Abstract
Background
Radiation therapy (RT) for breast cancer (BC) can result in subtle cardiac dysfunction that can occur early after treatment. In 2022, the European Society of Cardiology (ESC) published the first guidelines in cardio-oncology with a harmonized definition of cancer therapy-related cardiac dysfunction (CTRCD). The aim of this study was to evaluate CTRCD occurrence over 24 months of follow-up after RT in BC patients and to analyze the association with cardiac radiation exposure.
Methods
The prospective monocentric BACCARAT study included BC patients treated with RT without chemotherapy, aged 40–75 years, with conventional and 2D Speckle tracking echocardiography performed before RT, 6 and 24 months after RT. Based on ESC cardio-oncology guidelines, CTRCD and corresponding severity were defined with left ventricle ejection fraction and global longitudinal strain decrease, occurring at 6 or 24 months after RT. Dosimetry for whole heart, left ventricle (LV) and left coronary artery (left anterior descending and circumflex arteries (CX)) was considered to evaluate the association with CTRCD, based on logistic regressions (Odds Ratio – OR and 95% confidence interval – 95%CI). Youden index based on receiver operating characteristic curve analysis was used to identify the optimal threshold of dose-volume parameters for predicting CTRCD.
Results
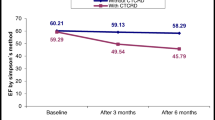
The study included 72 BC patients with a mean age of 58 ± 8.2 years. A total of 32 (44%) patients developed CTRCD during follow-up: 20 (28%) mild CTRCD, 7 (9%) moderate CTRCD, and 5 (7%) severe CTRCD. Cardiac radiation doses were generally higher among patients with CTRCD rather than non-CTRCD. Dose-response relationships were significant for mean CX dose (OR = 2.48, 95%CI (1.12–5.51), p = 0.02) and marginally significant for V2 of LV (OR = 1.03 95%CI (1.00-1.06), p = 0.05). V2 of LV ≥ 36% and mean CX dose ≥ 1.40 Gy thresholds were determined to be optimal for predicting CTRCD.
Conclusion
For BC patients treated with RT without chemotherapy, CTRCD can be observed in an important proportion of the population over 24 months after treatment. Left ventricle and circumflex coronary artery exposure were found to be associated with CTRCD and could be used for the prediction of such cardiotoxicity. Further research remains needed to confirm these results.
Trial Registration
ClinicalTrials.gov Identifier- NCT02605512.
Similar content being viewed by others
Explore related subjects
Discover the latest articles, news and stories from top researchers in related subjects.Background
Breast cancer (BC) is the most frequent cancer in women [1]. Its management has evolved significantly over the years, with multimodal approaches, including surgery, chemotherapy, endocrine therapy, target agents, immune therapy, and radiation therapy (RT), playing pivotal roles in achieving favorable outcomes. Among these modalities, RT has been widely used to target microscopic residual disease as an adjuvant treatment after surgery, reduce local recurrence rates, and improve overall survival [2,3,4].
While RT has proven effective in treating BC, prior studies have shown that RT can impact the heart. Cardiac radiation exposure can lead to pathophysiological changes that may result in short- and long-term radiation-induced cardiac toxicities. Such toxicities, covering a broad spectrum of possible manifestation, can cause substantial morbidity and may manifest clinically in the weeks to years after the completion of treatment, such as coronary artery disease, one of the most frequent RT-induced cardiac toxicity occurring several years after RT for BC, established as dose-dependent [5,6,7,8].
Echocardiography is essential in early identification and monitoring of cardiotoxicity related to cancer treatment [9]. It has been the primary modality to monitor left ventricular systolic dysfunction in cancer patients, and the parameter examined in the context of cardiotoxicity was the left ventricular ejection fraction (LVEF). However, LVEF has many limitations in recognizing subtle variations in LV function. The global longitudinal strain (GLS) assessment using speckle tracking echocardiography is used for detecting and quantifying subtle disturbances in the global long-axis LV systolic function [10, 11]. Long before the onset of potential LV dysfunction and heart failure, several studies based on populations of BC patients treated with RT could observe a statistically significant decrease in GLS during follow-up with a timeframe ranging from the end of RT to 6, 12 or 36 months after RT [12,13,14,15,16,17,18]. In contrast, most of these studies observed no significant change in LVEF. In a previous 6-month follow-up analysis of the BACCARAT study, the occurrence of subclinical LV dysfunction (defined as relative decrease of GLS > 10%) was associated with whole heart and LV doses received during RT for BC [18]. These results were confirmed in the European MEDIRAD EARLY-HEART study [19]. However, limited results from other studies were provided regarding the impact of cardiac doses. Moreover, several definitions were used to describe LV dysfunction, which led to potential discrepancies between studies.
In 2022, the first ESC guidelines in cardio-oncology introduced the descriptive term Cancer Therapy-Related Cardiac Dysfunction (CTRCD), combining both information on LVEF and GLS decrease from baseline [20]. There is still a lack of literature data about the effect of RT on this newly defined CTRCD. Understanding the interplay between RT for BC and CTRCD is, however, essential for improved patient care as it could guide preventive measures and enhance knowledge in cardio-oncology towards more personalized and effective BC management.
In this new analysis of the BACCARAT cohort, with an enhanced follow-up of 24 months, we aimed to evaluate the occurrence of CTRCD (early-term six months after RT and/or mid-term 24 months after RT) and its association with radiotherapy-induced cardiac exposure, and also to detect the most suitable dosimetry predictors of subsequent CTRCD.
Methods
Study design and population
The protocol of the study has been described elsewhere [21]. Briefly, between October 2015 and December 2017, this prospective monocentric study included 113 BC female patients aged 40 to 75 years, with left or right unilateral BC, who underwent RT after breast-conserving surgery, without chemotherapy, at the Clinique Pasteur (Toulouse, France). Patients with metastatic BC, prior thoracic RT, history of severe cardiovascular disease such as coronary artery disease including acute myocardial ischemia or infarction, renal failure, allergies to iodinated contrast injection, and pregnancy were excluded.
Patients were included at baseline before RT with follow-up visits at 6 and 24 months after RT, and the follow-up period of the last patient ended in January 2020. The patient’s medical history was collected at baseline, and cardiac examinations, particularly echocardiography examinations, were performed by cardiologists during the programmed visit at baseline (i.e. before RT), 6 and 24 months after RT. After the exclusion of the patients with incomplete echocardiographic follow-up data, the study population presented here consisted of 72 patients with complete echography measurements (both LVEF, and GLS measurements) at baseline, six months, and 24 months after RT, as well as cardiac radiation dosimetry data.
Radiotherapy
All patients were treated with three Dimensional Conformal Radiation Therapy (3D-CRT) with 6 and 25 MV photon beams by tangential fields second to the surgical treatment. Two modalities of the planning target volume dose were applied. The first modality was to administer 50 Gy (Gy) delivered in 25 daily fractions of 2 Gy over five weeks or 47 Gy delivered in 20 daily fractions of 2.35 Gy over five weeks. This second fractionation choice was only driven by the need to slightly limit the number of sessions per patient due to the technical problems that arose in one 3D-CRT machine from January 2016 to May 2016. Six MV photons were used for most patients, except for a few patients with big breast sizes, where 25 MV additional photons were used. An extra 9–15 Gy boost might be applied to the tumor site using photon/electron beams with energies ranging from 6 MeV to 18 MeV. The treatment planning system (TPS) applied to conduct dose calculations was Eclipse™ with the Analytical Anisotropic Algorithm (AAA version 13.6) (Varian Medical System, Palo Alto, CA, USA). Each patient’s RT was planned in a manner in which the dose distribution was optimized and normalized to the International Commission on Radiation Units and Measurements (ICRU) reference point of the breast cancer and to achieve QUANTEC dose constraints to organs at risk, including the whole heart [22]. Deep inspiration breath hold was used for very few patients treated for left-sided breast cancer with heart very close to the anterior chest wall and for QUANTEC dose constraints achievement (volume of heart receiving at least 25 Gy (V25) less than 10%).
Cardiac dosimetry
The methods of evaluating cardiac radiation doses in the BACCARAT cohort were already described elsewhere [21, 23]. Clinic Pasteur RT department provided the Dose-Volume Histograms (DVHs) for the whole heart. The delineation of the other cardiac structures (left ventricle (LV) and left coronary artery, including left anterior decreasing coronary artery (LAD), circumflex coronary artery (Cx), and right coronary artery (RCA)) were performed manually (Fig. 1A). Using the 3D dose matrix made during planning treatment and the delineated substructure, DVH for cardiac structures was produced with ISOGray TPS by the dosimetry department of IRSN in partnership with the Clinic Pasteur RT department. This study focussed on left ventricular cardiac dysfunction (CTRCD), and we thus focussed on the following heart substructures: the whole heart, left ventricle, and the left coronary artery that carries blood to the left part of the heart (LAD and CX) (Fig. 1B). The following absorbed dose metrics for the cardiac substructures were collected: Dmean (in Gy), the volume-weighted mean dose; D2 (in Gy), the minimal dose absorbed by the most irradiated 2% of the structure volume, which can be considered as a substitute for maximum dose; V2 (in %) the relative volumes exposed to at least to 2 Gy.
A Three-dimensional coronary segments view with the Left Anterior Descending Coronary artery (LAD) and the circumflex coronary artery (CX). B CT dose-planned left tangential breast irradiation showing isodoses and delineated structures: Heart, left ventricle (LV), Left anterior descending coronary artery (LAD) and Circumflex coronary artery (CX)
Clinical evaluation and echocardiography
Cardiotoxicity related to cancer treatment was assessed through a combination of clinical evaluation and imaging techniques performed at baseline before RT, 6 and 24 months after RT. During clinical evaluation with cardiologist: Medical history included detailed patient history including pre-existing cardiovascular conditions, family history of heart disease, and lifestyle factors (e.g., smoking, alcohol use); Symptoms included inquiry about symptoms such as chest pain, shortness of breath, fatigue, palpitations, and swelling in the legs or abdomen; Physical Examination included assessment of heart rate, blood pressure, jugular venous pressure, peripheral edema, and auscultation for heart sounds and murmurs. Imaging techniques, further described below, was based on echocardiography to monitor heart function where Left Ventricular Ejection Fraction (LVEF) was a primary measure for assessing systolic function and Global Longitudinal Strain (GLS) was used to detect early myocardial dysfunction before a decrease in LVEF is apparent.
An exhaustive 2-dimensional (2D) echocardiography examination was done at baseline before RT, at six months, and 24 months after RT using a commercially available ultrasound Acuson S2000 (Siemens Medical Solutions USA, Inc. Malvern, USA), using a 3 MHz cardiologic probe transducer. The echographic data were independently analyzed by a single-blinded cardiologist unaware of the patient’s medical history and clinical examination. Biplane Simpson’s method from apical two- and four-chamber windows was used to measure LV ejection fraction (LVEF). Strains were calculated using 2D speckle tracking echocardiography (2D-STE) and the automated function imaging technique for tracking acoustic markers (speckles) [24]. We used the 2DSTE vendor offline Syngo Velocity Vector Imaging 2.0 software (Siemens Medical Solutions USA, Inc., Moutain View, Calif, USA). Based on the American Society of Echocardiography guidelines, images were analyzed in a 16-segment model [25]. All segmental values were averaged GLS, in %.
CTRCD events definition
Based on ESC guidelines in cardio-oncology [20], symptomatic CTRCD was defined by the presence of heart failure (incapacity of the heart to pomp enough blood to the tissues) and asymptomatic CTRCD was defined as follows:
-
Severe CTRCD: New LVEF reduction to < 40%.
-
Moderate CTRCD: New LVEF reduction by ≥ 10% points to an LVEF of 40–49% OR New LVEF reduction by < 10% points to an LVEF of 40–49% AND new relative decline in GLS by > 15% from baseline
-
Mild CTRCD: LVEF ≥ 50% AND new relative decline in GLS by > 15% from baseline.
In the present study, “CTRCD” was defined for any grade asymptomatic CTRCD observed at 6 or 24 months after RT; “early CTRCD” for any grade asymptomatic CTRCD observed at six months and “mid-term CTRCD” for any grade asymptomatic CTRCD observed at 24 months. Grade of CTRCD (“mild CTRCD”; “moderate CTRCD”; “severe CTRCD”) was defined as the highest grade of asymptomatic CTRCD observed during the follow-up period.
Statistical analysis
Continuous variables are presented with mean and standard deviation or median and range values. Student’s t-test or Wilcoxon-Mann-Whitney non-parametric test was used to compare continuous variables when appropriate. Categorical variables are reported as counts and percentages. The χ2 and Fisher’s exact tests were used to compare categorical variables, as applicable. To analyze the associations between CTRCD and radiation and non-radiation factors in univariable analysis, we used binary logistic regressions (odds ratios (ORs), 95% confidence intervals (CI), p-values). Cardiac radiation exposure factors included the laterality of BC, Dmean, D2, and V2 for the whole heart, left ventricle, left anterior decreasing coronary artery (LAD), and circumflex coronary artery (Cx). Non-radiation factors included age, body mass index (BMI), smoking status, hypertension, diabetes mellitus, hypercholesterolemia, menopausal status, and endocrine therapy administered sequentially after RT. For multivariable analysis, we only considered for adjustment the non-radiation variables with p-value < 0.20 in univariable analysis. For dosimetry parameters significantly associated with the risk of CTRCD, receiver operating characteristic (ROC) curves were constructed, and the maximum Youden Index was utilized to determine the optimal cut-off values and thresholds for predicting CTRCD. A p-value < 0.05 was considered statistically significant in all two-sided statistical tests. All analyses were performed using SAS 9.4 and STATA 14.2 software.
Results
Patients’ characteristics
A total of 72 patients with echocardiographic data available for the time points (before RT, RT + 6, and RT + 24 months) and dosimetry measurements were included in this analysis. A description of their baseline characteristics is presented in Table 1. The mean age at RT was approximately 58 ± 8.2 years old, and less than 20% of patients presented cardiovascular risk factors such as diabetes, hypertension, or current smoking. Patients mostly had left-sided BC (82%) and received endocrine treatment (75%). The majority of the patients had locally invasive BC (82%). Mean heart dose and mean left ventricle doses for the whole population were 2.5 ± 1.5 Gy and 5.1 ± 3.6 Gy, respectively, with doses much higher for left-sided BC than right-sided BC (Supplementary Material 1). LAD was the most exposed cardiac substructure for left-sided BC with a mean dose of 15.8 ± 7.6 Gy. Among other coronary arteries, the mean dose for CX was 1.4 ± 0.9 Gy with D2 that could reach a maximum of 48.7 Gy among left-sided BC.
CTRCD events during follow-up
No patient developed symptomatic CTRCD, defined by the presence of heart failure, during the two-year follow-up.
Overall, 32 patients (44%) developed new asymptomatic CTRCD during the two-year follow-up: 22 (31%) occurred 6 months after RT corresponding to early CTRCD, and 14 (19%) occurred 24 months after RT corresponding to mid-term CTRCD (Table 2). Mild CTRCD was the most frequent grade of CTRCD during the two-year follow-up with 20 patients; 7 and 5 patients experienced moderate or severe CTRCD, respectively (Supplementary Material 2).
Association between CTRCD and radiation doses
Dose distribution according to the CTRCD status is presented in Fig. 2. The doses were generally higher among patients with CTRCD than those without CTRCD. However, differences were statistically significant only for whole heart-V2, LV-V2, and CX-Dmean, CX-D2, and CX-V2. After Holm-Bonferroni correction for multiple tests, only LV-V2, CX-Dmean, and CX-V2 remained statistically significant.
Boxplots and comparisons of dose distribution according to CTRCD status D2: dose to 2% of the structure mostly exposed (in Gy); V2: volume of the structure receiving dose ≥ 2 Gy (in %); Dmean: mean absorbed dose (in Gy); LAD: Left Anterior Descending Coronary artery; CX: Circumflex Coronary Artery; p-values for comparison of dose metrics distributions between CTRCD and no CTRCD groups
Among non-radiation factors, BMI, smoking status, hypertension, hypercholesterolemia, and endocrine therapy reached a p-value of less than 0.20 and were considered in further multivariable analysis for adjustment (Table 3).
BMI Body Mass Index: Variables with a p-value in bold (< 0.20) were considered for multivariable analysis for the association between cardiac exposure and CTRCD.
Univariable and multivariable CTRCD analyses by dose-volume parameters is presented in Table 4. Left-sided BC presented a higher risk of CTRCD but the association was not statistically significant (OR = 3.22, p = 0.09). None of the dose parameters for the whole heart and the LAD were significantly associated with CTRCD. For LV, only V2 was significantly associated with CTRCD (OR = 1.03, p = 0.01), and this result remained marginally significant in the adjusted analysis (OR = 1.03, p = 0.05). For CX, both Dmean, D2, and V2 were significantly associated with CTRCD, but only Dmean remained significantly associated with CTRCD in multivariable analysis (OR = 2.48, p = 0.02). Exploratory analysis for early CTRCD occurring at six months and mid-term CTRCD occurring at 24 months presented similar results for dose responses (Supplementary Material 3).
Analysis of CTRCD risk according to dose category divided into 33th and 66th percentile showed a non-significant 4-fold increase in the adjusted OR for CTRCD for the most exposed category of LV-V2 (> 53%) as compared with the less exposed category (< 34%). For the Dmean of the CX, highly exposed patients with doses > 1.48 Gy presented an OR of 7.95 (p < 0.05) compared to lowly-exposed patients with Dmean < 1.08 Gy (Supplementary Material 4).
An exploratory analysis of the association between cardiac radiation doses and CTRCD grade was conducted (Supplementary Material 5). Patients with either severe or moderate CTRCD were all left-sided BC. The association between cardiac radiation doses and severe CTRCD was more robust than for overall CTRCD, reaching statistical significance for all cardiac structures (whole heart Dmean and V2, LV V2, LAD Dmean, and CX Dmean, D2, and V2). In particular, the association with CX-Dmean was three times higher than for overall CTRCD (OR = 6.42, p = 0.001, compared to severe CTRCD OR = 2.44, p = 0.008). No association for mild or moderate CTRD reached statistical significance, except CX-D2 associated with mild CTRCD (OR = 1.87, p = 0.01). We did not perform an adjusted analysis because of the limited number of events.
ROC analysis
A ROC curve analysis was performed to diagnose the performance of V2 of the LV and Dmean of CX level in identifying CTRCD (Fig. 3). The optimal cut-off value of LV-V2 in the detection of CTRCD was 36% (area under the curve: 0.68, 50% sensitivity, 81% specificity). The optimal cut-off value of CX-Dmean was 1.40 Gy (area under the curve: 0.72, 80% sensitivity, 66% specificity).
Discussion
The role of 2D speckle tracking echocardiography in early detection and quantification of subtle disturbances in left ventricular systolic function after RT for breast cancer was previously investigated, and radiotherapy-induced alterations in global longitudinal strain (GLS) were observed previously [26]. However, heterogeneous definitions of such GLS alterations in studies lead to discrepancies between results. A harmonized definition of CTRCD has recently been published in ESC cardio-oncology guidelines [20], either symptomatic in presence of heart failure, or asymptomatic based on a reduction in LVEF and/or relative change in GLS, but there was a lack of literature about the effect of RT on such CTRCD. In this study, we aimed to evaluate the occurrence and the radiation-induced risk of CTRCD in a population of BC patients treated with RT without chemotherapy during a follow-up of 24 months.
No symptomatic CTRCD was observed in our population, but asymptomatic CTRCD occurred in 32 patients, representing 44% of the study population, with almost two-thirds of them detected six months after RT and one-third detected 24 months after RT. Mild CTRCD was the most frequent grade of severity (28%), followed by moderate CTRCD (9%) and severe CTRCD (7%). Previous studies on BC patients showed that RT could impair LV systolic function, as measured by GLS decrease without LVEF change or slight LVEF decrease remaining in normal range [12,13,14,15,16,17,18]. Compared to baseline GLS, a statistically significant reduction in GLS could be observed at the end of RT [16], six weeks after RT [27], six months after RT [28], 12 months after RT [13], 14 months after RT [12] and even three years after RT [14], [29]. However, these studies were mainly based on GLS analyzed as a continuous variable, and few reported the occurrence of relative GLS decrease above a threshold considered clinically relevant. Six months after RT, we previously reported 47% of patients with a relative GLS decrease > 10% in the BACCARAT study [18]. The MEDIRAD Early-Heart study reported 12% of patients with a relative GLS decrease > 15% [19], below the 28% of mild CTRCD we observed in our study. However, in Early-Heart study, mean heart dose was lower compared to ours (mean heart dose = 1.72 Gy, vs. 2.5 Gy). With mean heart doses similar to ours, Tuohinen et al. reported that 30% of patients with a relative GLS decrease by > 15% three years after RT, which is consistent with our findings, even if no data on GLS decrease before RT + 3 years, in particular at RT + 2 years, was provided in their study [14].
Previous studies analyzed mild forms of CTRCD but did not consider moderate or severe forms as we did. Moreover, it is difficult to compare studies providing results from cross-sectional evaluations at different time points because of the possible reversibility of subtle cardiac systolic dysfunction measured with GLS. Such reversibility in GLS decrease was observed in the present study, with patients presenting CTRCD at six months who recovered at 24 months, as this was also observed in patients treated with chemotherapy [30]. Possible changes in measurement conditions during follow-up may affect cardiac volumes, LVEF, and GLS quantification [31] and explain such reversibility in systolic function. However, Tuohinen et al. [14] found that worsening diastolic function was detectable even in patients without significant changes in systolic function determined by GLS, illustrating that potential reversibility of GLS alterations after RT could still cause some damage to the heart. Similarly, BC patients treated with RT presented a risk of heart failure despite preserved LVEF [32].
Data showing the relationship between radiation dose to heart substructures and subsequent cardiac dysfunction is scarce. The laterality of BC or mean heart dose was often used as a exposure measurement proxy for the irradiation of cardiac substructures. Some studies could observe an enhanced decrease in GLS after RT for left-sided BC [12] or higher mean heart doses [18, 19], but some others did not [16, 27, 28, 33]. In our study, despite the higher frequency of left-sided BC or higher mean heart doses for patients with CTRCD, the association of these parameters with CTRCD did not reach statistical significance (OR = 3.34, p = 0.14 and OR = 1.17, p = 0.46 respectively). It is worth noting that for severe CTRCD, all patients were left-sided, and heart exposure (Dmean and V2) was significantly associated with severe CTRCD. However, this result was based on only five patients and would require further investigation in a larger population. Our study focused on RT alone and did not provide data on cardiovascular outcome of concomitant radiotherapy and chemotherapy. In a retrospective cohort study [34], left-side irradiation showed limited effect on the incidence of cardiovascular events. However, the effect of left-side irradiation on the incidence of cardiovascular events was stronger in patients with a cumulative doxorubicin dose ≥ 250 mg/m2, illustrating that additive or synergistic effect of chemotherapy on radiotherapy could induce higher risk of cardiac injury, such as CTRCD.
Considering dose to the cardiac substructures in cardiotoxicity risk analysis rather than using laterality or mean absorbed dose to the whole heart as a proxy has already been discussed [23]. In the context of CTRCD, cardiac dysfunction is characterized by left ventricular dysfunction. Therefore, exposure to cardiac structures in the left part of the heart was a priori relevant, and we investigated exposure to the left ventricle but also the left coronary artery that carries blood to the left part of the heart (divided into left anterior descending artery (LAD) and circumflex artery (CX)). We found dose-response relationships for V2 of the left ventricle (OR = 1.03, p = 0.05). Such association with LV exposure was previously observed [18, 19]. We also found dose-response relationships for the mean dose to the CX (OR = 2.48, p = 0.02). This is the first study to show an association with CX exposure. This result is surprising because LAD can receive doses much higher than CX. However, the mean absorbed dose to CX may be a better proxy of exposure to explain the risk of CTRCD than LAD exposure. This was also suggested in our analysis of atherosclerotic plaques [8]. Another explanation could be linked with anatomy and function of coronary arteries. While the LAD supplies blood to the anterior portion of the left ventricle, the CX supplies blood to the lateral wall of the left ventricle and sometimes to the posterior inferior part of the ventricle. GLS is an average measure of segmental strains for the whole left ventricle, but in a previous analysis of territorial longitudinal strains, we found a decrease in strain not only for segments related to LAD territory, but also for segments related to CX territory [17], illustrating that CX exposure could also play a central role in asymptomatic moderate and mild CTRCD occurrence driven by change in CX-related segmental strain.
Finally, we evaluated two optimal cut-off values in V2 of LV and Dmean of CX to predict CTRCD. V2 of LV above 36% (AUC = 0.68, sensitivity = 50%, specificity = 81%) and mean dose to CX > 1.40 Gy (AUC = 0.72, sensitivity = 80%, specificity = 66%) were associated with an increased possibility of CTRCD development than lower values. Our study highlighted LV and CX cardiac substructure exposure regarding the risk of CTRCD. Still, these results remain to be confirmed by other studies, and recommendations that the dose to the heart has to be as low as possible remain relevant. To enhance knowledge on radiation-induced CTRCD after RT, an effort will be required for accurate segmentation and dose estimation for cardiac substructures, including coronary arteries, not limited to the LAD. The coronary arteries are difficult to delineate because of their small volumes and inherent contouring variability, but some atlas and auto-segmentation tools exist [35,36,37] and will be further developed. By making the segmentation task of all these cardiac substructures more accessible, detailed dosimetry data of several potential “targets” regarding cardiotoxicity will be available and fruitful to enhance the understanding of radiation-induced cardiovascular dysfunction, a central concern of cardio-oncology.
Limitations
First, despite being one of the largest studies with 72 patients, our sample size was small and from a single center, limiting our ability to adjust for multiple confounders, our power to detect significant associations and our findings’ generalizability. However, our multivariable models were adjusted for serial confounders, including BMI, hypertension, smoking, hypercholesterolemia, and endocrine therapy, and we observed a significant dose-response relationship for some dose parameters, which remained significant even after adjustment. Some patients with poor-quality echocardiography at baseline, six-month, or 24-month examinations had missing GLS measurements and should be excluded from this analysis. No alternative examination, such as cardiac magnetic resonance imaging (CMR), was performed in our study to assess cardiac dysfunction, particularly LVEF or GLS. This was conducted in the multicenter European MEDIRAD Early-Heart study, which included 250 BC patients treated with RT and should have provided complementary results [38]. No data on diastolic functional indices (e.g., E/e’, tissue Doppler) and right ventricular (RV) function was available for this analysis. For a fuller picture, this could be the subject of a supplementary study if we evaluate these indices using the echocardiography that have all been preserved for the patients. We only observed asymptomatic CTRCD, but the clinical adverse effects usually appear several years or decades after RT. Moreover, some patients with CTRCD were observed six months after RT and recovered 24 months after RT. We did not have follow-up clinical information for the patients regarding pharmacological and non-pharmacological cardio-protective measures, which could explain the reversibility of CTRCD found in our study. Longer follow-up time and more extensive studies remain needed to understand the clinical implications of these findings on early or mid-term occurrence of CTRCD.
Conclusion
To our knowledge, this is the first study that investigated the occurrence and radiation-risk analysis of CTRCD based on its latest definition published in ESC cardio-oncology guidelines, in a population of BC patients treated with RT. With a follow-up of 24 months, no patient developed symptomatic CTRCD, but we observed that more than 40% of BC patients developed asymptomatic CTRCD, mainly mild CTRCD, but also, in less frequent cases, moderate or severe grades. Left ventricle and circumflex coronary artery exposure were found to be associated with CTRCD risk and could be used for the prediction of such cardiotoxicity. Further research remains needed to confirm this result and propose prevention recommendations to adapt and personalize RT for BC patients.
Data availability
No datasets were generated or analysed during the current study.
Abbreviations
- AUC:
-
Area Under the Curve
- BC:
-
Breast cancer
- CTRCD:
-
Cancer therapy-related cardiac dysfunction
- CX:
-
Circumflex coronary artery
- GLS:
-
Global Longitudinal Strain
- OR:
-
Odds Ratio
- RT:
-
Radiotherapy
- LV:
-
Left Ventricle
- LVEF:
-
Left Ventricular Ejection Fraction
References
Bray F, et al. Global cancer statistics 2018: GLOBOCAN estimates of incidence and mortality worldwide for 36 cancers in 185 countries. CA Cancer J Clin. 2018;68(6):394–424.
Clarke M, et al. Effects of radiotherapy and of differences in the extent of surgery for early breast cancer on local recurrence and 15-year survival: an overview of the randomised trials. Lancet. 2005;366(9503):2087–106.
Darby S, et al. Effect of radiotherapy after breast-conserving surgery on 10-year recurrence and 15-year breast cancer death: meta-analysis of individual patient data for 10,801 women in 17 randomised trials. Lancet. 2011;378(9804):1707–16.
McGale P, et al. Effect of radiotherapy after mastectomy and axillary surgery on 10-year recurrence and 20-year breast cancer mortality: meta-analysis of individual patient data for 8135 women in 22 randomised trials. Lancet. 2014;383(9935):2127–35.
Darby SC, et al. Risk of ischemic heart disease in women after radiotherapy for breast cancer. N Engl J Med. 2013;368(11):987–98.
van den Bogaard VA, et al. Validation and modification of a prediction model for Acute Cardiac events in patients with breast Cancer treated with Radiotherapy based on three-dimensional dose distributions to Cardiac substructures. J Clin Oncol. 2017;35(11):1171–8.
Honaryar MK et al. Early coronary artery calcification progression over two years in breast Cancer patients treated with Radiation Therapy: Association with Cardiac exposure (BACCARAT study). Cancers (Basel), 2022. 14(23).
Honaryar MK et al. Early Development of atherosclerotic plaques in the coronary arteries after Radiotherapy for breast Cancer (BACCARAT study). J Cardiovasc Dev Dis, 2023. 10(7).
Slawinski G et al. Global longitudinal strain in Cardio-Oncology: a review. Cancers (Basel), 2023. 15(3).
Lancellotti P, et al. Expert consensus for multi-modality imaging evaluation of cardiovascular complications of radiotherapy in adults: a report from the European Association of Cardiovascular Imaging and the American Society of Echocardiography. Eur Heart J Cardiovasc Imaging. 2013;14(8):721–40.
Plana JC, et al. Expert consensus for multimodality imaging evaluation of adult patients during and after cancer therapy: a report from the American Society of Echocardiography and the European Association of Cardiovascular Imaging. J Am Soc Echocardiogr. 2014;27(9):911–39.
Erven K, et al. Subclinical cardiotoxicity detected by strain rate imaging up to 14 months after breast radiation therapy. Int J Radiat Oncol Biol Phys. 2013;85(5):1172–8.
Trivedi SJ, et al. Persistent reduction in global longitudinal strain in the longer term after radiation therapy in patients with breast cancer. Radiother Oncol. 2019;132:148–54.
Tuohinen SS, et al. 3-Year Follow-Up of Radiation-Associated changes in diastolic function by Speckle Tracking Echocardiography. JACC CardioOncol. 2021;3(2):277–89.
Tuohinen SS, et al. Left Ventricular Speckle Tracking Echocardiography Changes among early-stage breast Cancer patients three years after Radiotherapy. Anticancer Res. 2019;39(8):4227–36.
Tuohinen SS, et al. Radiotherapy-induced global and regional differences in early-stage left-sided versus right-sided breast cancer patients: speckle tracking echocardiography study. Int J Cardiovasc Imaging. 2017;33(4):463–72.
Walker V, et al. Myocardial deformation after radiotherapy: a layer-specific and territorial longitudinal strain analysis in a cohort of left-sided breast cancer patients (BACCARAT study). Radiat Oncol. 2020;15(1):201.
Walker V, et al. Early detection of subclinical left ventricular dysfunction after breast cancer radiation therapy using speckle-tracking echocardiography: association between cardiac exposure and longitudinal strain reduction (BACCARAT study). Radiat Oncol. 2019;14(1):204.
Locquet M, et al. Subclinical left ventricular dysfunction detected by Speckle-Tracking Echocardiography in breast Cancer patients treated with Radiation Therapy: a six-Month Follow-Up analysis (MEDIRAD EARLY-HEART study). Front Oncol. 2022;12:883679.
Lyon AR, et al. 2022 ESC guidelines on cardio-oncology developed in collaboration with the European Hematology Association (EHA), the European Society for Therapeutic Radiology and Oncology (ESTRO) and the International Cardio-Oncology Society (IC-OS). Eur Heart J. 2022;43(41):4229–361.
Jacob S, et al. Early detection and prediction of cardiotoxicity after radiation therapy for breast cancer: the BACCARAT prospective cohort study. Radiat Oncol. 2016;11:54.
Gagliardi G, et al. Radiation dose-volume effects in the heart. Int J Radiat Oncol Biol Phys. 2010;76(3 Suppl):S77–85.
Jacob S, et al. Is mean heart dose a relevant surrogate parameter of left ventricle and coronary arteries exposure during breast cancer radiotherapy: a dosimetric evaluation based on individually-determined radiation dose (BACCARAT study). Radiat Oncol. 2019;14(1):29.
Voigt JU, et al. Definitions for a common standard for 2D speckle tracking echocardiography: consensus document of the EACVI/ASE/Industry Task Force to standardize deformation imaging. J Am Soc Echocardiogr. 2015;28(2):183–93.
Lang RM, et al. Recommendations for chamber quantification: a report from the American Society of Echocardiography’s guidelines and Standards Committee and the Chamber Quantification Writing Group, developed in conjunction with the European Association of Echocardiography, a branch of the European Society of Cardiology. J Am Soc Echocardiogr. 2005;18(12):1440–63.
Xu X, et al. Role of global longitudinal strain in evaluating radiotherapy–induced early cardiotoxicity in breast cancer: a meta-analysis. Kardiol Pol. 2023;81(1):58–60.
Lo Q, et al. Strain imaging detects dose-dependent Segmental Cardiac Dysfunction in the Acute Phase after breast irradiation. Int J Radiat Oncol Biol Phys. 2017;99(1):182–90.
Yu AF, et al. Assessment of Early Radiation-Induced changes in left ventricular function by myocardial strain imaging after breast Radiation Therapy. J Am Soc Echocardiogr. 2019;32(4):521–8.
Skytta T, et al. Adjuvant radiotherapy-induced cardiac changes among patients with early breast cancer: a three-year follow-up study(). Acta Oncol. 2019;58(9):1250–8.
Mele D, et al. Reversibility of left ventricle longitudinal strain alterations Induced by Adjuvant Therapy in early breast Cancer patients. Ultrasound Med Biol. 2016;42(1):125–32.
Yingchoncharoen T, et al. Normal ranges of left ventricular strain: a meta-analysis. J Am Soc Echocardiogr. 2013;26(2):185–91.
Saiki H, et al. Risk of heart failure with preserved ejection fraction in older women after contemporary radiotherapy for breast Cancer. Circulation. 2017;135(15):1388–96.
Sritharan HP, et al. Evaluation of traditional and novel echocardiographic methods of cardiac diastolic dysfunction post radiotherapy in breast cancer. Int J Cardiol. 2017;243:204–8.
Kim DY, et al. Cardiovascular outcome of breast cancer patients with concomitant radiotherapy and chemotherapy: a 10-year multicenter cohort study. J Cardiol. 2019;74(2):175–81.
Feng M, et al. Development and validation of a heart atlas to study cardiac exposure to radiation following treatment for breast cancer. Int J Radiat Oncol Biol Phys. 2011;79(1):10–8.
Duane F, et al. A cardiac contouring atlas for radiotherapy. Radiother Oncol. 2017;122(3):416–22.
Olloni A, et al. An open source auto-segmentation algorithm for delineating heart and substructures - development and validation within a multicenter lung cancer cohort. Radiother Oncol. 2024;191:110065.
Walker V, et al. Early Detection of Cardiovascular Changes after Radiotherapy for breast Cancer: protocol for a European Multicenter prospective cohort study (MEDIRAD EARLY HEART Study). JMIR Res Protoc. 2018;7(10):e178.
Acknowledgements
Not applicable.
Funding
This research was funded by Fédération Française de Cardiologie (FFC), Electricité de France (EDF) and the H2020 Euratom research and training program 2014–2018 under grant agreement No 755523 in the frame of the MEDIRAD project.
Author information
Authors and Affiliations
Contributions
Conceptualization: S.J., G.J., O.L. and J.F.; methodology: M.K.H., M.L, R.A., F.d.V.; software: M.K.H., D.B., J.C.; validation: S.J., R.A, O.L, L.P., F.d.V., J.F.; formal analysis: M.K.H, M.L., R.A, S.J.; investigation: M.L., O.L, L.P, S.J.; resources: M.L., G.J, J.C., D.B., O.L, L.P; data curation, M.K.H, M.L.; writing—original draft preparation: M.K.H. and S.J.; writing—review and editing: all authors.; supervision: S.J.; project administration: S.J.; funding acquisition: S.J. and J.F. All authors read and approved the final manuscript.
Corresponding author
Ethics declarations
Ethics approval and consent to participate
This study obtained ethical approval from the French South West Committee for Protection of Persons (ID: CPP2015/66/2015-A00990–69) and the National Agency for Medical and Health Products Safety (Reference: 150873B-12). Written informed consent forms were obtained from all the study participants.
Consent for publication
Not applicable.
Competing interests
The authors declare no competing interests.
Additional information
Publisher’s Note
Springer Nature remains neutral with regard to jurisdictional claims in published maps and institutional affiliations.
Electronic supplementary material
Below is the link to the electronic supplementary material.
Rights and permissions
Open Access This article is licensed under a Creative Commons Attribution-NonCommercial-NoDerivatives 4.0 International License, which permits any non-commercial use, sharing, distribution and reproduction in any medium or format, as long as you give appropriate credit to the original author(s) and the source, provide a link to the Creative Commons licence, and indicate if you modified the licensed material. You do not have permission under this licence to share adapted material derived from this article or parts of it. The images or other third party material in this article are included in the article’s Creative Commons licence, unless indicated otherwise in a credit line to the material. If material is not included in the article’s Creative Commons licence and your intended use is not permitted by statutory regulation or exceeds the permitted use, you will need to obtain permission directly from the copyright holder. To view a copy of this licence, visit http://creativecommons.org/licenses/by-nc-nd/4.0/.
About this article
Cite this article
Honaryar, M.K., Locquet, M., Allodji, R. et al. Cancer therapy-related cardiac dysfunction after radiation therapy for breast cancer: results from the BACCARAT cohort study. Cardio-Oncology 10, 54 (2024). https://doi.org/10.1186/s40959-024-00255-9
Received:
Accepted:
Published:
DOI: https://doi.org/10.1186/s40959-024-00255-9