Abstract
Background
Linezolid causes hematological toxicity, mostly thrombocytopenia, which leads to treatment discontinuation and failure. Recent studies revealed that during linezolid therapy, the incidence of treatment-related hematological toxicity is significantly higher in patients with decreased renal function (DRF) than in those with normal renal function. Linezolid monitoring is necessary due to the high frequency of hematological toxicity in patients with DRF and the relationship between blood concentration and safety. We performed a systematic review and meta-analysis to evaluate the safety correlation between DRF and trough monitoring.
Methods
Articles published before June 24, 2022, on MEDLINE, Web of Sciences, Cochrane Register of Controlled Trials, and ClinicalTrials.gov were systematically analyzed. Odds ratios (ORs) and 95% confidence intervals (CIs) were calculated using the Mantel–Haenszel method and the variable effects model.
Results
The incidence of hematological toxicity was significantly higher in patients with DRF than in those without DRF (OR = 2.37; p < 0.001). Subgroup analysis, performed according to hematotoxicity classification, including thrombocytopenia, anemia, and pancytopenia, revealed a significantly higher incidence of thrombocytopenia (OR = 2.45; p < 0.001) and anemia (OR = 2.31; p = 0.006) in patients with DRF than in those without; pancytopenia (OR = 1.41; p = 0.80) incidences were not significantly higher. Based on a systematic review, linezolid trough concentrations > 6–7 μg/mL may be associated with an increased incidence of thrombocytopenia. However, no confidential threshold values for the development of thrombocytopenia were found in the area under the concentration curve values for children or adults.
Conclusion
We observed a high frequency of hematological toxicity during linezolid therapy in patients with DRF. To ensure safety, linezolid trough concentrations should be ≤6–7 μg/mL.
Similar content being viewed by others
Introduction
Linezolid is an oxazolidinone antibiotic used to treat infectious diseases caused by drug-resistant gram-positive bacteria, such as methicillin-resistant Staphylococcus aureus and vancomycin-resistant Enterococci. Linezolid inhibits bacterial protein synthesis by binding to ribosomal RNA (30S and 50S ribosomal subunits) [1]. This unique mechanism prevents cross-resistance to existing antimicrobial agents of other classes [2]. However, the major treatment-related adverse event of linezolid therapy is hematological toxicity, mostly thrombocytopenia, which leads to treatment discontinuation and failure [3,4,5]. Generally, linezolid and its primary metabolites are excreted via non-renal (approximately 65%) and renal mechanisms [6]; therefore, dose adjustment is not required in patients with decreased renal function (DRF) [2, 7, 8]. However, recent studies have revealed that during linezolid therapy, the incidence of treatment-related hematological toxicity is significantly higher in patients with DRF than in those with normal renal function [9,10,11,12,13].
To avoid hematological toxicity, some studies have suggested that linezolid dose optimization based on its plasma concentration may be effective [14,15,16]. The pharmacokinetic (PK)/pharmacodynamic parameter of linezolid associated with effectiveness is the area under the concentration curve (AUC)/minimum inhibitory concentration [17, 18]. However, details of the concentrations and PK parameters associated with the safety evaluation of linezolid have not been clarified. In general, the trough concentration or AUC is used to evaluate the safety of antimicrobials. Although association of the trough concentration or AUC with the safety of linezolid has been frequently reported, it is unclear whether trough concentration or AUC is a suitable PK parameter for safety evaluation; furthermore, the appropriate range has yet to be determined. Systematic reviews and meta-analyses have recommended using vancomycin for safety monitoring cases with an AUC of 400–600 mg × h/L [19, 20]. However, no systematic review or meta-analysis has explored the concentrations or PK indices associated with linezolid safety.
Therefore, this meta-analysis aimed to determine whether hematological toxicity has a high incidence in patients with DRF. To avoid adverse events, we also performed a systematic review to evaluate linezolid’s monitoring parameters and ranges.
Methods
Search strategies
Search strategy for the evaluation of linezolid-associated hematotoxicity in patients with DRF
PubMed, Web of Sciences, Cochrane Register of Controlled Trials, and ClinicalTrials.gov databases were searched for relevant studies published before June 24, 2022. Two of four reviewers (MA, CI, RS, and TN) independently searched databases for literature using the following research terms: “linezolid,” “renal,” “kidney,” “thrombocytopenia,” “anemia,” “neutropenia,” “myelosuppression,” “leucopenia,” and “hematotoxicity.” The publication language was limited to English, and there was no restriction on the publication year. Duplicate articles were excluded.
Search strategy for the evaluation of linezolid monitoring and ranges
We similarly searched PubMed, Web of Sciences, Cochrane Register of Controlled Trials, and ClinicalTrials.gov databases for relevant studies published before June 24, 2022. Two of the four reviewers (MA, CI, RS, and TN) independently searched for literature using the following research terms: “linezolid,” “monitoring,” “area under the curve,” “trough,” and “therapeutic drug monitoring.” The publication language was limited to English, and there was no restriction on the publication year. Duplicate articles were excluded from the study.
Study selection
Study selection for the evaluation of linezolid-associated hematotoxicity in patients with DRF
Two of the four reviewers (XL, MA, SO, and RS) independently screened the extracted literature. A study was considered eligible for evaluation in this meta-analysis provided that it met the following inclusion criteria: (1) the study included patients with and without DRF; (2) the study included patients who received linezolid treatment; and (3) the study revealed outcomes corresponding to hematotoxicity (thrombocytopenia, anemia, neutropenia, myelosuppression, and leukopenia). Studies that met the following criteria were excluded: (1) studies involving cells or animal models; and (2) case reports, case series, or reviews.
Study selection for the evaluation of linezolid monitoring and ranges
Two of the four reviewers (XL, MA, SO, and TN) independently screened the literature. A study was considered eligible for evaluation in this systematic review provided that it met the following inclusion criteria: (1) the study revealed the AUC or trough values of patients; (2) the study included patients who received treatment with linezolid; and (3) the study revealed the outcomes of thrombocytopenia.
Data extraction
Data extraction for the evaluation of linezolid-associated hematotoxicity in patients with DRF
Two of the four reviewers (XL, SO, CI, and RS) independently extracted data from the studies. The study period, study design, country of the study, age and weight of the patients, definition of hematotoxicity, definition of DRF, and patients with and without DRF (patients with or without hematotoxicity were counted separately) were extracted according to the predefined eligibility criteria.
Data extraction for the evaluation of linezolid monitoring and ranges
Two of the four reviewers (XL, SO, CI, and RA) independently extracted data from the studies. The study period, study design, country of study, age of the patients, and AUC or trough values were extracted.
Outcome analysis
Outcome analysis for the evaluation of linezolid-associated hematotoxicity in patients with DRF
The primary outcome was the incidence rate of hematotoxicity. The rate of hematotoxicity was defined according to each study’s definition. Subgroup analysis was performed according to the classification of hematotoxicity, including thrombocytopenia, anemia, pancytopenia, and myelosuppression.
Outcome analysis for the evaluation of linezolid monitoring and ranges
The primary outcome was the incidence of thrombocytopenia determined according to AUC24 (calculated by AUC12 if unavailable) and Cmin (minimum blood plasma concentration) in children and adults.
Assessment of the risk of bias
Two of the four reviewers (XL, SO, CI, and RA) independently assessed the risk of bias based on Cochrane Collaboration (Risk Of Bias In Non-Randomized Studies of Interventions, ROBINS-I) [21]. Discrepancies were resolved by discussion or consultation with the third reviewer (YE).
Assessment of quality of evidence
The GRADE handbook was used to rate the grade quality of the meta-analysis [22]. GRADE specifies that the quality of the evidence can be classified into four categories according to the corresponding evaluation criteria: (1) high (⊕⊕⊕⊕); (2) moderate (⊕⊕⊕⊖); (3) low (⊕⊕⊖⊖); and (4) very low (⊕⊖⊖⊖).
Analysis of the results and statistical analyses
The Review Manager for Windows (RevMan, Version 5.4, Copenhagen: The Nordic Cochrane Centre, The Collaboration, 2020) was used for data analysis and the preparation of forest plots. We used random-effects model for pooling study results. We calculated odds ratios (OR) with 95% confidence intervals (CIs) for discrete variables. To assess heterogeneity, I2 was calculated. Finally, funnel plots were constructed to assess potential publication bias.
Protocol registration
The present study was not registered with Prospero or elsewhere.
Results
Search results
In the database search for the evaluation of linezolid-associated hematotoxicity, 1213 articles were screened after duplicates were extracted (Fig. 1A). Twenty-five articles [9,10,11,12,13, 23,24,25,26,27,28,29,30,31,32,33,34,35,36,37,38,39,40,41,42] were included for the evaluation of linezolid-associated hematotoxicity.
In the database search for the evaluation of linezolid monitoring and ranges, 1087 articles were screened after exclusion of duplicates (Fig. 1B). Twenty-seven articles [16, 23, 25, 43,44,45,46,47,48,49,50,51,52,53,54,55,56,57,58,59,60,61,62,63,64,65,66] were included in the evaluation of linezolid monitoring strategies.
Characteristics
The characteristics of the 25 studies included in the meta-analysis for evaluating linezolid-associated hematotoxicity are shown in Table 1. These studies included 3831 patients, 1240 of whom had DRF. The definitions of DRF and hematotoxicity in each study are shown in Table 1. Most studies were conducted in Asian countries (16 of 25 studies). Twenty-three studies were retrospective, and two studies [25, 37] were prospective studies with a small number of cases conducted in Japan. Thrombocytopenia, anemia, pancytopenia, and reduction in neutrophils corresponded to hematotoxicity.
The characteristics of the 27 systematically reviewed studies are shown in Tables 2, 3, 4 and 5. Tables 2 and 3 show studies that evaluated the incidence of thrombocytopenia associated with AUC values in children and adults, respectively. In the analysis of AUC values associated with thrombocytopenia, two studies involved children (Table 2), and 15 studies involved adults (Table 3). A total of 230 patients (including eight children) were included in the analysis. All studies analyzing AUC values associated with thrombocytopenia in children were prospective studies. Of the 15 adult studies, two were retrospective studies, while 12 were prospective studies, on the analysis of AUC values associated with thrombocytopenia in adults. The National Institute of Allergy and Infectious Diseases (NIAID) study in 2018 was a clinical trial.
Tables 4 and 5 list studies that evaluated the incidence of thrombocytopenia associated with Cmin in children and adults, respectively. In the analysis of Cmin associated with thrombocytopenia, three studies included children (Table 4), and 17 studies included adults (Table 5). Two of the three studies were prospective in the analysis of Cmin associated with thrombocytopenia in children. Twelve of the 14 studies were prospective studies that analyzed Cmin associated with thrombocytopenia in adults.
Outcome analysis for the evaluation of linezolid-associated hematotoxicity in patients with DRF
Twenty-three retrospective studies and two prospective studies with 1240 patients with DRF and 2591 patients without DRF were enrolled in the meta-analysis. Compared with patients without DRF, patients with DRF had a significantly higher incidence of hematotoxicity (OR = 2.37; 95% CI: 1.93–2.90; p < 0.001; I2 = 33%) (Fig. 2).
Forest plot of the hematotoxicity associated with linezolid treatment with or without decreased renal function. Vertical line indicates no significant differences between the groups. Diamond shapes and horizontal lines indicate odds ratios and 95% confidence intervals, respectively. Squares indicate point estimates and the size of each square indicates the weight of each study
We also conducted a subgroup analysis based on the classification of hematotoxicity. The incidences of thrombocytopenia (OR = 2.45; 95% CI: 1.95–3.09; p < 0.001; I2 = 36%) and anemia (OR = 2.31; 95% CI: 1.27–4.21; p = 0.006; I2 = 29%) were significantly higher in patients with DRF than in those without DRF (Fig. 3A and C). However, no significant differences were observed in the incidence of pancytopenia (OR = 1.41; 95% CI: 0.10–20.72; p = 0.80, I2 = 65%) in patients with and without DRF (Fig. 3B).
Forest plot of the subgroup analysis of the hematotoxicity classification associated with linezolid treatment with or without decreased renal function. Vertical line indicates no significant differences between the groups. Diamond shapes and horizontal lines indicate odds ratios and 95% confidence intervals, respectively. Squares indicate point estimates and the size of each square indicates the weight of each study. Subgroup analysis of A anemia; B pancytopenia; and C thrombocytopenia
Outcome analysis for AUC values and the incidence of thrombocytopenia
No confidential threshold values for the development of thrombocytopenia were found in AUC values for children or adults (Tables 2 and 3). Only four studies reported the AUC values for patients with thrombocytopenia, and the values were 180.5 [44] 243 [49], 280.74 [16], and 175.0 or 345.8 [66] mg × h/L. Thrombocytopenia did not occur when the mean or median AUC24 (calculated by AUC12 if it was not available) was within 95.2–328.3 mg × h/L in adults (Table 3).
Outcome analysis for Cmin and the incidence of thrombocytopenia
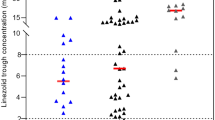
Twelve studies reported the incidence of thrombocytopenia. In the analysis for children, two studies revealed the incidence of thrombocytopenia, and the Cmin values of thrombocytopenia and non-thrombocytopenia were 4.7–7.17 and 0.1–4.6 μg/mL, respectively. One patient with a Cmin value of 4.7 μg/mL received high-dose methotrexate in combination treatment. In the adult analysis, 10 studies revealed the incidence of thrombocytopenia, and the Cmin values of thrombocytopenia and non-thrombocytopenia were 4.28–67.7 and 0.2–5.8 μg/mL, respectively. In seven studies, Cmin for patients without thrombocytopenia was not determined. Except for a Cmin of 4.28 μg/mL, thrombocytopenia occurred at Cmin values of > 6–7 μg/mL.
Publication bias
Funnel plots of the incidence of hematotoxicity are shown in Fig. 4. The funnel plots were symmetric and did not suggest the presence of publication bias in favor of a positive study for all outcomes.
Assessment of the risk of bias
The results of the assessment of the risk of bias are presented in Figs. S1 and S2. A high risk of confounding bias was found in the study by Hiraki et al. [25]. Information regarding selection bias was unavailable for most studies; few studies identified bias issues. No problems in intervention bias were identified, and moderate missing data bias was identified in the study by Choi 2019. Three studies [30, 33, 40] had a moderate risk of measurement of outcome bias. No information was available for deviation from the intended intervention and reporting biases.
Quality of the evidence
The results of the quality evaluation according to the GRADE guideline are shown in Table 6. This meta-analysis consisted primarily of observational studies, so there was a low initial rating. Some problems in the risk of bias downgraded the quality of evidence by one level, while a large magnitude of effect upgraded the quality of evidence by one level. The low final grade of the evidence shows that our confidence in the effect estimate is limited.
Discussion
In this meta-analysis of retrospective and prospective studies, the incidence of hematotoxicity was significantly higher in patients with DRF than in those without. In addition, subgroup analysis revealed a significant difference in the incidence of thrombocytopenia and anemia, but there was no significant difference in the incidence of pancytopenia (Fig. 3A–C). These results suggest that linezolid should be cautiously administered in patients with DRF while monitoring for hematotoxicity, especially thrombocytopenia and anemia.
Clinical phase III trials have reported a 2.4% incidence of thrombocytopenia in patients receiving linezolid therapy [67]. In our meta-analysis, the incidence of thrombocytopenia in patients with and without DRF ranged between 28.9 and 78.6% (except for the study by Hiraki et al. [25]) and 10.5 and 42.9%, respectively, which were higher than those previously reported. Nearly all the patients included in this meta-analysis were from Asian countries, such as Japan, China, and Korea, and had lower body weights than those of individuals from Western countries. Previously, lower body weight was considered a risk factor for thrombocytopenia [23]. Generally, linezolid was administered twice daily (600 mg × 2) and the dose was not adjusted by body weight. A comparison of the median weights among the groups that received linezolid treatment showed that the median weight was 80 kg when the AUC was 95.2 mg × h/L [53] and 58.3 kg when the AUC was 291.6 mg × h/L [45]. The difference in AUC values may be accounted for by the difference in the dose per body weight. Additionally, advanced age [68] and the duration of administration [69] are also considered risk factors; therefore, this difference in the patients’ backgrounds may explain the higher incidence of hematotoxicity.
A major reason for the higher incidence of thrombocytopenia in patients with DRF than in patients without DRF is the delayed excretion of linezolid and increased blood linezolid concentrations. Approximately 30% of linezolid is excreted by the kidneys of patients with normal renal function [70]. Furthermore, Matsumoto et al. evaluated the clearance of linezolid with renal function and reported a correlation between linezolid and creatinine clearance or blood urea nitrogen [69]. Therefore, we hypothesized that linezolid overexposure or higher Cmin is associated with decreased renal function [59, 71].
In this meta-analysis, no significant differences were observed in the incidence of pancytopenia. This result does not indicate the absence of a relationship between DRF and the incidence of pancytopenia, as the number of cases included in the systematic review was notably smaller than that of thrombocytopenia. In addition, many studies have focused on thrombocytopenia, which occurs most frequently among the different forms of hematotoxicity (Sheldon et al. 2003 [5];). Therefore, it might have been easier to identify significant differences in thrombocytopenia. If more studies on pancytopenia are published in the future, significant differences in the incidence of pancytopenia will be found.
The incidence of thrombocytopenia was higher when the Cmin of linezolid exceeded 6–7 μg/mL (Tables 4 and 5). Previous studies revealed the efficacy and safety ranges of linezolid trough values as 2–8 μg/mL [15, 16, 62, 72], 3.6–8.2 μg/mL [61], and 2–7 μg/mL [73]. In this study, we conducted a systematic review of the incidence of thrombocytopenia and Cmin in children and adults, as determined by the extracted Cmin threshold; the incidence of thrombocytopenia was higher when the Cmin exceeded 6–7 μg/mL. However, this systematic review could not determine the clinically relevant threshold of linezolid in terms of the AUC (Tables 2 and 3). Matsumoto et al. reported a strong correlation between AUC and trough concentrations [61]. Only four studies reported the AUC values for patients with thrombocytopenia in this study.
Further studies are required to determine the target AUC that correlates with thrombocytopenia. However, it is difficult to measure the AUC in clinical settings; therefore, Cmin may be a surrogate index of AUC in clinical practice. Consequently, we believe that therapeutic drug monitoring should be performed for linezolid administration from the perspective of safety and that the dose should be controlled to achieve a target trough value of < 6–7 μg/mL.
The previous meta-analysis showed that impaired renal function was associated with an increased risk of linezolid-induced thrombocytopenia [74]. Based on this knowledge, finding an association between hematotoxicity and patients with DRF, we classified hematotoxicity and performed a subgroup analysis, which showed that thrombocytopenia and anemia were significantly higher in patients with DRF than in those without DRF. We also conducted a systematic review and determined that hematotoxicity was higher when Cmin exceeded 6–7 μg/mL. This finding is a strength of the current study. To our knowledge, this study is the first systematic review to explore the association of Cmin with linezolid safety. This result may serve as an indication for the implementation of therapeutic drug monitoring and provide insights for further clinical trials.
This study had several limitations. First, most of the analyzed studies were observational studies. Therefore, the patient characteristics and study designs contained various types of bias, hindering their results’ generalizability. Second, the definitions of thrombocytopenia were different in these studies. Third, the estimation method of AUC differed in each study. This might have led to a misunderstanding of our results. However, this analysis did not clarify the target AUC due to the limited number of studies.
Conclusion
Decreased renal function correlates with an increased risk of thrombocytopenia and anemia due to overexposure. To maximize the efficacy and minimize the toxicity of linezolid, therapeutic drug monitoring should be recommended, using evidence-based thresholds in patients on long-term linezolid treatment or in patients with DRF.
Availability of data and materials
The datasets used and/or analyzed during the current study are available from the corresponding author upon reasonable request.
Abbreviations
- DRF:
-
Decreased renal function
- ORs:
-
Odds ratios
- CIs:
-
Confidence intervals
- PK:
-
Pharmacokinetics
- AUC:
-
Area under the concentration curve
- Cmin :
-
Minimum blood plasma concentration
References
Batts DH. Linezolid-a new option for treating gram-positive infections; 2000. p. 23–9.
Paladino JA. Linezolid: an oxazolidinone antimicrobial agent. Am J Health Syst Pharm. 2002;59:2413–25.
Attassi K, Hershberger E, Alam R, Zervos MJ. Thrombocytopenia associated with linezolid therapy. Clin Infect Dis. 2002;34:695–8.
Gerson SL, Kaplan SL, Bruss JB, Le V, Arellano FM, Hafkin B, et al. Hematologic effects of linezolid: summary of clinical experience. Antimicrob Agents Chemother. 2002;46:2723–6.
Kohno S, Yamaguchi K, Aikawa N, Sumiyama Y, Odagiri S, Aoki N, et al. Linezolid versus vancomycin for the treatment of infections caused by methicillin-resistant Staphylococcus aureus in Japan. J Antimicrob Chemother. 2007;60:1361–9.
Dryden MS. Linezolid pharmacokinetics and pharmacodynamics in clinical treatment. J Antimicrob Chemother. 2011;66(SUPPL. 4):7–15.
Moellering RC. Linezolid: the first oxazolidinone antimicrobial. Ann Intern Med. 2003;138:135–42.
MacGowan AP. Pharmacokinetic and pharmacodynamic profile of linezolid in healthy volunteers and patients with gram-positive infections. J Antimicrob Chemother. 2003;51:17ii–25.
Choi GW, Lee J-Y, Chang MJ, Kim YK, Cho Y, Yu YM, et al. Risk factors for linezolid-induced thrombocytopenia in patients without haemato-oncologic diseases. Basic Clin Pharmacol Toxicol. 2019;124:228–34.
Crass RL, Cojutti PG, Pai MP, Pea F. Reappraisal of linezolid dosing in renal impairment to improve safety. Antimicrob Agents Chemother. 2019;63(8):e00605–19.
Giunio-Zorkin N, Brown G. Real-life frequency of new-onset thrombocytopenia during linezolid treatment. Can J Hosp Pharm. 2019;72:133–8.
Kim HS, Lee E, Cho YJ, Lee YJ, Rhie SJ. Linezolid-induced thrombocytopenia increases mortality risk in intensive care unit patients, a 10 year retrospective study. J Clin Pharm Ther. 2019;44:84–90.
Lima LS, Brito EDCA, Mattos K, Parisotto EB, Perdomo RT, Weber SS. A retrospective cohort study to screen linezolid-induced thrombocytopenia in adult patients hospitalized in the Midwestern Region of Brazil. Hematol Transfus Cell Ther. 2020;42:230–7.
Richards GA, Brink AJ. Therapeutic drug monitoring: linezolid too? Crit Care. 2014;18:525.
Pea F, Furlanut M, Cojutti P, Cristini F, Zamparini E, Franceschi L, et al. Therapeutic drug monitoring of linezolid: a retrospective monocentric analysis. Antimicrob Agents Chemother. 2010;54:4605–10.
Pea F, Viale P, Cojutti P, Del pin B, Zamparini E, Furlanut M. Therapeutic drug monitoring may improve safety outcomes of long-term treatment with linezolid in adult patients. J Antimicrob Chemother. 2012;67:2034–42.
Alsultan A. Determining therapeutic trough ranges for linezolid. Saudi Pharm J. 2019;27:1061–3.
Rayner CR, Forrest A, Meagher AK, Birmingham MC, Schentag JJ. Clinical pharmacodynamics of linezolid in seriously ill patients treated in a compassionate use programme. Clin Pharmacokinet. 2003;42:1411–23.
Tsutsuura M, Moriyama H, Kojima N, Mizukami Y, Tashiro S, Osa S, et al. The monitoring of vancomycin: a systematic review and meta-analyses of area under the concentration-time curve-guided dosing and trough-guided dosing. BMC Infect Dis. 2021;21:1–15.
Aljefri DM, Avedissian SN, Rhodes NJ, Postelnick MJ, Nguyen K, Scheetz MH. Vancomycin area under the curve and acute kidney injury: a meta-analysis. Clin Infect Dis. 2019;69:1881–7.
Higgins J. Cochrane Handbook for systematic reviews of interventions version 5.1.0 (updated March 2011). 2011. http://training.cochrane.org/handbook.
Holger S, Jan B, Gordon G, Andrew O. Handbook for grading the quality of evidence and the strength of recommendations using the GRADE approach (Updated October 2013). 2013. https://gdt.gradepro.org/app/handbook/handbook.html
Dong HY, Xie J, Chen LH, Wang TT, Zhao YR, Dong YL. Therapeutic drug monitoring and receiver operating characteristic curve prediction may reduce the development of linezolid-associated thrombocytopenia in critically ill patients. Eur J Clin Microbiol Infect Dis. 2014;33:1029–35.
Fujii S, Takahashi S, Makino S, Kunimoto Y, Nakata H, Noda N, et al. Impact of vancomycin or linezolid therapy on development of renal dysfunction and thrombocytopenia in Japanese patients. Chemotherapy. 2014;59:319–24.
Hiraki Y, Tsuji Y, Hiraike M, Misumi N, Matsumoto K, Morita K, et al. Correlation between serum linezolid concentration and the development of thrombocytopenia. Scand J Infect Dis. 2012;44:60–4.
Hirano R, Sakamoto Y, Tachibana N, Ohnishi M. Retrospective analysis of the risk factors for linezolid-induced thrombocytopenia in adult Japanese patients. Int J Clin Pharm. 2014;36:795–9.
Jones SJ, Nichols KR, DeYoung HL, Cox EG, Knoderer CA. Linezolid-associated thrombocytopenia in children with renal impairment. J Pediatric Infect Dis Soc. 2015;4:272–5.
Lin YH, Wu VC, Tsai IJ, Ho YL, Hwang JJ, Tsau YK, et al. High frequency of linezolid-associated thrombocytopenia among patients with renal insufficiency. Int J Antimicrob Agents. 2006;28:345–51.
Moraza L, Leache L, Aquerreta I, Ortega A. Linezolid-induced haematological toxicity. Farm Hosp. 2015;39:320–32.
Plachouras D, Giannitsioti E, Athanassia S, Kontopidou F, Papadopoulos A, Kanellakopoulou K, et al. No effect of pyridoxine on the incidence of myelosuppression during prolonged linezolid treatment. Clin Infect Dis. 2006;43:e89–91.
Rabon AD, Fisher JP, MacVane SH. Incidence and risk factors for development of thrombocytopenia in patients treated with linezolid for 7 days or greater. Ann Pharmacother. 2018;52:1162–4.
Takahashi Y, Takesue Y, Nakajima K, Ichiki K, Tsuchida T, Tatsumi S, et al. Risk factors associated with the development of thrombocytopenia in patients who received linezolid therapy. J Infect Chemother. 2011;17:382–7.
Wu V-C, Wang Y-T, Wang C-Y, Tsai I-J, Wu K-D, Hwang J-J, et al. High frequency of linezolid-associated thrombocytopenia and anemia among patients with end-stage renal disease. Int J Antimicrob Agents. 2006;28:345–51.
Han X, Wang J, Zan X, Peng L, Nie X. Risk factors for linezolid-induced thrombocytopenia in adult inpatients. Int J Clin Pharm. 2022;44:330–8.
Hsu Y-C, Chen S-Y, Hung Y-J, Huang Y-W. Renal replacement therapy and concurrent fluconazole therapy increase linezolid-related thrombocytopenia among adult patients. Sci Rep. 2022;12:9894.
Kawasuji H, Tsuji Y, Ogami C, Kimoto K, Ueno A, Miyajima Y, et al. Proposal of initial and maintenance dosing regimens with linezolid for renal impairment patients. BMC Pharmacol Toxicol. 2021;22:13.
Komatsu T, Nakamura M, Uchiyama K, Inoue G, Sakanoue K, Kawamura A, et al. Initial trough concentration may be beneficial in preventing linezolid-induced thrombocytopenia. J Chemother. 2022;34:375–80.
Maray I, Rodríguez-Ferreras A, Álvarez-Asteinza C, Alaguero-Calero M, Valledor P, Fernández J. Linezolid induced thrombocytopenia in critically ill patients: risk factors and development of a machine learning-based prediction model. J Infect Chemother. 2022;28:1249–54.
Qin Y, Liu Y, Chen Z, Cao M, Shen Y, Ye Y. A risk factor-based predictive model for linezolid-induced anaemia: a 7-year retrospective study. J Clin Pharm Ther. 2021;46:1591–9.
Sato Y, Iguchi M, Kato Y, Morioka H, Hirabayashi A, Tetsuka N, et al. Number of concomitant drugs with thrombocytopenic adverse effects and the extent inflammatory response resolution are risk factors for thrombocytopenia in patients treated with linezolid for more than 14 days. Nagoya J Med Sci. 2020;82:407–14.
Thirot H, Briquet C, Frippiat F, Jacobs F, Holemans X, Henrard S, et al. Clinical use and adverse drug reactions of linezolid: a retrospective study in four Belgian hospital centers. Antibiotics. 2021;10(5):530.
Wu F, Zhang X-S, Dai Y, Zhou Z-Y, Zhang C-H, Han L, et al. Dosage strategy of linezolid according to the trough concentration target and renal function in Chinese critically ill patients. Front Pharmacol. 2022;13:844567.
Kosaka T, Kokufu T, Shime N, Sugioka N, Kato R, Hamaoka K, et al. Pharmacokinetics and tolerance of linezolid for meticillin-resistant Staphylococcus aureus mediastinitis in paediatric patients. Int J Antimicrob Agents. 2009;33:368–70.
Matsumoto K, Shigemi A, Takeshita A, Watanabe E, Yokoyama Y, Ikawa K, et al. Linezolid dosage in pediatric patients based on pharmacokinetics and pharmacodynamics. J Infect Chemother. 2014;21:70–3.
Alffenaar JWC, Van Altena R, Harmelink IM, Filguera P, Molenaar E, Wessels AMA, et al. Comparison of the pharmacokinetics of two dosage regimens of linezolid in multidrug-resistant and extensively drug-resistant tuberculosis patients. Clin Pharmacokinet. 2010;49:559–65.
Beer R, Engelhardt KW, Pfausler B, Broessner G, Helbok R, Lackner P, et al. Pharmacokinetics of intravenous linezolid in cerebrospinal fluid and plasma in neurointensive care patients with staphylococcal ventriculitis associated with external ventricular drains. Antimicrob Agents Chemother. 2007;51:379–82.
NCT00396084, National Institute of A, Infectious D. Early Bactericidal Activity of Linezolid, Gatifloxacin, Levofloxacin, Isoniazid (INH) and Moxifloxacin in HIV Negative Adults With Initial Episodes of Sputum Smear-Positive Pulmonary Tuberculosis. 2006. http://clinicaltrials.gov/show/NCT00396084.
Bhalodi AA, Papasavas PK, Tishler DS, Nicolau DP, Kuti JL. Pharmacokinetics of intravenous linezolid in moderately to morbidly obese adults. Antimicrob Agents Chemother. 2013;57:1144–9.
Boak LM, Rayner CR, Grayson ML, Paterson DL, Spelman D, Khumra S, et al. Clinical population pharmacokinetics and toxicodynamics of linezolid. Antimicrob Agents Chemother. 2014;58:2334–43.
Conte JE, Golden JA, Kipps J, Zurlinden E. Intrapulmonary pharmacokinetics of linezolid. Antimicrob Agents Chemother. 2002;46:1475–80.
Eslam RB, Burian A, Vila G, Sauermann R, Hammer A, Frenzel D, et al. Target site pharmacokinetics of linezolid after single and multiple doses in diabetic patients with soft tissue infection. J Clin Pharmacol. 2014;54:1058–62.
Gee T, Ellis R, Marshall G, Andrews J, Ashby J, Wise R. Pharmacokinetics and tissue penetration of linezolid following multiple oral doses. Antimicrob Agents Chemother. 2001;45:1843–6.
Luque S, Grau S, Alvarez-Lerma F, Ferrández O, Campillo N, Horcajada JP, et al. Plasma and cerebrospinal fluid concentrations of linezolid in neurosurgical critically ill patients with proven or suspected central nervous system infections. Int J Antimicrob Agents. 2014;44:409–15.
Myrianthefs P, Markantonis SL, Vlachos K, Anagnostaki M, Boutzouka E, Panidis D, et al. Serum and cerebrospinal fluid concentrations of linezolid in neurosurgical patients. Antimicrob Agents Chemother. 2006;50:3971–6.
Swoboda S, Ober MC, Lichtenstern C, Saleh S, Schwenger V, Sonntag HG, et al. Pharmacokinetics of linezolid in septic patients with and without extended dialysis. Eur J Clin Pharmacol. 2010;66:291–8.
Traunmüller F, Schintler MV, Spendel S, Popovic M, Mauric O, Scharnagl E, et al. Linezolid concentrations in infected soft tissue and bone following repetitive doses in diabetic patients with bacterial foot infections. Int J Antimicrob Agents. 2010;36:84–6.
Cojutti P, Maximova N, Crichiutti G, Isola M, Pea F. Pharmacokinetic/pharmacodynamic evaluation of linezolid in hospitalized paediatric patients: a step toward dose optimization by means of therapeutic drug monitoring and Monte Carlo simulation. J Antimicrob Chemother. 2015;70:198–206.
Cojutti PG, Merelli M, Bassetti M, Pea F. Proactive therapeutic drug monitoring (TDM) may be helpful in managing long-term treatment with linezolid safely: findings from a monocentric, prospective, open-label, interventional study. J Antimicrob Chemother. 2019;74:3588–95.
Fang J, Chen C, Wu Y, Zhang M, Zhang Y, Shi G, et al. Does the conventional dosage of linezolid necessitate therapeutic drug monitoring?—experience from a prospective observational study. Ann Transl Med. 2020;8:493.
Luque S, Muñoz-Bermudez R, Echeverría-Esnal D, Sorli L, Campillo N, Martínez-Casanova J, et al. Linezolid dosing in patients with liver cirrhosis: standard dosing risk toxicity. Ther Drug Monit. 2019;41:732–9.
Matsumoto K, Shigemi A, Takeshita A, Watanabe E, Yokoyama Y, Ikawa K, et al. Analysis of thrombocytopenic effects and population pharmacokinetics of linezolid: a dosage strategy according to the trough concentration target and renal function in adult patients. Int J Antimicrob Agents. 2014;44:242–7.
Morata L, Cuesta M, Rojas JF, Rodriguez S, Brunet M, Casals G, et al. Risk factors for a low linezolid trough plasma concentration in acute infections. Antimicrob Agents Chemother. 2013;57:1913–7.
Nukui Y, Hatakeyama S, Okamoto K, Yamamoto T, Hisaka A, Suzuki H, et al. High plasma linezolid concentration and impaired renal function affect development of linezolid-induced thrombocytopenia. J Antimicrob Chemother. 2013;68:2128–33.
Tsuji Y, Hiraki Y, Matsumoto K, Mizoguchi A, Kobayashi T, Sadoh S, et al. Thrombocytopenia and anemia caused by a persistent high linezolid concentration in patients with renal dysfunction. J Infect Chemother. 2011;17:70–5.
Alffenaar J-WC, Kosterin JGW, van Altena R, van der Werf TS, Uges DRA, Proost JH. Limited sampling strategies for therapeutic drug monitoring of amikacin and kanamycin in patients with multidrug-resistant tuberculosis. Ther Drug Monit. 2010;32:97–101.
Blackman AL, Jarugula P, Nicolau DP, Chui SH, Joshi M, Heil EL, et al. Evaluation of linezolid pharmacokinetics in critically ill obese patients with severe skin and soft tissue infections. Antimicrob Agents Chemother. 2021;65(2):e01619–20.
Rubinstein E, Isturiz R, Standiford HC, Smith LG, Oliphant TH, Cammarata S, et al. Worldwide assessment of linezolid’s clinical safety and tolerability: comparator-controlled phase III studies. Antimicrob Agents Chemother. 2003;47:1824–31.
Cattaneo D, Gervasoni C, Cozzi V, Castoldi S, Baldelli S, Clementi E. Therapeutic drug management of linezolid: a missed opportunity for clinicians?. Int J Antimicrob Agents. 2016;48(6):728–31 S0924857916302783. https://doi.org/10.1016/j.ijantimicag.2016.08.023.
Matsumoto K, Takeshita A, Ikawa K, Shigemi A, Yaji K, Shimodozono Y, et al. Higher linezolid exposure and higher frequency of thrombocytopenia in patients with renal dysfunction. Int J Antimicrob Agents. 2010;36:179–81.
Slatter JG, Stalker DJ, Feenstra KL, Welshman IR, Bruss JB, Sams JP, et al. Pharmacokinetics, metabolism, and excretion of linezolid following an oral dose of [14C]linezolid to healthy human subjects. Drug Metab Dispos. 2001;29:1136–45.
Alici G, Valerio M, Munoz P, Alcala L, Xandra Garcia G, Burillo A, et al. Crossm systematic therapeutic drug monitoring. Antimicrob Agents Chemother. 2017;61:1–6.
Cattaneo D, Gervasoni C, Cozzi V, Castoldi S, Baldelli S, Clementi E. Therapeutic drug management of linezolid: a missed opportunity for clinicians? Int J Antimicrob Agents. 2016;48:728–31.
Pea F, Cojutti P, Dose L, Baraldo M. A 1 year retrospective audit of quality indicators of clinical pharmacological advice for personalized linezolid dosing: one stone for two birds? Br J Clin Pharmacol. 2016;81:341–8.
Shi C, Xia J, Ye J, Xie Y, Jin W, Zhang W, et al. Effect of renal function on the risk of thrombocytopaenia in patients receiving linezolid therapy: a systematic review and meta-analysis. Br J Clin Pharmacol. 2022;88:464–75.
Acknowledgments
We would like to thank Editage (www.editage.com) for English language editing.
Funding
This study did not receive any grants from funding agencies in the public, commercial, or not-for-profit sectors.
Author information
Authors and Affiliations
Contributions
YE organized and coordinated the study. KM was the chief investigator and data analyst. XL, MA, SO, CI, RS, TN, and KT designed the study. XL was a major contributor to writing the manuscript. All authors contributed to the writing of the final manuscript, approved its publication, and agreed to be accountable for all aspects of the work in ensuring that questions related to the accuracy or integrity of any part of the work were appropriately investigated and resolved.
Corresponding author
Ethics declarations
Ethics approval and consent to participate
Not applicable.
Consent for publication
Not applicable.
Competing interests
XL, MA, SO, CI, RS, TN, YE, and KT report no conflicts of interest. KM received a research grant from Meiji Seika Pharma Co. Ltd.
Additional information
Publisher’s Note
Springer Nature remains neutral with regard to jurisdictional claims in published maps and institutional affiliations.
Supplementary Information
Additional file 1: Fig. S1.
Assessment of the risks of bias for studies included in meta-analysis. Fig. S2. Assessment of the risks of bias for studies included in systematic review.
Rights and permissions
Open Access This article is licensed under a Creative Commons Attribution 4.0 International License, which permits use, sharing, adaptation, distribution and reproduction in any medium or format, as long as you give appropriate credit to the original author(s) and the source, provide a link to the Creative Commons licence, and indicate if changes were made. The images or other third party material in this article are included in the article's Creative Commons licence, unless indicated otherwise in a credit line to the material. If material is not included in the article's Creative Commons licence and your intended use is not permitted by statutory regulation or exceeds the permitted use, you will need to obtain permission directly from the copyright holder. To view a copy of this licence, visit http://creativecommons.org/licenses/by/4.0/. The Creative Commons Public Domain Dedication waiver (http://creativecommons.org/publicdomain/zero/1.0/) applies to the data made available in this article, unless otherwise stated in a credit line to the data.
About this article
Cite this article
Liu, X., Aoki, M., Osa, S. et al. Safety of linezolid in patients with decreased renal function and trough monitoring: a systematic review and meta-analysis. BMC Pharmacol Toxicol 23, 89 (2022). https://doi.org/10.1186/s40360-022-00628-9
Received:
Accepted:
Published:
DOI: https://doi.org/10.1186/s40360-022-00628-9