Abstract
Introduction
Exposure to endocrine disrupting chemicals (EDCs) can result in alterations of natural hormones in the body. The aim of this review article is to highlight the knowledge about EDCs and obesity.
Methods
A scoping review of the electronic literature was performed using PubMed platform for studies on EDCs and obesity published between the years 2013–2023. A total of 10 systematic reviews and meta-analysis studies met our inclusion criteria on more prominent EDCs focusing mainly on bisphenols, including parabens, triclosan, and phthalates, and their association with obesity.
Design
Scoping review.
Results
EDCs, mostly bisphenols and phthalates, are related to health effects, while there is less information on the impact of parabens and triclosan. A series of negative physiological effects involving obesogenic, diabetogenic, carcinogenic, and inflammatory mechanisms as well as epigenetic and microbiota modulations was related to a prolonged EDCs exposure. A more profound research of particular pollutants is required to illuminate the accelerating effects of particular EDCs, mixtures or their metabolites on the mechanism of the development of obesity.
Conclusion
Considering the characteristics of EDCs and the heterogeneity of studies, it is necessary to design specific studies of effect tracking and, in particular, education about daily preventive exposure to EDCs for the preservation of long-term public health.
Similar content being viewed by others
Introduction
Excess body weight as well as the environmental excesses of different contaminants are accompanied by different cardiovascular and/or reproductive health problems [2], [64, 65, 73]. The environmental pollutants interrupting the endocrine system known as endocrine disrupting chemicals (EDCs) are associated with reproductive complications, hormone-sensitive cancers, problems with thyroid function, changes in neuroendocrine systems and microbiota, diabetes, and obesity [23, 47]. The general objective to minimize EDC exposure and body burden is one of the central goals of the European regulation [4, 17, 42]. Recent findings suggest that EDCs, especially bisphenol A (BPA), correlate during all life stages with increased body weight and/or body mass index, adipogenesis, adipose tissue inflammation, lipids, and glucose dysregulation, thus contributing to weight gain and pathophysiology of obesity [13, 37, 42, 43, 47, 52, 55, 58].
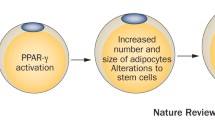
Environmental stressors such as EDCs are classified as potential diabetogenic, obesogenic, carcinogenic substances related to obesity, but more profound conclusions based on experimental human studies are required to understand possible mechanisms of related disease development later in life [29, 47]. Studies have demonstrated that the EDCs can bind to different hormone receptors, mostly those for estrogens, with specific agonistic or antagonistic effects and responses [14], [35]. The estrogens preserve the integrity of energy homeostasis at central and peripheral levels via nuclear and extranuclear pathways in both females and males [46]. Moreover, some studies have confirmed that BPA exerts its disrupting effects on the classical nuclear receptors such as estrogen receptors alpha and beta (ERa and ERb), the non-classical membrane estrogen receptor (ncmER), the estrogen-related receptor gamma (ERRg), the G protein-coupled receptor 30 (GPR30), and the aryl hydrocarbon receptor (AhR) [1], [52]. Through these interactions, EDCs are assumed to be involved in the onset of the metabolic dysfunction [4, 42] through different responses in the human body (Fig. 1).
Moreover, structural similarities of BPA with other bisphenols such as (BP)S have raised several concerns and emphasized the need for evaluation of potential toxicity at different doses [6]. This review identifies several environmental EDCs presented in daily life (such as bisphenols—bisphenol A (BPA), bisphenol F (BPF), and bisphenol S (BPS), parabens, phthalates, and triclosan), which have already been included in the studies on obesity development, potentially also weight-loss regimen (Fig. 2).
This review article focuses on the following endocrine disrupting chemicals:
Bisphenols
Bisphenol A (BPA; 4,4′-(Propane-2,2-diyl) diphenol; C15H16O2) is a synthetic chemical compound which was developed in the 1890s and its effect on estrogen activity was recognized in the 1930s [60]. BPA has been used in many consumer products including plastic (as a polymer), polycarbonate plastic (PVC), food packaging, dental sealants, and thermal receipts. Humans are exposed to BPA through their diet, inhalation of household dust, and dermal exposure [10, 72]. Moreover, due to health concerns BPA has largely been replaced by bisphenol F (BPF; 4,4′-Methylenediphenol; C13H12O2) and bisphenol S (BPS; 4,4′-Sulfonyldiphenol; C12H10O4S), which has resulted in the increased production of BPF and BPS over the last few decades [18]. However, it has been found that other bisphenols are toxic and have the potential to interrupt the metabolic action just as BPA, with some even exceeding that of BPA [68], [6], [42].
Parabens
Parabens (PBs), chemically a series of parahydroxybenzoates or esters of parahydroxybenzoic acid (also known as 4-hydroxybenzoic acid) include a group of chemicals which are used as preservatives in the food, cosmetic and pharmaceutical industries [11]. Their estrogenic effects were associated with the endocrine organs and other tissues, including adipose tissue. Several of these chemicals are known to cause obesogenic effects. In some experimental studies, it has been found that there are some important connections between methylparaben exposure and adipsin, affecting energy balance in the body and metabolic health, thus indicating its obesogenic potential [36]. In human biomonitoring, PB levels were associated with canned food and the use of personal care products such as makeup and skin products [62, 65].
Triclosan
Triclosan (TCS; 5-Chloro-2-(2,4-dichlorophenoxy) phenol; C12H7Cl3O2) developed in 1966 (Boyce & Pittet, 2002) is usually found in personal care products as an antibacterial agent. In animal studies, TCS exposure was associated with androgenic and thyroid disturbance, contact dermatitis, and skin irritation [66]. Beside the estrogenic and androgenic effects in mammals, some studies suggest the potential of TCS to trigger antibiotic resistance [22, 61].
Phthalates
Phthalates, esters of phthalic acid (C6H4(CO2H)2), are chemicals used to improve the utility of plastic and personal care products in daily life [38]. High-molecular-weight phthalates can enable flexibility of plastic and are used as components in toys, building materials, medical devices, and paints. Meanwhile, low-molecular-weight phthalates are usually used as components of personal care products and cosmetics such as shampoos, lotions, nail care products, and other personal hygiene products. In general, phthalates or even more a prominent sum of phthalate metabolites are the well-known EDCs with possible estrogenic and/or anti-androgenic reproductive effects [27], [40],). Moreover, the influence of combined phthalates has been related to metabolic disturbances that may interfere with obesity development (Milošević et al., 2019; [51]).
Materials and methods
This scoping review was performed in accordance with the Preferred Reporting Items for Systematic reviews and Meta-Analyses extension for Scoping Reviews (PRISMA, Fig. 3) guideline [67]. We conducted an electronic literature search utilizing the National Library of Medicine (PubMed) database. The following medical subject heading (MESH) keywords were used to search for studies reporting on the role of environmental endocrine disruptors in the occurrence of obesity: “endocrine disrupting chemicals” AND “obesity”.
Selected literature
The focus of literature selection was to investigate the effects of EDCs such as BPA, parabens, phthalates and triclosan in studies. The database was searched for studies published in the decade from December 2013 to December 2023. The studies included were meta-analyses and systematic reviews. Due to numerous numbers of experimental studies on different EDCs which are methodologically heterogenic and need to be interpreted with caution, we focused only on systematic review studies and meta-analyses involving the selected EDCs (bisphenols, parabens, phthalates, triclosan) and obesity associations found through the inclusion criteria. The studies published in languages other than English were excluded from this review. We also performed a hand search of the reference lists of full-text articles that met our criteria in the primary literature search. All manuscripts and abstracts were independently reviewed by investigators for possible study inclusion regarding their subject and quality. Studies and manuscripts were confirmed by all researchers included.
Data extraction
After the primary search, we identified 249 potentially eligible citations (Full text, Clinical Trial, Meta-Analysis, Randomized Controlled Trial, Review, Systematic Review, Humans, English, from 2013/12/31–2023/12/31). Among those, 238 were Review studies and the rest were meta-analyses and systematic review studies (n = 20) which we included in the detailed review. Based on our inclusion criteria, we selected 10 studies which were meta-analyses and systematic review studies for detailed screening. By reviewing the reference lists of selected studies, we detected no additional and/or potentially eligible citations. Studies that met our inclusion criteria identified possibble association of exposure to main EDCs (bisphenols, parabens, triclosans and phthalates) with obesity and health related risks (Table 1). Figure 3 presents a PRISMA flow diagram of the association of EDCs with obesity.
Results
The studies have identified possible association of obesity with the exposure to endocrine disrupting chemicals (EDCs). The data on systematic review studies and meta -analyses are presented in Table 1.
Literature identification
The possible effects of endocrine disrupting chemicals on obesity in humans described in systematic reviews and meta-analysis are summarized in Table 1.
EDCs Endocrine disrupting chemicals; T2DM-Type 2 diabetes mellitus, BMI Body mass index.
The main conclusions of the 10 reviewed studies indicate that obesity indications might be related to EDC exposure [20, 33, 59], [3, 25, 69, 75], [19], Maqbool et al., 2016). In addition, researchers also suggested that mainly BPA and phthalates might be related to obesity and cardiometabolic risk factors and adverse health effects in children [19, 40], [3], [25]).
Discussion
Exposure to a wide range of EDCs, mainly bisphenols (BPA, BPs) and phthalates, is inevitable and inadvertent. Those EDCs are environmental endocrine disruptors which may interact in the development of obesity, specifically anthropometric alterations, metabolic disorders, reproductive system damage, and hormone-sensitive cancers [59], [3, 69, 75], [33]. EDC exposure observations proved the association of a vulnerable prenatal group with exposure to environmental pollutants, which may have important health consequences later in life [19, 40], [25] [20]. Therefore, it is extremely important to pay attention even to environmental factors in the prevention of obesity, which, with long-term exposure, can affect the development and course of the hormonal state and can inhibit body weight regulation through various mechanisms (Maqbool et al., 2016). Several meta-analyses and systematic reviews have analyzed and evaluated the relationship of mainly bisphenols and phthalates while less is yet discovered regarding other EDCs, such as triclosans and parabens.
Environmental stressors such as EDCs with broad health implications, including the potential development of obesity related diseases and performance limitations, should not be neglected. This review argues the importance of environmental exposure control as a tool to promote obesogenic as well as diabetogenic, and carcinogenic health prevention. Research findings emphasize close relation of the most prominent environmental pollutants, their mixtures and metabolites as a potential additional obesogenic substance. Assessing the full impact of human exposure to EDCs is challenging because the adverse effects develop latently and manifest at different ages, although preclinical and clinical evidence suggests that developing fetuses and neonates are most vulnerable to endocrine disruption (Modica et al., 2023). Therefore, the growing evidence supports the urgent need to reduce exposure to EDCs with healthy choices on a daily basis and at the same time to decrease the EDCs’ negative effects.
The importance of EDCs for vulnerable health groups
As obesity increases, the incidence of cardiovascular disease, type 2 diabetes, obesity-related cancers, osteoarthritis, and psychological disorders increases [16], [45]. Obesity clearly has a measurable impact on physical and mental health and the quality of life, and causes significant direct and indirect costs. Obesity is also associated with environmental pollutants such as bisphenols, mainly BPA, which disturb the hormonal balance and can be passed on to future generations in the vulnerable health group in the reproductive period [34] through epigenetic programming, during the sensitive time for obesogenic action in the womb and early childhood [31]. Moreover, prenatal life exposure to EDCs was associated with low body mass index (BMI) in newborns [40] with increased susceptibility to diseases or disfunctions and susceptibility to gain more weight than babies with normal body weight across the life course [56], [28]; [30, 71]. Metabolic disturbances related to obesity development induced by a mixture of chemical compounds people are daily exposed to have the capacity to interfere with the endocrine axis at different levels and might even have carcinogenic effects. While some EDCs have already been linked to the development of breast and thyroid cancer (like dioxin and cadmium), prostate cancer (arsenic, asbestos, and dioxin), and testicular cancer (organochlorines/organohalogens), new evidence supports the role of the remaining EDCs as possible carcinogens, thus it is recommended for pregnant women to avoid the risk area and exposure [54]. Moreover, researchers showed that BPA exposure was significantly associated with higher thyroid stimulating hormone levels in overweight/obese subjects suggesting that BPA exposure is a risk factor for thyroid cancer in overweight/obese subjects [47]. The effect modification observed in the group with body mass index (BMI) ≥ 25 kg/m2 confirms the relation between body weight and the importance of normal BMI control. Furthermore, malignancy risk for breast cancer was associated with parabens [26].
EDC evaluations and monitoring of effects
The European Union reports (ANSES, 2014; EFSA, 2015; ECHA, 2015) have already assessed the effect on BPA on metabolism, obesity and metabolic disorders, confirming diabetogenic and obesogenic effects [3]. The similarity of the bisphenol BPA with other bisphenols, such as BPS, also causes great concern in the European Union. Indeed, toxicokinetic data showed a 100-fold higher oral bioavailability of BPS than BPA compared to BPA in a pig model [6]. The data observed with adverse effects for BPS at much lower doses calls for the protection of humans by proposing reference values. In the determination of environmental doses the need to link the exposure to human diseases and to include exposomics in the characterization of environment-related pathologies together with genomics and other omics has already been expressed [5, 31]. Additionally, evidence pointing to the fact that low-level exposure to multiple chemicals can be associated with metabolic disturbances under conditions where no effect would be expected when considering the concentration of each individual chemical in the mixture, means that assessment procedures must also consider the possibility of a mixture of pollutants, i.e. the so-called cocktail effect [42]. Furthermore, EDCs, especially phthalates, can exhibit synergistic and additive effects due to their similar molecular structure and mechanism of action, therefore mixtures of phthalates increase the disruptive effects, indicating that the sum of phthalate metabolites is more important than the individual ones and may be associated with oestrogenic and/or anti-androgenic reproductive effects in adolescent girls [27]. Moreover, the evidence that combined phthalates may influence glucose and lipid metabolism which can also increase insulin resistance highlights the association of EDCs with obesity [53]. It has also been shown that increased concentrations of phthalate metabolites can influence obesity, glucose and lipid impairment in women with polycystic ovary syndrome. The authors demonstrated that urinary concentrations of total phthalate metabolites were positively associated with BMI, waist circumference, waist-to-height ratio, serum leptin and lipid storage product levels, and visceral fat index, which further emphasizes the importance of BMI awareness for obesity prevention [51].
EDCs during weight loss protocols
Obesity and overweight identification, treatment, and prevention present major public health challenges in multiple perspectives on a global scale [74]. The physiopathology and clinical impacts of excess body fat are understood insufficiently, and there are many difficulties in developing safe and effective long-term therapeutic strategies [32]. Specifically, in metabolically unhealthy obese persons higher blood levels of EDCs were observed compared to metabolically healthy obese persons,the levels were not changed after weight loss protocols [15], or they even increased as weight loss occurred [9]. The results of a higher level of EDCs associated with greater weight regain and greater decrease in resting metabolic rate [44] reflects the association of the importance to avoid EDCs to prevent obesity. The association of EDCs, specifically BPA, with obesity can also be explained by evidence of the presence of molecular changes which could cause metabolic disorders and affect the action of estrogen in metabolism [50]. For the purpose of controlling body weight, potential vitamin supplements have also been tested to reduce the toxic effects of EDCs, which needs further research [24]. The conclusions of review studies suggest the involvement of EDCs in the development of obesity [33], [15], [20], Riberiro et al., 2020, Wasseraar & Legler, 2018) observed by generalized and abdominal obesity (Ribeiro, 2020).
EDCs as a lipophilic environmental toxicant
EDCs may have short half-lives. Some are lipophilic and can as such bio-accumulate in adipose tissues, thus presenting increasing health hazards in individuals with excess body weight [9]. Weight loss has been related to the release and redistribution of organic pollutants to other lipid-rich organs such as the brain, kidney, and liver, which needs further investigation [9]. It has been suggested that even weight control interventions should be considered to limit organ exposure to pollutants when planning weight loss protocols [9], [12]. Moreover, in diet induced weight-loss higher baseline plasma EDCs concentrations have significantly been associated with greater weight regain, especially in women, accompanied by a slower regression of resting metabolic rate [44]. It is necessary to understand the complexity of the mechanisms involved in differentiation of fat cells and the influence of EDCs in the adipogenesis and the etiology of obesity [21]. The findings indicate a significant association between exposure to BPA and obesity in adults, but are insufficient to support that EDCs cause obesity “per se” in humans due to the cross-sectional design of most included studies [59]. González-Casanova et al. [21] reported that EDCs can alter lipid metabolism, promote fat accumulation, and interfere with processes such as adipogenesis through the immunomodulation effects and other mechanisms of action, such as microbiota and epigenetics. Findings on immune and inflammatory responses of animals support the idea that further investigations are needed in humans to better understand the health consequences of EDCs exposure.
BPA in obesity prevention
Humans may be exposed to more EDCs at the same time and exposure varies over time, which makes it impossible to evaluate their potential synergistic or antagonistic effects. Furthermore, it is important for EDCs to have sometimes a short urinary elimination half-life in the human body, and sometimes they don’t. Therefore, it may be necessary to consider whether a single urine or blood sample is representative of the overall exposure of an individual, including the subjective level of daily environmental exposure. EDCs act via various hormone receptors through a variety of known and unknown mechanisms including epigenetic modifications. They differ from classic toxins in several ways such a slow-dose effect (time after exposure), non-monotonic dose and trans-generational effects. Therefore, more studies are warranted to elucidate the mechanisms functioning as the link between EDC exposure and metabolic health [44]. In addition, trans-generational inheritance and bioaccumulation of EDCs are among clear obstacles for new research evidence [39]. Specifically, the results of reviewed studies have suggested that mainly BPA is associated with obesity development which may negatively affect the metabolic functions in various ways. Some epidemiological studies using data from the National Health and Nutrition Examination Survey (NHANES) have reported that higher urine BPA concentrations tend to be correlated with greater obesity risk and waist circumference [63], [7]. Researchers confirmed a significant relationship between BPA and childhood obesity [35]. They also described the role of BPA in modulating canonical endocrine function, which regulates the metabolism during childhood. It was reported that BPA at environmental doses acts in mature adipocytes by modulating gene expression which leads to molecular changes promoting metabolic disorders in children. The persistence of BPA in the environment and in the human adipose tissue might be an important risk factor and a strong argument for further research of the development of obesity [50].
Phthalates and parabens in obesity prevention
Wassenaar & Legler [71] reported that early-life exposure of animals to phthalates is associated with increased fat weight, while non-significant negative association was related to body weight. On the other hand, studies on the connection between exposure to phthalates in childhood and obesity were inconsistent. Specifically, prenatal phthalate exposure may disturb the normal growth of children by decreased BMI rather than causing obesity, as hypothesized by previous studies [40]. Moreover, it has recently been discovered that BPA and phthalates are cardiovascular disruptors and BPA by itself could have a direct relationship with systemic inflammation regardless of obesity or insulin resistance.
Parabens were related to obesogenic potential affecting energy balance and metabolic health [36]. While contradictory results were obtained regarding triclosan and obesity, one of the recent animal studies has emphasized the underlying toxic obesogenic mechanism of triclosan which is based on gut-brain axis [70]. However, due to inconclusive outcomes regarding phthalates and parabens further research is needed.
Future prospectives to prevent EDCs' negative health effects
Increased exposure to EDCs could pose long-term health risk effects. EDCs are ubiquitous contaminants with a broad spectrum of effects. The chemicals such as BPA, parabens, triclosan and phthalates are common environmental pollutants incriminated for metabolic as well as reproductive disorders. Therefore, most published literature concerning EDCs and obesity focuses mainly on BPA with diabetogenic, obesogenic and carcinogenic effects which need further investigation regarding their mechanisms. Therefore, human research combining biomonitoring, analyses of biological samples, and a structured questionnaire on the quality of life, food and physical activity to evaluate the association between lifestyle variables potentially related to different EDCs, mixtures of EDCs and their metabolites could present one of the possible designs of a more profound strategy to understand the long-term association of EDCs and obesity.
Collectively, the data indicates that endocrine disruptors may interfere with mechanisms regulating body weight through various physiological pathways, potentially leading to obesity. The conclusions might thus serve as a precaution to avoid the exposure of EDCs in daily life. This is particularly important in the current worldwide scenario of ongoing exposure of children and adults to EDCs, not only to chemicals which are still used for a wide range of purposes, but also to compounds which were banned in many countries but have a persistent and ubiquitous occurrence in the environment. To better illuminate the environmental triggers of obesity, further studies on larger populations are needed.
Summary
In this review, we summarized the knowledge on the association of EDCs such as BPA, parabens, triclosan and phthalates with obesity in the last 10 years. Revealed studies, methodologically heterogenic, have reported a potential association between prolonged exposure to EDCs and obesity. However, the main findings of this review need to be interpreted with caution due to the used methodology investigating EDCs and obesity, the selected database, and heterogenous experimental studies that were not included. Recent research indicates that there is a specific need to combine human biomonitoring with other investigating methods to evaluate the quality of life including exposure to environmental triggers such as plastic and personal hygiene products of the observed population. More studies on molecular effects are needed to understand the association of EDCs with metabolic diseases, particularly human obesity. The exposure of environmental triggers and possible associations with human performance and disease prevention should not be neglected.
Limitations
The literature search scoping review was limited to a single database and to publications available entirely in the English language, potentially excluding valuable research conducted in other languages and from other databases. Lastly, our results are only up to date as of December 2023, and any subsequent studies beyond this timeframe are not incorporated into our review.
Conclusion
Regardless of some inconsistences in the findings across summarized studies, involuntary exposure to EDCs might be related with obesity development. Further studies including multicentric studies are needed to delineate exact mechanisms through which EDC exposure causes obesity, so that efficient preventative measures could be implemented. The lack of public knowledge about the effects of EDCs on human health is concerning, which should encourage health care providers to inform especially a specific health population or ergonomically demanding populations to avoid the excess body weight and possible EDC exposure, thus empowering them to make healthier choices.
Availability of data and materials
No datasets were generated or analysed during the current study.
References
Alonso-Magdalena P, Ropero AB, Soriano S, García-Arévalo M, Ripoll C, et al. Bisphenol-A acts as a potent estrogen via non-classical estrogen triggered pathways. Mol Cell Endocrinol. 2012;355(2):201–7. https://doi.org/10.1016/j.mce.2011.12.012.
Amato AA, Wheeler HB, Blumberg B. Obesity and endocrine-disrupting chemicals. Endocr Connect. 2021;10(2):R87–105. https://doi.org/10.1530/EC-20-0578.
Andújar N, Gálvez-Ontiveros Y, Zafra-Gómez A, Rodrigo L, Álvarez-Cubero MJ, Aguilera M, Monteagudo C, Rivas AA. Bisphenol a analogues in food and their hormonal and obesogenic effects: a review. Nutrients. 2019;11(9):2136. https://doi.org/10.3390/nu11092136.PMID:31500194;PMCID:PMC6769843.
Anses. Annex XV Restriction report—proposal for a restriction–4.4’-isopropylidenediphenol (Bisphenol a; BPA), 2014.
Barouki R, Samson M, Blanc EB, Colombo M, Zucman-Rossi J, Lazaridis KN, Miller GW, Coumoul X. The exposome and liver disease-how environmental factors affect liver health. J Hepatol. 2023;79(2):492–505.
Beausoleil C, Le Magueresse-Battistoni B, Viguié C, Babajko S, Canivenc-Lavier MC, Chevalier N, Emond C, Habert R, Picard-Hagen N, Mhaouty-Kodja S. Regulatory and academic studies to derive reference values for human health: the case of bisphenol S. Environ Res. 2022;1(204):112233.
Bhandari R, Xiao J, Shankar A. Urinary bisphenol A and obesity in US children. Am J Epidemiol. 2013;177(11):1263–70. https://doi.org/10.1093/aje/kws391.
Boyce JM, Pittet D. Healthcare Infection Control Practices Advisory, Committee.; HICPAC/SHEA/APIC/IDSA hand hygiene task, force. "Guideline for Hand Hygiene in Health-Care Settings. Recommendations of the Healthcare Infection Control Practices Advisory Committee and the HICPAC/SHEA/APIC/IDSA Hand Hygiene Task Force. Society for Healthcare Epidemiology of America/Association for Professionals in Infection Control/Infectious Diseases Society of America". MMWR. Recommendations and Reports. 2002; 51(RR-16): 1–45, quiz CE1–4.
Brown RH, Ng DK, Steele K, Schweitzer M, Groopman JD. Mobilization of environmental toxicants following bariatric surgery. Obesity (Silver Spring). 2019;27(11):1865–73. https://doi.org/10.1002/oby.22618.
Carwile JL, Luu HT, Bassett LS, Driscoll DA, Yuan C, et al. Polycarbonate bottle use and urinary bisphenol A concentrations. Environ Health Perspect. 2009;117(9):1368–72. https://doi.org/10.1289/ehp.0900604.
Cashman AL, Warshaw EM. Parabens: a review of epidemiology, structure, allergenicity, and hormonal properties. Dermatitis. 2005;16(2):57–66. https://doi.org/10.2310/derm.1.2005.2050.
Dabas J, Shunmukha Priya S, Alawani A, Budhrani P. What could be the reasons for not losing weight even after following a weight loss program? J Health Popul Nutr. 2024;43(1):37. https://doi.org/10.1186/s41043-024-00516-4.PMID:38429842;PMCID:PMC10908186.
Darbre PD. Endocrine disruptors and obesity. Curr Obes Rep. 2017;6(1):18–27. https://doi.org/10.1007/s13679-017-0240-4.
De Coster S, Van Larebeke N. Endocrine-disrupting chemicals: associated disorders and mechanisms of action. J Environ Public Health. 2012; 713696. https://doi.org/10.1155/201
Dirinck EL, Dirtu AC, Govindan M, Covaci A, Jorens PG, et al. Endocrine-disrupting polychlorinated biphenyls in metabolically healthy and unhealthy obese subjects before and after weight loss: difference at the start but not at the finish. Am J Clin Nutr. 2016;103(4):989–98. https://doi.org/10.3945/ajcn.115.119081.
Dixon JB. The effect of obesity on health outcomes. Mol Cell Endocrinol. 2010;316(2):104–8. https://doi.org/10.1016/j.mce.2009.07.008. (Epub 2009 Jul 21 PMID: 19628019).
EP, Endocrine Disruptors: from Scientific Evidence to Human Health Protection (2019–2023). Policy department for citizens' rights and constitutional affairs. European Parliament; [Accessed 2023, Oct 2].2019; https://www.europarl.europa.eu/RegData/etudes/STUD/2019/608866/IPOL_STU(2019)608866_EN.pdf
Fürhacker M, Scharf S, Weber H. Bisphenol A: emissions from point sources. Chemosphere. 2000;41(5):751–6. https://doi.org/10.1016/s0045-6535(99)00466-x.
Gao H, Wang YF, Wang ZW, Wang Y, Tao FB. Prenatal phthalate exposure associated with age-specific alterations in markers of adiposity in offspring: a systematic review. Ecotoxicol Environ Saf. 2022;1(232):113247. https://doi.org/10.1016/j.ecoenv.2022.113247.
Golestanzadeh M, Riahi R, Kelishadi R. Association of exposure to phthalates with cardiometabolic risk factors in children and adolescents: a systematic review and meta-analysis. Environ Sci Pollut Res Int. 2019;26(35):35670–86. https://doi.org/10.1007/s11356-019-06589-7.
González-Casanova JE, Pertuz-Cruz SL, Caicedo-Ortega NH, Rojas-Gomez DM. Adipogenesis regulation and endocrine disruptors: emerging insights in obesity. Biomed Res Int. 2020;18:7453786. https://doi.org/10.1155/2020/7453786.
Goodman M, Naiman DQ, LaKind JS. Systematic review of the literature on triclosan and health outcomes in humans. Crit Rev Toxicol. 2018;48(1):1–51. https://doi.org/10.1080/10408444.2017.1350138.
Gore AC, Chappell VA, Fenton SE, Flaws JA, Nadal A, et al. EDC-2: the endocrine society’s second scientific statement on endocrine-disrupting chemicals. Endocr Rev. 2015;36(6):1–150. https://doi.org/10.1210/er.2015-1010.
Guo W, Huen K, Park JS, Petreas M, Crispo et al. Vitamin C intervention may lower the levels of persistent organic pollutants in blood of healthy women—a pilot study. Food Chem Toxicol. 2016; 92:197–204. https://doi.org/10.1016/j.fct.2016.04.006
Gutiérrez-Torres DS, Barraza-Villarreal A, Hernandez-Cadena L, Escamilla-Nuñez C, Romieu I. Prenatal exposure to endocrine disruptors and cardiometabolic risk in preschoolers: a systematic review based on cohort studies. Ann Glob Health. 2018;84(2):239–49. https://doi.org/10.29024/aogh.911.PMID:30873814;PMCID:PMC6748211.
Hager E, Chen J, Zhao L. Minireview: parabens exposure and breast cancer. Int J Environ Res Public Health. 2022;19(3):1873. https://doi.org/10.3390/ijerph19031873.PMID:35162895;PMCID:PMC8834979.
Hart R, Doherty DA, Frederiksen H, Keelan JA, Hickey M, Sloboda D, Pennell CE, Newnham JP, Skakkebaek NE, Main KM. The influence of antenatal exposure to phthalates on subsequent female reproductive development in adolescence: a pilot study. Reproduction. 2014;147(4):379–90. https://doi.org/10.1530/REP-13-0331. (PMID: 24025997).
Haugen AC, Schug TT, Collman G, Heindel JJ. Evolution of DOHaD: the impact of environmental health sciences. J Dev Orig Health Dis. 2015;6(2):55–64. https://doi.org/10.1017/S2040174414000580.
Haverinen E, Fernandez MF, Mustieles V, Tolonen H. Metabolic syndrome and endocrine disrupting chemicals: an overview of exposure and health effects. Int J Environ Res Public Health. 2021;18(24):13047. https://doi.org/10.3390/ijerph182413047.
Heindel JJ, Blumberg B, Cave M, Machtinger R, Mantovani A, Mendez MA, Nadal A, Palanza P, Panzica G, Sargis R, Vandenberg LN, Vom Saal F. Metabolism disrupting chemicals and metabolic disorders. Reprod Toxicol. 2017;68:3–33. https://doi.org/10.1016/j.reprotox.2016.10.001.
Heindel JJ, Howard S, Agay-Shay K, Arrebola JP, Audouze K, Babin PJ, Barouki R, Bansal A, Blanc E, Cave MC, Chatterjee S, Chevalier N, Choudhury M, Collier D, Connolly L, Coumoul X, Garruti G, Gilbertson M, Hoepner LA, Holloway AC, Howell G, Kassotis CD, Kay MK, Kim MJ, Lagadic-Gossmann D, Langouet S, Legrand A, Li Z, Le Mentec H, Lind L, Monica Lind P, Lustig RH, Martin-Chouly C, Munic Kos V, Podechard N, Roepke TA, Sargis RM, Starling A, Tomlinson CR, Touma C, Vondracek J, Vom Saal F, Blumberg B. Obesity II: establishing causal links between chemical exposures and obesity. Biochem Pharmacol. 2022;199:115015. https://doi.org/10.1016/j.bcp.2022.115015.
Heymsfield SB, Wadden TA. Mechanisms, pathophysiology, and management of obesity. N Engl J Med. 2017;376(15):1492. https://doi.org/10.1056/NEJMc1701944.
Hwang S, Lim JE, Choi Y, Jee SH. Bisphenol A exposure and type 2 diabetes mellitus risk: a meta-analysis. BMC Endocr Disord. 2018;18(1):81. https://doi.org/10.1186/s12902-018-0310-y.
Kahn LG, Philippat C, Nakayama SF, Slama R, Trasande L. Endocrine-disrupting chemicals: implications for human health. Lancet Diabetes Endocrinol. 2020;8(8):703–18. https://doi.org/10.1016/S2213-8587(20)30129-7.PMID:32707118;PMCID:PMC7437820.
Kim JT, Lee HK. Childhood obesity and endocrine disrupting chemicals. Ann Pediatr Endocrinol Metab. 2017;22:219–25. https://doi.org/10.6065/apem.2017.22.4.219.
Kolatorova L, Sramkova M, Vitku J, Vcelak J, Lischkova O, et al. Parabens and their relation to obesity. Physiol Res. 2018;67(3):S465–72.
Kumar M, Sarma DK, Shubham S, Kumawat M, Verma V, et al. Environmental endocrine-disrupting chemical exposure: role in non-communicable diseases. Front Public Health. 2020;24(8):553850. https://doi.org/10.3389/fpubh.2020.553850.
Kumari M, Pulimi M. Phthalate esters: occurrence, toxicity, bioremediation, and advanced oxidation processes. Water Sci Technol. 2023;87(9):2090–115. https://doi.org/10.2166/wst.2023.119.
Lauretta R, Sansone A, Sansone M, Romanelli F, Appetecchia M. Endocrine disrupting chemicals: effects on endocrine glands. Front Endocrinol. 2019;10:178. https://doi.org/10.3389/fendo.2019.00178.
Lee DW, Lim HM, Lee JY, Min KB, Shin CH, et al. Prenatal exposure to phthalate and decreased body mass index of children: a systematic review and meta-analysis. Sci Rep. 2022;12(1):8961. https://doi.org/10.1038/s41598-022-13154-9.
Le Magueresse-Battistoni B, Multigner L, Beausoleil C, Rousselle C. Effects of bisphenol A on metabolism and evidences of a mode of action mediated through endocrine disruption. Mol Cell Endocrinol. 2018;5(475):74–91. https://doi.org/10.1016/j.mce.2018.02.009. (Epub 2018 Feb 23 PMID: 29481862).
Le Magueresse-Battistoni B, Vidal H, Naville D. Environmental pollutants and metabolic disorders: the multi-exposure scenario of Life. Front Endocrinol (Lausanne). 2018;2(9):582. https://doi.org/10.3389/fendo.2018.00582.PMID:30333793;PMCID:PMC6176085.
Legeay S, Faure S. Is bisphenol A an environmental obesogen? Fundam Clin Pharmacol. 2017;31(6):594–609. https://doi.org/10.1111/fcp.12300.
Liu G, Dhana K, Furtado JD, Rood J, Zong G, et al. Perfluoroalkyl substances and changes in body weight and resting metabolic rate in response to weight-loss diets: a prospective study. PLoS Med. 2018;15(2): e1002502. https://doi.org/10.1371/journal.pmed.1002502.
Lustig RH, Collier D, Kassotis C, Roepke TA, Kim MJ, Blanc E, Barouki R, Bansal A, Cave MC, Chatterjee S, Choudhury M, Gilbertson M, Lagadic-Gossmann D, Howard S, Lind L, Tomlinson CR, Vondracek J, Heindel JJ. Obesity I: overview and molecular and biochemical mechanisms. Biochem Pharmacol. 2022;199:115012. https://doi.org/10.1016/j.bcp.2022.115012.
Mahboobifard F, Pourgholami MH, Jorjani M, Dargahi L, Amiri M. Estrogen as a key regulator of energy homeostasis and metabolic health. Biomed Pharmacother. 2022;156: 113808. https://doi.org/10.1016/j.biopha.2022.113808.
Marotta V, Grumetto L, Neri I, Russo G, Tortora A, Izzo G, Panariello I, Rocco D, Pezzullo L, Vitale M. Exposure to Bisphenol A increases malignancy risk of thyroid nodules in overweight/obese patients. Environ Pollut. 2023;316(Pt1):120478. https://doi.org/10.1016/j.envpol.2022.120478.
Maqbool F, Mostafalou S, Bahadar H, Abdollahi M. Review of endocrine disorders associated with environmental toxicants and possible involved mechanisms. Life Sci. 2016;15(145):265–73. https://doi.org/10.1016/j.lfs.2015.10.022. (Epub 2015 Oct 21 PMID: 26497928).
Mendes V, Ribeiro C, Delgado I, Peleteiro B, Aggerbeck M, et at. The association between environmental exposures to chlordanes, adiposity and diabetes-related features: a systematic review and meta-analysis. Sci Rep. 2021; 11(1):14546. https://doi.org/10.1038/s41598-021-93868-4
Menale C, Piccolo MT, Cirillo G, Calogero RA, Papparella A. Bisphenol A effects on gene expression in adipocytes from children: association with metabolic disorders. J Mol Endocrinol. 2015;54(3):289–303. https://doi.org/10.1530/JME-14-0282.
Milankov A, Milanović M, Milošević N, Sudji J, Pejaković S, Milić N, Bjelica A, Medić SM. The effects of phthalate exposure on metabolic parameters in polycystic ovary syndrome. Clin Chim Acta. 2023;1(540):117225. https://doi.org/10.1016/j.cca.2023.117225.
Milanović M, Milošević N, Milić N, Stojanoska MM, Petri E, Filipović JM. Food contaminants and potential risk of diabetes development: a narrative review. World J Diabetes. 2023;14(6):705–23. https://doi.org/10.4239/wjd.v14.i6.705.PMID:37383596;PMCID:PMC10294057.
Milošević N, Milanović M, Sudji J, Bosić Živanović D, Stojanoski S, Vuković B, Milić N, Medić SM. Could phthalates exposure contribute to the development of metabolic syndrome and liver disease in humans? Environ Sci Pollut Res Int. 2020;27(1):772–84. https://doi.org/10.1007/s11356-019-06831-2. (Epub 2019 Dec 6 PMID: 31808097).
Modica R, Benevento E, Colao A. Endocrine-disrupting chemicals (EDCs) and cancer: new perspectives on an old relationship. J Endocrinol Invest. 2023;46(4):667–77. https://doi.org/10.1007/s40618-022-01983-4. (Epub 2022 Dec 16 PMID: 36526827).
Naomi R, Yazid MD, Bahari H, Keong YY, Rajandram R. Bisphenol A (BPA) leading to obesity and cardiovascular complications: a compilation of current in vivo study. Int J Mol Sci. 2022;23(6):2969. https://doi.org/10.3390/ijms23062969.
Newbold RR, Padilla-Banks E, Snyder RJ, Phillips TM, Jefferson WN. Developmental exposure to endocrine disruptors and the obesity epidemic. Reprod Toxicol. 2007;23(3):290–6. https://doi.org/10.1016/j.reprotox.2006.12.010.
Predieri B, Bruzzi P, Bigi E, Ciancia S, Madeo SF, et al. Endocrine disrupting chemicals and type 1 diabetes. Int J Mol Sci. 2020;21(8):2937. https://doi.org/10.3390/ijms21082937.
Ribeiro E, Ladeira C, Viegas S. EDCs mixtures: a stealthy hazard for human health? Toxics. 2017;5(1):5. https://doi.org/10.3390/toxics5010005.PMID:29051438;PMCID:PMC5606671.
Ribeiro CM, Beserra BTS, Silva NG, Lima CL, Rocha PRS, et al. Exposure to endocrine-disrupting chemicals and anthropometric measures of obesity: a systematic review and meta-analysis. BMJ Open. 2020;10(6): e033509. https://doi.org/10.1136/bmjopen-2019-033509.
Richter CA, Birnbaum LS, Farabollini F, Newbold RR, Rubin BS, et al. In vivo effects of bisphenol A in laboratory rodent studies. Reprod Toxicol. 2007;24(2):199–224. https://doi.org/10.1016/j.reprotox.2007.06.004.
Runkel AA, Mazej D, Snoj Tratnik J, Tkalec Ž, Kosjek T, et al. Exposure of men and lactating women to environmental phenols, phthalates, and DINCH. Chemosphere. 2022;286(3): 131858. https://doi.org/10.1016/j.chemosphere.2021.131858.
Sanchis Y, Coscollà C, Yusà V. Analysis of four parabens and bisphenols A, F, S in urine, using dilute and shoot and liquid chromatography coupled to mass spectrometry. Talanta. 2019;202:42–50. https://doi.org/10.1016/j.talanta.2019.04.048.
Shankar A, Teppala S, Sabanayagam C. Bisphenol A and peripheral arterial disease: results from the NHANES. Environ Health Perspect. 2012;120(9):1297–300. https://doi.org/10.1289/ehp.1104114.
Srnovršnik T, Virant-Klun I, Pinter B. Polycystic ovary syndrome and endocrine disruptors (bisphenols, parabens, and triclosan)—a systematic review. Life (Basel). 2023;13(1):138. https://doi.org/10.3390/life13010138.
Tkalec Ž, Kosjek T, Snoj Tratnik J, Stajnko A, Runkel AA, et al. Exposure of Slovenian children and adolescents to bisphenols, parabens and triclosan: urinary levels, exposure patterns, determinants of exposure and susceptibility. Environ Int. 2021;146: 106172. https://doi.org/10.1016/j.envint.2020.106172.
Thompson A, Griffin P, Stuetz R, Cartmell E. The fate and removal of triclosan during wastewater treatment. Water Environ Res. 2005;77(1):63–7. https://doi.org/10.2175/106143005X41636.
Tricco AC, Lillie E, Zarin W, O’Brien KK, Colquhoun H, Levac D, Moher D, Peters MDJ, Horsley T, Weeks L, Hempel S, Akl EA, Chang C, McGowan J, Stewart L, Hartling L, Aldcroft A, Wilson MG, Garritty C, Lewin S, Godfrey CM, Macdonald MT, Langlois EV, Soares-Weiser K, Moriarty J, Clifford T, Tunçalp Ö, Straus SE. PRISMA extension for scoping reviews (PRISMA-ScR): checklist and explanation. Ann Intern Med. 2018;169(7):467–73. https://doi.org/10.7326/M18-0850. (Epub 2018 Sep 4 PMID: 30178033).
Viñas R, Watson CS. Bisphenol S disrupts estradiol-induced nongenomic signaling in a rat pituitary cell line: effects on cell functions. Environ Health Perspect. 2013;121(3):352–8. https://doi.org/10.1289/ehp.1205826.
Wang Y, Hollis-Hansen K, Ren X, Qiu Y, Qu W. Do environmental pollutants increase obesity risk in humans? Obes Rev. 2016;17(12):1179–97. https://doi.org/10.1111/obr.12463. (Epub 2016 Oct 5 PMID: 27706898).
Wang Y, Song J, Wang X, Qian Q, Wang H. Study on the toxic-mechanism of triclosan chronic exposure to zebrafish (Danio rerio) based on gut-brain axis. Sci Total Environ. 2022;2022844: 156936. https://doi.org/10.1016/j.scitotenv.2022.156936.
Wassenaar PNH, Legler J. Systematic review and meta-analysis of early life exposure to di(2-ethylhexyl) phthalate and obesity related outcomes in rodents. Chemosphere. 2017;188:174–81. https://doi.org/10.1016/j.chemosphere.2017.08.165.
Wetherill YB, Akingbemi BT, Kanno J, McLachlan JA, Nadal A, et al. In vitro molecular mechanisms of bisphenol A action. Reprod Toxicol. 2007;24(2):178–98. https://doi.org/10.1016/j.reprotox.2007.05.010.
Woodruff TJ, Charlesworth A, Zlatnik MG, Pandipati S, DeNicola N, et al. Code OB: we need urgent action on climate change and toxic chemicals. Int J Gynaecol Obstet. 2023;160(2):363–5. https://doi.org/10.1002/ijgo.14566.
WHO, 2021. Obesity and overweight. 2021–2023. World Health Organization; [Accessed 2023, Oct 2]. https://www.who.int/news-room/fact-sheets/detail/obesity-and-overweight/.
Wu Q, Li G, Zhao CY, Na XL, Zhang YB. Association between phthalate exposure and obesity risk: a meta-analysis of observational studies. Environ Toxicol Pharmacol. 2023;102: 104240. https://doi.org/10.1016/j.etap.2023.104240. (Epub 2023 Aug 5 PMID: 37549759).
Funding
This research did not receive any specific grant from funding agencies in the public, commercial, or not-for-profit sectors.
Author information
Authors and Affiliations
Contributions
All authors have contributed to the article, read manuscript, agreed with the content, and approved the submitted version. Authors confirm that the manuscript is original and has not been published in a journal and is not currently under consideration by another journal. MA collected the data for paper, coordinated and contributed at all stages of the review and critically reviewed the final review. TK contributed to the writing of the review and contributed to the strategy development and in all stages of the review. IVK contributed to the writing of the paper and participated in all stages of data collection.
Corresponding author
Ethics declarations
Competing interests
The authors declare no competing interests.
Consent for publication
Not applicable.
Additional information
Publisher's Note
Springer Nature remains neutral with regard to jurisdictional claims in published maps and institutional affiliations.
Rights and permissions
Open Access This article is licensed under a Creative Commons Attribution-NonCommercial-NoDerivatives 4.0 International License, which permits any non-commercial use, sharing, distribution and reproduction in any medium or format, as long as you give appropriate credit to the original author(s) and the source, provide a link to the Creative Commons licence, and indicate if you modified the licensed material. You do not have permission under this licence to share adapted material derived from this article or parts of it. The images or other third party material in this article are included in the article’s Creative Commons licence, unless indicated otherwise in a credit line to the material. If material is not included in the article’s Creative Commons licence and your intended use is not permitted by statutory regulation or exceeds the permitted use, you will need to obtain permission directly from the copyright holder. To view a copy of this licence, visit http://creativecommons.org/licenses/by-nc-nd/4.0/.
About this article
Cite this article
Amon, M., Kek, T. & Klun, I.V. Endocrine disrupting chemicals and obesity prevention: scoping review. J Health Popul Nutr 43, 138 (2024). https://doi.org/10.1186/s41043-024-00627-y
Received:
Accepted:
Published:
DOI: https://doi.org/10.1186/s41043-024-00627-y