Abstract
Background
Bronchial bifurcation abnormalities are often discovered incidentally on chest computed tomography or bronchoscopy. As this condition is asymptomatic, it has little effect on the disease course of patients with lung cancer. However, this abnormality must be considered when performing lung resection.
Case presentation
Patient 1 was a 73-year-old man with suspected simultaneous triple lung cancers [cT1c (3) N0M0, Stage IA3] in the right and left upper lobes. He was initially scheduled to undergo right upper lobectomy and systematic nodal dissection. Chest computed tomography revealed a displaced B3 that arose from the right middle lobe bronchus. V1+2 was transected first, followed by the superior truncus of the pulmonary artery, and B1+2, respectively. After the branches of V3 were ligated, B3 was identified smoothly. Finally, the incomplete interlobar fissure between the upper and middle lobes was separated using an auto-stapler. No vascular abnormalities were observed. Patient 2 was a 62-year-old woman with suspected lung cancer (cT1cN0M0, Stage IA3) in the right upper lobe, and was scheduled to undergo right upper lobectomy and lobe-specific nodal dissection. Chest computed tomography revealed a right top pulmonary vein and a displaced B1 that arose from the right main bronchus independently. Because V1+3 was resected simultaneously during upper and middle lobe resection during robot-assisted thoracic surgery, the procedure was cool-converted to video-assisted thoracic surgery. An independently A1 was observed, followed by A2b and A3, which branched off as a common stem. A right top pulmonary vein was smoothly detected. Each blood vessel was transected using an auto-stapler. B2+3 was transected first using an auto-stapler, followed by B1.
Conclusions
The displaced anomalous bronchus is often accompanied by pulmonary arterial or venous abnormalities and an incomplete interlobar fissure. A “hilum first, fissure last” technique is often useful. Preoperative evaluation and surgical planning are important.
Similar content being viewed by others
Explore related subjects
Discover the latest articles, news and stories from top researchers in related subjects.Background
Owing to the development and widespread application of imaging technology, such as three-dimensional (3D) computed tomography (CT), thoracic surgeons can obtain a precise understanding of the anatomical structures of the patient’s lungs and detect bronchial bifurcation abnormalities or branching anomalies of pulmonary vessels preoperatively [1, 2]. A displaced right upper bronchus (DRUB) is asymptomatic and has little effect on the progress of lung disease. However, these abnormalities must be considered when performing lung resection. Abnormal branching of the pulmonary vessels and bronchi is often encountered during pulmonary resections, and it is extremely important to discuss any abnormalities observed preoperatively on 3D-CT with the surgical team and formulate a proper surgical plan [2]. Herein, we report two cases of right upper lobectomy associated with DRUB.
Case presentation
Patient 1
A 73-year-old male patient presented with an abnormal chest shadow during a routine health checkup. Adenocarcinoma was detected by sputum cytology. He was a former smoker, but otherwise had no notable medical history. Chest CT revealed a 25-mm solid nodule in the right ventral segment (S3), a 12-mm part-solid nodule in the apical segment (S1), and a 28-mm part-solid nodule in the left apicodorsal segment (S1+2). Therefore, simultaneous triple lung cancers were suspected. 3D-CT broncho-angiography (BAG) (Fig. 1) and virtual bronchoscopy (VB) detected an abnormality wherein the right ventral bronchus (B3) originated from the right middle lobe bronchus (MLB) and the apicodorsal bronchus (B1+2) from the right main bronchus (RMB). Transbronchial lung biopsy (TBLB) revealed adenocarcinoma in the nodule in the right S3. Right upper lobectomy (RUL) and systematic nodal dissection were performed using video-assisted thoracic surgery (VATS). Lobulation between the upper and middle lobes was incomplete. At the pulmonary hilum, the common trunk of the apical and dorsal veins, superior truncus of the pulmonary artery (PA), and B1+2 were transected using an auto-stapler. Next, the branches of the ventral veins were ligated. B3 was detected smoothly (Fig. 2) with light assistance from bronchoscopy. However, since the branch that was thought to be B3 and transected using an auto-stapler was actually B3b, B3 was transected in more central direction from the stump of B3b. Finally, near-infrared fluorescence imaging was performed by administering 5 mg of indocyanine green intravenously to visualize the demarcation line of the upper and middle lobes, which were separated using an auto-stapler. No complications were observed during surgery. The patient was diagnosed with simultaneous lung cancer, S3 adenocarcinoma (pT1cN0M0, Stage IA3) and S1 adenocarcinoma (pT1aN0M0, Stage IA1). The drainage tube was removed on postoperative day (POD) 2. He developed atrial fibrillation on POD 3 and underwent direct current defibrillation. He was discharged on POD 9. Left upper division segmentectomy and lobe-specific nodal dissection were performed on POD 35. The patient was diagnosed with a left upper lobe adenocarcinoma (pT1bN0M0, Stage IA2). The patient did not wish to receive adjuvant chemotherapy. Ten months after the initial lung surgery, the patient was undergoing outpatient follow-up.
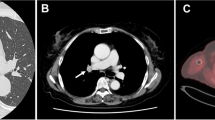
Preoperative findings of Patient 1. Chest computed tomography (CT) revealed: a an apicodorsal bronchus (B1+2) from the right main bronchus (RMB) and b a right ventral bronchus (B3) originating from the right middle lobe bronchus (MLB). c Three dimensional-CT broncho-angiography detected a displaced B3 originating from the right MLB and B1+2 from the RMB
Patient 2
A 62-year-old woman presented with an abnormal chest shadow detected during a routine health checkup. She was a never smoker and had no notable medical history. CT revealed a 27-mm irregularly shaped nodule in the right dorsal segment (S2). 3D-CT BAG and VB imaging detected a displaced right apical bronchus (B1) and a common stem of the dorsal and ventral bronchus (B2+3) that arose from the RMB independently and a right top pulmonary vein (RTPV) (Fig. 3a, b). TBLB revealed that the nodule in the right S2 was adenocarcinoma. Right upper lobectomy and lobe-specific nodal dissection were performed using robot-assisted thoracic surgery (RATS). Lobulation between the upper and middle lobes was existent but poor. After exposing the main trunk of the PA at the inter-lobe, the upper and middle lobes were dissected using an auto-stapler. During interlobar resection, the apicoventral vein (V1+3) was simultaneously resected incidentally. Because the stump of V1 + 3 was involved in the staple line of the middle lobe, we decided to perform a cool conversion from RATS to VATS. The apical artery (A1) branched independently, followed by the horizontal subsegmental artery (A2b) and ventral artery (A3), which branched off as a common stem (Fig. 4a). Each branch was transected using an auto-stapler. While dissecting A2b + A3, the interlobar lymph node (LN) (#11 s) around B2+3 was subjected to intraoperative frozen section biopsy, which tested negative. The RTPV was smoothly detected and transected using an auto-stapler (Fig. 4b). Thereafter, the upper and lower lobes were dissected using an auto-stapler. Finally, the B2+3 and B1 were transected by auto-stapler in this order. The patient was diagnosed with lung adenocarcinoma with segmental LN (#13) metastasis (pT1cN1M0, Stage IIB). The patient underwent adjuvant chemotherapy with cisplatin–docetaxel followed by osimertinib. Six months after surgery, the patient was undergoing follow-up without recurrence.
Intraoperative findings of Patient 2. a An apical artery (A1), with a common stem of the horizontal subsegmental artery (A2b) and ventral artery (A3), and dorsal subsegmental artery (A2a) branched separately. b At the dorsal side of the hilum, a right top pulmonary vein (RTPV) and displaced apical bronchus (B1) were detected
Discussion
Bronchial bifurcation abnormalities are often discovered incidentally on chest CT or bronchoscopy. Previous studies reported that the incidence of displaced bronchi is 0.64–0.76% [3, 4]. Further, 75–84.8% of tracheobronchial anomalies are reportedly found in the right upper lobe [3, 4]. Ohta et al. found that only 0.0045% (59 of 13,222) of cases showed a displaced segmental bronchus; of these, 10 cases (16.9%) had B3 branching off from the MLB, as in Patient 1, and 8 cases (13.6%) had B1 branching off alone from the RMB, as in Patient 2 [3]. Yaginuma analyzed the chest CT scans of 6,072 patients and reported that a displaced bronchus was observed in 46 cases (0.76%). He further classified DRUB into 4 types: (i) the “right upper lobar type”, where the right upper bronchus arises from the lateral wall of the trachea; (ii) the “right B1 type”, where B1 arises from the lateral wall of the trachea or RMB; (iii) the “right B2 type”, where B2 arises from the bronchus intermedius; and (iv) the “right B3 type”, where B3 is absent from the right upper bronchus and arises from the right MLB [5].
Tables 1 and 2 summarize previously published case reports of lung resection for lung tumors associated with a displaced B3 from the RMB [6,7,8,9,10] and a displaced B1 from the right main bronchus in Japan [11,12,13,14,15], respectively. To date, displaced B3 and displaced B1 has been reported in seven cases each, including the current study. In the cases with displaced B3, no association was found with PV anomalies. An incomplete interlobar fissure (IF) between the upper and middle lobes was observed in all patients. Therefore, in right upper lobectomy, a “hilum first, fissure last” or “no-touch fissure” technique was often chosen [9], and the approach of transecting the incomplete IF first was not adopted [6,7,8,9,10]. It is reportedly important to transect the incomplete IF after preparing the bronchus in order to prevent postoperative air leakage and to confirm that the resected part does not include a displaced bronchus [10]. An incomplete IF was observed in Patient 1. Transecting B1+2 made it easier to unfold the upper lobe dorsoinferiorly, and B3 became easier to dissect. By first transecting the bronchus, it was easier to transect the incomplete IF. If it is difficult to identify the bifurcation of B3, it may be easier to dissect it by transecting the peripheral branches such as B3b first. Among the cases with a displaced B1, 3 cases were complicated by the presence of the RTPV. Several studies did not provide a description of the lobulation between the upper and middle lobes, and the details were unknown; however, one case had incomplete lobulation and the lobulation in Patient 2 in this study was also poor. Previous studies have reported that bronchial abnormalities are accompanied by anomalies in PAs and PVs [4, 14,15,16,17]. Yaginuma et al. reported that patients with a RTPV have a high frequency of displaced bronchus in addition to incomplete fissure [4]. Katsumata et al. have reported cases in which A1, dorsal subsegmental artery (A2a), A2b, lateral subsegmental artery (A3a), and medial subsegmental artery (A3b) diverged individually [11]. In the present Patient 1, in addition to the superior truncus (A1 + recurrent A2), A3a and A3b branched separately. In present Patient 2, the A1, the common stem of A2b and A3, and A2a branched separately.
In our institution, between April 2016 and March 2024, 1,069 lung cancer surgeries were performed general anesthesia, including pleural biopsy and diagnostic thoracoscopy, of which 10 cases (0.9%) were found to have DRUB (Table 3). DRUB was classified according to Yaginuma's classification [5]. Chest CT was performed to assess the presence of the RTPV and mediastinal branches of the left PA. RTPV was found in 3 of 10 cases. The mediastinal branch of the left PA was also found in 3 of 10 cases and one case included a branch to the lower lobe. We compared the RTPV, left mediastinal lingular branch of the PA, and left mediastinal inferior lobar branch of PA in patients with or without a DRUB (Table 4). The Chi-square test was used for analysis, and p < 0.05 was considered significant. Patients with a DRUB were significantly more likely to have an RTPV (p < 0.001). Although it is not possible to draw a definitive conclusion due to the small sample size, it was suggested that patients with a DRUB may also have a left mediastinal inferior lobar branch of the PA (p < 0.001). When a DRUB is present, attention must be paid to the abnormal vascular course not only during surgery on the right side but also in surgery on the left side. When performing anatomical lung resections, such as lobectomy and segmentectomy in pulmonary surgery, it is extremely important to use 3D-CT to visualize branching abnormalities in the PAs, PVs, and bronchi [2]. Furthermore, it is important to share this information with the surgeons and anesthesiologists before surgery. This is because anesthesiologists must generally perform intraoperative management, such as bronchial toilets and selective segmental inflation [18], during lung resection.
Conclusions
We performed right upper lobectomies for lung cancer associated with a DRUB. When DRUB is present, attention must be paid to the incomplete IF and abnormal vascular course, especially RTPVs. A “hilum first, fissure last” technique is often useful. It is extremely important to discuss any abnormalities using 3D-CT before surgery within the team for proper surgical planning.
Availability of data and materials
All data generated or analyzed during this study are included in this published article.
Abbreviations
- 3D:
-
Three-dimensional
- CT:
-
Computed tomography
- DRUB:
-
Displaced right upper bronchus
- S3 :
-
Ventral segment
- S1 :
-
Apical segment
- S1+2 :
-
Apicodorsal segment
- B3 :
-
Ventral bronchus
- BAG:
-
Broncho-angiography
- VB:
-
Virtual bronchoscopy
- B1+2 :
-
Apicodorsal bronchus
- MLB:
-
Middle lobe bronchus
- RMB:
-
Right main bronchus
- TBLB:
-
Transbronchial lung biopsy
- RUL:
-
Right upper lobectomy
- VATS:
-
Video-assisted thoracic surgery
- PA:
-
Pulmonary artery
- POD:
-
Postoperative day
- B1 :
-
Apical bronchus
- S2 :
-
Dorsal segment
- B2+3 :
-
Common stem of dorsal and ventral bronchus
- A1 :
-
Apical artery
- A2b:
-
Horizontal subsegmental artery
- A3 :
-
Ventral artery
- RTPV:
-
Right top pulmonary vein
- RATS:
-
Robot assisted thoracic surgery
- PV:
-
Pulmonary vein
- A2a:
-
Dorsal subsegmental artery
- A3a:
-
Lateral subsegmental artery
- A3b:
-
Medial subsegmental artery
References
Ikeda N, Yoshimura A, Hagiwara M, Akata S, Saji H. Three dimensional computed tomography lung modeling is useful in simulation and navigation of lung cancer surgery. Ann Thorac Cardiovasc Surg. 2013;19:1–5.
Iijima Y, Kinoshita H, Nakajima Y, Kurihara Y, Akiyama H, Hirata T. Branching anomaly of the pulmonary ventrobasal and laterobasal arteries from the mediastinal lingular pulmonary artery. Gen Thorac Cardiovasc Surg. 2020;68:1558–61.
Ohta S, Saito Y, Usuda K, Kanma K, Sagawa M, Sato M, et al. Tracheobronchial anomalies: report of 71 cases. J Jpn Soc Respir Endosc. 1986;8:122–30.
Yaginuma H. Investigation of displaced bronchi using multidetector computed tomography: Associated abnormalities of lung lobulations, pulmonary arteries and veins. Gen Thorac Cardiovesc Surg. 2020;68:342–9.
Yaginuma H, Takano K, Umea M. Right top pulmonary veins associated with lung incomplete fissure and displaced bronchus: a retrospective study using multidetector computed tomography. Gen Thorac Cardiovesc Surg. 2021;69:290–6.
Ohno K, Ozeki M, Negishi H, Minegishi K, Maki M, Tsubochi H, et al. Right upper lobectomy for lung cancer patient with a right displaced B3 bronchus. J Jpn Soc Respir Endosc. 2021;43:16–20.
Yamada T, Mori T. Resection of right upper lobe lung cancer with a displaced anomalous right B3 bronchus: a case report. J Jpn Soc Respir Endosc. 2023;45:130–4.
Tanaka N, Fujimori H, Ida A. A case of complete video-assisted thoracoscopic middle lobectomy for lung cancer with displaced B3. J Jpn Soc Respir Endosc. 2023;45:189–92.
Nakanishi K, Kuroda H, Nakada T, Ueno H, Sakakura N. Thoracoscopic lobectomy using indocyanine green fluorescence to detect the interlobar fissure in a patient with displaced B3 and absence of fissure: a case report. Thorac Cancer. 2019;10:1654–6.
Fukutomi T, Yoshizu A. Right S3+4+5 resection for lung cancer with displaced B3. Kyobu Geka. 2020;73:1072–5.
Katsumata S, Miyoshi T, Tane K, Samejima J, Aokage K, Tsuboi M. A case of right upper lobectomy for a patient with lung cancer with a displaced B2+3 bronchus. J Jpn Soc Respir Endosc. 2022;44:54–8.
Hara K, Matsuda Y, Furukawa N, Miyamoto H, Kimura T, Okabe K. Right upper lobectomy for lung cancer patient with a displaced right bronchus. Respir Med Case Rep. 2022;38: 101689.
Sakaguchi K, Horio H. Lung cancer right upper lobe with displaced anomalous bronchi: a report of two cases. J Jpn Soc Respir Endosc. 2013;35:666–70.
Tajima K, Uchida N, Sasamoto H, Okada T, Kohri T, Mogi A, et al. Lung adenocarcinoma with anomalous bronchi and pulmonary veins preoperatively identified by computed tomography. Thorac Cancer. 2016;7:599–601.
Mun M, Goto H, Matsuura Y, Nakao M. Thoracoscopic right upper lobectomy in a patient with bronchial and pulmonary vein anomalies. JTCVS Tech. 2020;4:316–8.
Tatematsu T, Saito Y, Kasugai T, Satake A, Yamakawa Y. Resection of right upper lung cancer with a displaced anomalous B2 bronchus: a case report. Jpn J Chest Surg. 2017;31:116–21.
Sakai E, Nakahara K, Kina S, Miyanaga S. Thoracoscopic right upper and middle bilobectomy for lung cancer with a displaced right upper bronchus and incomplete lobulation. Jpn J Chest Surg. 2021;35:76–81.
Okada M, Mimura T, Ikegaki J, Katoh H, Itoh H, Tsubota N. A novel video-assisted anatomic segmentectomy technique: Selective segmental inflation via bronchofiberoptic jet followed by cautery cutting. J Thorac Cardiovasc Surg. 2007;133:753–8.
Acknowledgements
We would like to thank Editage (www.editage.jp) for English language editing.
Funding
Not applicable.
Author information
Authors and Affiliations
Contributions
Y.I. participated in the surgery, and conceived and conducted the study. Y.I., T.M., and M.I. performed the literature search. Y.I., S.I., and N.M. participated in the surgery. N.M. and H.U. supervised the manuscript preparation and critically revised the manuscript. All authors have read and approved the final manuscript.
Corresponding author
Ethics declarations
Ethics approval and consent to participate
Not applicable.
Consent for publication
Written informed consent was obtained from the patients for the publication of this report and its accompanying images.
Competing interests
All authors declare that they have no competing interests.
Additional information
Publisher's Note
Springer Nature remains neutral with regard to jurisdictional claims in published maps and institutional affiliations.
Rights and permissions
Open Access This article is licensed under a Creative Commons Attribution 4.0 International License, which permits use, sharing, adaptation, distribution and reproduction in any medium or format, as long as you give appropriate credit to the original author(s) and the source, provide a link to the Creative Commons licence, and indicate if changes were made. The images or other third party material in this article are included in the article's Creative Commons licence, unless indicated otherwise in a credit line to the material. If material is not included in the article's Creative Commons licence and your intended use is not permitted by statutory regulation or exceeds the permitted use, you will need to obtain permission directly from the copyright holder. To view a copy of this licence, visit http://creativecommons.org/licenses/by/4.0/.
About this article
Cite this article
Iijima, Y., Mizoguchi, T., Ishikawa, M. et al. Right upper lobectomy for lung cancer associated with a displaced anomalous bronchus: two case reports. surg case rep 10, 187 (2024). https://doi.org/10.1186/s40792-024-01986-8
Received:
Accepted:
Published:
DOI: https://doi.org/10.1186/s40792-024-01986-8