanderbilt Technologies for Advanced Genomics) at Vanderbilt University, Nashville, TN. Sequencing libraries were prepared with the NEBNext® Small RNA Library Prep Set for Illumina® (New England Biolabs, Ipswich, MA, USA) and sequencing was assayed in a NovaSeq 6000 Sequencing System (Illumina, San Diego, CA, USA) to produce a minimum of 10 million reads per sample (Fig. 1B).
Raw sequences were processed for the removal of adapters with Trimmomatic [61] and alignment to the bovine genome (Bos_taurus.ARS-UCD1.2.104) using bowtie2 (v.2.3.5.1) [62] using the “–very-sensitive” option. Next, Samtools (v 1.10) [63] was used to retain only primary alignments. Lastly, featurecounts (v 2.0.1) [64] was used to count reads according to the Ensemble annotation (Bos_taurus.ARS-UCD1.2.104) [65, 66]. Counts per million (CPM) was calculated with the function “cpm” from the “edgeR” package [67, 68] in R software. Lastly, only annotated small RNAs with > 50 sequences across all samples were retained for downstream analysis.
Statistical analyses
Transcript abundance of miRNAs was compared using the R packages ‘edgeR’ [67, 68], with the quasi-likelihood test, and ‘DEseq2’ [69], using the Wald’s and likelihood test. The nominal P values of both tests were corrected for multiple hypothesis testing using the false discovery rate (FDR) method [70]. Differential transcript abundance was assumed when FDR < 0.05 for both tests.
Enrichment tests for Gene Ontology categories were carried out using “goseq” package [71] in R software. In all tests, the genes whose transcript abundances were estimated for the samples being tested were used as the background. The nominal P value was adjusted for multiple hypothesis testing by controlling the familywise error rate (FWER) following the method proposed by Holm [72] using the function “p.adjust” from the ‘stats’ R package. Significance was inferred when FWER ≤ 0.01.
Transcript abundance from mRNA data were obtained from EET and endometrium collected from the same uteri used to collect ULF EVs (GSE232489 [73], Fig. 1C). Co-expression analysis is an analytical approach to identify quantitative relationships between transcript abundances, and can be measured using a correlation coefficient [74]. Given the experimental design in our study, we were able to conduct a co-expression analysis between miRNAs and mRNAs for which we adopted the procedures recommended by Johnson and Krishnan [75]. All miRNAs and protein coding genes that passed our filtering for lowly expressed genes were used in this analysis. For each group and sample type (i.e., EET AI), CPM metric was calculated using the trimmed mean of M values method [76]. Then, CPM was transformed using the arcsine transformation \(Log\left(x+ \sqrt{\left({x}^{2}+1\right)}\right)\) using the “asinh” function in R. Lastly, the Pearson coefficient of correlation and the corresponding P value were calculated using the function “corAndPvalue” from the package “WGCNA” [77] for each pair of genes (miRNA and mRNA). Empirical values of FDR (eFDR) [21, 78]were estimated with 10,000 randomizations of the data. Co-expression was inferred when eFDR < 0.00004 (equivalent to nominal P value < 0.00001).
miRNA predicted targets
In order to assess if a specific protein coding genes would be a target of a specific miRNA, we obtained mRNA predicted targets for miRNAs from humans and cattle from the miRWalk database [79] (http://mirwalk.umm.uni-heidelberg.de/resources/) on November 26th 2021, including untranslated regions and coding sequences.
Results
Following the nanoparticle tracking analysis with finite track length adjustment [80], the mode size distribution of the EV 90 and 97, with an average and standard deviation of the particle sizes of 148 ± 102 and 168 ± 120 nm, respectively. An assay of eight proteins that are common markers of EV showed a high abundance of ALIX in our EV preparation. Other proteins with signal but at a lower abundance were ANXA5, TSG101, ICAM and FLOT1. There were faint signals of CD63 and GM130 (Fig. 2A). Next we extracted RNAs from EVs out of which, most were miRNAs (Fig. 2B).
Small RNA sequencing of EVs collected from the ULF was performed for 19 samples from gestation d 18 (AI, n = 7; IVP-ET, n = 7; NCP, n = 5). Altogether, over 537 million reads were produced from the small RNAs obtained from the EVs in the ULF, out of which 9,082,232 sequences matched to miRNAs on the Ensembl annotation averaging of 478,012 sequences per samples. After filtering for annotated miRNAs that had more than 50 reads across all samples, there were 263 annotated miRNAs (Additional file 1). Notably, the top 40 miRNAs present in the ULF flushed from pregnant uterus on d 18 of gestation accounted for 92% of the total reads produced and mapped to annotated miRNAs (Fig. 3A).
miRNAs in the ULF. A Top 40 most abundant miRNAs in the ULF EVs of pregnant heifers (AI group) on gestation d 18. B miRNAs with differential abundance in the ULF EVs of d 18 pregnant heifers (AI group) versus NCP counterparts. C miRNAs with differential abundance in the ULF EVs of pregnant heifers harboring an IVP-ET conceptus versus an AI conceptus. AI: artificial insemination; IVP-ET: in vitro produced and embryo transfer; NCP: no conceptus present after ET
There were 24 miRNAs with differential abundance in the EVs obtained from ULF in the AI group versus the NCP group (greater abundance in AI: bta-mir-130b, bta-mir-15b, bta-mir-17, bta-mir-2285aa, bta-mir-2315, bta-mir-2387, bta-mir-302a, bta-mir-302b, bta-mir-371, bta-mir-500, bta-mir-503, bta-mir-6120, bta-mir-7-3, bta-mir-7857-1, bta-mir-7857-2, bta-mir-93, MIR18A, MIR378A, MIR7-1; lower abundance in AI: bta-mir-181b-2, bta-mir-320a-1, bta-mir-320a-2, MIR34B, FDR < 0.05, Fig. 3B, Additional file 2). Four miRNAs were more abundant in the EVs obtained from ULF of the AI group (bta-mir-17, bta-mir-7-3, MIR18A, MIR7-1, FDR < 0.05, Fig. 3C, Additional file 3) relative to those in the IVP-ET group.
Because miRNAs mostly degrade mRNAs, we tested whether the four miRNAs with higher abundance in the ULF EVs from AI group as compared to IVP-ET group could contribute to lower abundance of target mRNAs in conceptus and endometrial tissues collected from the same uteri (GSE232489 [73]). According to target predictions for humans and cattle present in the miRWalk database [79], the mature forms of bta-mir-17, bta-mir-7-3, MIR18A, MIR7-1, could be targeting 116, 397, 453 genes that have lower abundance in extra-embryonic tissues, caruncular and intercaruncular areas of the endometrium, respectively. Although there was no significant enrichment, the biological functions with the greatest number of genes with reduced transcript abundance in extra-embryonic tissues were “signal transduction” (5 genes), “regulation of transcription by RNA polymerase II” (5 genes), and “transmembrane transport” (4 genes) (Additional file 4). In the caruncular areas of the endometrium, most genes were annotated with “regulation of DNA-templated transcription” (32 genes), “signal transduction” (27 genes), and “protein phosphorylation” (25 genes) (Additional file 5). By contrast, the biological process “cilium movement” (7 genes) was significantly enriched (FWER = 0.0787) for intercaruncular areas of the endometrium. The categories with the greatest number of genes were “regulation of transcription by RNA polymerase II” (23 genes), “protein phosphorylation” (18 genes), and “transmembrane transport” (16 genes) (Additional file 6).
We also interrogated the data to understand whether miRNAs present in the ULF of d 18 pregnant heifers would have a co-expression pattern with genes expressed in the conceptus. Our analysis showed that 20 genes (AMMECR1L, APEX1, CNIH1, CREG1, DDX52, EIF1AD, GNA12, MGST1, NPC2, PARP6, POLE3, PSMD14, PURB, TMEM218, TRAPPC4, WBP4, etc.) had transcripts co-expressing with 15 miRNAs in the ULF of heifers pregnant by AI (bta-mir-143, bta-mir-145, bta-mir-155, bta-mir-16b, bta-mir-19b-2, bta-mir-335, bta-mir-429, bta-mir-532, bta-mir-9-2, MIR129-2, MIR140, etc.) (FDR < 0.0004, Fig. 4A, Additional file 7). Notably, the co-expressing pairs of protein-coding genes (n = 36) and miRNAs (n = 27) present in the ULF of heifers harboring a conceptus produced in vitro were different from those identified in pregnancies initiated by AI (genes: AFDN, B4GALT4, C2CD2, DBT, DMPK, EIF4G2, FBL, FGGY, GGA1, IDH3B, IRF2BP2, KCNK1, MINDY1, MINDY3, MTX1, MYO9B, NDUFA8, NDUFS1, NQO2, NUFIP2, PTPRA, RAPGEF1, RASGRP2, RMDN3, RMND1, RPS5, SERPINB1, TPX2, USP20, ZWILCH; miRNAs: bta-let-7a-3, bta-mir-100, bta-mir-106b, bta-mir-139, bta-mir-141, bta-mir-155, bta-mir-184, bta-mir-194-1, bta-mir-194-2, bta-mir-2387, bta-mir-30a, bta-mir-365-1, bta-mir-378-2, bta-mir-499, bta-mir-652, bta-mir-7-3, bta-mir-7859, MIR185, MIR197, MIR200B, MIR378A, MIR99B, FDR < 0.0004, Fig. 4A, Additional file 7). Twelve pairs of miRNAs (7 in the AI group and 5 in the IVP-ET group) and protein-coding genes with inverted correlation were supported by the miRWalk database [79] of miRNA targets (Fig. 4B).
We also identified co-expressing pairs between protein-coding genes expressed in the endometrium and miRNAs present in the ULF. In caruncular areas of the endometrium of pregnancies harboring a conceptus produced by AI, there were 20 genes (B3GNT6, CBY1, CUX1, ENG, FAAP100, GPATCH3, GUCY1A1, MFSD4A, MMP11, NSMF, RIC8A, SYMPK, SYNGR1, TPM1, UBE2O, UBLCP1, ZNF202, etc.) forming co-expression pairs with 16 miRNAs (bta-mir-146a, bta-mir-192, bta-mir-204, bta-mir-2285b-1, bta-mir-29b-1, bta-mir-29b-2, bta-mir-30b, bta-mir-32, bta-mir-3596, bta-mir-454, bta-mir-6119, bta-mir-7861, bta-mir-92a-2, MIR128-1, MIR29A, MIR455) (FDR < 0.0004, Fig. 5A, Additional file 8). By contrast, only 8 genes (CD3E, CNDP2, HADHA, MAP4K1, SLC5A11, STX5, TMEM171, etc.) formed co-expression with 6 miRNAs (bta-let-7f-2, bta-mir-196a-1, bta-mir-204, bta-mir-324, bta-mir-500, MIR494) in pregnancies initiated by the transfer of an in vitro-produced embryo (FDR < 0.0004, Fig. 5A, Additional file 8). Only six pairs of miRNAs and protein-coding genes with inverted correlation in pregnancies initiated by AI were supported by the miRWalk database [79] of miRNA targets (Fig. 5B).
In intercaruncular areas of the endometrium, there were 16 genes (A2M, ACE, ADPRH, BOLA-DQA5, C25H16orf71, C9H6orf118, CLIC5, CXCL16, FAP, FNDC5, HENMT1, HGH1, NDUFB2, PGAM5, PPIC, TSPAN17) forming co-expression pairs with 18 miRNAs (bta-mir-10174, bta-mir-1307, bta-mir-133a-1, bta-mir-144, bta-mir-187, bta-mir-190a, bta-mir-196a-1, bta-mir-221, bta-mir-2285bc, bta-mir-28, bta-mir-30f, bta-mir-490, bta-mir-7857-1, MIRLET7C, etc.) (FDR < 0.0004, Fig. 6A, Additional file 9). By contrast, only 17 genes (ABCD1, ASGR2, BPGM, CHCHD3, ECPAS, FARSB, GAS8, ILDR1, POLR2M, SF3B2, SLC66A1, SPINDOC, STX18, STX1A, TNMD, etc.) formed co-expression with 17 miRNAs (bta-mir-196a-1, bta-mir-2285bc, bta-mir-2387, bta-mir-2887-1, bta-mir-302a, bta-mir-320a-1, bta-mir-429, bta-mir-484, bta-mir-505, bta-mir-95, MIR183, MIR18A, MIR197, MIR200B, MIR491, etc.) in pregnancies initiated by the transfer of an in vitro-produced embryo (FDR < 0.0004, Fig. 6A, Additional file 9). We identified six pairs of miRNAs and protein-coding genes with inverted correlation supported by the database of miRNA targets (Fig. 6B).
Discussion
The ULF contains EVs during the cycle and early pregnancy in all studied mammals including cattle [27, 81,82,83,84]. The ULF EVs contain small RNAs, and their contents change throughout pregnancy [42,43,44,45]. Trophoblast and epithelial cells in the endometrium can uptake these EVs and their content [42, 43, 45, 46], supporting the idea that small RNAs present in the ULF have a role in pregnancy establishment. The particle size analysis confirmed that the purification with polyethylene glycol [54] enriched the pellet with EVs (30–150 nm [85], 30–200 nm [86]), while avoiding apoptotic bodies or cells. The protein ALIX has been identified in EVs isolated from conceptuses [37, 41], but this result does not eliminate that those EVs could also have come from the endometrium. Notably, however, is that ALIX may have a role into enriching miRNAs into EVs during their biogenesis [87]. Collectively, most, if not all, of miRNAs reported in this study were present in EVs originated from the conceptus and endometrium.
Our study has shortcomings. First our experimental design did not allow us to identify the origin of the EVs, hence the discussion addresses miRNAs present in EVs in the ULF without attempting to sort out their origin. Second, we did not measure progesterone nor IFNT, which are important elements in the establishment of pregnancy. Third, we did not carry out specific mechanistic studies to evaluate the impact of disturbing miRNAs on conceptuses or endometrium nor corresponding cell lines. Such shortcomings hinder the establishment of causation [88] and origin of the EVs. However, the careful hypothesis-driven data analysis of a rich and unique dataset overlapped with a reputable database that pairs miRNA and their targets provided important biological insights into the importance of miRNA cargo in EVs present in the ULF.
Our comprehensive analysis of miRNAs in the ULF of cyclic and pregnant heifers on gestation d 18 confirms that miRNA profiles in the ULF change in consequence of pregnancy. Four miRNAs were downregulated in the ULF on gestation d 18, and there is a compelling body of evidence supporting that the downregulation of these miRNAs is necessary for appropriate attachment of the conceptus to the endometrium. In rats, the abundance of mir-320 was lower on gestation d 5 in the endometrium [89]. The hsa-miR-320a is two-fold more abundant in the ULF of women with recurrent implantation failure compared to healthy fertile women [90]. The upregulation of mir-320a inhibits the growth and invasion of human extravillous trophoblast cell line HTR-8/SVneo by targeting interleukin 4 [91]. Also in humans, the upregulation of miR-34b in the endometrium is associated recurrent implantation failure [92]. In vitro experiments indicate that miR-34b inhibits cell proliferation by targeting Wnt/β-catenin [93] or Notch 1 [94] signaling pathways. In mice, the entire mir-181 family members, including mir-181b, which targets Leukemia Inhibitory Factor mRNA, were downregulated in the uterus on d 4 of pregnancy [95]. Taken together, the lower abundance of the miRNAs bta-mir-181b-2, bta-mir-320a-1, bta-mir-320a-2, and miR-34B in the uterine lumen creates an environment that is permissive to trophoblast proliferation and differentiation, which are essential for conceptus development during attachment.
Twenty miRNAs had greater transcript abundance in the ULF of a pregnant uterus relative to the non-pregnant counterparts. The bta-mir-302a and bta-mir-302b were among the topmost significant miRNAs, with greater fold change in pregnant versus not-pregnant uterus. mir-302a-3p was detected in porcine trophoblast but not in the endometrium [96], thus it is possible that the trophectoderm is the source of this miRNA in the ULF. miR-302 can induce and maintain pluripotency in trophoblast cells [97], and its expression is reduced upon pharmacological induction of differentiation of trophoblast cells [98]. Also of note, miR-15b is one of the three miRNAs that can stimulate the differentiation of mouse embryonic stem cells into trophoblast-like cells and sustain self-renewal properties [97]. In humans, miR-15b-5p stimulates trophoblast cell growth and migration [99]. The miRNA miR-503 is also highly expressed in mouse-differentiated trophoblast cells [100]. Collectively, these miRNAs with greater abundance in the ULF on gestation d 18 of pregnancy may have a role in maintaining the balance of stemness and differentiation of the trophoblast cells.
Four miRNAs were upregulated in the ULF of the pregnant uterus and were also down-regulated in the ULF when conceptuses were produced in vitro (mir-7-1, mir-7-3, mir-17, mir-18a). Interestingly, mir-7 downregulates the TGF-β-SMAD family member 2 pathway [101], promoting proliferating extravillous trophoblast cells [102, 103]. mir-17 inhibits trophoblast differentiation by regulating hGCM1 and hCYP19A1 [104]. miR-18a was detected in both placental trophoblasts and endothelial cells [105,106,107] and promotes trophoblast cell differentiation [30] and invasion [105, 108, 109]. Collectively, these data would suggest that mir-7-1, mir-7-3, mir-17, mir-18a have an important role in the balance between proliferation and differentiation of trophoblast cells. It is important to note, that the lower abundance of mir-7-1, mir-7-3, mir-17, mir-18a in the ULF of pregnancies initiated by the transfer of an in vitro produced conceptus may be impactful to the differentiation of binucleate cells, which are less abundant in the chorion of some pregnancies harboring an in vitro produced conceptus [110].
Most miRNAs execute their roles by promoting mRNA degradation and/or inhibiting translation [111, 112]. To that end, we analyzed our dataset in conjunction with mRNA transcriptome obtained from the same reproductive tracts [73] to investigate co-expression between miRNA and mRNAs. The inverted co-expression, inferred from our data, of predicted miRNA—mRNA target pairs, from mirWalk database, support the hypothesis that miRNAs present in the ULF regulate transcript abundance of protein-coding genes in the conceptus and endometrium during the early stages of pregnancy. This could be explained by exchange of miRNAs between cells [29] where miRNAs can be enclosed into EVs [113], exported to the ULF and be up taken by either the conceptus or endometrium, thus a potential mechanism of signaling between conceptus and endometrium. While we could not determine the origin of the miRNA, co-expression analysis provided clues about the tissue where they are exerting their action.
The results also showed that the co-expression between miRNAs in the ULF and mRNAs (EET and endometrium) were remarkably different based on whether the conceptus was produced in vitro or generated by AI. Because the origin of the miRNAs was not determined in our experiments, we cannot determine whether those differences were caused by the conceptus or by the endometrial differential biosensing [25, 114, 115] of the conceptus’ origin. However, this differential co-expression adds another layer of complexity to the myriad of individualized [21] molecular interaction between conceptus and endometrium at attachment.
Notably, mir-143 showed co-expression with multiple target genes in EET obtained from pregnancies initiated by AI. In pigs, mir-143-3p is present in the luminal fluid and is taken by trophoblast cells promoting cell proliferation and migration [116]. Although there was no co-expression between mir-143 and genes expressed in the endometrium in our analysis, mir-143 may also have a role in endometrial cells. In mice, mir-143 is highly expressed in the subluminal stroma at implantation sites [117]. In rats, endometrial cells express mir-143 on gestation d 5–8, and experiments carried out in human endometrial stromal cells showed that mir-143 inhibits cell proliferation, migration, and invasion [118]. In women, mir-143 expression in the endometrial epithelium is induced by progesterone, and mir-143 inhibits the proliferation of endometrial cancer cells [119]. Among the co-expression pattern inferred in the EET of pregnancies with an in vitro produced conceptus, miR-141 is also expressed in trophoblast cells, and can regulate trophoblastic cell viability and proliferation [120].
One interesting observation about the co-expression between miRNA and mRNAs in the endometrium is that there were less pairs of co-expressing genes relative to the EET. Second, the co-expressing pairs detected in caruncular and inter-caruncular areas of the endometrium were different, which is not a surprising finding because these areas have genes with differential transcript abundance [25], reflecting their anatomical and physiological differences [10, 23, 121, 122]. Notably, mir-30b had a co-expression pattern with two genes (B3GNT6, GUCY1A1). In women, mir-30b [123, 124] and mir-30d [123] (14th most abundant miRNA in the ULF) may participate in the regulation of endometrial receptivity, although the functional mechanisms remain unknown. The miRNA mir-28, which formed a co-expression pattern with PPIC in inter-caruncular areas of the endometrium, had greater transcript abundance in implantation sites relative to inter-implantation sites in mice on d 5 of pregnancy [125]. Also, in the inter-caruncular area of the endometrium, MIR18A showed a co-expression with ABCD1, revealing a potential role of this miRNA in the endometrium. It is also notable that there was a greater transcript abundance of this miRNA in the ULF of pregnancies initiated by AI relative to non-pregnant or pregnancies initiated by the transfer of an in vitro cultured embryo.
The results of co-expression between miRNAs in the ULF and mRNAs of target genes is a strong indication of the participation of those miRNAs in the ULF in the regulation of transcript abundance in EET and endometrium. However, it is important to highlight that our experiment did not determine the impact of that regulation on the conceptus, endometrium, or pregnancy health. For instance, the excessive abundance of mir-143 [90] and mir-145 [126, 127] have been associated with pregnancy failure in women and mice. A high abundance of mir-29b has been associated with preeclampsia in women [128], and the ULF of women with recurrent implantation failure had 2.7-fold more transcripts of mir-491 relative to the ULF of healthy fertile women [90].
Conclusions
In summary, the results of this study support the idea that EVs present in the ULF of cows on d 18 of gestation contain a myriad of miRNAs. The alteration in the abundance of specific miRNAs in pregnant uterus versus non-pregnant ones indicates a role of specific miRNAs in the regulation of trophoblast health, endometrial remodeling, and the establishment of pregnancy. The presence of a conceptus produced in vitro was associated with the failure to increase the abundance of 4 miRNAs and suggests another layer of complexity that contributes to the lower success of pregnancy establishment of in vitro-produced embryos.
Availability of data and materials
The datasets supporting the conclusions of this article are available in the Gene Omnibus Repository under the identifier GSE232489.
Abbreviations
- AI:
-
Artificial insemination
- bta:
-
Bos taurus
- CIDR:
-
Controlled intravaginal drug release
- CPM:
-
Counts per million
- EET:
-
Extra-embryonic tissue
- eFDR:
-
Empirical false discovery rate
- ET:
-
Embryo transfer
- EV:
-
Extracellular vesicles
- FDR:
-
False discovery rate
- FWER:
-
Family wise error rate
- HEPES:
-
4-(2-Hydroxyethyl)-1-piperazine ethanesulfonic acid
- IFNT:
-
Interferon tau
- IVF:
-
In vitro fertilization
- IVP:
-
In vitro produced
- IU:
-
International units
- LE:
-
Luminal epithelium
- miRNA:
-
MicroRNA
- mRNA:
-
Messenger ribonucleic acid
- n :
-
Sample size
- NCP:
-
No conceptus present
- P :
-
Probability
- sCMOS:
-
Scientific Complementary Metal–Oxide–Semiconductor
- TALP:
-
Tyrode’s albumin lactate pyruvate
- ULF:
-
Uterine lumen fluid
References
Wooding P, Burton G. Comparative placentation: structures, functions and evolution. Heidelberg: Springer Berlin; 2008. p. 301.
Carson DD, Bagchi I, Dey SK, Enders AC, Fazleabas AT, Lessey BA, et al. Embryo implantation. Dev Biol. 2000;223(2):217–37. https://doi.org/10.1006/dbio.2000.9767.
Hernandez-Ledezma JJ, Sikes JD, Murphy CN, Watson AJ, Schultz GA, Roberts RM. Expression of bovine trophoblast interferon in conceptuses derived by in vitro techniques. Biol Reprod. 1992;47(3):374–80. https://doi.org/10.1095/biolreprod47.3.374.
Bazer FW. Pregnancy recognition signaling mechanisms in ruminants and pigs. J Anim Sci Biotechnol. 2013;4:23. https://doi.org/10.1186/2049-1891-4-23.
Hansen TR, Sinedino LDP, Spencer TE. Paracrine and endocrine actions of interferon tau (IFNT). Reproduction. 2017;154(5):F45–59. https://doi.org/10.1530/REP-17-0315.
Betteridge KJ, Flechon JE. The anatomy and physiology of pre-attachment bovine embryos. Theriogenology. 1988;29(1):155–87. https://doi.org/10.1016/0093-691X(88)90038-6.
Robinson RS, Fray MD, Wathes DC, Lamming GE, Mann GE. In vivo expression of interferon tau mRNA by the embryonic trophoblast and uterine concentrations of interferon tau protein during early pregnancy in the cow. Mol Reprod Dev. 2006;73:470–4. https://doi.org/10.1002/mrd.20431.
Hue I, Degrelle SA, Turenne N. Conceptus elongation in cattle: genes, models and questions. Anim Reprod Sci. 2012;134(1–2):19–28. https://doi.org/10.1016/j.anireprosci.2012.08.007.
Spencer TE, Hansen TR. Implantation and establishment of pregnancy in ruminants. Adv Anat Embryol Cell Biol. 2015;216:105–35. https://doi.org/10.1007/978-3-319-15856-3_7.
King GJ, Atkinson BA, Robertson HA. Development of the intercaruncular areas during early gestation and establishment of the bovine placenta. J Reprod Fertil. 1981;61:469–74.
Wooding FBP. The ruminant placental trophoblast binucleate cell: an evolutionary breakthrough. Biol Reprod. 2022;107(3):705–16. https://doi.org/10.1093/biolre/ioac107.
Wooding FB. Current topic: the synepitheliochorial placenta of ruminants: binucleate cell fusions and hormone production. Placenta. 1992;13:101–13.
Chavatte-Palmer P, Guillomot M. Comparative implantation and placentation. Gynecol Obstet Invest. 2007;64:166–74. https://doi.org/10.1159/000101742.
Lee KY, DeMayo FJ. Animal models of implantation. Reproduction. 2004;128:679–95. https://doi.org/10.1530/rep.1.00340.
Wydooghe E, Vandaele L, Heras S, De Sutter P, Deforce D, Peelman L, et al. Autocrine embryotropins revisited: how do embryos communicate with each other in vitro when cultured in groups? Biol Rev Camb Philos Soc. 2017;92(1):505–20. https://doi.org/10.1111/brv.12241.
Sponchiado M, Gomes NS, Fontes PK, Martins T, Del Collado M, Pastore AA, et al. Pre-hatching embryo-dependent and -independent programming of endometrial function in cattle. PLoS One. 2017;12(4):e0175954. https://doi.org/10.1371/journal.pone.0175954.
Sponchiado M, Gonella-Diaza AM, Rocha CC, Turco EGL, Pugliesi G, Leroy J, et al. The pre-hatching bovine embryo transforms the uterine luminal metabolite composition in vivo. Sci Rep. 2019;9:8354. https://doi.org/10.1038/s41598-019-44590-9.
Forde N, Carter F, Spencer TE, Bazer FW, Sandra O, Mansouri-Attia N, et al. Conceptus-induced changes in the endometrial transcriptome: how soon does the cow know she is pregnant? Biol Reprod. 2011;85:144–56. https://doi.org/10.1095/biolreprod.110.090019.
Bauersachs S, Ulbrich SE, Reichenbach HD, Reichenbach M, Büttner M, Meyer HHD, et al. Comparison of the effects of early pregnancy with human interferon, alpha 2 (IFNA2), on gene expression in bovine endometrium. Biol Reprod. 2012;86(2):46. https://doi.org/10.1095/biolreprod.111.094771.
Mamo S, Mehta JP, Forde N, McGettigan P, Lonergan P. Conceptus-endometrium crosstalk during maternal recognition of pregnancy in cattle. Biol Reprod. 2012;87(1):61–9. https://doi.org/10.1095/biolreprod.112.099945.
Biase FH, Hue I, Dickinson SE, Jaffrezic F, Laloe D, Lewin HA, et al. Fine-tuned adaptation of embryo-endometrium pairs at implantation revealed by transcriptome analyses in Bos taurus. PLoS Biol. 2019;17(4):e3000046. https://doi.org/10.1371/journal.pbio.3000046.
Aitken CE, Marshall RA, Puglisi JD. An oxygen scavenging system for improvement of dye stability in single-molecule fluorescence experiments. Biophys J. 2008;94:1826–35.
King GJ, Atkinson BA, Robertson HA. Development of the bovine placentome from days 20 to 29 of gestation. J Reprod Fertil. 1980;59(1):95–100.
Moraes JGN, Behura SK, Geary TW, Hansen PJ, Neibergs HL, Spencer TE. Uterine influences on conceptus development in fertility-classified animals. P Natl Acad Sci USA. 2018;115(8):E1749–58. https://doi.org/10.1073/pnas.1721191115.
Biase FH, Rabel C, Guillomot M, Hue I, Andropolis K, Olmstead CA, et al. Massive dysregulation of genes involved in cell signaling and placental development in cloned cattle conceptus and maternal endometrium. P Natl Acad Sci USA. 2016;113(51):14492–501. https://doi.org/10.1073/pnas.1520945114.
Jose AM. Movement of regulatory RNA between animal cells. Genesis. 2015;53:395–416. https://doi.org/10.1002/dvg.22871.
Guzewska MM, Szuszkiewicz J, Kaczmarek MM. Extracellular vesicles: focus on peri-implantation period of pregnancy in pigs. Mol Reprod Dev. 2023;90(7):634–45. https://doi.org/10.1002/mrd.23664.
Lo Cicero A, Stahl PD, Raposo G. Extracellular vesicles shuffling intercellular messages: for good or for bad. Curr Opin Cell Biol. 2015;35:69–77. https://doi.org/10.1016/j.ceb.2015.04.013.
Valadi H, Ekström K, Bossios A, Sjöstrand M, Lee JJ, Lötvall JO. Exosome-mediated transfer of mRNAs and microRNAs is a novel mechanism of genetic exchange between cells. Nat Cell Biol. 2007;9:654–9. https://doi.org/10.1038/ncb1596.
Liang L, Chen Y, Wu C, Cao Z, Xia L, Meng J, et al. MicroRNAs: key regulators of the trophoblast function in pregnancy disorders. J Assist Reprod Genet. 2023;40(1):3–17. https://doi.org/10.1007/s10815-022-02677-9.
Hume L, Edge JC, Tinning H, Wang D, Taylor AS, Ovchinnikov V, et al. MicroRNAs emerging coordinate with placental mammals alter pathways in endometrial epithelia important for endometrial function. iScience. 2023;26(4):106339. https://doi.org/10.1016/j.isci.2023.106339.
Goharitaban S, Abedelahi A, Hamdi K, Khazaei M, Esmaeilivand M, Niknafs B. Role of endometrial microRNAs in repeated implantation failure (mini-review). Front Cell Dev Biol. 2022;10:936173. https://doi.org/10.3389/fcell.2022.936173.
Omeljaniuk WJ, Laudanski P, Miltyk W. The role of miRNA molecules in the miscarriage process. Biol Reprod. 2023;109(1):29–44. https://doi.org/10.1093/biolre/ioad047.
Melo-Baez B, Wong YS, Aguilera CJ, Cabezas J, Mancanares ACF, Riadi G, et al. MicroRNAs from extracellular vesicles secreted by bovine embryos as early biomarkers of developmental competence. Int J Mol Sci. 2020;21(23):8888. https://doi.org/10.3390/ijms21238888.
Pavani KC, Meese T, Pascottini OB, Guan X, Lin X, Peelman L, et al. Hatching is modulated by microRNA-378a-3p derived from extracellular vesicles secreted by blastocysts. P Natl Acad Sci USA. 2022;119(12):e2122708119. https://doi.org/10.1073/pnas.2122708119.
Mellisho EA, Briones MA, Velasquez AE, Cabezas J, Castro FO, Rodriguez-Alvarez L. Extracellular vesicles secreted during blastulation show viability of bovine embryos. Reproduction. 2019;158(6):477–92. https://doi.org/10.1530/Rep-19-0233.
De Bem THC, Bridi A, Tinning H, Sampaio RV, Malo-Estepa I, Wang D, et al. Biosensor capability of the endometrium is mediated in part, by altered miRNA cargo from conceptus-derived extracellular vesicles. FASEB J. 2024;38(10):e23639. https://doi.org/10.1096/fj.202302423RR.
Koh YQ, Peiris HN, Vaswani K, Reed S, Rice GE, Salomon C, et al. Characterization of exosomal release in bovine endometrial intercaruncular stromal cells. Reprod Biol Endocrinol. 2016;14(1):78. https://doi.org/10.1186/s12958-016-0207-4.
Mazzarella R, Canon-Beltran K, Cajas YN, Hamdi M, Gonzalez EM, da Silveira JC, et al. Extracellular vesicles-coupled miRNAs from oviduct and uterus modulate signaling pathways related to lipid metabolism and bovine early embryo development. J Anim Sci Biotechnol. 2024;15:51. https://doi.org/10.1186/s40104-024-01008-5.
Zhai Y, Shi Q, Chu Q, Chen F, Feng Y, Zhang Z, et al. miRNA profiling in intrauterine exosomes of pregnant cattle on day 7. Front Vet Sci. 2022;9:1078394. https://doi.org/10.3389/fvets.2022.1078394.
Bridi A, Andrade GM, Del Collado M, Sangalli JR, de Avila A, Motta IG, et al. Small extracellular vesicles derived from in vivo- or in vitro-produced bovine blastocysts have different miRNAs profiles-implications for embryo-maternal recognition. Mol Reprod Dev. 2021;88(9):628–43. https://doi.org/10.1002/mrd.23527.
Nakamura K, Kusama K, Hori M, Imakawa K. The effect of bta-miR-26b in intrauterine extracellular vesicles on maternal immune system during the implantation period. Biochem Biophys Res Commun. 2021;573:100–6. https://doi.org/10.1016/j.bbrc.2021.08.019.
Nakamura K, Kusama K, Ideta A, Kimura K, Hori M, Imakawa K. Effects of miR-98 in intrauterine extracellular vesicles on maternal immune regulation during the peri-implantation period in cattle. Sci Rep. 2019;9:20330. https://doi.org/10.1038/s41598-019-56879-w.
Kusama K, Nakamura K, Bai R, Nagaoka K, Sakurai T, Imakawa K. Intrauterine exosomes are required for bovine conceptus implantation. Biochem Biophys Res Commun. 2018;495(1):1370–5. https://doi.org/10.1016/j.bbrc.2017.11.176.
Nakamura K, Kusama K, Bai R, Sakurai T, Isuzugawa K, Godkin JD, et al. Induction of IFNT-stimulated genes by conceptus-derived exosomes during the attachment period. PLoS One. 2016;11(6):e0158278. https://doi.org/10.1371/journal.pone.0158278.
Wang X, Li Q, Xie T, Yuan M, Sheng X, Qi X, et al. Exosomes from bovine endometrial epithelial cells ensure trophoblast cell development by miR-218 targeting secreted frizzled related protein 2. J Cell Physiol. 2021;236(6):4565–79. https://doi.org/10.1002/jcp.30180.
Ortega MS, Wohlgemuth S, Tribulo P, Siqueira LG, Cole JB, Hansen PJ. A single nucleotide polymorphism in COQ9 affects mitochondrial and ovarian function and fertility in Holstein cows. Biol Reprod. 2017;96(3):652–63. https://doi.org/10.1093/biolre/iox004.
Ortega MS, Moraes JGN, Patterson DJ, Smith MF, Behura SK, Poock S, et al. Influences of sire conception rate on pregnancy establishment in dairy cattle. Biol Reprod. 2018;99(6):1244–54. https://doi.org/10.1093/biolre/ioy141.
Tribulo P, Rivera RM, Ortega Obando MS, Jannaman EA, Hansen PJ. Production and culture of the bovine embryo. Methods Mol Biol. 2019;2006:115–29. https://doi.org/10.1007/978-1-4939-9566-0_8.
Bó G, Mapletoft R. Evaluation and classification of bovine embryos. Anim Reprod. 2018;10(3):344–8.
Gomez E, Carrocera S, Martin D, Perez-Janez JJ, Prendes J, Prendes JM, et al. Efficient one-step direct transfer to recipients of thawed bovine embryos cultured in vitro and frozen in chemically defined medium. Theriogenology. 2020;146:39–47. https://doi.org/10.1016/j.theriogenology.2020.01.056.
Thomas JM, Locke JWC, Bishop BE, Abel JM, Ellersieck MR, Yelich JV, et al. Evaluation of the 14-d CIDR-PG and 9-d CIDR-PG protocols for synchronization of estrus in Bos indicus-influenced and Bos taurus beef heifers. Theriogenology. 2017;92:190–6. https://doi.org/10.1016/j.theriogenology.2017.01.020.
Reith S, Hoy S. Review: behavioral signs of estrus and the potential of fully automated systems for detection of estrus in dairy cattle. Animal. 2018;12(2):398–407. https://doi.org/10.1017/S1751731117001975.
Weng Y, Sui Z, Shan Y, Hu Y, Chen Y, Zhang L, et al. Effective isolation of exosomes with polyethylene glycol from cell culture supernatant for in-depth proteome profiling. Analyst. 2016;141(15):4640–6. https://doi.org/10.1039/c6an00892e.
Welsh JA, Goberdhan DCI, O’Driscoll L, Buzas EI, Blenkiron C, Bussolati B, et al. Minimal information for studies of extracellular vesicles (MISEV2023): from basic to advanced approaches. J Extracell Vesicles. 2024;13(2):e12404. https://doi.org/10.1002/jev2.12404.
Chugh PE, Sin SH, Ozgur S, Henry DH, Menezes P, Griffith J, et al. Systemically circulating viral and tumor-derived microRNAs in KSHV-associated malignancies. PLoS Pathog. 2013;9(7):e1003484. https://doi.org/10.1371/journal.ppat.1003484.
Sohel MMH, Hoelker M, Noferesti SS, Salilew-Wondim D, Tholen E, Looft C, et al. Exosomal and non-exosomal transport of extra-cellular microRNAs in follicular fluid: implications for bovine oocyte developmental competence. PLoS One. 2013;8: e78505. https://doi.org/10.1371/journal.pone.0078505.
Umezu T, Ohyashiki K, Kuroda M, Ohyashiki JH. Leukemia cell to endothelial cell communication via exosomal miRNAs. Oncogene. 2013;32(22):2747–55. https://doi.org/10.1038/onc.2012.295.
Rio DC, Ares M Jr, Hannon GJ, Nilsen TW. Purification of RNA using TRIzol (TRI reagent). Cold Spring Harb Protoc. 2010;2010(6):pdb.prot5439. https://doi.org/10.1101/pdb.prot5439.
Biase FH. Isolation of high-quality total RNA and RNA sequencing of single bovine oocytes. STAR Protoc. 2021;2(4):100895. https://doi.org/10.1016/j.xpro.2021.100895.
Bolger AM, Lohse M, Usadel B. Trimmomatic: a flexible trimmer for illumina sequence data. Bioinformatics. 2014;30(15):2114–20. https://doi.org/10.1093/bioinformatics/btu170.
Langmead B, Salzberg SL. Fast gapped-read alignment with Bowtie 2. Nat Methods. 2012;9:357–9. https://doi.org/10.1038/nmeth.1923.
Li H, Handsaker B, Wysoker A, Fennell T, Ruan J, Homer N, et al. The sequence Alignment/Map format and SAMtools. Bioinformatics. 2009;25(16):2078–9. https://doi.org/10.1093/bioinformatics/btp352.
Liao Y, Smyth GK, Shi W. featureCounts: an efficient general purpose program for assigning sequence reads to genomic features. Bioinformatics. 2014;30(7):923–30. https://doi.org/10.1093/bioinformatics/btt656.
Flicek P, Amode MR, Barrell D, Beal K, Billis K, Brent S, et al. Ensembl 2014. Nucleic Acids Res. 2014;42(DI):D749-55. https://doi.org/10.1093/nar/gkt1196.
Kinsella RJ, Kähäri A, Haider S, Zamora J, Proctor G, Spudich G, et al. Ensembl BioMarts: a hub for data retrieval across taxonomic space. Database. 2011;2011:bar030. https://doi.org/10.1093/database/bar030.
McCarthy DJ, Chen Y, Smyth GK. Differential expression analysis of multifactor RNA-Seq experiments with respect to biological variation. Nucleic Acids Res. 2012;40:4288–97. https://doi.org/10.1093/nar/gks042.
McCarthy DJ, Smyth GK. edgeR: a Bioconductor package for differential expression analysis of digital gene expression data. Bioinformatics. 2010;26:139–40. https://doi.org/10.1093/bioinformatics/btp616.
Love MI, Huber W, Anders S. Moderated estimation of fold change and dispersion for RNA-seq data with DESeq2. Genome Biol. 2014;15:550. https://doi.org/10.1186/PREACCEPT-8897612761307401.
Benjamini Y, Hochberg Y. Controlling the false discovery rate - a practical and powerful approach to multiple testing. J Roy Stat Soc B Met. 1995;57(1):289–300.
Young MD, Wakefield MJ, Smyth GK, Oshlack A. Gene ontology analysis for RNA-seq: accounting for selection bias. Genome Biol. 2010;11(2):R14. https://doi.org/10.1186/gb-2010-11-2-r14.
Holm S. A simple sequentially rejective multiple test procedure. Scand Stat Theory Appl. 1979;6(2):65–70.
Biase FH, Moorey SE, Schnuelle JG, Rodning S, Ortega MS, Spencer TE. Extensive rewiring of the gene regulatory interactions between in vitro-produced conceptuses and endometrium during attachment. PNAS Nexus. 2023;2(9):pgad284. https://doi.org/10.1093/pnasnexus/pgad284.
Horvath S. Correlation and gene co-expression networks. In: Weighted network analysis: applications in genomics and systems biology. New York: Springer; 2011. p. 91–121. https://doi.org/10.1007/978-1-4419-8819-5_5.
Johnson KA, Krishnan A. Robust normalization and transformation techniques for constructing gene coexpression networks from RNA-seq data. Genome Biol. 2022;23:1. https://doi.org/10.1186/s13059-021-02568-9.
Robinson MD, Oshlack A. A scaling normalization method for differential expression analysis of RNA-seq data. Genome Biol. 2010;11:R25. https://doi.org/10.1186/gb-2010-11-3-r25.
Langfelder P, Horvath S. WGCNA: an R package for weighted correlation network analysis. BMC Bioinformatics. 2008;9:559. https://doi.org/10.1186/1471-2105-9-559.
Storey JD, Tibshirani R. Statistical significance for genomewide studies. P Natl Acad Sci USA. 2003;100(16):9440–5. https://doi.org/10.1073/pnas.1530509100.
Sticht C, De La Torre C, Parveen A, Gretz N. miRWalk: an online resource for prediction of microRNA binding sites. PLoS One. 2018;13(10):e0206239. https://doi.org/10.1371/journal.pone.0206239.
Kestens V, Bozatzidis V, De Temmerman PJ, Ramaye Y, Roebben G. Validation of a particle tracking analysis method for the size determination of nano- and microparticles. J Nanopart Res. 2017;19(8):271. https://doi.org/10.1007/s11051-017-3966-8.
O’Neil EV, Burns GW, Ferreira CR, Spencer TE. Characterization and regulation of extracellular vesicles in the lumen of the ovine uterus. Biol Reprod. 2020;102(5):1020–32. https://doi.org/10.1093/biolre/ioaa019.
Burns GW, Brooks KE, Spencer TE. Extracellular vesicles originate from the conceptus and uterus during early pregnancy in sheep. Biol Reprod. 2016;94(3):56. https://doi.org/10.1095/biolreprod.115.134973.
Burns G, Brooks K, Wildung M, Navakanitworakul R, Christenson LK, Spencer TE. Extracellular vesicles in luminal fluid of the ovine uterus. PLoS One. 2014;9(3):e90913. https://doi.org/10.1371/journal.pone.0090913.
Hu Q, Zang X, Ding Y, Gu T, Shi J, Li Z, et al. Porcine uterine luminal fluid-derived extracellular vesicles improve conceptus-endometrial interaction during implantation. Theriogenology. 2022;178:8–17. https://doi.org/10.1016/j.theriogenology.2021.10.021.
Sidhom K, Obi PO, Saleem A. A review of exosomal isolation methods: is size exclusion chromatography the best option? Int J Mol Sci. 2020;21(18):6466. https://doi.org/10.3390/ijms21186466.
Gurung S, Perocheau D, Touramanidou L, Baruteau J. The exosome journey: from biogenesis to uptake and intracellular signalling. Cell Commun Signal. 2021;19(1):47. https://doi.org/10.1186/s12964-021-00730-1.
Iavello A, Frech VS, Gai C, Deregibus MC, Quesenberry PJ, Camussi G. Role of Alix in miRNA packaging during extracellular vesicle biogenesis. Int J Mol Med. 2016;37(4):958–66. https://doi.org/10.3892/ijmm.2016.2488.
Casadevall A, Fang FC. Mechanistic science. Infect Immun. 2009;77(9):3517–9. https://doi.org/10.1128/IAI.00623-09.
Xia HF, Jin XH, Song PP, Cui Y, Liu CM, Ma X. Temporal and spatial regulation of miR-320 in the uterus during embryo implantation in the rat. Int J Mol Sci. 2010;11(2):719–30. https://doi.org/10.3390/ijms11020719.
von Grothusen C, Frisendahl C, Modhukur V, Lalitkumar PG, Peters M, Faridani OR, et al. Uterine fluid microRNAs are dysregulated in women with recurrent implantation failure. Hum Reprod. 2022;37(4):734–46. https://doi.org/10.1093/humrep/deac019.
Xie N, Jia Z, Li L. miR-320a upregulation contributes to the development of preeclampsia by inhibiting the growth and invasion of trophoblast cells by targeting interleukin 4. Mol Med Rep. 2019;20(4):3256–64. https://doi.org/10.3892/mmr.2019.10574.
Choi Y, Kim HR, Lim EJ, Park M, Yoon JA, Kim YS, et al. Integrative analyses of uterine transcriptome and MicroRNAome reveal compromised LIF-STAT3 signaling and progesterone response in the endometrium of patients with recurrent/repeated implantation failure (RIF). PLoS One. 2016;11(6):e0157696. https://doi.org/10.1371/journal.pone.0157696.
Ye K, Xu C, Hui T. MiR-34b inhibits the proliferation and promotes apoptosis in colon cancer cells by targeting Wnt/beta-catenin signaling pathway. Biosci Rep. 2019;39(10):BSR-2019-1799_RET. https://doi.org/10.1042/BSR20191799.
Sun D, Wu Y, Zhang S, Han Y, Shen J, Zheng W, et al. Distinct roles of miR-34 family members on suppression of lung squamous cell carcinoma. Biomed Pharmacother. 2021;142:111967. https://doi.org/10.1016/j.biopha.2021.111967.
Chu B, Zhong L, Dou S, Wang J, Li J, Wang M, et al. miRNA-181 regulates embryo implantation in mice through targeting leukemia inhibitory factor. J Mol Cell Biol. 2015;7(1):12–22. https://doi.org/10.1093/jmcb/mjv006.
Reliszko ZP, Gajewski Z, Kaczmarek MM. Signs of embryo-maternal communication: miRNAs in the maternal serum of pregnant pigs. Reproduction. 2017;154(3):217–28. https://doi.org/10.1530/REP-17-0224.
Nosi U, Lanner F, Huang T, Cox B. Overexpression of trophoblast stem cell-enriched MicroRNAs promotes trophoblast fate in embryonic stem cells. Cell Rep. 2017;19(6):1101–9. https://doi.org/10.1016/j.celrep.2017.04.040.
Viswanathan SR, Mermel CH, Lu J, Lu CW, Golub TR, Daley GQ. microRNA expression during trophectoderm specification. PLoS One. 2009;4(7):e6143. https://doi.org/10.1371/journal.pone.0006143.
Xu Y, Wu D, Liu J, Huang S, Zuo Q, Xia X, et al. Downregulated lncRNA HOXA11-AS affects trophoblast cell proliferation and migration by regulating RND3 and HOXA7 expression in PE. Mol Ther Nucleic Acids. 2018;12:195–206. https://doi.org/10.1016/j.omtn.2018.05.007.
Saha S, Ain R. MicroRNA regulation of murine trophoblast stem cell self-renewal and differentiation. Life Sci Alliance. 2020;3(11):e202000674. https://doi.org/10.26508/lsa.202000674.
Shih JC, Lin HH, Hsiao AC, Su YT, Tsai S, Chien CL, et al. Unveiling the role of microRNA-7 in linking TGF-beta-Smad-mediated epithelial-mesenchymal transition with negative regulation of trophoblast invasion. FASEB J. 2019;33(5):6281–95. https://doi.org/10.1096/fj.201801898RR.
Xu J, Sivasubramaniyam T, Yinon Y, Tagliaferro A, Ray J, Nevo O, et al. Aberrant TGFbeta signaling contributes to altered trophoblast differentiation in preeclampsia. Endocrinology. 2016;157(2):883–99. https://doi.org/10.1210/en.2015-1696.
Haider S, Lackner AI, Dietrich B, Kunihs V, Haslinger P, Meinhardt G, et al. Transforming growth factor-beta signaling governs the differentiation program of extravillous trophoblasts in the developing human placenta. P Natl Acad Sci USA. 2022;119(28):e2120667119. https://doi.org/10.1073/pnas.2120667119.
Kumar P, Luo Y, Tudela C, Alexander JM, Mendelson CR. The c-Myc-regulated microRNA-17~92 (miR-17~92) and miR-106a~363 clusters target hCYP19A1 and hGCM1 to inhibit human trophoblast differentiation. Mol Cell Biol. 2013;33(9):1782–96. https://doi.org/10.1128/MCB.01228-12.
Xu P, Zhao Y, Liu M, Wang Y, Wang H, Li YX, et al. Variations of microRNAs in human placentas and plasma from preeclamptic pregnancy. Hypertension. 2014;63(6):1276–84. https://doi.org/10.1161/HYPERTENSIONAHA.113.02647.
Bidarimath M, Edwards AK, Wessels JM, Khalaj K, Kridli RT, Tayade C. Distinct microRNA expression in endometrial lymphocytes, endometrium, and trophoblast during spontaneous porcine fetal loss. J Reprod Immunol. 2015;107:64–79. https://doi.org/10.1016/j.jri.2014.11.004.
Su L, Liu R, Cheng W, Zhu M, Li X, Zhao S, et al. Expression patterns of microRNAs in porcine endometrium and their potential roles in embryo implantation and placentation. PLoS One. 2014;9(2):e87867. https://doi.org/10.1371/journal.pone.0087867.
Xu P, Li Z, Wang Y, Yu X, Shao X, Li YX, et al. miR-18a contributes to preeclampsia by downregulating Smad2 (full length) and reducing TGF-beta signaling. Mol Ther Nucleic Acids. 2020;22:542–56. https://doi.org/10.1016/j.omtn.2020.09.019.
Zhu X, Yang Y, Han T, Yin G, Gao P, Ni Y, et al. Suppression of microRNA-18a expression inhibits invasion and promotes apoptosis of human trophoblast cells by targeting the estrogen receptor alpha gene. Mol Med Rep. 2015;12(2):2701–6. https://doi.org/10.3892/mmr.2015.3724.
Miles JR, Farin CE, Rodriguez KF, Alexander JE, Farin PW. Effects of embryo culture on angiogenesis and morphometry of bovine placentas during early gestation. Biol Reprod. 2005;73(4):663–71. https://doi.org/10.1095/biolreprod.105.040808.
Bushati N, Cohen SM. microRNA functions. Annu Rev Cell Dev Biol. 2007;23:175–205. https://doi.org/10.1146/annurev.cellbio.23.090506.123406.
Huang Y, Shen XJ, Zou Q, Wang SP, Tang SM, Zhang GZ. Biological functions of microRNAs: a review. J Physiol Biochem. 2011;67(1):129–39. https://doi.org/10.1007/s13105-010-0050-6.
Lee YJ, Shin KJ, Chae YC. Regulation of cargo selection in exosome biogenesis and its biomedical applications in cancer. Exp Mol Med. 2024;56(4):877–89. https://doi.org/10.1038/s12276-024-01209-y.
Mansouri-Attia N, Sandra O, Aubert J, Degrelle S, Everts RE, Giraud-Delville C, et al. Endometrium as an early sensor of in vitro embryo manipulation technologies. P Natl Acad Sci USA. 2009;106(14):5687–92. https://doi.org/10.1073/pnas.0812722106.
Bauersachs S, Ulbrich SE, Zakhartchenko V, Minten M, Reichenbach M, Reichenbach HD, et al. The endometrium responds differently to cloned versus fertilized embryos. P Natl Acad Sci USA. 2009;106(14):5681–6. https://doi.org/10.1073/pnas.0811841106.
Ding Y, Hu Q, Gan J, Zang X, Gu T, Wu Z, et al. Effect of miR-143–3p from extracellular vesicles of porcine uterine luminal fluid on porcine trophoblast cells. Animals (Basel). 2022;12(23):3402. https://doi.org/10.3390/ani12233402.
Hu SJ, Ren G, Liu JL, Zhao ZA, Yu YS, Su RW, et al. MicroRNA expression and regulation in mouse uterus during embryo implantation. J Biol Chem. 2008;283(34):23473–84. https://doi.org/10.1074/jbc.M800406200.
Tian S, Su X, Qi L, Jin XH, Hu Y, Wang CL, et al. MiR-143 and rat embryo implantation. Biochim Biophys Acta. 2015;1850(4):708–21. https://doi.org/10.1016/j.bbagen.2014.11.023.
Yuan DZ, Lei Y, Zhao D, Pan JL, Zhao YB, Nie L, et al. Progesterone-induced miR-145/miR-143 inhibits the proliferation of endometrial epithelial cells. Reprod Sci. 2019;26(2):233–43. https://doi.org/10.1177/1933719118768687.
Ospina-Prieto S, Chaiwangyen W, Herrmann J, Groten T, Schleussner E, Markert UR, et al. MicroRNA-141 is upregulated in preeclamptic placentae and regulates trophoblast invasion and intercellular communication. Transl Res. 2016;172:61–72. https://doi.org/10.1016/j.trsl.2016.02.012.
King GJ, Atkinson BA, Robertson HA. Development of the bovine placentome during the second month of gestation. J Reprod Fertil. 1979;55(1):173–80. https://doi.org/10.1530/jrf.0.0550173.
Atkinson BA, King GJ, Amoroso EC. Development of the caruncular and intercaruncular regions in the bovine endometrium. Biol Reprod. 1984;30(3):763–74.
Altmae S, Martinez-Conejero JA, Esteban FJ, Ruiz-Alonso M, Stavreus-Evers A, Horcajadas JA, et al. MicroRNAs miR-30b, miR-30d, and miR-494 regulate human endometrial receptivity. Reprod Sci. 2013;20(3):308–17. https://doi.org/10.1177/1933719112453507.
Li R, Qiao J, Wang L, Li L, Zhen X, Liu P, et al. MicroRNA array and microarray evaluation of endometrial receptivity in patients with high serum progesterone levels on the day of hCG administration. Reprod Biol Endocrinol. 2011;9:29. https://doi.org/10.1186/1477-7827-9-29.
Geng Y, He J, Ding Y, Chen X, Zhou Y, Liu S, et al. The differential expression of microRNAs between implantation sites and interimplantation sites in early pregnancy in mice and their potential functions. Reprod Sci. 2014;21(10):1296–306. https://doi.org/10.1177/1933719114525273.
Liu X, Zhao H, Li W, Bao H, Qu Q, Ma D. Up-regulation of miR-145 may contribute to repeated implantation failure after IVF-embryo transfer by targeting PAI-1. Reprod Biomed Online. 2020;40(5):627–36. https://doi.org/10.1016/j.rbmo.2020.01.018.
Kang YJ, Lees M, Matthews LC, Kimber SJ, Forbes K, Aplin JD. MiR-145 suppresses embryo-epithelial juxtacrine communication at implantation by modulating maternal IGF1R. J Cell Sci. 2015;128(4):804–14. https://doi.org/10.1242/jcs.164004.
Wang L, Li Y. MiR-29b-3p affects growth and biological functions of human extravillous trophoblast cells by regulating bradykinin B2 receptor. Arch Med Sci. 2022;18(2):499–522. https://doi.org/10.5114/aoms.2019.91512.
Acknowledgements
We thank Mr. Barney Wilborn and his team from The Lambert-Powell Meat Laboratory at Auburn University for their assistance in the collection of reproductive tracts.
Funding
This project was supported by Agriculture and Food Research Initiative Competitive Grant no. 2018-67015-31936 from the USDA National Institute of Food and Agriculture.
Author information
Authors and Affiliations
Contributions
FHB conceptualized the research, supervised the reproductive management, breeding, embryo transfers, collected and processed samples, carried out data analysis, and wrote the manuscript. SEM managed the breeding and embryo transfers, collected and processed samples, JGS conducted the embryo transfers, SR supervised the herd and reproductive management, MSO produced embryos in vitro for transfer. TES supervised in vitro embryo production and production of RNA-sequencing data. FHB and TES obtained funding for this work.
Corresponding author
Ethics declarations
Ethics approval and consent to participate
All animal procedures for live handling were approved by the Institutional Animal Care and Use Committee, Auburn University, under protocol 2016-2874. Consent to participate is not applicable.
Competing interests
The authors declare competing interests.
Supplementary Information
Additional file 1.
Annotated miRNAs present in the uterine luminal fluid.
Additional file 2.
Analysis of differential abundance of miRNA in the EVs obtained from uterine luminal fluid in the AI pregnant group versus the 'no conceptus present' group.
Additional file 3.
Analysis of differential abundance of miRNA in the EVs obtained from uterine luminal fluid in the AI pregnant group versus the ET pregnant group.
Additional file 4.
Annotation of biological processes of the genes that can be targets of bta-mir-17, bta-mir-7-3, MIR18A, MIR7-1 and are also down regulated in extra-embryonic tissues of pregnancies initiated by artificial insemination versus pregnancies initiated by the transfer of an in vitro produced embryo.
Additional file 5
. Annotation of biological processes of the genes that can be targets of bta-mir-17, bta-mir-7-3, MIR18A, MIR7-1 and are also down regulated in caruncular areas of endometrium of pregnancies initiated by artificial insemination versus pregnancies initiated by the transfer of an in vitro produced embryo.
Additional file 6
. Annotation of biological processes of the genes that can be targets of bta-mir-17, bta-mir-7-3, MIR18A, MIR7-1 and are also down regulated in inter-caruncular areas of endometrium of pregnancies initiated by artificial insemination versus pregnancies initiated by the transfer of an in vitro produced embryo.
Additional file 7
. Coexpression between miRNAs in the uterine luminal fluid and the genes expressed in extra-embryonic tissue.
Additional file 8
. Coexpression between miRNAs in the uterine luminal fluid and the genes expressed in caruncular areas of the endometrium.
Additional file 9
. Coexpression between miRNAs in the uterine luminal fluid and the genes expressed in intercaruncular areas of the endometrium.
Rights and permissions
Open Access This article is licensed under a Creative Commons Attribution 4.0 International License, which permits use, sharing, adaptation, distribution and reproduction in any medium or format, as long as you give appropriate credit to the original author(s) and the source, provide a link to the Creative Commons licence, and indicate if changes were made. The images or other third party material in this article are included in the article's Creative Commons licence, unless indicated otherwise in a credit line to the material. If material is not included in the article's Creative Commons licence and your intended use is not permitted by statutory regulation or exceeds the permitted use, you will need to obtain permission directly from the copyright holder. To view a copy of this licence, visit http://creativecommons.org/licenses/by/4.0/. The Creative Commons Public Domain Dedication waiver (http://creativecommons.org/publicdomain/zero/1.0/) applies to the data made available in this article, unless otherwise stated in a credit line to the data.
About this article
Cite this article
Biase, F.H., Moorey, S.E., Schnuelle, J.G. et al. Altered microRNA composition in the uterine lumen fluid in cattle (Bos taurus) pregnancies initiated by artificial insemination or transfer of an in vitro produced embryo. J Animal Sci Biotechnol 15, 130 (2024). https://doi.org/10.1186/s40104-024-01083-8
Received:
Accepted:
Published:
DOI: https://doi.org/10.1186/s40104-024-01083-8