Abstract
Immunotherapy has emerged as a pivotal modality in cancer treatment, with immune checkpoint inhibitors effectively combating malignancies by impeding crucial pathways within the immune system and stimulating patients’ immune responses. Soluble forms of immune checkpoints exhibit a remarkable diversity and can be readily tracked in circulation, holding immense potential as biomarkers for cancer treatment. An increasing number of studies focused on soluble immune checkpoints in cancer have emerged thanks to technological advancements. In this systematic review, we comprehensively summarized the recent studies on soluble immune checkpoints in human cancer risk prediction, outcome prediction, therapeutic applications, and potential molecular mechanisms, which demonstrated the promising future of soluble immune checkpoints in clinical applications. The clinical relevance of soluble immune checkpoints has been recognized in multiple cancers, yet the therapeutic applications and mechanisms remain obscure. Interpreting the impacts and mechanisms of soluble immune checkpoints could shed a light on the novel strategies of cancer screening, treatments, and outcome prediction.
Similar content being viewed by others
Introduction
Immunotherapy has been revolutionizing cancer treatments, especially immune checkpoint inhibitors (ICIs), bringing significant efficacy in solid tumor treatments. Immune checkpoint molecules, like programmed cell death protein 1 and its ligand (PD-1/PD-L1), cytotoxic T-lymphocyte antigen-4 (CTLA-4), could play substantial roles in both maintaining immune tolerance and eliminating tumors. ICIs have been applied in cancer immunotherapy, which demonstrated significant efficacy in multiple types of cancer by activating adaptive immunity.
The efficacy of ICIs mainly depends on the levels of immune checkpoint molecules in tumors [1]. For example, PD-L1 expression on tumor cells has become a common biomarker in selecting lung adenocarcinoma [2] and bladder cancer [3] patients for ICI therapy. However, limited volumes of biopsy samples and intra-tumor heterogeneity restrain the prediction of response to ICIs [4, 5]. Therefore, to identify sensitive, easily acquired and minimally invasive biomarkers is urgently needed.
Soluble immune checkpoint molecules are soluble isoforms of immune checkpoint molecules in circulation, which play distinct roles during carcinogenesis. Soluble immune checkpoint molecules could interact with their receptors/ligands in tumors, thereby affecting anti-tumor immunity. Thus, they have been utilized as biomarkers for predicting disease risks, outcomes, and treatment responses in various cancers. For example, soluble (s)PD-1/PD-L1have a potential role in the prediction of prognosis and treatment of pancreatic cancer (PDCA) [6]. In addition, sPD-L1 and sPD-1 levels could serve as unfavorable prognostic factors for ovarian cancer (OC), and sCTLA-4 is also considered as a potential biomarker in the diagnosis of OC [7]. Despite this, soluble immune checkpoint molecules remain difficult to be detected due to their low levels in the blood. In recent years, multiple technical advances have enabled us to precisely detect the levels of these biomarkers, like sPD-1 and sPD-L1 [8, 9]. Therefore, soluble immune checkpoint molecules could be promising biomarkers in cancer screening, prognosis prediction and treatment.
Here, we systematically review the recent literatures regarding the roles of soluble immune checkpoint molecules in cancer. We elucidate the applications of soluble immune checkpoint molecules in disease risk, outcome prediction and therapeutic potentiality in cancer, reflecting current researches and prospectives. We further illustrate the molecular mechanisms of soluble immune checkpoint molecules in tumor microenvironment (TME), highlighting their crosstalk with key signaling pathways in cancer.
Soluble immune checkpoint molecules in cancer screening
Soluble immune checkpoint molecules are identified as biomarkers in cancer screening and early detection in multiple cancers. Soluble PD-1, PD-L1, CD28 family of receptors, B7 ligands families, LAG3, etc. all have reported associations with cancer susceptibility. A summary of the information gathered on the soluble immune checkpoints is shown in Table 1.
Soluble PD-1
PD-1 is the most extensively studied co-inhibitory immune checkpoint receptor in T cells, binding to its ligands PD-L1 and PD-L2. PD-1 could generate soluble isoforms through alternative splicing, which are served as predictive biomarkers in cancer screening.
Elevated sPD-1 levels are significantly associated with increased susceptibility of cancer. A case-control study revealed that with a 1 pg/ml increase in sPD-1 levels, the risk of HBV-associated hepatocellular carcinoma (HCC) increased 2.02-fold in a multivariate logistic regression model [10]. The sPD-1 levels were significantly elevated in triple-negative breast cancer (TNBC) patients before neoadjuvant chemotherapy (NAC) compared to the healthy group (mean ± SD; 549.3 ± 58.76 vs. 379.2 ± 17.30 pg/mL) [11]. Similarly, sPD-1 levels were found significantly higher among the patients compared to the matched healthy donors in lung adenocarcinoma, [11] aggressive prostate cancer (PCa), [12] papillary thyroid cancer, [13] and classical Hodgkin lymphoma (cHL) [14].
However, opposite results were also reported. A case-control study involving 100 gastric cancer (GC) patients and 60 healthy donors found that sPD-1 levels were significantly lower in the former group, while the sPD-1 levels were not associated with cancer risk [15]. Another study showed that the mean levels of sPD-1 were 53.07 ± 24.23 pg/mL in the healthy donors and 47.99 ± 39.32 pg/mL in the group of colorectal cancer (CRC) patients [16]. Similar results were also reported in breast cancer (BC), [17] renal cell carcinoma (RCC), [18] and non-small cell lung cancer (NSCLC) [19].
Soluble PD-L1
PD-L1 and PD-L2 are major ligands of PD-1, playing substantial roles in ICI therapy. sPD-L1 could also be utilized as a screening biomarker for patients with various cancers, including HCC, GC, lung cancer (LC) and bladder cancer [20].
sPD-L1 has been reported to be associated with disease susceptibility in multiple cancers. In a study of small cell lung cancer (SCLC), the mean sPD-L1 level in the SCLC patients was 1.74 ± 0.82 ng/ml, while its level was 0.59 ± 0.33 ng/ml in the healthy control group [21]. In a prospective cohort study, the preoperative median sPD-L1 levels in the GC patients (71.69 pg/mL) were significantly higher than the healthy controls (35.34 pg/mL), and the area under the curve (AUC) for GC diagnosis based on sPD-L1 was 0.96 (95% confidence interval (CI): 0.93–0.99) [22]. sPD-L1 levels were also found to be significantly elevated in the patients with relapsed/refractory multiple myeloma and bone tumors compared to the healthy donors [23, 24]. Similar results were also observed in studies of aggressive PCa, [12] papillary thyroid cancer, [13] BC, [17] lymphoma, [25,26,27] cervical cancer, [28] OC, [29] endometrial cancer, [30] mesothelioma, [31] pancreatic cancer, [32] and NSCLC [33].
However, decreased sPD-L1 levels were found in the patients with CRC, [16] RCC, [18] BC, [34] and OC [35]. Heterogeneity among cancer sites, race disparity, retrospective design and different methodologies may influence the findings, a multi-center based prospective study could help address the role of sPD-L1 in cancer.
The CD28 family of receptors
Soluble CTLA-4 and soluble CD28
CTLA-4 competes with CD28 to bind the common ligands B7-1 (CD80) and B7-2 (CD86), constituting the most definitely characterized regulatory T cell pathway [36]. Therefore, CTLA-4 and CD28 soluble isoforms play vital roles in anti-tumor immune responses.
Several studies reported the roles of soluble CTLA-4 and CD28 in cancer screening. One study reported that the median plasma levels of CTLA-4 and CD28 in patients with early-stage BC were both significantly lower than that in the healthy controls [34]. The median sCTLA-4 levels in metastatic melanoma patients were also slightly lower than that in the healthy donors, but the difference was not statistically significant [37]. Interestingly, another case-control study demonstrated that the CTLA-4 levels in patients with basal cell carcinoma (BCC) were significantly increased compared with the healthy individuals, with the AUC of 0.757 (95% CI: 0.597–0.859) for the BCC prediction model [38]. Similar results were also observed in GC [39].
Soluble BTLA
B and T lymphocyte attenuator (BTLA) is another substantial co-inhibitory receptor on T cells, and its ligand is herpesvirus entry mediator (HVEM). BTLA/HVEM axis is a promising target for cancer immunotherapy. It was reported that sBTLA levels exhibited a significant increase in the pancreatic ductal adenocarcinoma (PDAC) patients compared to that in the healthy donors. Multivariable logistic regression model indicated that sBTLA was significantly associated with PDAC risk (odds ratio (OR) = 1.46, 95% CI: 1.01–2.17) [40].
The B7 family of ligands
The B7 family of ligands, belonging to the immunoglobulin superfamily, bind to the CD28 family of receptors on lymphocytes and regulate immune responses through co-stimulatory or co-inhibitory signals [41]. B7 family members, including B7-1/CD80, B7-2/CD86, PD-L2, and B7-H2 play critical roles in cell proliferation, cytokine secretion and TME regulation [42]. A few studies focused on sCD80 and sCD86 found that the median levels of sCD80 (1613.27 vs. 2329.77 pg/mL) and sCD86 (11199.42 vs. 14297.09 pg/mL) were significantly lower in the early-stage BC patients compared with the healthy donors [34].
Interestingly, sB7-H5 levels in the GC, CRC, LC and PDAC patients were significantly increased compared with the healthy controls, which showed a diagnostic value for these cancers [32, 43]. In addition, a retrospective study revealed that sB7-H4 levels gradually increased from cervicitis to cervical cancer, and decreased after treatment [44].
Soluble LAG-3
Lymphocyte activation gene-3 (LAG-3) is a novel immunosuppressive receptor which is abnormally expressed in various TMEs, and is a substantial immune checkpoint molecule in tumor immune response. sLAG-3 was identified as a promising serum biomarker for the early detection of BCC, [38] and lymphatic leiomyoma [45]. Similarly, Li et al. found that the median sLAG-3 levels in patients with cervical cancer were significantly lower than that in the healthy controls (3.76 vs. 8.36 ng/mL), and low sLAG-3 level was an independent predictor of cervical cancer [46]. However, sLAG-3 was significantly positively associated with PDAC risk (OR = 1.52, 95% CI: 1.04–2.28) in a multivariate logistic regression model [40]. Additionally, increased sLAG-3 levels were also associated with the increased susceptibility in advanced clear cell RCC (ccRCC) [47] and NSCLC [48].
Soluble TIM-3
T cell immunoglobulin mucin-3 (TIM-3) is a negatively regulated immune checkpoint protein, which inhibits the activation and proliferation of T cells and leads to the immune escape of tumor cells. Therefore, sTIM-3 could be used as a biomarker in cancer screening.
The median levels of sTIM-3 were significantly elevated in BCC patients (7978 pg/mL) compared to the healthy controls (1129 pg/mL), with the AUC of 0.848 (95% CI: 0.721–0.919) in the sTIM-3 incorporated model, suggesting that sTIM-3 could be an effective predictor of BCC susceptibility [38]. Moreover, another study showed that the median sTIM-3 levels were significantly elevated in 45 PDAC patients compared with 50 non-PDAC participants (4585 vs. 2026.5 pg/mL) [47]. Similar results were also found in NSCLC [48]. In addition, sTIM-3, sLAG-3 and sCD137 based signature could help improving the accuracy of NSCLC diagnosis [48].
The TNF superfamily
The tumor necrosis factor (TNF) superfamily currently comprises 19 ligands and 29 receptors, some of which are expressed on immune cells and participate in the development of tumor-specific immune responses. These molecules also have splicing variants, resulting in soluble isomers that can be traced in body fluids like serum. For example, sGITR, sGITRL, sCD27 and sCD40 were significantly decreased in the patients with early-stage BC [34].
In a prospective and exploratory cohort study, sCD40 levels were significantly elevated in the elderly GC patients compared with the healthy elderly individuals, whereas sCD40L levels were significantly decreased [49]. sCD40 was also considered as a non-invasive biomarker for PDAC diagnosis (AUC = 0.795) [49]. In addition, elevated plasma levels of sOX40 could be used as biomarkers for the diagnosis of acute T-cell leukemia [50].
sHVEM is the soluble isoform of dual immune checkpoint HVEM. One study revealed a significant increase of sHVEM levels in the BC patients (mean ± SD; 4612 ± 2329 vs. 2946 ± 1857 pg/mL) and GC patients (mean ± SD; 4528 ± 1915 vs. 2946 ± 1857 pg/mL) compared to the control group, although this change of sHVEM levels in the CRC patients was not statistically significant [51]. Similar results were obtained in another GC study, where sHVEM levels of GC patients were significantly higher than the non-ulcer dyspepsia patients [52]. By contrast, the early-stage BC patients in another study had relatively lower sHVEM levels compared with the healthy individuals (1866.92 vs. 2290.19 pg/mL) [34].
Other soluble immune checkpoints
Several soluble immune checkpoint molecules under investigation, like sGARP, sMIC-A, sIDO, sICOS, sCD33, and sTLR-2, showed levels significantly variated in the cancer patients, but their potential of prediction in cancer screening still await further exploration [34, 53, 54, 55, 56, 57].
Soluble immune checkpoint molecules in cancer outcomes prediction
Soluble immune checkpoint molecules are associated with cancer outcomes, including survival, recurrence, and response to treatment. Understanding the predictive performance of these soluble immune checkpoints on cancer outcomes is conducive to screening the most suitable treatments for patients and monitoring disease development.
Soluble PD-1
sPD-1 was reported associating with the prognosis of multiple cancers, though the conclusions of some studies remain controversial.
Studies showed that higher baseline sPD-1 levels were associated with poorer prognosis in the patients with diffuse large B cell lymphoma, [58] OC, [7] PDAC, [59, 60] PCa, [55] ccRCC, [18, 61] and CRC [16]. In a multicenter prospective study of 439 GC patients treated with nivolumab, [62] higher sPD-1 levels were associated with the worse overall survival (OS). Melanoma patients with higher baseline sPD-1 levels also experienced the worse OS after ICI therapy [63]. For HCC patients who underwent liver transplantation, Hwang et al. found that higher sPD-1 level was an independent risk factor of recurrence [64]. Patients with TNBC in complete or partial remission to NAC had significantly decreased sPD-1 levels compared to the patients who did not respond well [65]. And increased sPD-1 levels after anti-PD-1 antibody therapy were also found correlating with the accelerated progression of solid tumors [66].
However, other studies demonstrated that sPD-1 could be a favorable prognostic factor for patients with cancers. A Japanese study reported that higher sPD-1 levels were associated with the improved OS in patients with NSCLC receiving anti-PD-1 immunotherapy, [67] which was consistent with another prospective study [68] and a case-control study [69]. Higher sPD-1 levels were also shown to be associated with the better OS in patients with nasopharyngeal carcinoma (NPC) after definitive intensity-modulated radiotherapy, [70] and in GC patients after gastrectomy [71]. In a study of HCC, [72] researchers found that sPD-1 was a favorable independent predictive factor for disease-free survival (DFS) (hazard ratio (HR) = 0.32, 95% CI: 0.14–0.74) and OS (HR = 0.54, 95% CI: 0.30–0.98). Metastatic melanoma patients treated with ICIs were revealed to have increased sPD-1 levels which was correlated to superior progression-free survival (PFS) [73]. And other researchers also found the association between higher baseline sPD-1 levels and better PFS in metastatic ccRCC patients treated with nivolumab [74]. In a study of advanced head & neck cancer (HNC), patients with higher baseline sPD-1 levels responded better to anti-PD-1 treatment than patients with lower concentrations, and these patients experienced prolonged PFS [68].
The intriguing role of sPD-1 in survival prediction of multiple cancers may derived from the interaction between sPD-1 and its ligands in TME. sPD-1 could compete with membrane-bound PD-1 from binding with PD-L1 in vivo, which in turn enhance the anti-tumor immunity [75, 76]. In contrast, sPD-1 could also impair the T cell proliferation and IL2 production through combining with PD-L1 on dendritic cells in vitro [77]. The complicated interaction between PD-1 and its ligands resulted in the alteration of anti-tumor immunity, subsequently affect the survival of cancer patients. However, the mechanisms underlying remain obscure for inconsistent findings between in vivo and in vitro studies, more investigation is warranted to illustrate the mechanisms.
Soluble PD-L1 and soluble PD-L2
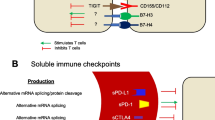
Soluble PD-L1
As one of the most well-studied soluble immune checkpoint ligands, sPD-L1 was considered an unfavorable prognostic factor in a wide variety of cancers by most studies. To briefly summarize current studies on sPD-L1, we depicted Fig. 1 to show its impact on cancer prognosis.
sPD-L1 level was associated with impeded anti-tumor immunity and poor outcomes in multiple cancers. The sPD-L1 could bind with the PD-1 receptor on T cells, thereby inducing T cell exhaustion and inhibiting T cell functions, eventually leading to immune evasion. Elevated levels of sPD-L1 are reported to be associated with the poor outcomes in multiple cancers
First, studies revealed that baseline sPD-L1 level was an independent adverse predictor of OS for multiple cancers [78,79,80,81]. In prospective studies of NSCLC patients treated with ICIs, patients with higher levels of circulating sPD-L1 had poorer OS [67, 82]. Also, patients with higher sPD-L1 levels had shorter OS than patients with lower levels in a retrospective study of 120 advanced NSCLC patients, [83] and similar conclusions were strongly agreed in several meta-analyses [84,85,86,87]. A worse OS was observed in mesothelioma patients with higher baseline sPD-L1 levels [31]. In a cohort of 219 NPC patients, higher sPD-L1 levels appeared to be associated with poorer OS [88]. The relationship between higher baseline sPD-L1 levels and shorter OS had been revealed in many other types of cancers, including esophageal cancer, [89, 90], GC, [22, 62, 91, 92, 93, 94] HCC, [72, 95, 96, 97, 98, 99, 100] biliary tract cancer, [101] RCC, [18, 102] upper tract urothelial carcinoma, [103] lymphoma, [104,105,106,107] OC, [7, 108] CRC, [109, 110] soft tissue sarcoma (STS),[111, 112] glioma, [113, 114] and PDAC [59, 115].
Second, higher baseline sPD-L1 levels could also be a biomarker of poor PFS, DFS, or time to progress in cancer patients [79,80,81]. For example, preoperative circulating sPD-L1 levels were negatively correlated with recurrence-free survival (RFS) [109] and DFS [110] in the CRC patients. A high level of plasma sPD-L1 could be an independent unfavorable prognostic factor of PFS in the patients with metastatic ccRCC [61]. STS patients with higher sPD-L1 levels from the PEMBROSARC basket study tended to experience shorter PFS [111]. Similar results were observed in other cancers, like LC, [67, 69, 82, 83, 84, 85, 86, 87116117] HNC, [88, 118] esophageal cancer, [89, 90] GC, [62, 91, 92, 93, 119] HCC, [72, 95, 96, 97, 99] OC, [7] lymphoma, [104,105,106] glioma, [114] and PDAC [115].
Third, cancer patients with poorer response to treatments tended to have higher baseline sPD-L1 levels than those who had ideal response. For instance, the serum levels of sPD-L1 were significantly higher in ICIs non-responsive HNC patients than that in the responders [100]. In a cohort of esophageal cancer patients treated with anti-PD-1/PD-L1 monotherapy, patients with higher baseline sPD-L1 levels displayed a remarkably increased disease control rate versus that of the lower subgroup [90]. As for the metastatic RCC patients treated with PD-1 inhibitor nivolumab, however, higher baseline sPD-L1 levels were correlated to higher rate of progressive disease [102, 120]. For chemotherapy-treated patients with lymphoma, both lower basal sPD-L1 levels [25, 106] and the reduction of sPD-L1 levels after treatment [107] were associated with higher response rate. Patients with LC,[87, 116, 121, 122] or other solid tumors [80, 90, 123] who have higher baseline sPD-L1 levels also tend to experience adverse clinical response. Meanwhile, sPD-L1 levels could also be used as a risk biomarker for the occurrence of cancer metastasis in patients with CRC, [16, 124] upper tract urothelial carcinoma, [103] STS, [112] NPC, [88] and ccRCC [18].
However, a few studies indicated that higher baseline sPD-L1 levels were associated with the better treatment response or the longer PFS and OS in patients with cancer, such as lymphoma, [27] metastatic ccRCC, [74] and NSCLC [125].
In addition to baseline levels, dynamic changes of sPD-L1 levels during treatment were also reported associating with the prognosis of multiple cancers. In general, the reduction of sPD-L1 levels during treatment was predictive of better prognosis for a variety of cancers, [126] including GC, [91] metastatic ccRCC, [120] biliary tract cancer, [101] TNBC, [65] lymphoma,[26, 107, 127] pancreatic cancer, [115] CRC, [109] and NSCLC, [67] regardless to treatment modalities. However, other studies suggested that the decrease of sPD-L1 levels was associated with the poor prognosis in patients with LC, [21, 69] or mesothelioma [31].
Interestingly, sPD-L1 could also be combined with other biomarkers to enhance the accuracy of prognosis prediction in cancer. For instance, the combinations of sPD-L1 with PD-L1 in tumor cells [128] or PD-L1 positivity in tumor tissues [18] were more beneficial in assessing the postoperative prognosis and the OS of patients with NSCLC or ccRCC. sPD-L1 could also be combined with sPD-1,[69, 125, 129] sCTLA-4, [110] Epstein-Barr virus DNA, [88] CCL5, [90] and Glasgow prognostic score [62] to better predict cancer outcomes.
Therefore, sPD-L1 is a promising biomarker in predicting outcomes and treatment responses in cancer patients, though more prospective, independent validated studies are still warranted.
Soluble PD-L2
PD-L2 was another substantial ligand of PD-1, whose clinical significance remains obscure. Soluble PD-L2 was reported in several studies as prognostic biomarker in multiple cancers.
Higher baseline sPD-L2 levels were associated with the better clinical response to dendritic cell vaccine therapy in patients with advanced melanoma [130]. It was also associated with the higher risk of biochemical recurrence and progression in PCa patients [55]. A multicenter study revealed a significant positive correlation between baseline sPD-L2 levels and the occurrence of immune-related adverse events (irAEs) in cancer patients receiving immunotherapy [131].
Increased levels of sPD-L2 were significantly associated with higher risk of recurrence in patients with ccRCC [47] (HR = 2.51, 95%CI: 1.46–4.34) and higher risk of invasive disease in a cohort of NSCLC [132] patients (OR = 4.23, 95% CI: 1.20–17.70). And when combined with other variables like sCD27, the prediction performance of sPD-L2 was greatly improved [132].
The CD28 family of receptors
Soluble CTLA-4 and CD28
sCTLA-4 and sCD28 could be prognostic predictors for multiple cancers. Higher levels of baseline sCTLA-4 were associated with the shorter PFS in patients with cHL (HR = 4.30, 95%CI: 1.54–13.26) [133] or glioma (HR = 2.52, 95%CI: 1.01–6.28) [134]. Another cohort study suggested that both sCD28 and sCTLA-4 levels were predictors of biochemical recurrence in the PCa patients [55]. Similarly, higher sCTLA-4 levels at baseline were also significantly associated with the worse OS, DFS or disease progression in patients with GC, [62] CRC, [110] or HNC [135]. Besides baseline levels, dynamic changes of sCTLA-4 and sCD28 were also found associating with OS in the patients with HBV-related advanced HCC in a multicenter study [136]. Interestingly, for the HCC patients treated with radiofrequency ablation, higher baseline sCTLA-4 levels were linked to the shorter DFS of local recurrence (HR = 2.43, 95%CI: 1.03–5.75) but longer RFS of intrahepatic metastasis (HR = 0.19, 95%CI: 0.05–0.81), which showed the dual roles of sCTLA-4 in immune responses. And this performance of sCTLA-4 could be improved when combined with baseline alpha-fetoprotein levels [137].
Other members of the CD28 family
Besides sCTLA-4 and sCD28, soluble forms of other CD28 family members could also be served as biomarkers for cancer outcomes.
In a cohort of solid tumor treated with ICIs, researchers found that the patients with higher levels of baseline sBTLA had worse OS [138]. Likewise, PCa patients with higher baseline sBTLA levels had the higher risk of progression [55]. Similar correlations were also demonstrated in the patients with PDAC, [59] chronic lymphocytic leukemia, [139] ccRCC, [47] and advanced HCC [136, 140]. A multicenter observational study of 81 NSCLC patients [141] showed that elevated sICOS levels during treatment were linked to the improved OS and PFS.
The B7 family of ligands
As ligands of the CD28 family, the B7 family proteins play a crucial role in regulating T cell activation and tolerance through co-stimulatory and co-inhibitory pathways, thereby extensively involve in tumor immune evasion. Their soluble forms could be promising predictive factors of cancer outcomes.
Higher baseline levels of sCD80 were associated with the worse OS and PFS in patients with STS, [142] NSCLC, [143] and PCa [55]. In addition, studies showed that dynamic changes of sCD80 during treatment were associated with the OS of patients with HBV-related advanced HCC, [136] and the risk of invasive disease of NSCLC [132]. Higher level of sCD86 could be an independent predictor of poorer OS in the patients with multiple myeloma [144]. And both higher levels of sB7-H3 and sB7-H4 at baseline were found to be associated with the better OS (sB7-H3: HR = 0.33, 95%CI: 0.14–0.78; sB7-H4: HR = 0.42, 95%CI: 0.19–0.94) and PFS (sB7-H3: HR = 0.32, 95%CI: 0.17–0.64; sB7-H4: HR = 0.32, 95%CI: 0.16–0.64) in the patients with NSCLC [117].
Soluble LAG-3
Baseline sLAG-3 levels are associated with patients’ outcomes in multiple cancers, and dynamic changes of sLAG-3 levels could be applied in disease monitoring.
Baseline sLAG-3 levels were associated with poor response to immunotherapy in the patients of advanced PDAC, [60] and melanoma [130]. Moreover, studies showed that the increase of sLAG-3 during treatment might predict the worse OS and the clinical responses of patients with HBV-related advanced HCC treated with icariin, [136] and the patients with locally advanced cervical cancer after concurrent chemoradiotherapy [145]. Also, a significant positive correlation between basal circulating levels of sLAG-3 and the occurrence of irAEs in cancer patients receiving immunotherapy was reported in a multicenter study [131].
Soluble TIM-3
sTIM-3 could also be a biomarker for cancer outcomes. Higher baseline sTIM-3 levels were associated with higher recurrence risk of the ccRCC patients [47] and worse OS of the PDAC patients [146]. Despite this, changes of sTIM-3 levels during treatment could also be an unfavorable sign of the OS in patients with HCC [136] or the development of relapses to chimeric antigen receptor T-cell therapy in patients with mantle cell lymphoma (MCL) [127].
The TNF superfamily
Both soluble TNF receptors and ligands were reported as biomarkers of cancer outcomes and adverse reactions to cancer treatments.
For the patients with advanced HCC, dynamic changes of sTNF-α receptor I during Lenvatinib treatment were associated with the response to Lenvatinib treatment [147]. Elevated levels of baseline and post-treatment sTNF-R1 and sTNF-R2 were correlated with decreased OS in the patients with advanced urothelial carcinoma who treated with ICIs [148]. Higher levels of s4-1BB at baseline could also predict the poorer OS in patients with metastatic uveal melanoma [149] and the occurrence of irAEs in other type of cancers [131]. Baseline levels of s4-1BB might predict the risk of MCL patients’ recurrence [127] and the aggressiveness of NSCLC, [132] as well as the clinical response to 4-1BB agonist therapy [150].
Increased sCD27 levels were significantly associated with the higher risk of invasive disease in a NSCLC cohort [132]. In contrast, another study indicated that higher levels of sCD27 after ICI therapy could predict clinical benefit in the patients with advanced solid tumors [151]. Higher baseline levels of sHVEM might also indicate the higher risk of biochemical recurrence and progression in PCa patients [55]. In addition, higher baseline levels of sOX40, [152] sCD30, [148] sCD40, [153, 154] and sGITR [55] were associated with worse prognosis in cancer patients.
Being a soluble form of dual immune checkpoint HVEM, the basal circulating levels of sHVEM were positively correlated with the toxicity of irAEs for cancer patients receiving immunotherapy [131]. A multicenter study revealed a significant positive correlation between baseline sCD27 levels and the occurrence of irAEs [131].
On the other hand, as for the soluble forms of the TNF ligands, lower sCD95L levels in the OC patients could be independent poor prognostic factors for the risk of recurrence (HR = 2.63, 95% CI: 1.16–5.95) [155]. And higher sCD70 levels at baseline were found to be associated with better response and PFS in the NSCLC patients [68]. However, higher levels of sCD254 might be a marker of worse clinical response in the metastatic RCC patients treated with nivolumab [156].
Other soluble immune checkpoints
Other soluble immune checkpoints were also reported associating with cancer outcomes by researchers. For instance, higher soluble intercellular adhesion molecule 1 (sICAM-1) levels were associated with better PFS and OS in many types of cancers [123]. Despite this, higher baseline sICAM-1 levels could predict worse tumor-free survival in the HCC patients treated with radical hepatectomy, especially when combined with alpha-fetoprotein indicators [157].
In addition, although under-studied, higher baseline levels of many other soluble immune checkpoints including sIDO, [55, 60] sMIC-A,[57, 158] sCD8, [159] sCD73,[160, 161] sCD163, [148] and soluble urokinase plasminogen activator receptor [162] were found to be associated with poor prognosis in the patients with various types of cancers. Furthermore, Yoshida et al. found that an increase in sCD226 during chemotherapy might predict worse treatment response in the patients with esophageal cancer [163].
Signatures of soluble immune checkpoints
Interestingly, there are studies on solid tumors,[151, 164] locally advanced rectal cancer, [165] and PDAC, [40] focusing on the integration of multiple soluble immune checkpoints as composite signature. And these comprehensive predictive models tended to have a higher predictive value than a single molecule.
In summary, we summarized the role of some crucial soluble immune checkpoint molecules in cancer prognosis prediction (Table 2).
Therapeutic applications of soluble immune checkpoint molecules in cancer
We illustrated the successful applications of soluble immune checkpoints as biomarkers of cancer outcomes and therapeutic responses in multiple cancers. Further, soluble immune checkpoints could also serve as treatment targets or therapeutic modalities in cancer patients.
The potential therapeutic value of soluble immune checkpoints
On the one hand, soluble immune checkpoints can be potential therapeutic targets. A study revealed that the CRC patients who had scarce tumor-infiltrating lymphocytes (TILs) in tumor had significantly higher sOX40 levels compared to the patients with TILs, suggesting that targeting sOX40 might hold promise for immunotherapy [166]. Likewise, a recent study demonstrated that targeting sMIC alongside non-blocking antibodies could provide dual co-stimulation to antigen-specific CD8+ T cells through NKG2D and CD28, thereby improving the anti-tumor immunity [167]. Subsequently, researchers demonstrated combining anti-PD-L1 ICIs with antibody targeting sMIC significantly improved the survival rate of mice compared to monotherapy, suggesting potential therapeutic implications for patients with MIC+/sMIC+ metastatic melanoma [168].
On the other hand, changing the levels of soluble immune checkpoints and blocking the interactions between soluble immune checkpint proteins and membrane receptors or ligands have potential therapeutic values for cancers. For example, therapeutic plasma exchange in the melanoma patients could enhance the efficacy of immunotherapy by reducing the levels of sPD-L1 and extracellular vesicles PD-L1 [169, 170]. Moreover, a recent study demonstrated that the small molecule inhibitors CH-4 and its analogue CH-4.7 could effectively inhibit the PD-1/sPD-L1 interaction, thereby enhancing anti-tumor immunity in the T cell acute lymphoblastic leukemia model [171]. Similarly, the vaccinia virus M2 protein, capable of binding to CD80/CD86 and inhibiting their interactions with soluble CD28/CTLA-4, while promoting the binding of sPD-L1 and sCD80, exhibited potential as a novel immunosuppressive agent [172].
Soluble immune checkpoints as therapeutic modalities
Monotherapy
Soluble immune checkpoints may serve a similar function to membrane antibodies, and are therefore anticipated to be utilized in the treatment of cancer. For example, sPD-1 demonstrates a functional efficiency comparable to that of anti-PD-1 or anti-PD-L1 monoclonal antibodies (mAb), interfering the interaction between PD-L1 or PD-L2 ligands and their cognate receptor, membrane-bound PD-1 (mPD-1) on the surface of T lymphocytes. Therefore, sPD-1 could serve as an alternative “antibody” to mAb-based immunotherapy and promised preferable anti-tumor immune effects in OC [173] and BC [75] models. In addition, a study revealed that L3C7c, a high-affinity variant of human sPD-L1, could improve the ability of T cells to inhibit melanoma growth and showed promise as a new-generation tumor immunotherapy agent based on PD-1/PD-L1 axis blockade [174]. Similarly, sCD80 could also increase tumor-infiltrating T cells and significantly prolong the survival time of tumor-bearing mice [175]. Targeting alternative splicing also has the potential to be a novel cancer immunotherapy. Inhibiting serine arginine-rich splicing factor (SRSF1 and SRSF3) could regulate alternative splicing of PD-1 to generate sPD-1, thereby preventing T cell exhaustion [176, 177]. In conclusion, soluble immune checkpoints might be a novel therapy for cancer treatment.
Combined therapy
Construction of recombinant vector
Oncolytic viruses are an excellent platform for developing effective strategies in cancer immunotherapy. However, several challenges remain in the use of viro-immunotherapy for cancer. Therefore, some researchers combine viruses with soluble immune proteins to efficiently overcome several major hurdles. For example, NDV/Anh-TRAIL, a recombinant Newcastle disease virus (NDV) Anhinga strain capable of secreting soluble TNF-related apoptosis-inducing ligand (TRAIL), showed potential as a candidate drug for glioma treatment [178]. In China, Wei and his colleagues generated a recombinant adenovirus expressing a soluble fusion protein, sPD1/CD137L, which was effective in suppressing tumor growth and improving survival in the HCC mouse model [179].
Furthermore, soluble recombinant 4-1BBL protein generated by fusing the extracellular domains of murine 4-1BBL to a modified version of streptavidin, could inhibit the development of lung tumors induced by tobacco carcinogens in mice [180]. Similarly, a recombinant vector pMCSG7-hsTNF-R2 was constructed to generate human soluble TNF-R2 recombinant protein, which was expected to be used as an immunotherapy drug for TNF-R2+ cancer in an in vitro bioactivity evaluation [181].
Combined with other therapeutic strategies
First, several challenges remain in the use of immunotherapy for cancer, such as poor immune cell infiltration, insufficient co-activation signals, and negative regulation of immune checkpoints. Combine soluble immune checkpoints with immunotherapy might improve anti-tumor immunity. Recent studies mostly focused on combination with CAR T-cell immunotherapy. For example, Zhang et al. established modified CAR-T cells called sPD-1 CAR-T cells, which could secrete sPD-1 and had higher cytotoxicity against CD19+ PD-L1+ tumor cells in vitro compared with conventional CAR-T cells. The sPD-1 CAR-T cells could effectively reduce tumor burden and prolong the survival time of mice [182]. Similarly, researchers of another study engineered CAR T cells to secrete the soluble trimeric 4-1BBL fused to anti-PD-1 single-chain fragment variable region (αPD1-41BBL), and the CAR19.αPD1-41BBL T cell-treated mice displayed significant improved tumor growth control and OS [183]. Also, Xia et al. designed HER2-specific sPD-1-CAR-NK cells for BC treatment. These bio-engineered NK cells could transport sPD-1 specifically into cancer cells with high HER2 expression, thereby enhancing the anti-tumor effect of HER2-CAR-NK cells [184].
Second, soluble immune checkpoints could also be combined with other therapeutic strategies. In a study combined sPD-1-mediated immune checkpoint therapy with chlorin e6-assisted sonodynamic therapy, Tan et al. generated nanobubbles loaded simultaneously with sPD-1 and chlorin e6. Compared with monotherapy, the combined therapy showed the best immunotherapy effect on HCC [185]. Besides, targeting alternative splicing combined with adoptive cellular immunotherapy could enhance the levels of sPD-1 and reverse T cell exhaustion by disrupting mPD-1/PD-L1 interaction in effector T cells [186].
The above treatments were mostly tested in mice or cell lines. Encouragingly, there are already human clinical trials exploring the safety and efficacy of soluble immune checkpoints in combination with other therapies. Researchers in a study combined sLAG-3 with the PD-1 antagonist pembrolizumab to treat patients with metastatic melanoma and the results showed strong antitumor activity [187]. Later, Hans et al. combined sLAG-3 with paclitaxel in a treatment for metastatic HR+ BC patients and displayed a numerically improvement in OS, though not statistically significant [188].
Overall, we also summarized the applications of some crucial soluble immune checkpoint molecules in cancer treatment (Table 3).
Molecular mechanisms of soluble immune checkpoint molecules in cancer development
Soluble immune checkpoints can be produced by several molecular mechanisms: (1) ectodomains cleaved by proteolysis and excreted to extracellular space by enzyme release, (2) selective mRNA splicing, and (3) released as components of extracellular vesicles.189 These mechanisms prompt them to alter the body’s immunity through a plethora of mechanisms, which have an impact on the development of tumors. The interaction between soluble immune checkpoint molecules and membrane-bound immune checkpoints receptors/ligands in TME could significantly impact anti-tumor immunity and cancer outcomes. To make it clear, we depicted the interactions of mentioned soluble checkpoints and their membrane ligands/receptors in Fig. 2. Elucidating the fundamental mechanisms governing soluble immune checkpoints and their membrane counterparts in cancer could facilitate their utilization in guiding cancer therapeutic strategies.
First, soluble forms of co-suppressive immune checkpoints have different effects on cancer development. On the one hand, they could bind to the corresponding membrane-bound ligands/receptors, thereby hindering the inhibitory effect of membrane-bound ligands/receptors on immune cells, ultimately inhibiting tumor growth. For instance, sPD-1, retaining the function of full-length PD-1, is able to bind to mPD-1 ligands and thereby blocking their interaction with mPD-1 and increasing the effector function of T cells and NK cells [75, 189]. Similarly, sPD-L1 can act as a receptor antagonist, reversing T cell inhibition mediated by mPD-L1 [190]. Also, the soluble form of Siglec-5 (sSiglec-5) was found to intensify the cytotoxicity of T cells to cancer cells [191]. On the other hand, soluble forms of co-suppressive immune checkpoints could also inhibit the function of immune cells, thereby promoting cancer development. For example, in cHL cell lines, sPD-1 could induce PD-L1 reverse signaling, which was associated with inhibition of the mitogen-activated protein kinase (MAPK) pathway and reduced mitochondrial oxygen consumption, thereby promoting tumor growth, proliferation, and metabolism of cHL [14]. sPD-L1 has a similar inhibition to mPD-L1 on T effector cells in in vitro assays, which could induce regulatory B cell differentiation and inhibit peripheral T cells [192,193,194]. sCTLA-4 was also found to have immunosuppressive abilities like CTLA-4 [195]. Specifically, sCTLA-4 could restrict CD8+ T cells to a non-cytotoxic state and attenuate T cell activation, thereby inhibiting anti-tumor immunity and promoting tumor growth [196]. In BCC, sCD200 in TME could inhibit MAPK pathway signaling, resulting in the almost non-existence of tumor-infiltrating NK cells and further promoting tumor development [197].
Second, soluble forms of co-stimulatory immune checkpoints could also play different roles during carcinogenesis. Firstly, they could bind to corresponding membrane-bound ligands/receptors, thereby hindering the membrane-bound ligands/receptors from activating immune cells and ultimately promoting tumor growth. For instance, tumor-derived sMIC-A could bind to membrane-bound NKG2D receptors, thereby blocking the activation of NKG2D pathways, inhibiting the cytotoxicity of NK and T cells against tumor cells [57, 168]. Similarly, sCD160 could also exert immunosuppressive activity by binding to HLA molecules or HVEMs on target cells, thereby inhibiting the cytotoxicity of NK cells [198]. Secondly, soluble forms of co-stimulatory immune checkpoints could also promote the efficacy of immune cells, thereby inhibiting tumor development. For example, sCD80 could maintain T cell activity by simultaneously blocking PD-1 and binding to CD28. The activated T cells could increase the production of IFNγ and IL-2, which in turn boosting anti-tumor immunity via TCR and CD28 signaling [175].
As surface molecules on cancer cells or immune cells, membrane-bound immune checkpoints act through trans or cis interactions to modulate immune responses, depending on factors like expressing cells, relative expression levels, action forms, and downstream cells [199]. For example, trans-interaction of PD-L1 or PD-L2 with PD-1 on T cells can lead to inhibition of signaling, while the cis-interaction of PD-L1-CD80 can play a positive role in anti-tumor immunity. In contrast, soluble immune checkpoints could not only exhibit similar functional effects to membrane-bound immune checkpoints, but also have complicated impacts on the immune system due to their unique forms. Therefore, a comprehensive understanding of the roles of soluble immune checkpoints in TME is conducive to the development of immunotherapy in future.
Conclusion and prospective
Soluble immune checkpoint molecules have been a hotspot of research due to their pivotal roles of regulating immune responses in TME. In this review, we systematically reviewed the literatures regarding the major soluble immune checkpoint molecules in cancer screening, outcome prediction, and potential molecular mechanisms. Soluble immune checkpoint molecules could be easily detected in blood and tissues in multiple cancers, and they could be critical factors reflecting the risk of cancer susceptibility, prognosis, and the sensitivity to the treatment. Their interaction with corresponding receptor/ligand in the membrane of cells in TME also indicated potential therapeutic targets and molecular mechanisms (Fig. 3).
Soluble immune checkpoints in cancer risk prediction, outcomes prediction, therapeutic application, and molecular mechanism. Soluble immune checkpoints could be used as biomarkers for cancer surveillance strategies and targets for checkpoint blockade therapies, while also facilitating cancer immunotherapy and the exploration of immune mechanisms
Researches on soluble immune checkpoints in cancer are still expanding. sPD-1 and sPD-L1 could be the mainstream biomarkers of immunotherapy as well as the therapeutic targets interfering PD-1/PD-L1 binding in TME, though the molecular mechanisms remain unclear due to complicated splice/cleavage of the proteins. Further studies are also warranted to explore the predictive significance of other soluble immune checkpoints in cancer, like sLAG3 and sTIM3. Soluble immune proteins hold great promise for cancer treatment, either as monotherapy analogous to the function of monoclonal antibodies or in combination with other therapies to enhance overall antitumor activity and provide better treatment for patients. Therefore, more prospective clinical trials are required to provide more evidence of clinical applications of these soluble immune checkpoint molecules. In light of these explorations, we propose that soluble immune checkpoint molecules could be promising biomarkers and targets for cancer patients in the era of precise medicine.
Data availability
No datasets were generated or analysed during the current study.
Abbreviations
- BC:
-
Breast cancer
- BCC:
-
Basal cell carcinoma
- BTLA:
-
B and T lymphocyte attenuator
- ccRCC:
-
Clear cell renal cell carcinoma
- cHL:
-
Classical Hodgkin lymphoma
- CI:
-
Confidence interval
- CRC:
-
Colorectal cancer
- CTLA-4:
-
Cytotoxic T-lymphocyte antigen-4
- DFS:
-
Disease-free survival
- GC:
-
Gastric cancer
- HCC:
-
Hepatocellular carcinoma
- HLA-G:
-
Human leukocyte antigen-G
- HNC:
-
Head & neck cancer
- HR:
-
Hazard ratio
- HVEM:
-
Herpesvirus entry mediator
- ICIs:
-
Immune checkpoint inhibitors
- irAEs:
-
Immune-related adverse events
- LAG-3:
-
Lymphocyte activation gene-3
- LC:
-
Lung cancer
- mAb:
-
Monoclonal antibodies
- MAPK:
-
Mitogen-activated protein kinase
- MCL:
-
Mantle cell lymphoma
- MHC:
-
Major histocompatibility complex
- mPD-1:
-
Membrane-bound PD-1
- NAC:
-
Neoadjuvant chemotherapy
- NDV:
-
Newcastle disease virus
- NK:
-
Natural killer
- NPC:
-
Nasopharyngeal carcinoma
- NSCLC:
-
Non-small cell lung cancer
- OC:
-
Ovarian cancer
- OR:
-
Odds ratio
- OS:
-
Overall survival
- PCa:
-
Prostate cancer
- PD-1:
-
Programmed cell death protein 1
- PDAC:
-
Pancreatic ductal adenocarcinoma
- PD-L1:
-
Programmed cell death protein 1 ligand 1
- PFS:
-
Progression-free survival
- RCC:
-
Renal cell carcinoma
- RFS:
-
Recurrence-free survival
- SCLC:
-
small cell lung cancer
- sICAM-1:
-
Soluble intercellular adhesion molecule 1
- STS:
-
Soft tissue sarcoma
- TILs:
-
Tumor-infiltrating lymphocytes
- TIM-3:
-
T cell immunoglobulin mucin-3
- TME:
-
Tumor microenvironment
- TNBC:
-
Triple-negative breast cancer
- TNF:
-
Tumor necrosis factor
- TRAIL:
-
TNF-related apoptosis-inducing ligand
References
Morad G, Helmink BA, Sharma P, et al. Hallmarks of response, resistance, and toxicity to immune checkpoint blockade. Cell. 2021;184(21):5309–37. https://doi.org/10.1016/j.cell.2021.09.020. [published Online First: 2021/10/09].
Liu F, Zhang X, Lu M, et al. The association of genomic alterations with PD-L1 expression in Chinese patients with EGFR/ALK wild-type lung adenocarcinoma and potential predictive value of Hippo pathway mutations to immunotherapy. Cancer Med. 2024;13(3):e7038. https://doi.org/10.1002/cam4.7038. [published Online First: 2024/02/24].
Su X, Jin K, Guo Q, et al. Integrative score based on CDK6, PD-L1 and TMB predicts response to platinum-based chemotherapy and PD-1/PD-L1 blockade in muscle-invasive bladder cancer. Br J Cancer. 2024;130(5):852–60. https://doi.org/10.1038/s41416-023-02572-9. [published Online First: 2024/01/12].
Sankar K, Ye JC, Li Z, et al. The role of biomarkers in personalized immunotherapy. Biomark Res. 2022;10(1):32. https://doi.org/10.1186/s40364-022-00378-0. [published Online First: 2022/05/19].
Pesapane F, Suter MB, Rotili A, et al. Will traditional biopsy be substituted by radiomics and liquid biopsy for breast cancer diagnosis and characterisation? Med Oncol. 2020;37(4):29. https://doi.org/10.1007/s12032-020-01353-1. [published Online First: 2020/03/18].
Mucileanu A, Chira R, Mircea PA. PD-1/PD-L1 expression in pancreatic cancer and its implication in novel therapies. Med Pharm Rep. 2021;94(4):402–10. https://doi.org/10.15386/mpr-2116. [published Online First: 2022/09/16].
Świderska J, Kozłowski M, Nowak K, et al. Clinical relevance of Soluble forms of Immune Checkpoint molecules sPD-1, sPD-L1, and sCTLA-4 in the diagnosis and prognosis of Ovarian Cancer. Diagnostics (Basel). 2022;12(1). https://doi.org/10.3390/diagnostics12010189. [published Online First: 2022/01/22].
Goto M, Chamoto K, Higuchi K, et al. Analytical performance of a new automated chemiluminescent magnetic immunoassays for soluble PD-1, PD-L1, and CTLA-4 in human plasma. Sci Rep. 2019;9(1):10144. https://doi.org/10.1038/s41598-019-46548-3. [published Online First: 2019/07/14].
Wuethrich A, Rajkumar AR, Shanmugasundaram KB, et al. Single droplet detection of immune checkpoints on a multiplexed electrohydrodynamic biosensor. Analyst. 2019;144(23):6914–21. https://doi.org/10.1039/c9an01450k. [published Online First: 2019/10/28].
Ma Y, Hao YQ, Bi LQ. [Association of circulating levels of soluble PD-1, PD-1 gene polymorphisms with HBV infection and HBV infection-associated hepatocellular carcinoma]. Zhonghua Yu Fang Yi Xue Za Zhi. 2023;57(6):863–67. https://doi.org/10.3760/cma.j.cn112150-20220930-00947. [published Online First: 2023/06/26].
Wu Y, Guo H, Yue J, et al. Serum sPD1 and sPDL1 as biomarkers for evaluating the Immune State of Lung Adenocarcinoma patients. J Immunol Res. 2022;2022:9101912. https://doi.org/10.1155/2022/9101912. [published Online First: 2022/12/09].
Katongole P, Sande OJ, Reynolds SJ, et al. Soluble programmed death-ligand 1 (sPD-L1) is elevated in aggressive prostate Cancer Disease among African men. Oncol Ther. 2022;10(1):185–93. https://doi.org/10.1007/s40487-022-00184-6. [published Online First: 2022/02/08].
Wang G, He L, Wang S, et al. EV PD-L1 is correlated with clinical features and contributes to T cell suppression in Pediatric thyroid Cancer. J Clin Endocrinol Metab. 2020;105(8). https://doi.org/10.1210/clinem/dgaa309. [published Online First: 2020/05/28].
Jalali S, Price-Troska T, Bothun C, et al. Reverse signaling via PD-L1 supports malignant cell growth and survival in classical Hodgkin lymphoma. Blood Cancer J. 2019;9(3):22. https://doi.org/10.1038/s41408-019-0185-9. [published Online First: 2019/02/21].
Gershtein ES, Ognerubov NA, Chang VL, et al. [The content of the soluble forms PD-1 and PD-L1 in blood serum of patients with gastric cancer and their relationship with clinical and morphological characteristics of the disease]. Klin Lab Diagn. 2020;65(6):347–52. https://doi.org/10.18821/0869-2084-2020-65-6-347-352. [published Online First: 2020/05/28].
Kovaleva OV, Rashidova MA, Gratchev AN, et al. Immunosuppression factors PD-1, PD-L1, and IDO1 and colorectal Cancer. Dokl Biochem Biophys. 2021;497(1):66–70. https://doi.org/10.1134/s1607672921020095. [published Online First: 2021/04/26].
Gershtein ES, Korotkova EA, Vorotnikov IK, et al. Soluble forms of PD-1/PD-L immune checkpoint receptor and ligand in blood serum of breast cancer patients: association with clinical pathologic factors and molecular type of the tumor. Klin Lab Diagn. 2022;67(2):76–80. https://doi.org/10.51620/0869-2084-2022-67-2-76-80. [published Online First: 2022/02/23].
Larrinaga G, Solano-Iturri JD, Errarte P, et al. Soluble PD-L1 is an independent prognostic factor in Clear Cell Renal Cell Carcinoma. Cancers (Basel). 2021;13(4). https://doi.org/10.3390/cancers13040667. [published Online First: 2021/02/11].
Gu Y, Tang YY, Wan JX, et al. Sex difference in the expression of PD-1 of non-small cell lung cancer. Front Immunol. 2022;13:1026214. https://doi.org/10.3389/fimmu.2022.1026214. [published Online First: 2022/11/08].
Lee SH, Park HJ, Moon JY, et al. Soluble programmed cell death Ligand-1 (sPD-L1) levels in various Cancer types and normal populations. Clin Lab. 2023;69(4). https://doi.org/10.7754/Clin.Lab.2022.220701. [published Online First: 2023/04/15].
Lu F, Dong Y, Li Q, et al. The Change of Soluble Programmed Death Ligand 1 (sPD-L1) in plasma of small cell Lung Cancer and its clinical significance. Comput Math Methods Med. 2022;2022:8375349. https://doi.org/10.1155/2022/8375349. [published Online First: 2022/02/08].
Chivu-Economescu M, Herlea V, Dima S, et al. Soluble PD-L1 as a diagnostic and prognostic biomarker in resectable gastric cancer patients. Gastric Cancer. 2023;26(6):934–46. https://doi.org/10.1007/s10120-023-01429-7. [published Online First: 2023/09/05].
Young MH, Pietz G, Whalen E, et al. Immunomodulation by durvalumab and pomalidomide in patients with relapsed/refractory multiple myeloma. Sci Rep. 2021;11(1):16460. https://doi.org/10.1038/s41598-021-95902-x. [published Online First: 2021/08/14].
Kushlinskii NE, Alferov AA, Boulytcheva IV, et al. Comparative analysis of the levels of soluble forms of receptor and ligand of the immunity control point PD-1/PD-L1 in the blood serum of patients with typical bone osteosarcoma and chondrosarcoma. Klin Lab Diagn. 2020;65(11):669–75. https://doi.org/10.18821/0869-2084-2020-65-11-669-675. [published Online First: 2020/12/11].
Zhang X, Liu L, Zhou S, et al. Plasma soluble programmed death ligand 1 levels predict clinical response in peripheral T-cell lymphomas. Hematol Oncol. 2019;37(3):270–76. https://doi.org/10.1002/hon.2636. [published Online First: 2019/05/11].
Feng X, Luo X, Yang Y, et al. Expression of PD-1/PD-L1 in peripheral blood and tumor tissues of patients with classical Hodgkin’s lymphoma. Med (Baltim). 2023;102(44):e35757. https://doi.org/10.1097/md.0000000000035757. [published Online First: 2023/11/07].
Feng Y, Jing C, Yu X, et al. Predicting treatment response of patients with extranodal natural killer/T-cell lymphoma based on levels of PD-L1 mRNA and soluble PD-L1. Hematol Oncol. 2020;38(4):467–77. https://doi.org/10.1002/hon.2758. [published Online First: 2020/06/10].
Solorzano-Ibarra F, Alejandre-Gonzalez AG, Ortiz-Lazareno PC, et al. Immune checkpoint expression on peripheral cytotoxic lymphocytes in cervical cancer patients: moving beyond the PD-1/PD-L1 axis. Clin Exp Immunol. 2021;204(1):78–95. https://doi.org/10.1111/cei.13561. [published Online First: 2020/12/12].
Okła K, Rajtak A, Czerwonka A, et al. Accumulation of blood-circulating PD-L1-expressing M-MDSCs and monocytes/macrophages in pretreatment ovarian cancer patients is associated with soluble PD-L1. J Transl Med. 2020;18(1):220. https://doi.org/10.1186/s12967-020-02389-7. [published Online First: 2020/06/04].
Sulaiman R, De P, Aske JC, et al. Tumor-TME Bipartite Landscape of PD-1/PD-L1 in endometrial cancers. Int J Mol Sci. 2023;24(13). https://doi.org/10.3390/ijms241311079. [published Online First: 2023/07/14].
Chiarucci C, Cannito S, Daffinà MG, et al. Circulating levels of PD-L1 in Mesothelioma patients from the NIBIT-MESO-1 study: correlation with survival. Cancers (Basel). 2020;12(2). https://doi.org/10.3390/cancers12020361. [published Online First: 2020/02/09].
Wu W, Xia X, Cheng C, et al. Serum soluble PD-L1, PD-L2, and B7-H5 as potential diagnostic biomarkers of human pancreatic Cancer. Clin Lab. 2021;67(6). https://doi.org/10.7754/Clin.Lab.2021.210103. [published Online First: 2021/06/11].
Zamora Atenza C, Anguera G, Riudavets Melià M, et al. The integration of systemic and tumor PD-L1 as a predictive biomarker of clinical outcomes in patients with advanced NSCLC treated with PD-(L)1blockade agents. Cancer Immunol Immunother. 2022;71(8):1823–35. https://doi.org/10.1007/s00262-021-03107-y. [published Online First: 2022/01/06].
Rapoport BL, Steel HC, Hlatshwayo N, et al. Systemic Immune Dysregulation in early breast Cancer is Associated with decreased plasma levels of both Soluble Co-inhibitory and Co-stimulatory Immune Checkpoint molecules. Front Immunol. 2022;13:823842. https://doi.org/10.3389/fimmu.2022.823842. [published Online First: 2022/06/10].
Kovaleva OV, Belova TP, Kushlinsky DN, et al. Soluble forms of immune checkpoints in ovarian cancer. Klin Lab Diagn. 2021;66(2):80–6. [published Online First: 2021/03/19]. doi: 10.51620/0869-2084-2021-66-2-80-86.
Chakrabarti R, Kapse B, Mukherjee G. Soluble immune checkpoint molecules: serum markers for cancer diagnosis and prognosis. Cancer Rep (Hoboken). 2019;2(4):e1160. https://doi.org/10.1002/cnr2.1160. [published Online First: 2020/07/29].
Pistillo MP, Fontana V, Morabito A, et al. Soluble CTLA-4 as a favorable predictive biomarker in metastatic melanoma patients treated with ipilimumab: an Italian melanoma intergroup study. Cancer Immunol Immunother. 2019;68(1):97–107. https://doi.org/10.1007/s00262-018-2258-1. [published Online First: 2018/10/13].
Malinga NZ, Siwele SC, Steel HC, et al. Systemic levels of the soluble co-inhibitory immune checkpoints, CTLA-4, LAG-3, PD-1/PD-L1 and TIM-3 are markedly increased in basal cell carcinoma. Transl Oncol. 2022;19:101384. https://doi.org/10.1016/j.tranon.2022.101384. [published Online First: 2022/03/08].
Yan P, Kong S, Zheng Y, et al. Correlation of CTLA-4 polymorphism and the risk of gastric cancer in a Chinese Bai population. Int J Immunogenet. 2023;50(5):256–63. https://doi.org/10.1111/iji.12632. [published Online First: 2023/07/27].
Pan S, Zhao W, Li Y, et al. Prediction of risk and overall survival of pancreatic cancer from blood soluble immune checkpoint-related proteins. Front Immunol. 2023;14:1189161. https://doi.org/10.3389/fimmu.2023.1189161. [published Online First: 2023/05/31].
Collins M, Ling V, Carreno BM. The B7 family of immune-regulatory ligands. Genome Biol. 2005;6(6):223. https://doi.org/10.1186/gb-2005-6-6-223. [published Online First: 2005/06/18].
Xiao L, Guan X, Xiang M, et al. B7 family protein glycosylation: promising novel targets in tumor treatment. Front Immunol. 2022;13:1088560. https://doi.org/10.3389/fimmu.2022.1088560. [published Online First: 2022/12/24].
Shi T, Zhou S, Zhang T, et al. Establishment of a monoclonal antibody-based enzyme-linked immunosorbent assay to measure Soluble B7-H5 in patients with Cancer. J Immunol Res. 2022;2022:3013185. https://doi.org/10.1155/2022/3013185. [published Online First: 2022/08/16].
Qiu F, Yuan C, Xu J, et al. Role of B7-H4 in the progression and prognosis of cervical inflammation to Cancer after human papilloma virus infection. J Biomed Nanotechnol. 2019;15(5):1043–51. https://doi.org/10.1166/jbn.2019.2741. [published Online First: 2019/03/21].
Liu X, Xu Y, Wu X, et al. Soluble Immune-related proteins as new candidate serum biomarkers for the diagnosis and progression of Lymphangioleiomyomatosis. Front Immunol. 2022;13:844914. https://doi.org/10.3389/fimmu.2022.844914. [published Online First: 2022/03/19].
Li Y, Wang W, Tian J, et al. Clinical significance of Soluble LAG-3 (sLAG-3) in patients with Cervical Cancer determined via enzyme-linked immunosorbent assay with monoclonal antibodies. Technol Cancer Res Treat. 2023;22:15330338231202650. https://doi.org/10.1177/15330338231202650. [published Online First: 2023/11/16].
Wang Q, Zhang J, Tu H, et al. Soluble immune checkpoint-related proteins as predictors of tumor recurrence, survival, and T cell phenotypes in clear cell renal cell carcinoma patients. J Immunother Cancer. 2019;7(1):334. https://doi.org/10.1186/s40425-019-0810-y. [published Online First: 2019/12/01].
Peng Y, Zhang C, Rui Z, et al. A comprehensive profiling of soluble immune checkpoints from the sera of patients with non-small cell lung cancer. J Clin Lab Anal. 2022;36(2):e24224. https://doi.org/10.1002/jcla.24224. [published Online First: 2022/01/13].
Silva R, Torres LC, da Fonte EJA, et al. Analysis of physical activity and plasma levels of soluble CD40 and CD40L in older people with gastrointestinal tract cancer. Exp Gerontol. 2022;160:111677. https://doi.org/10.1016/j.exger.2021.111677. [published Online First: 2022/01/16].
Tanaka Y, Takahashi Y, Tanaka R, et al. Association of high levels of plasma OX40 with acute adult T-cell leukemia. Int J Hematol. 2019;109(3):319–27. https://doi.org/10.1007/s12185-018-02580-z. [published Online First: 2019/01/18].
Javadzadeh SM, Tehrani M, Keykhosravi M, et al. Can we consider soluble herpes virus entry mediator (sHVEM) as a tumor marker? Casp J Intern Med. 2022;13(4):693–98. https://doi.org/10.22088/cjim.13.4.693. [published Online First: 2022/11/25].
Azarafza M, Tehrani M, Valadan R, et al. Role of BTLA/HVEM network in development of gastric cancer. Hum Immunol. 2022;83(8–9):637–44. https://doi.org/10.1016/j.humimm.2022.07.003. [published Online First: 2022/08/02].
Metelli A, Wu BX, Riesenberg B, et al. Thrombin contributes to cancer immune evasion via proteolysis of platelet-bound GARP to activate LTGF-β. Sci Transl Med. 2020;12(525). https://doi.org/10.1126/scitranslmed.aay4860. [published Online First: 2020/01/10].
Arianfar E, Khandoozi SR, Mohammadi S, et al. Suppression of CD56(bright) NK cells in breast cancer patients is associated with the PD-1 and TGF-βRII expression. Clin Transl Oncol. 2023;25(3):841–51. https://doi.org/10.1007/s12094-022-02997-3. [published Online First: 2022/11/23].
Wang Q, Ye Y, Yu H, et al. Immune checkpoint-related serum proteins and genetic variants predict outcomes of localized prostate cancer, a cohort study. Cancer Immunol Immunother. 2021;70(3):701–12. https://doi.org/10.1007/s00262-020-02718-1. [published Online First: 2020/09/11].
Pillsbury CE, Dougan J, Rabe JL, et al. Siglec-15 promotes evasion of adaptive immunity in B-cell Acute Lymphoblastic Leukemia. Cancer Res Commun. 2023;3(7):1248–59. https://doi.org/10.1158/2767-9764.Crc-23-0056. [published Online First: 2023/07/19].
Luo Q, Luo W, Zhu Q, et al. Tumor-derived Soluble MICA obstructs the NKG2D pathway to restrain NK cytotoxicity. Aging Dis. 2020;11(1):118–28. https://doi.org/10.14336/ad.2019.1017. [published Online First: 2020/02/06].
Mortensen JB, Monrad I, Enemark MB, et al. Soluble programmed cell death protein 1 (sPD-1) and the soluble programmed cell death ligands 1 and 2 (sPD-L1 and sPD-L2) in lymphoid malignancies. Eur J Haematol. 2021;107(1):81–91. https://doi.org/10.1111/ejh.13621. [published Online First: 2021/03/16].
Bian B, Fanale D, Dusetti N, et al. Prognostic significance of circulating PD-1, PD-L1, pan-BTN3As, BTN3A1 and BTLA in patients with pancreatic adenocarcinoma. Oncoimmunology. 2019;8(4):e1561120. [published Online First: 2019/03/25].
Mahalingam D, Chen S, Xie P, et al. Combination of pembrolizumab and pelareorep promotes anti-tumour immunity in advanced pancreatic adenocarcinoma (PDAC). Br J Cancer. 2023;129(5):782–90. https://doi.org/10.1038/s41416-023-02344-5. [published Online First: 2023/07/14].
Montemagno C, Hagege A, Borchiellini D, et al. Soluble forms of PD-L1 and PD-1 as prognostic and predictive markers of sunitinib efficacy in patients with metastatic clear cell renal cell carcinoma. Oncoimmunology. 2020;9(1):1846901. [published Online First: 2020/12/11].
Kawakami H, Sunakawa Y, Inoue E, et al. Soluble programmed cell death ligand 1 predicts prognosis for gastric cancer patients treated with nivolumab: blood-based biomarker analysis for the DELIVER trial. Eur J Cancer. 2023;184:10–20. https://doi.org/10.1016/j.ejca.2023.02.003. [published Online First: 2023/03/09].
Incorvaia L, Rinaldi G, Badalamenti G, et al. Prognostic role of soluble PD-1 and BTN2A1 in overweight melanoma patients treated with nivolumab or pembrolizumab: finding the missing links in the symbiotic immune-metabolic interplay. Ther Adv Med Oncol. 2023;15:17588359231151845. https://doi.org/10.1177/17588359231151845. [published Online First: 2023/02/24].
Hwang S, Lee KJ, Moon DB, et al. Prognostic impact of serum soluble PD-1 and ADV score for living donor liver transplantation in patients with previously untreated hepatocellular carcinoma. Ann Surg Treat Res. 2022;102(1):46–54. [published Online First: 2022/01/25].
Li Y, Cui X, Yang YJ, et al., et al. Serum sPD-1 and sPD-L1 as biomarkers for evaluating the efficacy of Neoadjuvant Chemotherapy in Triple-negative breast Cancer patients. Clin Breast Cancer. 2019;19(5):326–e321. https://doi.org/10.1016/j.clbc.2019.03.008. [published Online First: 2019/06/10].
Ohkuma R, Ieguchi K, Watanabe M, et al. Increased plasma soluble PD-1 concentration correlates with Disease Progression in patients with Cancer treated with Anti-PD-1 antibodies. Biomedicines. 2021;9(12). https://doi.org/10.3390/biomedicines9121929. [published Online First: 2021/12/25].
Himuro H, Nakahara Y, Igarashi Y, et al. Clinical roles of soluble PD-1 and PD-L1 in plasma of NSCLC patients treated with immune checkpoint inhibitors. Cancer Immunol Immunother. 2023;72(8):2829–40. https://doi.org/10.1007/s00262-023-03464-w. [published Online First: 2023/05/16].
Lambert SL, Zhang C, Guo C, et al. Association of Baseline and pharmacodynamic biomarkers with outcomes in patients treated with the PD-1 inhibitor Budigalimab. J Immunother. 2022;45(3):167–79. https://doi.org/10.1097/cji.0000000000000408. [published Online First: 2022/01/17].
Tiako Meyo M, Jouinot A, Giroux-Leprieur E, et al. Predictive value of Soluble PD-1, PD-L1, VEGFA, CD40 Ligand and CD44 for Nivolumab Therapy in Advanced Non-small Cell Lung Cancer: a case-control study. Cancers (Basel). 2020;12(2). https://doi.org/10.3390/cancers12020473. [published Online First: 2020/02/23].
Ruan Y, Hu W, Li W, et al. Analysis of plasma EBV-DNA and Soluble Checkpoint Proteins in nasopharyngeal carcinoma patients after definitive intensity-modulated Radiotherapy. Biomed Res Int. 2019;2019:3939720. https://doi.org/10.1155/2019/3939720. [published Online First: 2019/06/14].
Wei H, Wu F, Mao Y, et al. Measurement of soluble PD-1 and soluble PD-L1 as well as PD-L1 and PD-1 from perioperative patients with gastric carcinoma. Jpn J Clin Oncol. 2022;52(4):331–45. https://doi.org/10.1093/jjco/hyab214. [published Online First: 2022/02/03].
Chang B, Huang T, Wei H, et al. The correlation and prognostic value of serum levels of soluble programmed death protein 1 (sPD-1) and soluble programmed death-ligand 1 (sPD-L1) in patients with hepatocellular carcinoma. Cancer Immunol Immunother. 2019;68(3):353–63. https://doi.org/10.1007/s00262-018-2271-4. [published Online First: 2018/12/07].
Pedersen JG, Sokac M, Sørensen BS, et al. Increased soluble PD-1 predicts response to Nivolumab plus Ipilimumab in Melanoma. Cancers (Basel). 2022;14(14). https://doi.org/10.3390/cancers14143342. [published Online First: 2022/07/28].
Incorvaia L, Fanale D, Badalamenti G, et al. Baseline plasma levels of soluble PD-1, PD-L1, and BTN3A1 predict response to nivolumab treatment in patients with metastatic renal cell carcinoma: a step toward a biomarker for therapeutic decisions. Oncoimmunology. 2020;9(1):1832348. [published Online First: 2020/11/13].
Mohammadzadeh S, Khanahmad H, Esmaeil N, et al. Producing Soluble Human programmed cell death Protein-1: a natural supporter for CD4 + T cell cytotoxicity and tumor cells apoptosis. Iran J Biotechnol. 2019;17(4):e2104. https://doi.org/10.30498/ijb.2019.85180. [published Online First: 2020/07/17].
He L, Zhang G, He Y, et al. Blockade of B7-H1 with sPD-1 improves immunity against murine hepatocarcinoma. Anticancer Res. 2005;25(5):3309–13. [published Online First: 2005/08/17].
Kuipers H, Muskens F, Willart M, et al. Contribution of the PD-1 ligands/PD-1 signaling pathway to dendritic cell-mediated CD4 + T cell activation. Eur J Immunol. 2006;36(9):2472–82. https://doi.org/10.1002/eji.200635978. [published Online First: 2006/08/19].
Széles Á, Fazekas T, Váncsa S, et al. Pre-treatment soluble PD-L1 as a predictor of overall survival for immune checkpoint inhibitor therapy: a systematic review and meta-analysis. Cancer Immunol Immunother. 2023;72(5):1061–73. https://doi.org/10.1007/s00262-022-03328-9. [published Online First: 2022/11/18].
Scirocchi F, Strigari L, Di Filippo A, et al. Soluble PD-L1 as a Prognostic Factor for Immunotherapy Treatment in Solid Tumors: systematic review and Meta-analysis. Int J Mol Sci. 2022;23(22). https://doi.org/10.3390/ijms232214496. [published Online First: 2022/11/27].
Oh SY, Kim S, Keam B, et al. Soluble PD-L1 is a predictive and prognostic biomarker in advanced cancer patients who receive immune checkpoint blockade treatment. Sci Rep. 2021;11(1):19712. https://doi.org/10.1038/s41598-021-99311-y. [published Online First: 2021/10/07].
Sun J, Hu S, Li X. Meta-analysis of the prognostic value of soluble programmed death ligand-1 (sPD-L1) in cancers. Biomarkers. 2023;28(6):477–85. https://doi.org/10.1080/1354750x.2023.2198168. [published Online First: 2023/04/06].
Mazzaschi G, Minari R, Zecca A, et al. Soluble PD-L1 and circulating CD8 + PD-1 + and NK cells enclose a Prognostic and Predictive Immune Effector score in Immunotherapy treated NSCLC patients. Lung Cancer. 2020;148:1–11. [published Online First: 2020/08/10].
Chmielewska I, Grenda A, Krawczyk P, et al. The influence of plasma sPD-L1 concentration on the effectiveness of immunotherapy in advanced NSCLC patients. Cancer Immunol Immunother. 2023;72(12):4169–77. https://doi.org/10.1007/s00262-023-03552-x. [published Online First: 2023/10/11].
Cui Q, Li W, Wang D, et al. Prognostic significance of blood-based PD-L1 analysis in patients with non-small cell lung cancer undergoing immune checkpoint inhibitor therapy: a systematic review and meta-analysis. World J Surg Oncol. 2023;21(1):318. https://doi.org/10.1186/s12957-023-03215-2. [published Online First: 2023/10/12].
Wang Y, He H. Prognostic value of soluble programmed cell death ligand-1 in patients with non-small-cell lung cancer: a meta-analysis. Immunotherapy. 2022;14(12):945–56. https://doi.org/10.2217/imt-2021-0238. [published Online First: 2022/07/14].
Liao G, Zhao Z, Qian Y, et al. Prognostic role of Soluble Programmed Death Ligand 1 in Non-small Cell Lung Cancer: a systematic review and Meta-analysis. Front Oncol. 2021;11:774131. https://doi.org/10.3389/fonc.2021.774131. [published Online First: 2022/01/11].
Cheng Y, Wang C, Wang Y, et al. Soluble PD-L1 as a predictive biomarker in lung cancer: a systematic review and meta-analysis. Future Oncol. 2022;18(2):261–73. https://doi.org/10.2217/fon-2021-0641. [published Online First: 2021/12/08].
Lu T, Chen Y, Li J, et al. High Soluble programmed death-ligand 1 predicts poor prognosis in patients with nasopharyngeal carcinoma. Onco Targets Ther. 2020;13:1757–65. https://doi.org/10.2147/ott.S242517. [published Online First: 2020/03/13].
Shiraishi T, Toyozumi T, Sakata H, et al. Soluble PD-L1 concentration is proportional to the expression of PD-L1 in tissue and is Associated with a poor prognosis in esophageal squamous cell carcinoma. Oncology. 2022;100(1):39–47. https://doi.org/10.1159/000518740. [published Online First: 2022/01/07].
Ji S, Chen H, Yang K, et al. Peripheral cytokine levels as predictive biomarkers of benefit from immune checkpoint inhibitors in cancer therapy. Biomed Pharmacother. 2020;129:110457. https://doi.org/10.1016/j.biopha.2020.110457. [published Online First: 2020/09/06].
Park W, Bang JH, Nam AR, et al. Prognostic Value of Serum Soluble Programmed Death-Ligand 1 and Dynamics during Chemotherapy in Advanced Gastric Cancer patients. Cancer Res Treat. 2021;53(1):199–206. https://doi.org/10.4143/crt.2020.497. [published Online First: 2020/10/20].
Shigemori T, Toiyama Y, Okugawa Y, et al. Soluble PD-L1 expression in circulation as a predictive marker for recurrence and prognosis in gastric Cancer: direct comparison of the clinical Burden between tissue and serum PD-L1 expression. Ann Surg Oncol. 2019;26(3):876–83. https://doi.org/10.1245/s10434-018-07112-x. [published Online First: 2018/12/20].
Shin K, Kim J, Park SJ, et al. Prognostic value of soluble PD-L1 and exosomal PD-L1 in advanced gastric cancer patients receiving systemic chemotherapy. Sci Rep. 2023;13(1):6952. https://doi.org/10.1038/s41598-023-33128-9. [published Online First: 2023/04/29].
Kushlinskii NE, Gershtein ES, Chang VL, et al. Prognostic significance of soluble forms of immune checkpoint PD-1/PDL1 receptor and ligand in blood plasma of gastric cancer patients. Klin Lab Diagn. 2021;66(3):139–46. https://doi.org/10.51620/0869-2084-2021-66-3-139-146. [published Online First: 2021/04/02].
Ma XL, Qu XD, Yang WJ, et al. Elevated soluble programmed death-ligand 1 levels indicate immunosuppression and poor prognosis in hepatocellular carcinoma patients undergoing transcatheter arterial chemoembolization. Clin Chim Acta. 2020;511:67–74. https://doi.org/10.1016/j.cca.2020.09.026. [published Online First: 2020/09/27].
Han X, Gu YK, Li SL, et al. Pre-treatment serum levels of soluble programmed cell death-ligand 1 predict prognosis in patients with hepatitis B-related hepatocellular carcinoma. J Cancer Res Clin Oncol. 2019;145(2):303–12. https://doi.org/10.1007/s00432-018-2758-6. [published Online First: 2018/09/30].
Mocan T, Ilies M, Nenu I, et al. Serum levels of soluble programmed death-ligand 1 (sPD-L1): a possible biomarker in predicting post-treatment outcomes in patients with early hepatocellular carcinoma. Int Immunopharmacol. 2021;94:107467. https://doi.org/10.1016/j.intimp.2021.107467. [published Online First: 2021/02/22].
El-Gebaly F, Abou-Saif S, Elkadeem M, et al. Study of Serum Soluble Programmed Death Ligand 1 as a prognostic factor in Hepatocellular Carcinoma in Egyptian patients. Curr Cancer Drug Targets. 2019;19(11):896–905. https://doi.org/10.2174/1568009619666190718141647. [published Online First: 2019/09/21].
Xue JS, Liu H, Meng GX, et al. Prognostic value of soluble programmed cell death-1 (sPD-1) and soluble programmed cell death ligand-1 (sPD-L1) for hepatocellular carcinoma: a systematic review and meta-analysis. Cancer Immunol Immunother. 2022;71(7):1633–44. https://doi.org/10.1007/s00262-021-03103-2. [published Online First: 2021/11/10].
Boschert V, Teusch J, Aljasem A, et al. HGF-Induced PD-L1 expression in Head and Neck Cancer: preclinical and clinical findings. Int J Mol Sci. 2020;21(22). https://doi.org/10.3390/ijms21228770. [published Online First: 2020/11/26].
Ha H, Bang JH, Nam AR, et al. Dynamics of Soluble Programmed Death-Ligand 1 (sPDL1) during chemotherapy and its prognostic implications in Cancer patients: Biomarker Development in Immuno-Oncology. Cancer Res Treat. 2019;51(2):832–40. https://doi.org/10.4143/crt.2018.311. [published Online First: 2018/10/13].
Wakita N, Hinata N, Bando Y, et al. Prognostic value of serum soluble PD-L1 in metastatic renal cell carcinoma patients treated with Nivolumab. Anticancer Res. 2023;43(2):841–47. https://doi.org/10.21873/anticanres.16226. [published Online First: 2023/01/26].
Széles Á, Kovács PT, Csizmarik A, et al. High pretreatment serum PD-L1 levels are Associated with muscle Invasion and shorter survival in Upper Tract Urothelial Carcinoma. Biomedicines. 2022;10(10). https://doi.org/10.3390/biomedicines10102560. published Online First: 2022/10/28.
Ding Y, Sun C, Hu L, et al. Prognostic value of soluble programmed cell death ligand-1 (sPD-L1) in lymphoma: a systematic review and meta-analysis. Ann Hematol. 2023;102(9):2425–34. https://doi.org/10.1007/s00277-023-05325-z. [published Online First: 2023/06/29].
Cho I, Lee H, Yoon SE, et al. Serum levels of soluble programmed death-ligand 1 (sPD-L1) in patients with primary central nervous system diffuse large B-cell lymphoma. BMC Cancer. 2020;20(1):120. https://doi.org/10.1186/s12885-020-6612-2. [published Online First: 2020/02/15].
Shen H, Ji Y, Zhou D, et al. Soluble programmed death-ligand 1 are highly expressed in peripheral T-cell lymphoma: a biomarker for prognosis. Hematology. 2019;24(1):392–98. [published Online First: 2019/03/21].
Cheng CL, Yao CY, Huang PH, et al. Cerebrospinal fluid soluble programmed death-ligand 1 is a useful prognostic biomarker in primary central nervous system lymphoma. Br J Haematol. 2023;201(1):75–85. https://doi.org/10.1111/bjh.18598. [published Online First: 2022/12/09].
Pawłowska A, Kwiatkowska A, Suszczyk D, et al. Clinical and prognostic value of Antigen-presenting cells with PD-L1/PD-L2 expression in Ovarian Cancer patients. Int J Mol Sci. 2021;22(21). https://doi.org/10.3390/ijms222111563. [published Online First: 2021/11/14].
Chen X, Du Z, Huang M, et al. Circulating PD-L1 is associated with T cell infiltration and predicts prognosis in patients with CRLM following hepatic resection. Cancer Immunol Immunother. 2022;71(3):661–74. https://doi.org/10.1007/s00262-021-03021-3. [published Online First: 2021/07/30].
Omura Y, Toiyama Y, Okugawa Y, et al. Prognostic impacts of tumoral expression and serum levels of PD-L1 and CTLA-4 in colorectal cancer patients. Cancer Immunol Immunother. 2020;69(12):2533–46. https://doi.org/10.1007/s00262-020-02645-1. [published Online First: 2020/06/25].
Spalato-Ceruso M, Bouteiller F, Guegan JP, et al. Pembrolizumab combined with low-dose cyclophosphamide and intra-tumoral injection of the toll-like receptor 4 agonist G100 in patients with advanced pretreated soft tissue sarcoma: results from the PEMBROSARC basket study. J Hematol Oncol. 2022;15(1):157. https://doi.org/10.1186/s13045-022-01377-2. [published Online First: 2022/10/29].
Asanuma K, Nakamura T, Hayashi A, et al. Soluble programmed death-ligand 1 rather than PD-L1 on tumor cells effectively predicts metastasis and prognosis in soft tissue sarcomas. Sci Rep. 2020;10(1):9077. https://doi.org/10.1038/s41598-020-65895-0. [published Online First: 2020/06/05].
Mair MJ, Pajenda S, Ilhan-Mutlu A, et al. Soluble PD-L1 is associated with local and systemic inflammation markers in primary and secondary brain tumours. ESMO Open. 2020;5(6):e000863. https://doi.org/10.1136/esmoopen-2020-000863. [published Online First: 2020/11/14].
Ding XC, Wang LL, Zhu YF, et al. The change of Soluble programmed cell death-ligand 1 in Glioma patients receiving Radiotherapy and its impact on clinical outcomes. Front Immunol. 2020;11:580335. https://doi.org/10.3389/fimmu.2020.580335. [published Online First: 2020/11/24].
Park H, Bang JH, Nam AR, et al. Prognostic implications of soluble programmed death-ligand 1 and its dynamics during chemotherapy in unresectable pancreatic cancer. Sci Rep. 2019;9(1):11131. https://doi.org/10.1038/s41598-019-47330-1. [published Online First: 2019/08/02].
Jia Y, Li X, Zhao C, et al. Soluble PD-L1 as a predictor of the response to EGFR-TKIs in Non-small Cell Lung Cancer patients with EGFR mutations. Front Oncol. 2020;10:1455. https://doi.org/10.3389/fonc.2020.01455. [published Online First: 2020/09/29].
Genova C, Tasso R, Rosa A, et al. Prognostic role of Soluble and Extracellular Vesicle-Associated PD-L1, B7-H3 and B7-H4 in Non-small Cell Lung Cancer patients treated with Immune Checkpoint inhibitors. Cells. 2023;12(6). https://doi.org/10.3390/cells12060832. [published Online First: 2023/03/30].
Molga-Magusiak M, Rzepakowska A, Żurek M, et al. Prognostic and predictive role of soluble programmed death ligand-1 in head and neck cancer. Braz J Otorhinolaryngol. 2023;89(3):417–24. https://doi.org/10.1016/j.bjorl.2023.02.005. [published Online First: 2023/03/04].
Li G, Wang G, Chi F, et al. Higher postoperative plasma EV PD-L1 predicts poor survival in patients with gastric cancer. J Immunother Cancer. 2021;9(3). https://doi.org/10.1136/jitc-2020-002218. [published Online First: 2021/03/24].
Mahoney KM, Ross-Macdonald P, Yuan L, et al. Soluble PD-L1 as an early marker of progressive disease on nivolumab. J Immunother Cancer. 2022;10(2). https://doi.org/10.1136/jitc-2021-003527. [published Online First: 2022/02/09].
Sui X, Jiang L, Teng H, et al. Prediction of clinical outcome in locally Advanced Non-small Cell Lung Cancer patients treated with chemoradiotherapy by plasma markers. Front Oncol. 2020;10:625911. https://doi.org/10.3389/fonc.2020.625911. [published Online First: 2021/03/09].
Sagawa R, Sakata S, Gong B, et al. Soluble PD-L1 works as a decoy in lung cancer immunotherapy via alternative polyadenylation. JCI Insight. 2022;7(1). https://doi.org/10.1172/jci.insight.153323. [published Online First: 2021/12/08].
Lin Z, Liu M, Xing W, et al. A near-infrared fluorescence-enhancing plasmonic biosensing microarray identifies soluble PD-L1 and ICAM-1 as predictive checkpoint biomarkers for cancer immunotherapy. Biosens Bioelectron. 2023;240:115633. https://doi.org/10.1016/j.bios.2023.115633. [published Online First: 2023/09/09].
He Y, Zhang X, Zhu M, et al. Soluble PD-L1: a potential dynamic predictive biomarker for immunotherapy in patients with proficient mismatch repair colorectal cancer. J Transl Med. 2023;21(1):25. https://doi.org/10.1186/s12967-023-03879-0. [published Online First: 2023/01/14].
He J, Pan Y, Guo Y, et al. Study on the expression levels and clinical significance of PD-1 and PD-L1 in plasma of NSCLC patients. J Immunother. 2020;43(5):156–64. https://doi.org/10.1097/cji.0000000000000315. [published Online First: 2020/03/14].
Ando K, Hamada K, Watanabe M, et al. Plasma levels of Soluble PD-L1 correlate with Tumor regression in patients with lung and gastric Cancer treated with Immune Checkpoint inhibitors. Anticancer Res. 2019;39(9):5195–201. https://doi.org/10.21873/anticanres.13716. [published Online First: 2019/09/15].
Jiang VC, Hao D, Jain P, et al. TIGIT is the central player in T-cell suppression associated with CAR T-cell relapse in mantle cell lymphoma. Mol Cancer. 2022;21(1):185. https://doi.org/10.1186/s12943-022-01655-0. [published Online First: 2022/09/27].
Teramoto K, Igarashi T, Kataoka Y, et al. Prognostic impact of soluble PD-L1 derived from tumor-associated macrophages in non-small-cell lung cancer. Cancer Immunol Immunother. 2023;72(11):3755–64. https://doi.org/10.1007/s00262-023-03527-y. [published Online First: 2023/08/30].
Lu L, Risch E, Halaban R, et al. Dynamic changes of circulating soluble PD-1/PD-L1 and its association with patient survival in immune checkpoint blockade-treated melanoma. Int Immunopharmacol. 2023;118:110092. https://doi.org/10.1016/j.intimp.2023.110092. [published Online First: 2023/04/03].
Santos PM, Adamik J, Howes TR, et al. Impact of checkpoint blockade on cancer vaccine-activated CD8 + T cell responses. J Exp Med. 2020;217(7). https://doi.org/10.1084/jem.20191369. [published Online First: 2020/05/06].
Botticelli A, Cirillo A, Pomati G, et al. Immune-related toxicity and soluble profile in patients affected by solid tumors: a network approach. Cancer Immunol Immunother. 2023;72(7):2217–31. https://doi.org/10.1007/s00262-023-03384-9. [published Online First: 2023/03/04].
Wang Q, He Y, Li W, et al. Soluble Immune checkpoint-related proteins in blood are Associated with Invasion and Progression in Non-small Cell Lung Cancer. Front Immunol. 2022;13:887916. https://doi.org/10.3389/fimmu.2022.887916. [published Online First: 2022/07/26].
Vera-Lozada G, Barros MHM, Alves P, et al. EOMES/TBET and soluble CTLA4/full length CTLA4 expression ratios impact on the therapeutic response in patients with classical Hodgkin lymphoma. Br J Haematol. 2019;184(6):1061–64. https://doi.org/10.1111/bjh.15253. [published Online First: 2018/05/10].
Liu J, Tian X, Wang Y, et al. Soluble cytotoxic T-lymphocyte-associated antigen 4 (sCTLA-4) as a potential biomarker for diagnosis and evaluation of the prognosis in Glioma. BMC Immunol. 2021;22(1):33. https://doi.org/10.1186/s12865-021-00422-y. [published Online First: 2021/05/20].
Clare P, Al-Fatyan F, Risheh B, et al. A novel role for the Soluble Isoform of CTLA-4 in normal, dysplastic and neoplastic oral and Oropharyngeal Epithelia. Cancers (Basel). 2023;15(6). https://doi.org/10.3390/cancers15061696. [published Online First: 2023/03/30].
Qin SK, Li Q, Ming Xu J, et al. Icaritin-induced immunomodulatory efficacy in advanced hepatitis B virus-related hepatocellular carcinoma: immunodynamic biomarkers and overall survival. Cancer Sci. 2020;111(11):4218–31. https://doi.org/10.1111/cas.14641. [published Online First: 2020/09/06].
Teng W, Jeng WJ, Chen WT, et al. Soluble form of CTLA-4 is a good predictor for tumor recurrence after radiofrequency ablation in hepatocellular carcinoma patients. Cancer Med. 2022;11(20):3786–95. https://doi.org/10.1002/cam4.4760. [published Online First: 2022/04/19].
Gorgulho J, Roderburg C, Heymann F, et al. Serum levels of soluble B and T lymphocyte attenuator predict overall survival in patients undergoing immune checkpoint inhibitor therapy for solid malignancies. Int J Cancer. 2021;149(5):1189–98. https://doi.org/10.1002/ijc.33610. [published Online First: 2021/04/24].
Sordo-Bahamonde C, Lorenzo-Herrero S, Gonzalez-Rodriguez AP, et al. BTLA/HVEM Axis induces NK Cell Immunosuppression and poor outcome in chronic lymphocytic leukemia. Cancers (Basel). 2021;13(8). https://doi.org/10.3390/cancers13081766. [published Online First: 2021/05/01].
Dong MP, Enomoto M, Thuy LTT, et al. Clinical significance of circulating soluble immune checkpoint proteins in sorafenib-treated patients with advanced hepatocellular carcinoma. Sci Rep. 2020;10(1):3392. https://doi.org/10.1038/s41598-020-60440-5. [published Online First: 2020/02/27].
Inoue Y, Inui N, Karayama M, et al. Serum immune modulators associated with immune-related toxicities and efficacy of atezolizumab in patients with non-small cell lung cancer. J Cancer Res Clin Oncol. 2023;149(7):2963–74. https://doi.org/10.1007/s00432-022-04193-w. [published Online First: 2022/07/15].
Matsuyama Y, Asanuma K, Yoshida K, et al. The role of soluble CD80 in patients with soft tissue tumors. J Orthop Surg Res. 2022;17(1):404. https://doi.org/10.1186/s13018-022-03283-2. [published Online First: 2022/09/06].
Raza A, Mohsen R, Kanbour A, et al. Serum immune mediators as novel predictors of response to anti-PD-1/PD-L1 therapy in non-small cell lung cancer patients with high tissue-PD-L1 expression. Front Immunol. 2023;14:1157100. https://doi.org/10.3389/fimmu.2023.1157100. [published Online First: 2023/05/31].
Kinoshita R, Ishibashi M, Handa H, et al. The levels of serum soluble CD86 are correlated with the expression of CD86 variant 3 gene and are prognostic indicators in patients with myeloma. Exp Hematol. 2023;121:38–e472. https://doi.org/10.1016/j.exphem.2023.01.006. [published Online First: 2023/02/17].
Liu C, Li X, Li A, et al. Concurrent Chemoradiotherapy increases the Levels of Soluble Immune Checkpoint Proteins in patients with locally Advanced Cervical Cancer. J Immunol Res. 2022;2022:9621466. https://doi.org/10.1155/2022/9621466. [published Online First: 2022/04/15].
Pan Y, Gao J, Lin J, et al. High-dimensional single-cell analysis unveils distinct immune signatures of peripheral blood in patients with pancreatic ductal adenocarcinoma. Front Endocrinol (Lausanne). 2023;14:1181538. https://doi.org/10.3389/fendo.2023.1181538. [published Online First: 2023/06/22].
Nagai H, Mukozu T, Kobayashi K, et al. Lenvatinib might induce activation of Host Immunity in patients with Hepatocellular Carcinoma. Oncology. 2023;101(1):32–40. https://doi.org/10.1159/000527306. [published Online First: 2022/10/04].
Shibata Y, Kishida T, Kouro T, et al. Immune mediators as predictive biomarkers for anti-PD-1 antibody therapy in urothelial carcinoma. Front Pharmacol. 2023;14:1269935. https://doi.org/10.3389/fphar.2023.1269935. [published Online First: 2023/11/29].
Rossi E, Zizzari IG, Di Filippo A, et al. Circulating immune profile can predict survival of metastatic uveal melanoma patients: results of an exploratory study. Hum Vaccin Immunother. 2022;18(3):2034377. https://doi.org/10.1080/21645515.2022.2034377. [published Online First: 2022/03/09].
Peper-Gabriel JK, Pavlidou M, Pattarini L, et al. The PD-L1/4-1BB bispecific antibody-Anticalin Fusion protein PRS-344/S095012 elicits strong T-Cell stimulation in a tumor-localized manner. Clin Cancer Res. 2022;28(15):3387–99. https://doi.org/10.1158/1078-0432.Ccr-21-2762. [published Online First: 2022/02/06].
Li Y, Wang J, Wu L, et al. Diversity of Dominant Peripheral T cell receptor clone and Soluble Immune Checkpoint proteins Associated with Clinical outcomes following Immune checkpoint inhibitor treatment in Advanced Cancers. Front Immunol. 2021;12:649343. https://doi.org/10.3389/fimmu.2021.649343. [published Online First: 2021/06/25].
Sawada R, Arai Y, Sagawa Y, et al. High blood levels of soluble OX40 (CD134), an immune costimulatory molecule, indicate reduced survival in patients with advanced colorectal cancer. Oncol Rep. 2019;42(5):2057–64. https://doi.org/10.3892/or.2019.7304. [published Online First: 2019/09/24].
Digomann D, Heiduk M, Reiche C, et al. Serum immune checkpoint profiling identifies soluble CD40 as a biomarker for pancreatic cancer. NPJ Precis Oncol. 2023;7(1):104. https://doi.org/10.1038/s41698-023-00459-9. [published Online First: 2023/10/15].
Meltzer S, Torgunrud A, Abrahamsson H, et al. The circulating soluble form of the CD40 costimulatory immune checkpoint receptor and liver metastasis risk in rectal cancer. Br J Cancer. 2021;125(2):240–46. https://doi.org/10.1038/s41416-021-01377-y. [published Online First: 2021/04/11].
De La Motte Rouge T, Corné J, Cauchois A, et al. Serum CD95L level correlates with Tumor Immune Infiltration and is a positive prognostic marker for Advanced High-Grade Serous Ovarian Cancer. Mol Cancer Res. 2019;17(12):2537–48. https://doi.org/10.1158/1541-7786.Mcr-19-0449. [published Online First: 2019/09/21].
Simonetti S, Iuliani M, Stellato M, et al. Extensive plasma proteomic profiling revealed receptor activator of nuclear factor kappa-Β ligand (RANKL) as emerging biomarker of nivolumab clinical benefit in patients with metastatic renal cell carcinoma. J Immunother Cancer. 2022;10(9). https://doi.org/10.1136/jitc-2022-005136. [published Online First: 2022/09/15].
Cao W, Chen Y, Han W, et al. Potentiality of α-fetoprotein (AFP) and soluble intercellular adhesion molecule-1 (sICAM-1) in prognosis prediction and immunotherapy response for patients with hepatocellular carcinoma. Bioengineered. 2021;12(2):9435–51. https://doi.org/10.1080/21655979.2021.1990195. [published Online First: 2021/10/27].
Cole CB, Morelli MP, Fantini M, et al. First-in-human phase 1 clinical trial of anti-core 1 O-glycans targeting monoclonal antibody NEO-201 in treatment-refractory solid tumors. J Exp Clin Cancer Res. 2023;42(1):76. https://doi.org/10.1186/s13046-023-02649-6. [published Online First: 2023/03/30].
Siemiątkowska A, Bryl M, Kosicka-Noworzyń K, et al. Low on-treatment levels of serum soluble CD8 (sCD8) predict better outcomes in advanced non-small cell lung cancer patients treated with atezolizumab. Cancer Immunol Immunother. 2023;72(6):1853–63. https://doi.org/10.1007/s00262-023-03377-8. [published Online First: 2023/01/24].
Turiello R, Capone M, Giannarelli D, et al. Serum CD73 is a prognostic factor in patients with metastatic melanoma and is associated with response to anti-PD-1 therapy. J Immunother Cancer. 2020;8(2). https://doi.org/10.1136/jitc-2020-001689. [published Online First: 2020/12/29].
Messaoudi N, Cousineau I, Arslanian E, et al. Prognostic value of CD73 expression in resected colorectal cancer liver metastasis. Oncoimmunology. 2020;9(1):1746138. https://doi.org/10.1080/2162402x.2020.1746138. [published Online First: 2020/05/05].
Loosen SH, Gorgulho J, Jördens MS, et al. Serum Levels of Soluble Urokinase Plasminogen Activator Receptor Predict Tumor Response and Outcome to Immune checkpoint inhibitor therapy. Front Oncol. 2021;11:646883. https://doi.org/10.3389/fonc.2021.646883. [published Online First: 2021/04/20].
Yoshida J, Ishikawa T, Doi T, et al. Clinical significance of soluble forms of immune checkpoint molecules in advanced esophageal cancer. Med Oncol. 2019;36(7):60. https://doi.org/10.1007/s12032-019-1285-x. [published Online First: 2019/05/28].
Botticelli A, Pomati G, Cirillo A, et al. The role of immune profile in predicting outcomes in cancer patients treated with immunotherapy. Front Immunol. 2022;13:974087. https://doi.org/10.3389/fimmu.2022.974087. [published Online First: 2022/11/22].
Liu C, Wang P, Sun Y, et al. Neoadjuvant Chemoradiotherapy Changes the Landscape of Soluble Immune Checkpoint molecules in patients with locally advanced rectal Cancer. Front Oncol. 2022;12:756811. https://doi.org/10.3389/fonc.2022.756811. [published Online First: 2022/05/10].
Zor DS, Hakan MT, Özgür E, et al. Plasma levels of Kynurenine, Soluble OX40 and Mir-138-5p are Associated with Tumor-infiltrating lymphocytes in Colorectal Cancer: an exploratory study. Anticancer Res. 2023;43(7):3281–88. https://doi.org/10.21873/anticanres.16503. [published Online First: 2023/06/23].
Zhang J, Larrocha PS, Zhang B, et al. Antibody targeting tumor-derived soluble NKG2D ligand sMIC provides dual co-stimulation of CD8 T cells and enables sMIC(+) tumors respond to PD1/PD-L1 blockade therapy. J Immunother Cancer. 2019;7(1):223. https://doi.org/10.1186/s40425-019-0693-y. [published Online First: 2019/08/27].
Basher F, Dhar P, Wang X, et al. Antibody targeting tumor-derived soluble NKG2D ligand sMIC reprograms NK cell homeostatic survival and function and enhances melanoma response to PDL1 blockade therapy. J Hematol Oncol. 2020;13(1):74. https://doi.org/10.1186/s13045-020-00896-0. [published Online First: 2020/06/11].
Orme JJ, Enninga EAL, Lucien-Matteoni F, et al. Therapeutic plasma exchange clears circulating soluble PD-L1 and PD-L1-positive extracellular vesicles. J Immunother Cancer. 2020;8(2). https://doi.org/10.1136/jitc-2020-001113. [published Online First: 2020/08/21].
Davidson TM, Foster N, Lucien F, et al. Rescuing Cancer immunity by plasma exchange in metastatic melanoma (ReCIPE-M1): protocol for a single-institution, open-label safety trial of plasma exchange to clear sPD-L1 for immunotherapy. BMJ Open. 2022;12(5):e050112. https://doi.org/10.1136/bmjopen-2021-050112. [published Online First: 2022/05/14].
Lu CH, Chung WM, Tsai CH, et al. In vitro characterization of a small molecule PD-1 inhibitor that targets the PD-l/PD-L1 interaction. Sci Rep. 2022;12(1):303. https://doi.org/10.1038/s41598-021-03590-4. [published Online First: 2022/01/09].
Kleinpeter P, Remy-Ziller C, Winter E, et al. By binding CD80 and CD86, the Vaccinia Virus M2 protein blocks their interactions with both CD28 and CTLA4 and potentiates CD80 binding to PD-L1. J Virol. 2019;93(11). https://doi.org/10.1128/jvi.00207-19. [published Online First: 2019/03/29].
Miao YR, Thakkar KN, Qian J, et al. Neutralization of PD-L2 is essential for overcoming Immune Checkpoint Blockade Resistance in Ovarian Cancer. Clin Cancer Res. 2021;27(15):4435–48. https://doi.org/10.1158/1078-0432.Ccr-20-0482. [published Online First: 2021/05/21].
Liang Z, Li Y, Tian Y, et al. High-affinity human programmed death-1 ligand-1 variant promotes redirected T cells to kill tumor cells. Cancer Lett. 2019;447:164–73. https://doi.org/10.1016/j.canlet.2019.01.016. [published Online First: 2019/01/25].
Ostrand-Rosenberg S, Horn LA, Ciavattone NG. Radiotherapy both promotes and inhibits myeloid-derived suppressor cell function: novel strategies for preventing the Tumor-Protective effects of Radiotherapy. Front Oncol. 2019;9:215. https://doi.org/10.3389/fonc.2019.00215. [published Online First: 2019/04/20].
Wahid M, Pratoomthai B, Egbuniwe IU, et al. Targeting alternative splicing as a new cancer immunotherapy-phosphorylation of serine arginine-rich splicing factor (SRSF1) by SR protein kinase 1 (SRPK1) regulates alternative splicing of PD1 to generate a soluble antagonistic isoform that prevents T cell exhaustion. Cancer Immunol Immunother. 2023;72(12):4001–14. https://doi.org/10.1007/s00262-023-03534-z. [published Online First: 2023/11/17].
Wang X, Yan L, Guo J, et al. An anti-PD-1 antisense oligonucleotide promotes the expression of soluble PD-1 by blocking the interaction between SRSF3 and an exonic splicing enhancer of PD-1 exon 3. Int Immunopharmacol. 2023;126:111280. https://doi.org/10.1016/j.intimp.2023.111280. [published Online First: 2023/12/04].
He J, An Y, Qi J, et al. The recombinant Newcastle Disease virus Anhinga strain expressing human TRAIL exhibit antitumor effects on a glioma nude mice model. J Med Virol. 2021;93(6):3890–98. https://doi.org/10.1002/jmv.26419. [published Online First: 2020/08/12].
Zhang Y, Zhang H, Wei M, et al. Recombinant adenovirus expressing a Soluble Fusion protein PD-1/CD137L subverts the suppression of CD8 T cells in HCC +. Mol Ther. 2019;27(11):1906–18. https://doi.org/10.1016/j.ymthe.2019.07.019. [published Online First: 2019/08/31].
Gulen AE, Rudraboina R, Tarique M, et al. A novel agonist of 4-1BB costimulatory receptor shows therapeutic efficacy against a tobacco carcinogen-induced lung cancer. Cancer Immunol Immunother. 2023;72(11):3567–79. https://doi.org/10.1007/s00262-023-03507-2. [published Online First: 2023/08/22].
Li A, Sun K, Wang J, et al. Recombinant expression, purification and characterization of human soluble tumor necrosis factor receptor 2. Protein Expr Purif. 2021;182:105857. https://doi.org/10.1016/j.pep.2021.105857. [published Online First: 2021/02/28].
Zhang A, Sun Y, Wang S, et al. Secretion of human soluble programmed cell death protein 1 by chimeric antigen receptor-modified T cells enhances anti-tumor efficacy. Cytotherapy. 2020;22(12):734–43. https://doi.org/10.1016/j.jcyt.2020.05.007. [published Online First: 2020/07/21].
Dunn ZS, Qu Y, MacMullan M, et al. Secretion of 4-1BB ligand crosslinked to PD-1 checkpoint inhibitor Potentiates Chimeric Antigen Receptor T Cell Solid Tumor Efficacy. Hum Gene Ther. 2023;34(21–22):1145–61. https://doi.org/10.1089/hum.2022.068. [published Online First: 2023/03/01].
Xia W, Chen J, Hou W, et al. Engineering a HER2-CAR-NK Cell secreting Soluble programmed cell death protein with Superior Antitumor Efficacy. Int J Mol Sci. 2023;24(7). https://doi.org/10.3390/ijms24076843. [published Online First: 2023/04/14].
Tan Y, Yang S, Ma Y, et al. Nanobubbles containing sPD-1 and Ce6 mediate Combination Immunotherapy and suppress Hepatocellular Carcinoma in mice. Int J Nanomed. 2021;16:3241–54. https://doi.org/10.2147/ijn.S305857. [published Online First: 2021/05/20].
Yousefi-Najafabadi Z, Mehmandoostli Z, Asgari Y, et al. Reversing T cell exhaustion by converting membrane PD-1 to its soluble form in Jurkat cells; applying the CRISPR/Cas9 exon skipping strategy. Cell J. 2023;25(9):633–44. https://doi.org/10.22074/cellj.2023.1999548.1269. [published Online First: 2023/09/18].
Atkinson V, Khattak A, Haydon A, et al. Eftilagimod alpha, a soluble lymphocyte activation gene-3 (LAG-3) protein plus pembrolizumab in patients with metastatic melanoma. J Immunother Cancer. 2020;8(2). https://doi.org/10.1136/jitc-2020-001681. [published Online First: 2020/11/22].
Wildiers H, Armstrong A, Cuypere E, et al. Paclitaxel plus Eftilagimod Alpha, a Soluble LAG-3 protein, in metastatic, HR + breast Cancer: results from AIPAC, a Randomized, Placebo controlled phase IIb trial. Clin Cancer Res. 2024;30(3):532–41. https://doi.org/10.1158/1078-0432.Ccr-23-1173. [published Online First: 2023/11/08].
Mariotti FR, Ingegnere T, Landolina N, et al. Analysis of the mechanisms regulating soluble PD-1 production and function in human NK cells. Front Immunol. 2023;14:1229341. https://doi.org/10.3389/fimmu.2023.1229341. [published Online First: 2023/08/28].
Ng KW, Attig J, Young GR, et al. Soluble PD-L1 generated by endogenous retroelement exaptation is a receptor antagonist. Elife. 2019;8. https://doi.org/10.7554/eLife.50256. [published Online First: 2019/11/16].
Vuchkovska A, Glanville DG, Scurti GM, et al. Siglec-5 is an inhibitory immune checkpoint molecule for human T cells. Immunology. 2022;166(2):238–48. https://doi.org/10.1111/imm.13470. [published Online First: 2022/03/16].
Liang Z, Chen W, Guo Y, et al. Soluble monomeric human programmed cell death-ligand 1 inhibits the functions of activated T cells. Front Immunol. 2023;14:1133883. https://doi.org/10.3389/fimmu.2023.1133883. [published Online First: 2023/06/02].
Li X, Du H, Zhan S, et al. The interaction between the soluble programmed death ligand-1 (sPD-L1) and PD-1(+) regulator B cells mediates immunosuppression in triple-negative breast cancer. Front Immunol. 2022;13:830606. https://doi.org/10.3389/fimmu.2022.830606. [published Online First: 2022/08/09].
Orme JJ, Jazieh KA, Xie T, et al. ADAM10 and ADAM17 cleave PD-L1 to mediate PD-(L)1 inhibitor resistance. Oncoimmunology. 2020;9(1):1744980. https://doi.org/10.1080/2162402x.2020.1744980. [published Online First: 2020/05/05].
Khanolkar RC, Zhang C, Al-Fatyan F, et al. TGFβ2 induces the Soluble Isoform of CTLA-4 - implications for CTLA-4 based checkpoint inhibitor antibodies in malignant melanoma. Front Immunol. 2021;12:763877. https://doi.org/10.3389/fimmu.2021.763877. [published Online First: 2022/01/25].
Kennedy PT, Saulters EL, Duckworth AD, et al. Soluble CTLA-4 attenuates T-cell activation and modulates anti-tumour immunity. Mol Ther. 2023. https://doi.org/10.1016/j.ymthe.2023.11.028. [published Online First: 2023/12/06].
Morgan HJ, Rees E, Lanfredini S, et al. CD200 ectodomain shedding into the tumor microenvironment leads to NK cell dysfunction and apoptosis. J Clin Invest. 2022;132(21). https://doi.org/10.1172/jci150750. [published Online First: 2022/09/09].
Gauci ML, Giustiniani J, Lepelletier C, et al. The soluble form of CD160 acts as a tumor mediator of immune escape in melanoma. Cancer Immunol Immunother. 2022;71(11):2731–42. https://doi.org/10.1007/s00262-022-03199-0. [published Online First: 2022/04/17].
He X, Xu C. Immune checkpoint signaling and cancer immunotherapy. Cell Res. 2020;30(8):660–69. https://doi.org/10.1038/s41422-020-0343-4. [published Online First: 2020/05/30].
Acknowledgements
Not applicable.
Funding
The authors have not declared a specific grant for this research from any funding agency in the public, commercial or not-for-profit sectors.
Author information
Authors and Affiliations
Contributions
Q.W contributed the concept and design the study. Y.C and L.C performed the systematic review and wrote the original draft. All authors revised and approved the final manuscript.
Corresponding author
Ethics declarations
Ethics approval and consent to participate
Not applicable.
Consent for publication
Not applicable.
Competing interests
The authors declare no competing interests.
Additional information
Publisher’s note
Springer Nature remains neutral with regard to jurisdictional claims in published maps and institutional affiliations.
Rights and permissions
Open Access This article is licensed under a Creative Commons Attribution 4.0 International License, which permits use, sharing, adaptation, distribution and reproduction in any medium or format, as long as you give appropriate credit to the original author(s) and the source, provide a link to the Creative Commons licence, and indicate if changes were made. The images or other third party material in this article are included in the article’s Creative Commons licence, unless indicated otherwise in a credit line to the material. If material is not included in the article’s Creative Commons licence and your intended use is not permitted by statutory regulation or exceeds the permitted use, you will need to obtain permission directly from the copyright holder. To view a copy of this licence, visit http://creativecommons.org/licenses/by/4.0/. The Creative Commons Public Domain Dedication waiver (http://creativecommons.org/publicdomain/zero/1.0/) applies to the data made available in this article, unless otherwise stated in a credit line to the data.
About this article
Cite this article
Chen, L., Chao, Y., Li, W. et al. Soluble immune checkpoint molecules in cancer risk, outcomes prediction, and therapeutic applications. Biomark Res 12, 95 (2024). https://doi.org/10.1186/s40364-024-00647-0
Received:
Accepted:
Published:
DOI: https://doi.org/10.1186/s40364-024-00647-0