Abstract
Background
Sacubitril/valsartan is an angiotensin receptor neprilysin inhibitor (ARNI) that inhibits the degradation of endogenous natriuretic peptides. Therefore, ARNIs may increase the efficacy of human atrial natriuretic peptide (hANP), a drug for acute heart failure, by mediating its pharmacological mechanism. This study was aimed at evaluating the effects of ARNIs on the pharmacological effects of hANP by using surrogate marker, such as urinary output, in patients with heart failure.
Methods
In this multicenter retrospective cohort study, adult patients with heart failure who were taking angiotensin II receptor blockers (ARB) or ARNIs combined with hANP were enrolled. Information on basic characteristics, clinical laboratory data, medical history, and severity of cardiac insufficiency were collected from electronic medical records. The primary outcome was the change in adjusted fluid balance, calculated by IN-volume (mL/day) – OUT-volume (mL/day) / daily hANP dosage (μg).
Results
Ninety-two and 62 patients in the ARB + hANP and ARNI + hANP groups, respectively, were eligible for analysis. The adjusted fluid balance in the ARNI + hANP group was significantly lower than that in the ARB + hANP group (p = 0.001). After propensity score matching, 27 patients from each group were included. Similarly, there was a significant reduction in adjusted fluid balance in the ARNI + hANP group after propensity score matching (p = 0.026).
Conclusions
These findings suggest that ARNIs may enhance the efficacy of hANP and the combination of the two may be effective in the treatment of heart failure.
Similar content being viewed by others
Background
Renin–angiotensin–aldosterone inhibitors, including angiotensin-converting enzyme inhibitors (ACEIs) and angiotensin II receptor blockers (ARBs), have been used for treating patients with heart failure (HF) with reduced ejection fraction (HFrEF) [1]. Furthermore, sacubitril/valsartan, an angiotensin receptor neprilysin inhibitor (ARNI), has been reported to reduce the risk of hospitalization and death associated with HF compared with those related to enalapril [2].Therefore, sacubitril/valsartan has been recommended for reducing morbidity and mortality in patients with HFrEF in the 2022 American Heart Association/American College of Cardiology/Heart Failure Society of America guideline [3].
Sacubitril/valsartan is decomposed into sacubitril and valsartan in the body [4]. While valsartan inhibits angiotensin II receptor-mediated vasoconstriction, leading to myocardial hypertrophy and fibrosis, and water and sodium reabsorption [5], sacubitril is further metabolized to the active neprilysin inhibitor. Neprilysin is a membrane-bound protease distributed in a wide range of tissues in the body and is responsible for the degradation of natriuretic peptides [6]. Atrial natriuretic peptides (ANPs) are known to contribute to a decrease in vascular tone, increase renally mediated excretion of electrolytes and water, and have antifibrotic and antihypertrophic effects in the heart [7].
In Japan, human ANPs (hANPs) have been administered intravenously to patients with acute HF to ensure urinary output [8]. Nougue et al. [9] reported that switching from an ACEI/ARB to an ARNI may increase blood ANP levels. Considering this evidence [9] and the pharmacological mechanism of ARNIs, neprilysin inhibition by sacubitril may elevate hANP concentrations in the body; however, the underlying mechanisms remain unknown.
Hence, this multicenter study was aimed at evaluating the effects of ARNIs on the pharmacological effects of hANPs based on the urinary output in patients with HF.
Methods
Study design
This multicenter, retrospective cohort study was conducted at seven hospitals belonging to the Tokai-Hokuriku Group of the National Hospital Organization (Mie Chuo Medical Center, Shizuoka Medical Center, Toyohashi Medical Center, Nagoya Medical Center, Kanazawa Medical Center, Nagara Medical Center, and National Center for Geriatrics and Gerontology).
Data collection and exclusion criteria
Data on adult patients with HF receiving ARBs, ARNIs, and hANPs at the seven hospitals from September 1, 2020, to March 31, 2023, were collected. The exclusion criteria were as follows: (1) receipt of concomitant therapy for only 1 day, (2) unavailability of IN-volume (fluid intake and infusion volume) or OUT-volume (urinary output) measurements; (3) unknown ARB, ARNI, or hANP dosage; and (4) use of dialysis. Clinical data on patient characteristics (sex, age, weight, height, body surface area, clinical laboratory data, New York Heart Association [NYHA] classification, medical history, and concomitant medications); ARB, ARNI, and hANP dosages; ARB, ARNI, and hANP initiation dates; discharge and death dates; IN-volume; and OUT-volume were collected from electronic medical records. The data on concomitant medications were evaluated immediately prior to the initiation of combination therapy with ARB/ARNI and hANP. Adjusted fluid balance was calculated using the following formulae:
Outcome
The design scheme used in this study is illustrated in Fig. 1. The start date of combination therapy with ARB/ARNI and hANP was defined as day 0. As the accurate time for conducting this combination therapy was unknown, and the diuretic may exert a maximal effect on day 1 [10], the fluid balance data were collected on day 1. The primary endpoint was the adjusted fluid balance. ARNIs increase endogenous ANP levels mediated by neprilysin inhibition, which leads to an increase in urinary output, without concomitant use of hANP [9, 11]. Whereas a diuretic effect of ARNIs was observed on the first day, this phenomenon was not observed on the fifth day [12]. Therefore, we evaluated the adjusted fluid balance in patients who received ARBs or ARNIs for more than 5 days, at the time of combination therapy with ARB/ARNI and hANP as sub-group analysis.
Statistical analysis
As continuous variables were abnormally distributed, the Mann–Whitney U test was used. The chi-square test was used to compare categorical variables. To remove the influence of confounding factors, 1:1 propensity score (PS) matching was performed between the ARB + hANP and ARNI + hANP groups. The PS was calculated using the following variables: age; body mass index (BMI); creatinine clearance; medical history (atrial fibrillation, hypertension, diabetes, hyperlipidemia, and myocardial infarction); NYHA classification; and concomitant drugs such as loop diuretics, thiazide diuretics, tolvaptan, mineralocorticoid receptor antagonists (MRAs), sodium-glucose cotransporter 2 (SGLT2) inhibitors, and nitrates. The ARB + hANP and ARNI + hANP pairs were matched 1:1 with a caliper of 0.2 of the standard deviation of the logit of the PS. After PS matching, the respective baseline patient characteristics were evaluated and standardized differences were calculated. All statistical analyses were performed using SPSS version 28 (IBM Japan, Tokyo, Japan), and statistical significance was set at p < 0.05.
Results
From September 2020 to March 2023, 125 patients received ARBs and hANPs, and 83 patients received ARNIs and hANPs (Fig. 2). Based on the exclusion criteria, 92 and 62 patients in the ARB + hANP and ARNI + hANP groups, were eligible for the present study, respectively. The characteristics of patients in the ARB + hANP and ARNI + hANP groups are listed in Table 1. There were no significant differences in basic characteristics, such as sex, age, and clinical laboratory data, between the two groups. A higher percentage of patients in the ARB + hANP group had hypertension. A gap was observed between the NYHA classification (p < 0.001). Whereas the value of diastolic blood pressure was significantly lower in the ARB + hANP group compared to the ARNI + hANP group (p < 0.001), no significant differences were observed in systolic blood pressure. Regarding concomitant drugs, the ARNI + hANP group was more likely to use MRAs and SGLT2 inhibitors. The differences in tolvaptan and loop diuretics dosage between the ARB + hANP and ARNI + hANP groups were not significant.
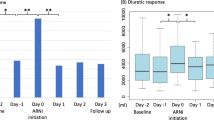
The adjusted fluid balance is shown in Fig. 3A. The adjusted fluid balance in the ARNI + hANP group was significantly lower than that in the ARB + hANP group (p = 0.001). In patients who received ARBs or ARNIs for more than 5 days, the adjusted fluid balance in the hANP + ARNI group also decreased on day 1 (p = 0.023) (Supplementary Fig. 1). In the sensitivity analysis, we compared the adjusted fluid balance after PS matching. After 1:1 PS matching, both ARB + hANP and ARNI + hANP groups included 27 patients each. Data on the basic characteristics of the patients after PS matching are shown in Table 2. There were no significant intergroup differences, and the standardized difference was less than 0.1, except for the BMI. A significant decrease in the adjusted fluid balance was observed in the ARNI + hANP group compared with that in the ARB + hANP group (p = 0.026) (Fig. 3B).
Fluid balance in the ARB + hANP and ARNI + hANP groups. A Adjusted fluid balance in the ARB + hANP and ARNI + hANP groups before PS matching. B Adjusted fluid balance in the ARB + hANP and ARNI + hANP groups after PS matching. Adjusted fluid balance was calculated as follows: In-volume (mL/day) – OUT-volume (mL/day) /daily hANP dosage. ARB, angiotensin II receptor blockers; ARNI, angiotensin receptor neprilysin inhibitor; hANP, human atrial natriuretic peptide; PS, propensity score
Discussion
To the best of our knowledge, this is the first study to demonstrate that concomitant use of ARNIs may increase urinary output compared to that of ARBs in patients treated with hANP. Moreover, the sensitivity analysis showed a decrease in the adjusted fluid balance, suggesting that the results of this study are highly reliable.
The ratio of hypertension, NYHA classification, diastolic blood pressure, MRA, and SGLT2 inhibitor were significantly different between the two groups (Table 1). The diuretic effect of MRA is relatively weak, and the beneficial effect of MRAs on HF has been attributed to non-diuretic effects [13]. On the other hand, SGLT2 inhibitors can increase urine output in HF patients [14]. Owing to these differences, we implemented PS matching to adjust for confounding factors, and differences in fluid levels were observed (Fig. 3B), suggesting that ARNI may potentially enhance the diuretic effect of hANP more effectively than ARB. In addition, this study included a large number of elderly patients (over the age of 80 years) compared with previous studies that investigated the background of patients with HF in Japan [15], indicating the efficacy of the ARNI and hANP combination in elderly patients with HF.
In sub-group analysis, we compared the adjusted fluid balance in patients who received ARBs or ARNIs for more than 5 days, at the time of combination therapy with ARB/ARNI and hANP to exclude the diuretic effect of ARNI alone. The adjusted fluid balance was lower in the ARNI + hANP group than in the ARB + hANP group, even after 5 days (Supplementary Fig. 1). Since hANP has an intramolecular neprilysin degradation site in its molecular structure [16, 17], it has been suggested that the suppression of hANP degradation by sacubitril results in an increased urinary output.
The present study has several limitations. First, the severity data of HF, such as clinical scenarios, serum ANPs, serum brain natriuretic peptides concentration, and ejection fraction data could not be elucidated because there are many missing values or descriptions. Second, although there were no significant differences in diuretic dosages among groups, the possibility that the combined effect of the diuretics influenced the fluid balance cannot be denied. Considering these limitations, future studies with larger sample sizes and greater statistical power should be conducted. Despite these limitations, the strength of this study was that it was a multicenter study, which minimized the scientific rigor and external validity biases.
Conclusions
This study indicates that ARNIs may potentiate the action of hANPs and that the combination of ARNIs and hANPs may be useful in the treatment of HF.
Availability of data and materials
The data supporting the findings of this study are available from the corresponding author upon reasonable request.
Abbreviations
- ACEI:
-
Angiotensin-converting enzyme inhibitors
- ANP:
-
Atrial natriuretic peptide
- ARB:
-
Angiotensin II receptor blockers
- ARNI:
-
Angiotensin receptor neprilysin inhibitor
- BMI:
-
Body mass index
- CABG:
-
Coronary artery bypass grafting
- hANP:
-
Human atrial natriuretic peptide
- HF:
-
Heart failure
- HFrEF:
-
HF with reduced ejection fraction
- MRA:
-
Mineralocorticoid receptor antagonist
- NYHA:
-
New York Heart Association
- PCI:
-
Percutaneous coronary intervention
- PMI:
-
Pacemaker implantation
- PS:
-
Propensity score
- SGLT2:
-
Sodium-glucose cotransporter 2
References
Tromp J, Ouwerkerk W, van Veldhuisen DJ, Hillege HL, Richards AM, van der Meer P, Anand IS, Lam CSP, Voors AA. A systematic review and network meta-analysis of pharmacological treatment of heart failure with reduced ejection fraction. JACC Heart Fail. 2022;10:73–84.
McMurray JJ, Packer M, Desai AS, Gong J, Lefkowitz MP, Rizkala AR, Rouleau JL, Shi VC, Solomon SD, Swedberg K, Zile MR. Angiotensin-neprilysin Inhibition versus enalapril in Heart Failure. N Engl J Med. 2014;371:993–1004.
Heidenreich PA, Bozkurt B, Aguilar D, Allen LA, Byun JJ, Colvin MM, Deswal A, Drazner MH, Dunlay SM, Evers LR, Fang JC, Fedson SE, Fonarow GC, Hayek SS, Hernandez AF, Khazanie P, Kittleson MM, Lee CS, Link MS, Milano CA, Nnacheta LC, Sandhu AT, Stevenson LW, Vardeny O, Vest AR, Yancy CW. 2022 AHA/ACC/HFSA guideline for the management of heart failure: Executive summary: A report of the American College of Cardiology/American Heart Association joint committee on clinical practice guidelines. Circulation. 2022;145:e876–94.
Murphy SP, Prescott MF, Camacho A, Iyer SR, Maisel AS, Felker GM, Butler J, Piña IL, Ibrahim NE, Abbas C, Burnett JC Jr, Solomon SD, Januzzi JL. Atrial natriuretic peptide and treatment with sacubitril/valsartan in heart failure with reduced ejection fraction. JACC Heart Fail. 2021;9:127–36.
Havakuk O, Elkayam U. Angiotensin receptor-neprilysin Inhibition. J Cardiovasc Pharmacol Ther. 2017;22:356–64.
Kuwahara K. The natriuretic peptide system in heart failure: Diagnostic and therapeutic implications. Pharmacol Ther. 2021;227:107863.
Nakagawa Y, Nishikimi T, Kuwahara K. Atrial and brain natriuretic peptides: Hormones secreted from the heart. Peptides. 2019;111:18–25.
Nogi K, Ueda T, Matsue Y, Sumita Y, Nishimura K, Kawakami R, Okura H, Miyamoto Y, Yasuda S, Tsutsui H, Komuro I, Ogawa H, Saito Y. Effect of carperitide on the 1 year prognosis of patients with acute decompensated heart failure. ESC Heart Fail. 2022;9:1061–70.
Nougué H, Pezel T, Picard F, Sadoune M, Arrigo M, Beauvais F, Launay JM, Cohen-Solal A, Vodovar N, Logeart D. Effects of sacubitril/valsartan on neprilysin targets and the metabolism of natriuretic peptides in chronic heart failure: A mechanistic clinical study. Eur J Heart Fail. 2019;21:598–605.
Suzuki S, Yoshihisa A, Yamaki T, Sugimoto K, Kunii H, Nakazato K, Abe Y, Saito T, Ohwada T, Suzuki H, Saitoh S, Kubota I, Takeishi Y. AVCMA investigators. Acute heart failure volume control multicenter randomized (AVCMA) trial: comparison of tolvaptan and carperitide. J Clin Pharmacol. 2013;12:1277–85.
Chatur S, Claggett BL, Vardeny O, Jering K, Desai AS, Pfeffer MA, Lefkowitz M, McMurray JJV, Solomon SD, Vaduganathan M. Sacubitril/valsartan and loop diuretic requirement in heart failure with preserved ejection fraction in the Paragon-HF trial. Eur J Heart Fail. 2023;25:87–94.
Ayalasomayajula S, Schuehly U, Pal P, Chen F, Zhou W, Sunkara G, Langenickel TH. Effect of the angiotensin receptor-neprilysin inhibitor sacubitril/valsartan on the pharmacokinetics and pharmacodynamics of a single dose of furosemide. Br J Clin Pharmacol. 2018;84:926–36.
Verbrugge FH, Damman K. Spironolactone: diuretic or disease-modifying drug in heart failure with preserved ejection fraction? Eur J Heart Fail. 2020;22(9):1611–4.
Carvalho PEP, Veiga TMA, Simões E Silva AC, Gewehr DM, Dagostin CS, Fernandes A, Nasi G, Cardoso R. Cardiovascular and renal effects of SGLT2 inhibitor initiation in acute heart failure: a meta-analysis of randomized controlled trials. Clin Res Cardiol. 2023;112(8):1044–55.
Ide T, Kaku H, Matsushima S, Tohyama T, Enzan N, Funakoshi K, Sumita Y, Nakai M, Nishimura K, Miyamoto Y, Tsuchihashi-Makaya M, Hatano M, Komuro I, Tsutsui H. Clinical characteristics and outcomes of hospitalized patients with heart failure from the large-scale Japanese registry of acute decompensated heart failure (JROADHF). Circ J. 2021;85:1438–50.
Ichiki T, Burnett JC. Atrial natriuretic peptide -Old but new therapeutic in cardiovascular diseases. Circ J. 2017;81:913–9.
Potter LR. Natriuretic peptide metabolism, clearance and degradation. FEBS J. 2011;278:1808–17.
Acknowledgements
We would like to thank Editage (www.editage.com) for English language editing.
Funding
The present study was funded by Grants from the Japan Society for the Promotion of Science (JSPS) Grants-in-Aid for Scientific Research (KAKENHI) (Grant No. 22K15331).
Author information
Authors and Affiliations
Contributions
Conceived of designed the study: TY, YA, YY, TY, and MT. Performed research: TY, NZ, SH, YK, SO, HK, and MA. Analyzed data: TY and YA. Wrote the paper: TY and YA. All authors read and approved the final manuscript.
Corresponding author
Ethics declarations
Ethics approval and consent to participate
This study was conducted in accordance with ethical guidelines for medical and health research involving human subjects. The study design was approved by Mie Chuo Medical Center (approval ref. MCERB-202252), Shizuoka Medical Center (approval ref. 2022-R33), Toyohashi Medical Center (approval ref. 4–21), Nagoya Medical Center (approval ref. 2022–055), Kanazawa Medical Center (approval ref. R04-066), Nagara Medical Center (approval ref. 2022–9), and the National Center for Geriatrics and Gerontology (approval ref. 22TB37). Owing to the retrospective study design, consent was obtained from each patient using an opt-out document posted on the website of the respective hospital.
Consent for publication
Not applicable.
Competing interests
The authors declare that there are no conflicts of interest.
Additional information
Publisher’s Note
Springer Nature remains neutral with regard to jurisdictional claims in published maps and institutional affiliations.
Supplementary Information
40780_2024_379_MOESM1_ESM.pptx
Supplementary Material 1: Fig. 1. Adjusted fluid balance in patients in whom the concomitant therapy was administered for more than 5 days. Adjusted fluid balance was calculated as follows: In-volume (mL/day) – OUT-volume (mL/day) /daily hANP dosage. ARB, angiotensin II receptor blockers; ARNI, angiotensin receptor neprilysin inhibitor; hANP, human atrial natriuretic peptide; PS, propensity score.
Rights and permissions
Open Access This article is licensed under a Creative Commons Attribution 4.0 International License, which permits use, sharing, adaptation, distribution and reproduction in any medium or format, as long as you give appropriate credit to the original author(s) and the source, provide a link to the Creative Commons licence, and indicate if changes were made. The images or other third party material in this article are included in the article's Creative Commons licence, unless indicated otherwise in a credit line to the material. If material is not included in the article's Creative Commons licence and your intended use is not permitted by statutory regulation or exceeds the permitted use, you will need to obtain permission directly from the copyright holder. To view a copy of this licence, visit http://creativecommons.org/licenses/by/4.0/. The Creative Commons Public Domain Dedication waiver (http://creativecommons.org/publicdomain/zero/1.0/) applies to the data made available in this article, unless otherwise stated in a credit line to the data.
About this article
Cite this article
Yanagawa, T., Asai, Y., Zakoji, N. et al. Changes in urinary output due to concomitant administration of sacubitril/valsartan and atrial natriuretic peptide in patients with heart failure: a multicenter retrospective cohort study. J Pharm Health Care Sci 10, 56 (2024). https://doi.org/10.1186/s40780-024-00379-1
Received:
Accepted:
Published:
DOI: https://doi.org/10.1186/s40780-024-00379-1