Abstract
Background
Gefitinib and erlotinib, are epidermal growth factor receptor tyrosine kinase inhibitors, and are currently recommended for non-small cell lung cancer stage IV in the elderly and in patients with decreased performance status in the Japanese Lung Cancer Society Guideline, but they occasionally caused severe hepatotoxicity requiring postponement or modification of treatment. However, little is known about the risk factors for hepatotoxicity in patients receiving gefitinib and erlotinib. In this study, we investigated the factors influencing hepatotoxicity in Japanese non-small cell lung cancer (NSCLC) patients treated with gefitinib or erlotinib monotherapy.
Methods
Japanese patients with NSCLC who started gefitinib or erlotinib monotherapy from January 2005 to December 2017 at Kanazawa University Hospital or Kanazawa Medical University Hospital were included in this study. Factors affecting hepatotoxicity were retrospectively investigated by multiple logistic regression analysis.
Results
A total of 102 patients who received gefitinib and 95 patients who received erlotinib were included in the analysis. In the gefitinib group, a body mass index (BMI) ≥ 25 was associated with an increased risk of hepatotoxicity (OR = 4.571, 95% CI = 1.486–14.056, P = 0.008). In the erlotinib group, concomitant use of acid-suppressing medications (AS), namely proton pump inhibitors or histamine-2 receptor antagonists, was associated with a reduced risk of hepatotoxicity (OR = 0.341, 95% CI = 0.129–0.900, P = 0.030).
Conclusions
BMI ≥ 25 in patients treated with gefitinib increased the risk of hepatotoxicity. In contrast, AS combination with erlotinib reduced the risk of hepatotoxicity. Thus, because different factors influence the risk of hepatotoxicity, monitoring for adverse events should take into account patient background factors and concomitant medications.
Similar content being viewed by others
Introduction
Chemotherapy for non-small cell lung cancer (NSCLC) patients includes molecular targeted inhibitors, cell-killing anticancer agents, and immune-checkpoint inhibitors represented by programmed death 1 (PD-1) / programmed death ligand 1 (PD-L1) inhibitors [1]. Among them, epidermal growth factor receptor tyrosine kinase inhibitors (EGFR-TKIs) such as gefitinib and erlotinib significantly prolong progression-free survival (PFS) in EGFR mutation-positive patients as monotherapy, compared with platinum-based combination therapy [2, 3]. Gefitinib and erlotinib are recommended in elderly patients with age ≥ 75 years and in patients with performance status (PS) decreased to ≥2 in The Japanese Lung Cancer Society Guideline for non-small cell lung cancer stage IV [1] because of their efficacy and safety [3,4,5,6], although the guidelines recommend osimertinib as first-line therapy in NSCLC patients with exon 19 deletions or L858R point mutations in exon 21 of EGFR [1].
Reported adverse events (AEs) caused by EGFR-TKIs include rash, diarrhea, and hepatotoxicity [7]. These AEs are associated with high exposure, namely increased area under the blood concentration-time curve (AUC) and increased serum trough concentration values [8, 9]. Among them, skin rash and diarrhea are mechanism-based AEs, and can be prevented, or at least prevented from becoming severe, by supportive care using moisturizers, topical steroids, and antidiarrheals [10, 11]. However, although there have been a few in vitro studies on the mechanism of EGFR-TKI-induced hepatotoxicity [12, 13], this has not yet yielded definitive prophylactic treatment or supportive care [14]. Veatch et al. reported that elevated bilirubin and aspartate aminotransferase (AST) levels of Grade ≥ 3 in patients were associated with increased treatment-related mortality [15]. In addition, Sakata et al. reported that when severe AEs occur, especially hepatotoxicity, switching EGFR-TKIs or temporary drug withdrawal may improve the prognosis of patients with EGFR mutation-positive NSCLC [16]. In a pooled analysis of 21 prospective clinical trials conducted between 2004 and 2014, the incidence of severe hepatotoxicity with elevated AST or alanine aminotransferase (ALT) of Grade ≥ 3 on the Common Terminology Criteria for Advanced Events (CTCAE) was 18.0% for gefitinib and 5.4% for erlotinib. Moreover, the incidence of hepatotoxicity with gefitinib was 18.5% in Asians vs. 3.2% in non-Asians [17]. There have been a few previous reports on factors associated with the risk of hepatotoxicity due to gefitinib and erlotinib. Factors identified so far include age < 65, exon 19 deletion mutations in EGFR, concomitant use of acid-suppressing medications (AS), namely proton pump inhibitors (PPIs) and histamine-2 receptor antagonists (H2RAs), and body mass index (BMI) ≥ 25 for gefitinib, and age ≥ 65, concomitant use of cytochrome P450 (CYP) 3A4 inducers and AS, and liver metastases for erlotinib [18,19,20]. However, further study is needed to accumulate evidence that would be helpful in the selection of appropriate EGFR-TKIs in order to minimize the risk of hepatotoxicity in individual patients.
Doses of EGFR-TKIs in a clinical setting are usually fixed according to recommendations in the package inserts without consideration of body size, and therefore differences in dose per body weight might be associated with the occurrence of AEs. Additionally, since the profile of AEs varies among different EGFR-TKIs, knowledge of these profiles is important for selecting the most appropriate EGFR-TKI and providing information to patients [21].
The aim of this study was to clarify risk factors related to hepatotoxicity in NSCLC patients receiving gefitinib or erlotinib monotherapy, currently used in the elderly and in patients with decreased PS according to the guideline [1], focusing on the influence of body size, concomitant medications, pharmacokinetics, and other factors, in order to help provide a rational basis for the management of AEs.
Materials & methods
Patients with NSCLC who started gefitinib or erlotinib monotherapy at Kanazawa University Hospital or Kanazawa Medical University Hospital between January 2005 and December 2017 were retrospectively studied using their electronic medical records. We excluded patients who started treatment at a reduced dose (less than 250 mg/day for gefitinib and less than 150 mg/day for erlotinib), those who were on concomitant therapy with other anticancer agents, and those with missing data (e.g., weight and height at the start of treatment, laboratory values required for evaluation of factors and hepatotoxicity, or patients who transferred to another hospital). If any of AST, ALT, total bilirubin (T-Bil), or alkaline phosphatase (ALP) as evaluated by the Common Terminology Criteria for Adverse Events (CTCAE) version 5.0 were Grade ≥ 2, or if liver metastasis was present at the time of initiation of treatment, those patients were also excluded. The following data were collected: age, sex, body surface area (BSA), BMI, type of EGFR mutation, smoking history, Eastern Cooperative Oncology Group Performance Status (ECOG PS), stages of cancer, treatment line, presence of metastasis, biochemical parameters, and concomitant medication. Age cutoff was defined based on guidelines and previous reports [1, 4, 6]. BSA was calculated according to the DuBois formula: BSA (m2) = [body weight (kg)]0.425 × [height (cm)]0.725 × 0.007184 [22]. BSA cutoff value was defined based on previous reports [19]. BMI was calculated according to the following equation: BMI (kg / m2) = body weight (kg) / height (m) × height (m). BMI cutoff values were set according to the World Health Organization (WHO) classification, with BMI ≥ 25 being overweight [23]. Concomitant medications, defined as a duration of concomitant use of ≥1 week for AS and CYP3A4 inhibitors, and of ≥2 weeks for CYP3A4 inducers [24,25,26] prior to the occurrence of hepatotoxicity, were investigated. Among AS concomitant medications, omeprazole, lansoprazole, rabeprazole, esomeprazole, and vonoprazan are categorized as PPIs, while cimetidine, famotidine, lafutidine, ranitidine, nizatidine, and roxatidine are categorized as H2RAs. CYP3A4 inhibitors include clarithromycin, erythromycin, fluconazole, voriconazole, itraconazole, and verapamil, CYP1A2 inhibitors include amiodarone, ciprofloxacin, and fluvoxamine. CYP3A4 and CYP1A2 inducers include phenobarbital, phenytoin, carbamazepine, and rifampicin. Based on the European Association for the Study of the Liver (EASL) Guidelines for Drug-induced Liver Disorders and previous reports [19, 27], hepatotoxicity was defined as the presence any of AST, ALT, T-Bil, or ALP of grade ≥ 2 as evaluated by CTCAE version 5.0. This clinical study was conducted with the approval of the Ethical Review Committees of Kanazawa University (Approval No. 2017–257) and Kanazawa Medical University (Approval No. H 189).
Statistical analysis
Patients’ background factors were analyzed by using Fisher’s exact test or the Mann-Whitney U test. Factors with P < 0.200 in univariate analysis and those considered of high clinical importance based on previous reports were included in subsequent multiple logistic regression analysis using the forced imputation method. The Kaplan-Meier method was used to analyze the time to first occurrence of hepatotoxicity, and the log-rank test was used to compare factors. All analyses were two-tailed, and P < 0.05 was considered statistically significant. Statistical analysis software used was IBM SPSS Statistics 27 (IBM Japan Ltd., Tokyo) or EZR (Saitama Medical Center, Jichi Medical University, Saitama, Japan), which is a graphical user interface for R (The R Foundation for Statistical Computing, Vienna, Austria, version 4.0.3). Specifically, EZR is a modified version of R commander (version 2.7–1), which was designed to add statistical functions frequently used in biostatistics [28].
Results
Patients
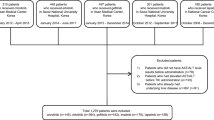
A total of 545 patients were included in the study. Three hundred forty-eight patients were excluded based on the exclusion criteria, and 197 patients were included in the analysis. Of these 197 patients, 102 received gefitinib, and 95 received erlotinib (Fig. 1), and their characteristics are summarized in Table 1. The variables that showed a significant difference between the two groups were age, age cut-off value of 75 years, BSA, and the treatment line.
Flow diagram of patient selection. The diagram shows the number of patients enrolled in the study and included in the analysis, as well as the number of patients excluded and the reasons for their exclusion (there may be more than one reason for exclusion). Liver dysfunction is defined as grade 2 or higher in any of AST, ALT, T-Bil, or ALP, as evaluated by CTCAE version 5.0
Univariate and multivariate analysis of factors affecting the development of hepatotoxicity with gefitinib
For univariate and multivariate analyses, patients were divided into two groups: those with and without hepatotoxicity. In the univariate analysis, six factors: BMI, BMI ≥ 25, treatment line, mutation of exon 19 deletion in EGFR, smoking history, and concomitant AS showed P < 0.200, suggesting a possible association with the occurrence of hepatotoxicity. Furthermore, based on the criteria mentioned in the Statistical Analysis section, BMI ≥ 25, mutation of exon 19 deletion of EGFR, and concomitant use of AS, were entered into the multivariate analysis (Table 2). The results of multivariate analysis showed that BMI ≥ 25 was an independent factor affecting gefitinib-induced hepatotoxicity (OR = 4.571, 95% CI = 1.486–14.056, P = 0.008).
Univariate and multivariate analysis of factors affecting the development of hepatotoxicity with erlotinib
As with gefitinib-treated patients, patients were divided into groups with and without hepatotoxicity. In the univariate analysis, both sex and concomitant use of AS showed P < 0.200, suggesting a possible association with the occurrence of hepatotoxicity. These two factors were included in the multivariate analysis (Table 3). The results of multivariate analysis showed that concomitant use of AS was an independent factor reducing the occurrence of hepatotoxicity (OR = 0.341, 95% CI = 0.129–0.900, P = 0.030).
Analysis of the association of first onset of hepatotoxicity and BMI in patients treated with gefitinib and erlotinib
In patients treated with gefitinib, the median time to first onset of hepatotoxicity was 13.5 months (95% CI = 1.97 to not applicable) in patients with BMI ≥ 25, and the median time was not reached during the observation period in patients with BMI < 25 (Fig. 2A). The log-rank test showed a significant difference in the time to onset of hepatotoxicity between the two BMI categories (P = 0.0498). On the other hand, in patients who received erlotinib, the median time to first onset of hepatotoxicity was not reached during the observation period in patients with BMI ≥ 25, while the median time in patients with BMI < 25 was 34.8 months (95% CI = 17.0 to not applicable), and there was no significant difference (P = 0.930) (Fig. 2B).
Discussion
In this study, multivariate analysis identified BMI ≥ 25 in patients treated with gefitinib and concomitant use of AS in patients treated with erlotinib as independent factors influencing the occurrence of hepatotoxicity.
BMI ≥ 25 in patients with gefitinib was associated with increased risk of hepatotoxicity, and furthermore, patients with BMI ≥ 25 had a shorter time to first onset of hepatotoxicity than those with BMI < 25. In addition, the median BMI was significantly higher in patients who developed hepatotoxicity than in patients who did not. Oda et al. also reported that BMI ≥ 25.0 was a risk factor for the occurrence of hepatotoxicity in patients with EGFR-mutated NSCLC undergoing gefitinib monotherapy [20], in accordance with the present results. In obese patients, it was commonly reported that the activity of CYP3A4 was decreased [29,30,31,32], although CYP2D6 and 1A2 were not affected [29, 30], and that the clearance of CYP3A4 substrates fentanyl and amiodarone was decreased [33, 34]. Since gefitinib is metabolized mainly by CYP3A4, and partially by CYP3A5, 2D6, 1A1, and 1A2 [35,36,37], reduced CYP3A4 activity in obese patients is expected to cause reduced clearance of gefitinib, which would lead to earlier occurrence of hepatotoxicity compared to patients with normal BMI. In contrast, BMI was not a risk factor for erlotinib. One reason for this may be that the contribution of CYP3A4 to metabolism of erlotinib is smaller than that in the case of gefitinib [35, 37, 38]. Thus, the contribution of CYP3A4 to metabolism of EGFR-TKIs may be a key determinant of hepatotoxicity in obese patients. On the other hand, although afatinib is hardly metabolized by CYPs, low BMI, low body weight, low BSA, female sex, and elderly status were risk factors for the occurrence of grade ≥ 3 diarrhea, and this is likely to be related to increased exposure to afatinib in patients with low BMI who receive the standard dose [39, 40]. Thus, the appropriate dosage of EGFR-TKIs appears to be dependent upon not only BMI, but also the type of EGFR-TKI, especially the contribution of CYP3A4 to the metabolism. Clinically, the perspective of considering weight and BMI in risk management for AEs is not well known, and even with gefitinib and erlotinib, dosage adjustment based on body size has not been performed. However, the results of this study, as well as the pattern for afatinib [39, 40], suggest that this would be important for risk management of AEs; especially, more careful liver function monitoring is desirable in patients with low or high BMI when initiating treatment with gefitinib. On the other hand, we could not investigate the influence of nonalcoholic fatty liver disease (NAFLD) and nonalcoholic steatohepatitis (NASH), which are associated with high BMI [41]. NSAH is itself a risk factor for liver dysfunction [41], and further study is needed to investigate the relationship of obesity-associated factors such as CYP3A4 or NAFLD/NASH with hepatotoxicity.
In patients treated with erlotinib, concomitant use of AS decreased the risk of hepatotoxicity. Gefitinib-treated patients also showed a reduced risk of hepatotoxicity in univariate analysis, although there was no statistically significant difference in multivariate analysis. Erlotinib and gefitinib exhibit pH-dependent solubility, and their AUCs decrease when they are used in combination with AS, such as PPIs and H2RAs [42,43,44]. Similar drug-drug interactions between AS and TKIs such as pazopanib and dasatinib have been reported [42,43,44]. For example, erlotinib showed a 46% decrease in AUC when combined with 40 mg of omeprazole daily, owing to a decrease in drug solubility [42]. Therefore, the reduced risk of hepatotoxicity with erlotinib is considered to be caused by the decrease in bioavailability and exposure due to concomitant use of AS. However, there is a conflicting report that concomitant use of PPIs or H2RAs increased the risk of hepatotoxicity in patients receiving erlotinib and gefitinib, and it was speculated that the mechanism might involve drug-drug interaction at ATP-binding cassette subfamily G member 2 (ABCG2) [18, 19]. Indeed, erlotinib and gefitinib are substrates of ABCG2, and PPIs inhibit ABCG2. However, the half-maximal inhibitory concentration (IC50) values of PPIs for ABCG2 in vitro are 50–200 times higher than the unbound concentration at the clinical dose [45], which suggests that the contribution of interaction between EGFR-TKIs and AS at ABCG2 might be negligible in the present study. From the viewpoint of clinical outcome, it has been reported that the concomitant use of EGFR-TKI and AS was associated with reduced OS and PFS [46, 47], but on the other hand, there are conflicting reports that concomitant use of AS did not affect OS and PFS [48, 49]. Kumarakulasinghe et al. considered that one of the reasons for the apparent discrepancies might be high sensitivity of EGFR-TKIs to EGFR mutation positivity [49]. In addition, various other factors affect OS and PFS, including the patient’s PS, metastasis to other organs, and treatment strategy [46,47,48,49]. Since OS and PFS could not be evaluated in this study, further work will be needed to clarify the effects of AS on OS and PFS as independent factors. It is desirable in clinical practice to evaluate the need for concomitant use of AS in individual patients depending upon the type of TKIs, with due consideration of its impact on efficacy. In the case of erlotinib, the risk of hepatotoxicity may fluctuate with concomitant use of AS, considering the results of this study and previous reports [19]. Therefore, we suggest that more attention be paid to liver function trends, especially when concomitant use of AS is initiated or discontinued.
Other reported risk factors for hepatotoxicity include age < 65 and exon 19 deletion mutations in EGFR for gefitinib and age ≥ 65 and concomitant use of CYP3A4 inducers for erlotinib [18, 19]. In patients with exon 19 deletion mutation of EGFR, PFS was significantly prolonged compared with cell-killing chemotherapy [2], and hepatotoxicity has been reported to increase with prolonged treatment [50]. In the gefitinib group in this study, exon 19 deletion mutation of EGFR was also found to be a significant risk factor for hepatotoxicity in univariate analysis, suggesting that the difference in the treatment duration was a confounding factor. Regarding age, generally, drug AEs are more frequently observed in elderly patients receiving polypharmacy or who have organ dysfunctions. On the other hand, some reports have described high efficacy and safety of erlotinib and gefitinib in elderly patients [4,5,6]. In an analysis of 9907 Japanese patients treated with erlotinib (2059 were age ≥ 75 years), the incidence of AEs, including hepatotoxicity, in elderly patients was similar to that in younger patients [6]. In the present study, we also examined age, but there was no significant difference. The concomitant use of CYP3A4 inducer was reported as a risk factor for hepatotoxicity with erlotinib, and it was suggested that CYP3A4-mediated active metabolite formation was involved. However, the results were obtained for a mixed population of NSCLC and pancreatic cancer, and risk factor analysis for each cancer type was not conducted. Another reason for the inconsistency may be the small number of cases in our study. On the other hand, it is generally considered that concomitant use of CYP3A4 inducers decreases the blood concentrations of many drugs [51,52,53], including those that cause hepatotoxicity, such as gefitinib and erlotinib.
There are several limitations in this study. First, we were unable to evaluate the blood concentration of the drugs, because this was a retrospective study. Future prospective studies will be needed to clarify the effects of BMI and AS on the pharmacokinetics of gefitinib and erlotinib and their contribution to hepatotoxicity. Secondly, since the mechanism of hepatotoxicity caused by gefitinib and erlotinib was not clear, we defined hepatotoxicity using AST, ALT, T-Bil, or ALP according to the published guidelines, which is a different definition compared to previous reports. Therefore, we performed a sensitivity analysis based on the use of only AST or ALT as a criterion of hepatotoxicity. Similar results were obtained in the gefitinib group (n = 26), i.e., that BMI ≥ 25 is a significant risk factor (P = 0.003) for hepatotoxicity. On the other hand, a similar trend could not be identified in the erlotinib group (n = 9), due to the small number of cases. Thirdly, concomitant medications and comorbidities may not have been adequately considered. In particular, the number of cases in which inducers or inhibitors of CYP3A4 and CYP1A2 were used was very small in our study, making it difficult to assess the risk of concomitant use. In addition, since the interaction of H2RA should be attenuated by separation of the timing of dosing of H2RA and EGFR-TKIs [42], evaluation of the appropriate dosing timing will also be necessary to clarify the effect of H2RA on the pharmacokinetics of EGFR-TKIs. Regarding comorbidities, we could not evaluate the effect of liver diseases, such as NAFLD/NASH in particular, which may have augmented the hepatotoxicity caused by TKIs. Finally, the sample size was small in our study, and there may have been some bias in the patients’ background factors.
According to the guideline [1], gefitinib and erlotinib are currently recommended for elderly patients and patients with decreased PS who are vulnerable and especially those who require intensive monitoring. In particular, careful management is needed to prevent severe disease in patients with a high BMI and when starting or discontinuing concomitant use of AS, as these were identified as risk factors of hepatotoxicity in this study. Further studies will be needed to examine the risk of AEs from newer drugs, as well as the role of other background factors.
Conclusion
We identified independent factors that influence the hepatotoxicity of gefitinib and erlotinib. BMI ≥ 25 increased the risk of hepatotoxicity in gefitinib monotherapy, and concomitant use of AS reduced the risk of hepatotoxicity in erlotinib monotherapy. Since different factors influence the risk of hepatotoxicity, our findings may be useful for assessing and managing the safety of continued treatment based on individual patient backgrounds and concomitant medications.
Availability of data and materials
All data generated or analyzed during this study are included in this published article.
Abbreviations
- ABCG2:
-
ATP-binding cassette subfamily G member 2
- AEs:
-
Adverse events
- ALP:
-
Alkaline phosphatase
- ALT:
-
Alanine aminotransferase
- AS:
-
Acid-suppressing medications
- AST:
-
Aspartate aminotransferase
- AUC:
-
Area under the blood concentration-time curve
- BMI:
-
Body mass index
- BSA:
-
Body surface area
- CI:
-
Confidence interval
- CTCAE:
-
Common Terminology Criteria for Advanced Events
- CYP:
-
Cytochrome P450
- ECOG PS:
-
Eastern Cooperative Oncology Group Performance Status
- EGFR-TKIs:
-
Epidermal growth factor receptor tyrosine kinase inhibitors
- H2RAs:
-
Histamine-2 receptor antagonists
- IC50 :
-
Half-maximal inhibitory concentration
- NAFLD:
-
Nonalcoholic fatty liver disease
- NASH:
-
Nonalcoholic steatohepatitis
- NSCLC:
-
Non-small cell lung cancer
- OR:
-
Odds ratio
- PD-1:
-
Programmed death 1
- PD-L1:
-
Programmed death ligand 1
- PFS:
-
Progression-free survival
- PPIs:
-
Proton pump inhibitors
- PS:
-
Performance status
- T-Bil:
-
Total bilirubin
- WHO:
-
World Health Organization
References
Akamatsu H, Ninomiya K, Kenmotsu H, Morise M, Daga H, Goto Y, et al. The Japanese lung Cancer society guideline for non-small cell lung cancer, stage IV. Int J Clin Oncol. 2019;24(7):731–70.
Mitsudomi T, Morita S, Yatabe Y, Negoro S, Okamoto I, Tsurutani J, et al. Gefitinib versus cisplatin plus docetaxel in patients with non-small-cell lung cancer harbouring mutations of the epidermal growth factor receptor (WJTOG3405): an open label, randomised phase 3 trial. Lancet Oncol. 2010;11(2):121–8.
Zhou C, Wu YL, Chen G, Feng J, Liu XQ, Wang C, et al. Erlotinib versus chemotherapy as first-line treatment for patients with advanced EGFR mutation-positive non-small-cell lung cancer (OPTIMAL, CTONG-0802): a multicentre, open-label, randomised, phase 3 study. Lancet Oncol. 2011;12(8):735–42.
Inoue A, Kobayashi K, Usui K, Maemondo M, Okinaga S, Mikami I, et al. First-line gefitinib for patients with advanced non-small-cell lung cancer harboring epidermal growth factor receptor mutations without indication for chemotherapy. J Clin Oncol. 2009;27(9):1394–400.
Kashiwabara K, Fujii S, Tsumura S, Sakamoto K. Overall survival of super-elderly (85 years or older) advanced non-small cell lung cancer patients with active epidermal growth factor receptor mutations receiving first-line gefitinib therapy: a single-institute retrospective study. J Cancer Res Clin Oncol. 2021;147(1):287–93.
Yoshioka H, Komuta K, Imamura F, Kudoh S, Seki A, Fukuoka M. Efficacy and safety of erlotinib in elderly patients in the phase IV POLARSTAR surveillance study of Japanese patients with non-small-cell lung cancer. Lung Cancer. 2014;86(2):201–6.
Takeda M, Nakagawa K. Toxicity profile of epidermal growth factor receptor tyrosine kinase inhibitors in patients with epidermal growth factor receptor gene mutation-positive lung cancer. Mol Clin Oncol. 2017;6(1):3–6.
Kobayashi H, Sato K, Niioka T, Miura H, Ito H, Miura M. Relationship among Gefitinib exposure, polymorphisms of its metabolizing enzymes and transporters, and side effects in Japanese patients with non-small-cell lung Cancer. Clin Lung Cancer. 2015;16(4):274–81.
Fukudo M, Ikemi Y, Togashi Y, Masago K, Kim YH, Mio T, et al. Population pharmacokinetics/pharmacodynamics of erlotinib and pharmacogenomic analysis of plasma and cerebrospinal fluid drug concentrations in Japanese patients with non-small cell lung cancer. Clin Pharmacokinet. 2013;52(7):593–609.
Melosky B, Leighl NB, Rothenstein J, Sangha R, Stewart D, Papp K, et al. Management of egfr tki-induced dermatologic adverse events. Curr Oncol. 2015 Apr;22(2):123–32.
Hirsh V, Blais N, Burkes R, Verma S, Croitoru K. Management of diarrhea induced by epidermal growth factor receptor tyrosine kinase inhibitors. Curr Oncol. 2014;21(6):329–36.
Li X, Kamenecka TM, Cameron MD. Bioactivation of the epidermal growth factor receptor inhibitor gefitinib: implications for pulmonary and hepatic toxicities. Chem Res Toxicol. 2009;22(10):1736–42.
Li X, Kamenecka TM, Cameron MD. Cytochrome P450-mediated bioactivation of the epidermal growth factor receptor inhibitor erlotinib to a reactive electrophile. Drug Metab Dispos. 2010;38(7):1238–45.
Mudd TW, Guddati AK. Management of hepatotoxicity of chemotherapy and targeted agents. Am J Cancer Res. 2021;11(7):3461–74.
Veatch JR, Sandhu V, Becker PS, Pagel JM, Appelbaum FR, Estey E. The NCI common toxicity criteria and treatment-associated mortality in acute myeloid leukemia. Blood. 2013;122(2):293–4.
Sakata Y, Kawamura K, Shingu N, Hiroshige S, Yasuda Y, Eguchi Y, et al. The effects of switching EGFR-TKI treatments for non-small cell lung cancer because of adverse events. Asia Pac J Clin Oncol. 2020;16(2):e113–7.
Takeda M, Okamoto I, Nakagawa K. Pooled safety analysis of EGFR-TKI treatment for EGFR mutation-positive non-small cell lung cancer. Lung Cancer. 2015;88(1):74–9.
Cho S, Yee J, Kim JY, Jeong Rhie S, Gwak HS. Effects of concomitant medication use on Gefitinib-induced hepatotoxicity. J Clin Pharmacol. 2018;58(2):263–8.
Kim MK, Yee J, Cho YS, Jang HW, Han JM, Gwak HS. Risk factors for erlotinib-induced hepatotoxicity: a retrospective follow-up study. BMC Cancer. 2018;18(1):988.
Oda N, Hotta K, Yoshioka H, Kudo K, Ichihara E, Kato Y, et al. Potential influence of being overweight on the development of hepatic dysfunction in Japanese patients with EGFR-mutated non-small cell lung cancer undergoing gefitinib monotherapy: the Okayama lung Cancer study group experience. Cancer Chemother Pharmacol. 2016;78(5):941–7.
Ding PN, Lord SJ, Gebski V, Links M, Bray V, Gralla RJ, et al. Risk of treatment-related toxicities from EGFR tyrosine kinase inhibitors: a Meta-analysis of clinical trials of Gefitinib, Erlotinib, and Afatinib in advanced EGFR-mutated non-small cell lung Cancer. J Thorac Oncol. 2017;12(4):633–43.
Du Bois D, Du Bois EF. Nutrition. A formula to estimate the approximate surface area if height and weight be known. Nutrition. 1989;5(5):303–11; discussion 312–3.
WHO Expert Consultation. Appropriate body-mass index for Asian populations and its implications for policy and intervention strategies. Lancet. 2004;363(9403):157–63.
Ward RM, Kearns GL. Proton pump inhibitors in pediatrics : mechanism of action, pharmacokinetics, pharmacogenetics, and pharmacodynamics. Paediatr Drugs. 2013;15(2):119–31.
Katzenmaier S, Markert C, Riedel KD, Burhenne J, Haefeli WE, Mikus G. Determining the time course of CYP3A inhibition by potent reversible and irreversible CYP3A inhibitors using a limited sampling strategy. Clin Pharmacol Ther. 2011;90(5):666–73.
Kapetas AJ, Sorich MJ, Rodrigues AD, Rowland A. Guidance for rifampin and midazolam dosing protocols to study intestinal and hepatic cytochrome P450 (CYP) 3A4 induction and De-induction. AAPS J. 2019;21(5):78.
European Association for the Study of the Liver. EASL clinical practice guidelines: drug-induced liver injury. J Hepatol. 2019;70(6):1222–61.
Kanda Y. Investigation of the freely available easy-to-use software 'EZR' for medical statistic. Bone Marrow Transplant. 2013;48(3):452–8.
Krogstad V, Peric A, Robertsen I, Kringen MK, Vistnes M, Hjelmesæth J, et al. Correlation of body weight and composition with hepatic activities of cytochrome P450 enzymes. J Pharm Sci. 2021;110(1):432–7.
Rodríguez-Morató J, Goday A, Langohr K, Pujadas M, Civit E, Pérez-Mañá C, et al. Short- and medium-term impact of bariatric surgery on the activities of CYP2D6, CYP3A4, CYP2C9, and CYP1A2 in morbid obesity. Sci Rep. 2019;9(1):20405.
Ulvestad M, Skottheim IB, Jakobsen GS, Bremer S, Molden E, Asberg A, et al. Impact of OATP1B1, MDR1, and CYP3A4 expression in liver and intestine on interpatient pharmacokinetic variability of atorvastatin in obese subjects. Clin Pharmacol Ther. 2013;93(3):275–82.
Brill MJ, Diepstraten J, van Rongen A, van Kralingen S, van den Anker JN, Knibbe CA. Impact of obesity on drug metabolism and elimination in adults and children. Clin Pharmacokinet. 2012;51(5):277–304.
Shibutani K, Inchiosa MA Jr, Sawada K, Bairamian M. Accuracy of pharmacokinetic models for predicting plasma fentanyl concentrations in lean and obese surgical patients: derivation of dosing weight ("pharmacokinetic mass"). Anesthesiology. 2004;101(3):603–13.
Fukuchi H, Nakashima M, Araki R, Komiya N, Hayano M, Yano K, et al. Effect of obesity on serum amiodarone concentration in Japanese patients: population pharmacokinetic investigation by multiple trough screen analysis. J Clin Pharm Ther. 2009;34(3):329–36.
Li J, Zhao M, He P, Hidalgo M, Baker SD, et al. Differential metabolism of gefitinib and erlotinib by human cytochrome P450 enzymes. Clin Cancer Res. 2007;13(12):3731–7.
McKillop D, McCormick AD, Millar A, Miles GS, Phillips PJ, Hutchison M. Cytochrome P450-dependent metabolism of gefitinib. Xenobiotica. 2005;35(1):39–50.
Luong TT, McAnulty MJ, Evers DL, Reinhardt BJ, Weina PJ. Pre-clinical drug-drug interaction (DDI) of gefitinib or erlotinib with cytochrome P450 (CYP) inhibiting drugs, fluoxetine and/or losartan. Curr Res Toxicol. 2021;2:217–24.
Rakhit A, Pantze MP, Fettner S, Jones HM, Charoin JE, Riek M, et al. The effects of CYP3A4 inhibition on erlotinib pharmacokinetics: computer-based simulation (SimCYP) predicts in vivo metabolic inhibition. Eur J Clin Pharmacol. 2008;64(1):31–41.
Hopkins AM, Nguyen AM, Karapetis CS, Rowland A, Sorich MJ. Risk factors for severe diarrhea with an Afatinib treatment of non-small cell lung Cancer: a pooled analysis of clinical trials. Cancers (Basel). 2018;10(10):384.
Wind S, Schnell D, Ebner T, Freiwald M, Stopfer P. Clinical pharmacokinetics and pharmacodynamics of Afatinib. Clin Pharmacokinet. 2017;56(3):235–50.
Liu W, Baker RD, Bhatia T, Zhu L, Baker SS. Pathogenesis of nonalcoholic steatohepatitis. Cell Mol Life Sci. 2016;73(10):1969–87.
Budha NR, Frymoyer A, Smelick GS, Jin JY, Yago MR, Dresser MJ, et al. Drug absorption interactions between oral targeted anticancer agents and PPIs: is pH-dependent solubility the Achilles heel of targeted therapy? Clin Pharmacol Ther. 2012;92(2):203–13.
Hussaarts KGAM, Veerman GDM, Jansman FGA, van Gelder T, Mathijssen RHJ, van Leeuwen RWF. Clinically relevant drug interactions with multikinase inhibitors: a review. Ther Adv Med Oncol. 2019;11:1758835918818347.
Gay C, Toulet D, Le Corre P. Pharmacokinetic drug-drug interactions of tyrosine kinase inhibitors: a focus on cytochrome P450, transporters, and acid suppression therapy. Hematol Oncol. 2017;35(3):259–80.
Suzuki K, Doki K, Homma M, Tamaki H, Hori S, Ohtani H, et al. Co-administration of proton pump inhibitors delays elimination of plasma methotrexate in high-dose methotrexate therapy. Br J Clin Pharmacol. 2009;67(1):44–9.
Nieves Sedano M, Manuel Caro Teller J, García Muñoz C, Fernandez Redondo D, Ponce Aix S, Menéndez Orenga M, et al. Clinical impact of gastric acid suppressing medication on the effectiveness of tyrosine kinase inhibitors in lung cancer patients. J BUON. 2018;23(3):647–53.
Chen YM, Lai CH, Chang HC, Chao TY, Tseng CC, Fang WF, et al. Antacid use and De novo brain metastases in patients with epidermal growth factor receptor-mutant non-small cell lung Cancer who were treated using first-line first-generation epidermal growth factor receptor tyrosine kinase inhibitors. PLoS One. 2016;11(2):e0149722.
Zenke Y, Yoh K, Matsumoto S, Umemura S, Niho S, Ohmatsu H, et al. Clinical impact of gastric acid-suppressing medication use on the efficacy of Erlotinib and Gefitinib in patients with advanced non-small-cell lung Cancer harboring EGFR mutations. Clin Lung Cancer. 2016;17(5):412–8.
Kumarakulasinghe NB, Syn N, Soon YY, Asmat A, Zheng H, Loy EY, et al. EGFR kinase inhibitors and gastric acid suppressants in EGFR-mutant NSCLC: a retrospective database analysis of potential drug interaction. Oncotarget. 2016;7(51):85542–50.
Wang J, Wu Y, Dong M, He X, Wang Z, Li J, et al. Observation of hepatotoxicity during long-term gefitinib administration in patients with non-small-cell lung cancer. Anti-Cancer Drugs. 2016;27(3):245–50.
Hakkola J, Hukkanen J, Turpeinen M, Pelkonen O. Inhibition and induction of CYP enzymes in humans: an update. Arch Toxicol. 2020;94(11):3671–722.
Molenaar-Kuijsten L, Van Balen DEM, Beijnen JH, Steeghs N, Huitema ADR. A review of CYP3A drug-drug interaction studies: practical guidelines for patients using targeted Oral anticancer drugs. Front Pharmacol. 2021;12:670862.
de Man FM, Goey AKL, van Schaik RHN, Mathijssen RHJ, Bins S. Individualization of irinotecan treatment: a review of pharmacokinetics, pharmacodynamics, and pharmacogenetics. Clin Pharmacokinet. 2018;57(10):1229–54.
Acknowledgments
We thank Fumiyuki Takase for assistance in data collection.
Funding
Not applicable.
Author information
Authors and Affiliations
Contributions
HN designed the protocol, carried out the study, and drafted the manuscript. YT, MN, HT and YY collected data from clinical records. HN collected, analyzed, and parsed data from clinical records at each site. HN collected data from clinical records at each site and analyzed the data. TS, ON, YT, AF, KN, KK and SY assisted in the analysis of the data. TS and YS designed and coordinated the study and assisted in drafting the manuscript. All authors read and approved the final manuscript.
Corresponding author
Ethics declarations
Ethics approval and consent for participation
The protocol of this study was approved by the Ethics Committee of Kanazawa University (Approval No. 2017–257) and the Ethics Committee of Kanazawa Medical University Hospital (Approval No. H 189). The study was conducted in accordance with the Declaration of Helsinki. The Ethics Committees of Kanazawa University and Kanazawa Medical University Hospital waived informed consent because data collection was from medical records and there was no opportunity for patient contact. However, in accordance with the Ethical Guidelines for Medical and Biological Research Involving Human Subjects in Japan, information regarding the conduct of this study was disclosed in the hospital and on the website as an opt-out approach to guarantee the opportunity for non-participation in the study and protection of personal information.
Consent for publication
Not applicable.
Competing interests
The authors declare that they have no competing interests.
Additional information
Publisher’s Note
Springer Nature remains neutral with regard to jurisdictional claims in published maps and institutional affiliations.
Rights and permissions
Open Access This article is licensed under a Creative Commons Attribution 4.0 International License, which permits use, sharing, adaptation, distribution and reproduction in any medium or format, as long as you give appropriate credit to the original author(s) and the source, provide a link to the Creative Commons licence, and indicate if changes were made. The images or other third party material in this article are included in the article's Creative Commons licence, unless indicated otherwise in a credit line to the material. If material is not included in the article's Creative Commons licence and your intended use is not permitted by statutory regulation or exceeds the permitted use, you will need to obtain permission directly from the copyright holder. To view a copy of this licence, visit http://creativecommons.org/licenses/by/4.0/. The Creative Commons Public Domain Dedication waiver (http://creativecommons.org/publicdomain/zero/1.0/) applies to the data made available in this article, unless otherwise stated in a credit line to the data.
About this article
Cite this article
Nagai, H., Shimada, T., Takahashi, Y. et al. Evaluation of factors affecting epidermal growth factor receptor tyrosine kinase inhibitor-induced hepatotoxicity in Japanese patients with non-small cell lung cancer: a two-center retrospective study. J Pharm Health Care Sci 8, 28 (2022). https://doi.org/10.1186/s40780-022-00258-7
Received:
Accepted:
Published:
DOI: https://doi.org/10.1186/s40780-022-00258-7