Abstract
Background
Mycoplasma hominis is a human commensal bacterium of the urogenital tract, and extragenital infection caused by M. hominis has rarely been reported. The identification of M. hominis is challenging, and surgeons are generally not aware that this bacteria can cause postoperative infection. Here, we report a rare case of postoperative mediastinitis caused by M. hominis after cardiac surgery in an immunocompetent patient.
Case presentation
A 54-year-old man presented with pain and purulent discharge from the wound after aortic valve replacement and patent foramen ovale closure. However, Gram staining and culture of bacteria from the purulent discharge was negative, and empiric sulbactam/ampicillin therapy was not effective. This patient developed mediastinitis and rupture of a pseudoaneurysm of the ascending aorta caused by mediastinitis, and re-operation was performed. Then, postoperative mediastinitis caused by M. hominis or Ureaplasma species was suspected and bacterial cultures targeting these pathogens were performed. M. hominis was identified from abscess and tissue obtained from the surgical site and urine. A final diagnosis of postoperative mediastinitis caused by M. hominis was determined. The patient was initially treated with levofloxacin and then with minocycline for 3 weeks. The patient’s clinical condition improved; the patient was transferred to another hospital.
Conclusion
The role of M. hominis as a cause of postoperative infection might be underestimated in cardiac surgery. M. hominis should be considered when culture-negative purulent discharge is observed or there is no response to standard empiric treatment of postoperative infections.
Similar content being viewed by others
Introduction
Mycoplasma hominis is a human commensal bacterium of the urogenital tract that is prevalent in sexually active adults [1] and typically causes urogenital infections [1, 2]. M. hominis rarely causes non-urogenital postoperative infection, including post-transplant infection after kidney, lung, and heart transplantation [3,4,5], mediastinitis after cardiac surgery [6], septic arthritis after joint replacement [7], and meningitis or brain abscess after neurosurgery [8, 9]. M. hominis cannot be detected by Gram staining, and its identification using conventional microbiological identification techniques is challenging. In addition, M. hominis is intrinsically resistant to antimicrobial agents such as beta-lactams, which are usually used during the perioperative period, because this organism lacks a cell wall. Because of these characteristics, postoperative infections caused by M. hominis might be underestimated, and the prevalence of postoperative mediastinitis caused by M. hominis is currently unknown. Here, we report a rare case of postoperative mediastinitis caused by M. hominis after cardiac surgery in an immunocompetent patient.
Case report
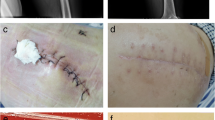
A 54-year-old man with severe aortic valve stenosis and patent foramen ovale (PFO) was hospitalized in our institution to undergo aortic valve replacement with a biological valve and PFO closure. This patient had a medical history of Ménierè syndrome and underwent surgery with prophylactic administration of cefazolin. The patient exhibited pain in the region of the surgical site, and purulent discharges from the wound were observed on postoperative day (POD) 11. Plain computed tomography (CT) showed no signs of postoperative mediastinitis such as retrosternal fluid and free air. Therefore, at that point, mediastinitis was not suspected. Blood culture and purulent discharges were submitted to culture, and sulbactam/ampicillin (3 g every 8 h) administration was empirically started. Microscopic examination of the purulent discharges showed no bacteria on Gram staining; however, many neutrophil aggregations were observed. The bacterial culture of purulent discharges and blood culture using the BacT/ALERT 3D system (bioMérieux, Marcy l’Etoile, France) was negative. The patient developed fever, and purulent discharges from the wound were observed despite the administration of sulbactam/ampicillin. On POD15, enhanced CT showed the appearance of a retrosternal fluid and free air, and mediastinitis was suspected (Fig. 1). Then, re-opening and vacuum-assisted closure for the sternotomy site was started. On POD16, the patient suddenly developed cardiopulmonary arrest, and transthoracic echocardiography showed cardiac tamponade. Extracorporeal membrane oxygenation (ECMO) support was started, and the patient was moved to the operating room to reopen the chest. Bleeding from the ascending aorta was detected, and rupture of a pseudo-aneurysm caused by mediastinitis was diagnosed. The pseudo-aneurysm originated primarily from the suture lines of a former aortic valve surgery. In addition, the infected aortic wall adjacent to the suture line had become vulnerable. The ascending aorta was repaired using a bovine pericardial patch, and debridement and omentopexy for mediastinitis was performed. The antimicrobial agent was changed to meropenem (1 g every 12 h) and vancomycin after surgery. The dose of antimicrobial agents was reduced due to renal dysfunction. Gram staining of the abscess and tissue sampled from the surgical site did not show any visible microorganisms; however, many neutrophil aggregations were observed. The cardiovascular surgeon consulted the department of infectious disease, and the infectious disease expert suspected postoperative mediastinitis caused by M. hominis or Ureaplasma species. On POD18, to identify M. hominis and Ureaplasma species, abscess and tissue obtained from POD16 and urine obtained on POD17 were cultured using urea-arginine LYO2 broth (bioMérieux). Then, the antimicrobial therapy was changed from meropenem and vancomycin to levofloxacin (250 mg every 24 h) and vancomycin. A color change in the broth was observed 24–48 h after the start of culture (Fig. 2). DNA was extracted from the broth using a MORA-EXTRACT DNA extraction kit (Kyokuto Pharmaceuticals Industrial Co., Ltd., Tokyo, Japan), and polymerase chain reaction (PCR) using M. hominis, Ureaplasma paruvum, and Ureaplasma urealyticum-specific primers was performed [10, 11]. All samples were positive for M. hominis, and a final diagnosis of postoperative mediastinitis caused by M. hominis was determined. No growth of other bacteria was detected from the abscess, and therefore, the antimicrobial agent was changed to only levofloxacin on POD21. The two sets of blood cultures sampled on POD16 were negative. The abscess, tissue, and urine samples were also cultured on Brucella HK agar plates (Kyokuto Pharmaceutical Industrial Co., Ltd.) under anaerobic conditions at 35 °C. After 4 days of anaerobic culture, numerous pinpoint colony formations became visible on the Brucella HK agar plates. M. hominis was identified from the colonies by PCR using M. hominis-specific primers. Matrix-assisted laser desorption/ionization time-of-flight mass spectrometry (MALDI-TOF MS) analysis was also performed with a BD MALDI Biotyper sirius system (Becton, Dickinson and Company, USA) using the MBT Compass 4.1 with reference database MBT Compass library: Ver.9.0.0.0 (8468MSPs) (Bruker Daltonik GmbH, Bremen, Germany). MALDI-TOF MS analysis using pure culture colonies, performed by direct transfer methods as previously described [12], identified the isolates as M. hominis with a high score value ≥ 2.000.
The minimal inhibitory concentration (MIC; μg/mL) values of clindamycin, levofloxacin, ciprofloxacin, and minocycline were determined using the E-test (bioMérieux) (Table 1). However, these MIC values were for reference only because the interpretation of MICs determined by the E-test is not mentioned in the criteria of Clinical and Laboratory Standard Institute (CLSI) M43-A [13].
After surgery, the postoperative course of mediastinitis was good. However, stenosis of the ascending aorta at the repair site was observed using transesophageal echocardiograph which showed that the maximum flow velocity at the stenosis site was 5.1 m/s. This patient could not be weaned from ECMO due to the stenosis. On POD23, thoracic endovascular aortic repair was performed for stenosis of the ascending aorta, and the intra-vertical hematoma was removed by re-opening the chest. After surgery, the patient was able to be weaned off ECMO. Although an abscess was not detected at the surgical site, the hematoma was cultured in urea-arginine LYO2 broth, and the broth changed color after 24 h. In addition, PCR using M. hominis-specific primers was positive. Although the MIC of levofloxacin determined by the E-test was not high, we suspected that the M. hominis isolate was resistant to levofloxacin, and the antimicrobial agent was changed to minocycline (100 mg every 12 h) on POD25. After re-surgery and changes to the antimicrobial agent, the postoperative course was good. Antimicrobial treatment with minocycline was continued for 3 weeks and the patient’s clinical condition improved; the patient was transferred to another hospital on POD80.
Discussion
We report a rare case of postoperative mediastinitis caused by M. hominis after cardiac surgery in an immunocompetent patient. Postoperative mediastinitis caused by M. hominis has rarely been reported, and Le Guern et al. reviewed 17 cases [6]. The median age was 55 years, and all reported patients were male. The median onset of clinical symptoms was 14 days after surgery. These clinical features were consistent with this patient. M. hominis colonizes the human urogenital tract in sexually active adults [1] and the respiratory tract, but this is less frequent [14]. Although a clear origin of the infection is difficult to recognize, it was hypothesized that invasive medical procedures such as urinary catheterization lead to bloodstream invasion of M. hominis and seeding of the surgical site [15]. In addition, recent reports have shown donor-derived M. hominis infection in lung transplant recipients [4]. In this study, the patient was not immunocompromised and urine culture was positive for M. hominis, but the septum was negative. Therefore, M. hominis colonized the urinary tract, and urinary catheterization during surgery might lead to bacteremia and seeding of the surgical site. As M. hominis is frequently found in the human urogenital tract [1] and catheterization is a common procedure during surgery, the possibility of postoperative M. hominis infections could be underestimated.
The identification of M. hominis infection is often challenging due to the slow growth of the colonies and the absence of a cell wall, which gives a negative Gram stain result. Therefore, it is difficult to detect M. hominis using standard microbiological methods without first suspecting them as a cause of postoperative infections. In addition, cardiovascular surgeons are generally not aware of the fact that M. hominis can cause postoperative infection. In this study, the infectious disease expert suspected M. hominis or Ureaplasma spp. because Gram staining and culture of the purulent discharges obtained from the surgical site were negative and sulbactam/ampicillin was not effective. In addition, Gram staining of the abscess and tissue sampled from the surgical site of re-operation was negative. To identify M. hominis, we used urea-arginine LYO2 broth and anaerobically cultured samples on Brucella HK agar plates for 4 days. As it is not popular to use urea-arginine LYO2 broth in clinical microbiological laboratories of general hospitals in Japan, it may be better to extend the culture period under 5% CO2 on blood agar plates or under anaerobic condition on Brucella HK agar plates to identify M. hominis because of its slow-growing nature. The identification of M. hominis is usually performed by 16S ribosomal DNA sequencing, PCR using M. hominis-specific primers, and MALDI-TOF MS [6, 9, 10]. In this study, we used PCR with M. hominis-specific primers and MALDI-TOF MS. Although MALDI-TOF MS has been reported to be a useful tool for the identification of human Mycoplasma species including M. hominis [16], M. hominis has not been included in the clinical use MALDI Biotyper database. In this case, we found that MALDI-TOF MS analysis performed by a MALDI Biotyper system with the reference database MBT Compass library: Ver.9.0.0.0 (8468MSPs) was very useful for the rapid identification of M. hominis. Because of its slow growth, 16S ribosomal DNA sequencing or PCR with M. hominis-specific primers from positive urea-arginine LYO2 broth might be faster than MALDI-TOF MS using bacterial colonies. Previous reports showed that 16S rDNA was sequenced directly from clinical samples and is a good method to identify M. hominis [6]. Further study is needed to evaluate if MALDI-TOF MS can directly identified M. hominis from positive urea-arginine LYO2 broth and positive blood culture bottles, as well as a pure cultured colony.
Postoperative mediastinitis is a major complication of cardiac surgery, with a low incidence but serious consequences in terms of morbidity and mortality [17]. Early diagnosis can lead to the early use of appropriate antimicrobial agents for M. hominis and avoid repeated surgical interventions. However, the identification of M. hominis is challenging under conventional microbiological identification techniques without suspecting them. Therefore, if a patient develops unexplained postoperative fever in cases of otherwise culture-negative infections, particularly if treated with beta-lactam antibiotics, and has a poor response, it is important to consider M. hominis infection as a differential diagnosis. When clinicians suspect M. hominis, they should inform the clinical microbiological laboratory and request to extend the culture period.
Beta-lactam antibiotics are generally used for antimicrobial prophylaxis in cardiac surgery [18]. In addition, broad-spectrum beta-lactam antibiotics and glycopeptides are usually used for empiric treatment of postoperative infection after cardiac surgery targeting gram-positive cocci, including methicillin-resistant Staphylococcus aureus and gram-negative bacilli [17]. However, beta-lactam antibiotics and glycopeptides are not effective against M. hominis because of the absence of a cell wall. M. hominis is generally susceptible to tetracyclines, clindamycin, and fluoroquinolones, but intrinsically resistant to clarithromycin and erythromycin [13]. M. hominis isolates resistant to these antimicrobial agents have also been reported; however, the resistance rate to fluoroquinolone, tetracyclines, and clindamycin of M. hominis varies by report [19,20,21]. In this case, we started levofloxacin after surgical debridement; however, the culture of the intra-vertical hematoma after 7 days of levofloxacin treatment was positive for M. hominis. Although the MIC of levofloxacin determined using the E-test was not high, we suspected that M. hominis was resistant to levofloxacin, and the antimicrobial agent was changed to minocycline. This condition might be associated with the short treatment duration of levofloxacin or residual intra-vertical hematoma. CLSI M43-A indicates that agar disk diffusion is not useful for testing mycoplasmas because there has been no correlation between inhibitory zones and MICs [13]. In addition, the method using the E-test was not mentioned in CLSI M43-A. However, broth microdilution and agar dilution, which are recommended methods to determine MICs by the CLSI M43-A, are not practical in clinical microbiological laboratories in Japanese hospitals. Therefore, the careful observation of clinical course after the administration of antimicrobial agents for M. hominis is needed.
Conclusion
We report a rare case of postoperative mediastinitis after cardiac surgery caused by M. hominis in an immunocompetent patient. The role of M. hominis as a cause of postoperative infections might be underestimated in cardiac surgery. M. hominis should be suspected, especially when culture-negative postoperative infections are observed or there is no response to standard empiric treatment of postoperative infections.
Availability of data and materials
Not applicable.
Abbreviations
- CLSI:
-
Clinical and Laboratory Standard Institute
- CT:
-
Computed tomography
- MALDI-TOF:
-
Matrix-assisted laser desorption/ionization time-of-flight mass spectrometry
- MIC:
-
Minimum inhibitory concentration
- PFO:
-
Patent foramen ovale
- POD:
-
Postoperative day
References
Horner P, Donders G, Cusini M, Gomberg M, Jensen JS, Unemo M. Should we be testing for urogenital Mycoplasma hominis, Ureaplasma parvum and Ureaplasma urealyticum in men and women?—a position statement from the European STI Guidelines Editorial Board. J Eur Acad Dermatol Venereol. 2018;32:1845–51.
Mori N, Takigawa A, Kagawa N, Kenri T, Yoshida S, Shibayama K, et al. Pelvic abscess due to Mycoplasma hominis following caesarean section. JMM Case Rep. 2016;3: e005059.
Okumura Y, Kajihara T, Koba Y, Onodera M, Hara T, Tahara H, et al. Multiple intraabdominal abscesses caused by Mycoplasma hominis infection following simultaneous pancreas-kidney transplantation. Ann Lab Med. 2018;38:381–3.
Smibert OC, Wilson HL, Sohail A, Narayanasamy S, Schultz MB, Ballard SA, et al. Donor-derived Mycoplasma hominis and an apparent cluster of M. hominis cases in solid organ transplant recipients. Clin Infect Dis. 2017;65:1504–8.
Hagiya H, Yoshida H, Yamamoto N, Kimura K, Ueda A, Nishi I, et al. Mycoplasma hominis periaortic abscess following heart-lung transplantation. Transpl Infect Dis. 2017;19: e12697.
Le Guern R, Loiez C, Loobuyck V, Rousse N, Courcol R, Wallet F. A new case of Mycoplasma hominis mediastinitis and sternal osteitis after cardiac surgery. Int J Infect Dis. 2015;31:53–5.
Xiang L, Lu B. Infection due to Mycoplasma hominis after left hip replacement: case report and literature review. BMC Infect Dis. 2019;19:50.
Reissier S, Masson R, Guérin F, Viquesnel G, Petitjean-Lecherbonnier J, Pereyre S, et al. Fatal nosocomial meningitis caused by Mycoplasma hominis in an adult patient: case report and review of the literature. Int J Infect Dis. 2016;48:81–3.
Pailhoriès H, Rabier V, Eveillard M, Mahaza C, Joly-Guillou ML, Chennebault JM, et al. A case report of Mycoplasma hominis brain abscess identified by MALDI-TOF mass spectrometry. Int J Infect Dis. 2014;29:166–8.
Blanchard A, Yáñez A, Dybvig K, Watson HL, Griffiths G, Cassell GH. Evaluation of intraspecies genetic variation within the 16S rRNA gene of Mycoplasma hominis and detection by polymerase chain reaction. J Clin Microbiol. 1993;31:1358–61.
Kong F, Ma Z, James G, Gordon S, Gilbert GL. Species identification and subtyping of Ureaplasma parvum and Ureaplasma urealyticum using PCR-based assays. J Clin Microbiol. 2000;38:1175–9.
Schulthess B, Brodner K, Bloemberg GV, Zbinden R, Böttger EC, Hombach M. Identification of Gram-positive cocci by use of matrix-assisted laser desorption ionization-time of flight mass spectrometry: comparison of different preparation methods and implementation of a practical algorithm for routine diagnostics. J Clin Microbiol. 2013;51:1834–40.
Waites KB, Bade DJ, Bébéar C, Brown SD, Davidson MK, Duffy LB, et al. Methods for antimicrobial susceptibility testing for human mycoplasmas; Approved Guideline. Wayne (PA): Clinical and Laboratory Standards Institute, Report No.: M43-A; 2011.
Patel KK, Salva PS, Webley WC. Colonization of paediatric lower respiratory tract with genital Mycoplasma species. Respirology. 2011;16:1081–7.
Fenollar F, Gauduchon V, Casalta JP, Lepidi H, Vandenesch F, Raoult D. Mycoplasma endocarditis: two case reports and a review. Clin Infect Dis. 2004;38:e21–4.
Pereyre S, Tardy F, Renaudin H, Cauvin E, Del Prá Netto Machado L, Tricot A, et al. Identification and subtyping of clinically relevant human and ruminant Mycoplasmas by use of matrix-assisted laser desorption ionization–time of flight mass spectrometry. J Clin Microbiol. 2013;51:3314–23.
Trouillet JL, Vuagnat A, Combes A, Bors V, Chastre J, Gandjbakhch I, et al. Acute poststernotomy mediastinitis managed with debridement and closed-drainage aspiration: factors associated with death in the intensive care unit. J Thorac Cardiovasc Surg. 2005;129:518–24.
Lador A, Nasir H, Mansur N, Sharoni E, Biderman P, Leibovici L, et al. Antibiotic prophylaxis in cardiac surgery: systematic review and meta-analysis. J Antimicrob Chemother. 2012;67:541–50.
Meygret A, Le Roy C, Renaudin H, Bébéar C, Pereyre S. Tetracycline and fluoroquinolone resistance in clinical Ureaplasma spp and Mycoplasma hominis isolates in France between 2010 and 2015. J Antimicrob Chemother. 2018;73:2696–703.
Lee JY, Yang JS. Prevalence and antimicrobial susceptibility of Mycoplasma hominis and Ureaplasma species in nonpregnant female patients in South Korea indicate an increasing trend of pristinamycin-resistant isolates. Antimicrob Agents Chemother. 2020;64:e01065-e1120.
Yang T, Pan L, Wu N, Wang L, Liu Z, Kong Y, et al. Antimicrobial resistance in clinical Ureaplasma spp. and Mycoplasma hominis and structural mechanisms underlying quinolone resistance. Antimicrob Agents Chemother. 2020;64: e02560-19.
Acknowledgements
We would like to thank Editage (www.editage.com) for English language editing.
Funding
No funding was obtained from the private or public sector for this research.
Author information
Authors and Affiliations
Contributions
HK was the principal investigator, responsible for the data collection and analysis, and drafted the manuscript. All authors shared in the study concept, data acquisition, critical revision of the manuscript for important intellectual content. All authors read and approved the final manuscript.
Corresponding author
Ethics declarations
Ethics approval and consent to participate
This study was performed in accordance with the principles of the Declaration of Helsinki. The study protocol was approved by the Ethical Committee for Epidemiology of Hiroshima University.
Consent for publication
Written informed consent was obtained from the patient for publication of this case report.
Competing interests
The authors declare that they do not have any competing interests.
Additional information
Publisher's Note
Springer Nature remains neutral with regard to jurisdictional claims in published maps and institutional affiliations.
Rights and permissions
Open Access This article is licensed under a Creative Commons Attribution 4.0 International License, which permits use, sharing, adaptation, distribution and reproduction in any medium or format, as long as you give appropriate credit to the original author(s) and the source, provide a link to the Creative Commons licence, and indicate if changes were made. The images or other third party material in this article are included in the article's Creative Commons licence, unless indicated otherwise in a credit line to the material. If material is not included in the article's Creative Commons licence and your intended use is not permitted by statutory regulation or exceeds the permitted use, you will need to obtain permission directly from the copyright holder. To view a copy of this licence, visit http://creativecommons.org/licenses/by/4.0/.
About this article
Cite this article
Kitagawa, H., Shimizu, H., Katayama, K. et al. Postoperative mediastinitis after cardiac surgery caused by Mycoplasma hominis: a case report. surg case rep 7, 248 (2021). https://doi.org/10.1186/s40792-021-01326-0
Received:
Accepted:
Published:
DOI: https://doi.org/10.1186/s40792-021-01326-0