Abstract
Background
[211At]PSMA-5 is a novel α-emitting therapeutic agent designed to target prostate-specific membrane antigen (PSMA), which is overexpressed in metastatic castration-resistant prostate cancer (mCRPC). Unlike β-emitting radioligands, [211At]PSMA-5 delivers highly localized cytotoxicity while minimizing damage to surrounding normal tissues. To enable clinical application, the objective of this study is to scale up the lab-scale synthesis to an automated manufacturing process that ensures high reproducibility and sufficient radioactivity for human administration.
Results
We developed and optimized a scalable automated synthesis method for [211At]PSMA-5 using the COSMiC-Mini VTRSC2 automated synthesizer. Optimization involved evaluating the recovery efficiency of 211At from the cold trap and reaction conditions, followed by automated synthesis under investigational new drug Good Manufacturing Practice conditions. Quality control of the synthesized [211At]PSMA-5 included assessment of radiochemical purity, radionuclide identity, impurity profile and sterility. Optimization with sodium hydrogen carbonate (7%) achieved over 90% recovery of 211At from the cold trap, and labeling rate of up to 93% were obtained using glass reaction vessels with stirring at 95 °C. Three automated syntheses were conducted using irradiated Bi targets containing 211At produced at two different facilities. Consistent radiochemical yield (approximately 30%) and high radiochemical purity (96 ± 1%) were achieved. Additional quality control confirmed the absence of impurities such as 210At, Bi residues, and iodide, as well as sterility and chemical stability suitable for intravenous administration.
Conclusions
This study successfully established an automated, scalable production process for [211At]PSMA-5 that meets clinical-grade quality requirements, enabling stable and reproducible manufacturing for investigator-initiated clinical trials in mCRPC. Equivalent radiochemical yields and consistent quality were obtained using irradiated Bi targets from two cyclotron facilities (RCNP and RIKEN), demonstrating site-independent robustness. This flexible system ensures a reliable supply and resilience to unexpected cyclotron downtime, representing a significant step toward clinical application of 211At-based PSMA-targeted alpha therapy.
Similar content being viewed by others
Background
Prostate-specific membrane antigen (PSMA) is highly expressed in advanced prostate cancer and has attracted considerable attention as a highly tumor-selective therapeutic target (Silver et al. 1997; Afshar-Oromieh et al. 2012). PSMA-targeted radioligand therapy (RLT) has rapidly advanced in clinical applications, particularly for the treatment of metastatic castration-resistant prostate cancer (mCRPC). Radioligands labeled with β-emitting radionuclides, such as 177Lu-PSMA-617, have already demonstrated significant efficacy and safety in both clinical trials and routine practice (Hofman et al. 2018; Sartor et al. 2021; Emmett et al. 2019). However, there remains a strong demand for the development of novel radioligands incorporating α-emitting nuclides to overcome resistance to β-emitter-based therapies and achieve more potent cytotoxic effects (Kratochwil et al. 2017; Morgenstern et al. 2020; Watabe et al. 2024).
Actinium-225 (225Ac), an α-emitting radionuclide, has already been applied in PSMA-targeted RLT (e.g., 225Ac-PSMA-617), demonstrating promising antitumor efficacy (Kratochwil et al. 2018). 225Ac possesses a relatively long half-life of 10 days and emits four α-particles during its decay, conferring high cytotoxic potential. Nevertheless, concerns have been raised regarding unintended accumulation in normal tissues due to the redistribution of daughter nuclides and prolonged systemic retention resulting from its long half-life (Parida et al. 2023). In contrast, astatine-211 (211At) has recently gained attention as an extremely promising α-emitter for clinical applications, alongside 225Ac and 212Pb. This interest arises from its simple decay scheme involving a single α-emission without subsequent emissions from redistributed daughter nuclides, as well as its feasible cyclotron-based production using natural bismuth targets (Zalutsky and Pruszynski 2011).
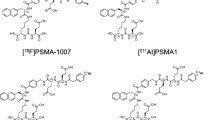
Recently, [211At]PSMA-5, an α-therapeutic agent developed at the University of Osaka based on [18F]PSMA-1007 as a model compound that targets PSMA, demonstrated excellent tumor growth inhibition with minimal toxicity to normal organs in a prostate cancer mouse model (Watabe et al. 2023; 2025a, b). Consequently, an investigator-initiated clinical trial has been launched to evaluate [211At]PSMA-5 as a novel targeted α-therapy for mCRPC (NCT06441994).
For preclinical studies, [211At]PSMA-5 was primarily synthesized manually on a “laboratory scale” conditions. To achieve reliable production conditions and radioactivity levels suitable for clinical trials, it is necessary to develop a scale-up, automated manufacturing process that ensures consistent radiochemical yield, product quality, aseptic formulation, and reproducible quality control. This study reports the development and optimization of an automated synthesis method for [211At]PSMA-5 that enables the supply more than 200 MBq of the investigational drug from the irradiated bismuth targets (> 1 GBq) in an investigator-initiated clinical trial based on preclinical findings.
Methods
Reagents for [211At]PSMA-5 synthesis
Dihydroxyboryl-PSMA-5 precursor (PSMA-5 precursor), a GMP-grade precursor for the radiolabeling of [211At]PSMA-5, was obtained from Peptide Institute (Osaka, Japan). Potassium Iodide (KI, 99.9%) was purchased from FUJIFILM Wako Pure Chemical (Osaka, Japan), and biotechnology-grade L(+)-ascorbic acid was obtained from Nacalai Tesque (Kyoto, Japan). Japanese Pharmacopoeia-grade sodium hydrogen carbonate (7%), water for injection (WFI) and ethanol were purchased from Otsuka Pharmaceuticals (Tokyo, Japan) and Merck (Darmstadt, Germany), respectively. Oasis HLB Plus Light Cartridge (30 mg Sorbent) were used for purification of [211At]PSMA-5.
Separation and purification of 211At from Bi target
Irradiated bismuth (Bi) plates containing 211At of identical specifications were produced from two facilities. One was produced using the K70 AVF cyclotron at the RIKEN RI Beam Factory (Saitama, Japan) (Sato et al. 2017), and the other was produced using the K140 AVF cyclotron at the Research Center for Nuclear Physics (RCNP), University of Osaka (Osaka, Japan). Both employed the nuclear reaction 209Bi(4He, 2n)211At.
Separation and purification of 211At from the irradiated Bi targets were performed using a dry distillation method as previously described, with the automated dry distillation unit of COSMiC-Mini VTRSC2 (NMP Business Support, Hyogo, Japan), which was installed in Investigational GMP facility at the University of Osaka Hospital after acceptance testing (Naka et al. 2024).
Optimization for scale-up production of [211At]PSMA-5
In preclinical studies, [211At]PSMA-5 manually synthesized as previously reported (Watabe et al. 2023). Briefly, 20 µL of a 0.1 mg/mL PSMA-5 precursor solution containing sodium hydrogen carbonate (7%) was mixed with 1–40 MBq (1–100 µL) of an aqueous 211At solution in a 1.5 mL fluoropolymer (PFA) tube. Subsequently, 20–40 µL of 0.1 mol/L KI solution was added, and the reaction mixture was heated at 80 °C for 45 min in a dry bath (Fig. 1). However, for stable production of [211At]PSMA-5 using an automated synthesizer required addressing two critical considerations. First, a procedure was investigated to recover 211At captured in the cold tube of the automated dry distillation system into the reaction vessel with high collection yield, using the minimum amount of solvent that avoid affecting the labeling reaction. Various volumes of WFI or sodium hydrogen carbonate (7%) were introduced manually into the cold trap, and recovery rates were evaluated for both with and without keeping. The recovery rate was calculated as the percentage of radioactivity recovered in the test solvent relative to the total radioactivity (sum of recovered and residual radioactivity in the cold trap). Second, modifying the reactor to adapt it to the automated synthesis system. Namely, the reaction vessel was modified from a 1.5 mL PFA tube to a 10 mL glass vial (SY-3, Nichiden Rika-Glass, Hyogo, Japan), which is commonly used for PET tracer radiolabeling. Using a 1 mL reaction mixture containing 211At in 700 µL of sodium hydrogen carbonate (7%), 10 µL of 1 mg/mL PSMA-5 precursor, and 290 µL of 0.1 M KI, we investigated optimal reaction temperature, stirring requirements by utilizing the dry bath and the reactor port of the synthesis unit, and adsorption of 211At to the glass after labeling. The labeling rate was evaluated by radio-TLC method (see Quality Control for [211At]PSMA-5). Adsorption to glass was calculated based on the difference between the radioactivity of the reaction vessel containing reaction solution and the radioactivity of the reaction vessel alone after removing it.
Automated production of [211At]PSMA-5 solution
The synthesis of [211At]PSMA-5 was performed using automated synthesis unit of COSMiC-Mini VTRSC2 (Fig. 2). The optimized conditions for the scale-up process were incorporated into the automated synthesis unit and its program (Fig. 3). Synthesis was performed as follows. After depressurizing the reaction vessel to − 26 to − 27 kPa, the flow path is switched, and 700 µL of sodium hydrogen carbonate (7%) filled in a syringe is drawn into the cold trap containing 211At separated and purified as describe above (Naka et al. 2024). After 5-min hold the sodium hydrogen carbonate (7%) in the cold trap, the entire contents were transferred under vacuum into the reaction vessel preloaded with 10 µL of 1 mg/mL PSMA-5 precursor, 290 µL of 0.1 M KI, and a stir bar. The labeling reaction was conducted at 95 °C for 45 min under stirring in a closed system connecting SLV103, SLV104 and the reaction vessel.
After labeling, the reaction mixture was transferred to a HLB cartridge. Subsequently, 1 mL of sodium hydrogen carbonate (7%) was passed through the HLB cartridge via the reaction vessel, followed by an additional 1 mL wash. The eluates were discarded into the waste vial. [211At]PSMA-5 was sequentially eluted with 0.5 mL WFI and 0.5 mL ethanol, and the eluate was collected into a dilution vial containing 14 mL of a 1% ascorbic acid–2.3% sodium hydrogen carbonate solution. The resulting solution was mixed thoroughly and passed through a sterile filtration 0.22-µm Millex GV filter (Merck Millipore, Burlington, MA) into a 20 mL sterile product vial (Mita Rika Kogyo, Hyogo, Japan). Throughout all procedures, helium flow and pressure were maintained at 20 mL/min and − 30 kPa, respectively.
Quality control for [211At]PSMA-5
Quality control items for [211At]PSMA-5 are summarized in Table 1. Radioactivity measurement, half-life, radionuclidic identity, contamination from other radionuclide, pH, sterility, endotoxin, concentration of ascorbic acid and filter integrity test were evaluated according to previously reported methods (Naka et al. 2024). The acceptance criteria for filter integrity test was ≥ 314 kPa, based on the bubble-point ratio obtained in preliminary testing (Merck Millipore, AN1505EN00 Ver 4). The identity and radiochemical purity of [211At]PSMA-5 were measured by radio thin layer chromatography (TLC) mini-Gita TLC analyzer (Elysia-Raytest, Straubenhardt, Germany) with silica gel 60 F254 glass plate and acetonitrile/water (2:1) as the mobile phase.
Chemical impurities, including PSMA-5 precursor, were quantified by high-performance liquid chromatography (HPLC) using the peak area of PSMA-5 precursor based on known concentration. The HPLC system (Shimadzu, Kyoto, Japan) comprised a CBM-20 A communications bus, SPD-20 A UV/VIS detector (wavelength: 254 nm), CTO-20AC column oven, SIL-20ACHT autosampler, LC-20AD pump, and DGU-20A5R degasser. The analysis column was a COSMOSIL 5C18-MS-II (150 mm × 4.6 mm; Nacalai Tesque), with a gradient elution of 0.5% formic acid (solvent A) and acetonitrile (solvent B), changing from 80% A to 30% A over 0–20 min. Flow rate and column temperature were set to 1.0 mL/min and 40 °C, respectively. Ethanol content was analyzed by gas chromatography (GC-2014, Shimadzu) equipped with a flame ionization detector (FID) a DB-624 column (30 m × 0.32 mm, 1.8 μm; Agilent, Santa Clara, CA, USA) using split injection (split ratio = 30:1). Helium served as carrier (2 mL/min) and makeup gas was (30 mL/min). The column, injector, and FID temperatures were 40 °C for 5 min to 200 °C (10 °C/min) for 1 min, 250 °C, and 250 °C, respectively. In addition to quality control items, the residual Bi from the target and iodine (I−) from the KI were quantified using inductively coupled plasma-mass spectrometry (ICP-MS 7800; Agilent) and HPLC, respectively. Contamination by 210At was evaluated by long-term (approximately 22 h) measurement using a Ge semiconductor detector (BE2020; Mirion Technologies Canberra, Meriden, CT). These analyses followed previously described methods (Naka et al. 2024). For I− measurement, the HPLC system (Shimadzu) consisted of a CBM-20 A communications bus, SPD-20 A UV/VIS detector (wavelength: 254 nm), CTO-20AC column oven, SIL-20AC autosampler, LC-20AD pump, and DGU-20A5R degasser, equipped with a HILICpak VT-50-2D column (150 mm × 2.0 mm; Resonac, Tokyo, Japan). The mobile phase was 50 mM formic acid/acetonitrile = 20/80, with a flow rate of 0.3 mL/min and a column oven temperature of 40 °C.
Identification of [211At]PSMA-5 using a liquid chromatography-mass spectrometry (LC-MS)
The obtained product was confirmed as [211At]PSMA-5 using LCMS-9030 systems (Shimadzu). High-concentration samples was generated by trapping of [211At]PSMA-5 on a HLB cartridge. The concentrated [211At]PSMA-5 was subsequently eluted with 0.5 mL of WFI and 0.5 mL ethanol, and used for LC-MS analysis. The system consisted of a CBM-40 system controller, SPD-M40 photodiode array detector, CTO-20AC column oven, SIL-20ACHT autosampler, LC-20AD pump and DGU-405 degasser, and a Gabi-Nova radioactivity detector (Raytest) positioned upstream of the MS inlet. HPLC conditions were identical to those used for related substance analysis, except that a 150 mm × 2.0 mm column was used and the flow rate was set to 0.2 mL/min.
Results
Optimization for scale-up production of [211At]PSMA-5
The recovery rate of 211At from the cold trap was 33% when using 500 µL of WFI, with more than 65% of the radioactivity remaining the cold trap. In contrast, when 1 mL of sodium hydrogen carbonate (7%) was used without retention it in the cold trap, the recovery rates increased to 86%. Furthermore, when the solution was allowed to remain in the cold trap for 1–5 min, recovery rates exceeding 90% were achieved regardless of the radioactivity level (Table 2).
The labeling reaction conducted in a glass reaction vessel yielded a labeling rate of approximately 75% after 45 min at 80 °C. Under the same reaction time but at 95 °C with stirring, labeling efficiencies ranged from 72 to 93% in the dry bath and reached 87% in the labeling port of synthesis unit (Table 3).
Production of [211At]PSMA-5 solution using 211At automated synthesis unit and quality control
Using irradiated Bi targets containing 211At obtained from both RIKEN and RCNP, 3-lot tests were performed per source (6-lots in total). After setting the Bi target into the electric furnace, [211At]PSMA-5 solutions were obtained with radioactivity of 134 ± 11 MBq and 183 ± 41 MBq, and radiochemical yield of 29 ± 3% and 30 ± 1%, respectively, at the end of synthesis (EOS), with an average synthesis time of 129 min. Radio-TLC analysis revealed that [211At]PSMA-5 exhibited a Rf value of 0.34–0.45, with a radiochemical purity of 96 ± 1% (Fig. 4a). Moreover, radiochemical purity remained above 90% even 6 h after EOS (Fig. 4-b), meeting the specification criteria. In HPLC analysis of chemical impurities, the sum of peaks above the quantification limit (0.05 µg/mL), including the PSMA-5 precursor, was 0.7 ± 0.1 µg/mL (Fig. 5a, b). In gamma-ray spectrometry, three peaks corresponding to 211At (76.9, 79.3 and 687.0 keV) were observed, with no additional peaks detected (Fig. 6). Ethanol concentration was 2.8 ± 0.3 v/v%, and all other quality control met the acceptance criteria. The detailed quality control results for the RIKEN and RCNP targets are summarized in Tables 4 and 5, respectively.
Typical HPLC UV and radio chromatograms for analysis of chemical impurities including PSMA-5 precursor. (a) Typical UV chromatograms of [211At]PSMA-5 solution. (b) Typical UV chromatograms of 1 µg/mL PSMA-5 precursor solution for system suitability test. (c) Typical radio chromatograms of [211At]PSMA-5 solution
In additional analyses performed for the 6-lot test, prolonged (22 h) gamma spectrometry confirmed that the 245.3 keV peak derived from 210At was below the detection limit. The radioactivity ratio of 210At/211At was estimated to be < 5 × 10− 4%. Residual Bi measured by ICP-MS was below the detection limit (< 40 ppb), and the residual I− concentration determined by HPLC UV-VIS was also below the quantification limit (< 1 ppm).
Identification of [211At]PSMA-5 by LC-MS
The peak region of [211At]PSMA-5 detected by the radioactivity detector connected immediately upstream of the MS detector, was averaged to generate mass spectra. The observed exact masses were 1236.3927 in positive ion mode ([M + H]⁺) and 1234.3752 in negative ion mode ([M-H]−), consistent with the theoretical exact masses of [211At]PSMA-5, [M + H]⁺ = 1236.3893 and [M-H]− = 1234.3737. Representative chromatograms from the radioactivity and MS detectors are shown in Fig. 7.
Typical HPLC chromatograms. (a), (c) Typical radioactivity chromatograms of [211At]PSMA-5. (b) Typical mass spectrum (m/z = 1236.3927 ± 10 ppm) chromatograms of [211At]PSMA-5 in positive ion mode. (d) Typical mass spectrum (m/z = 1234.3752 ± 10 ppm) chromatograms of [211At]PSMA-5 in negative ion mode
Discussion
This study successfully scaled up the production of [211At]PSMA-5 using an automated synthesis unit, transitioning from a laboratory-scale process to a manufacturing method suitable for an investigator-initiated clinical trial. The synthesized product met all quality criteria for intravenous administration in patients. Furthermore, by using irradiated Bi targets containing 211At produced at two independent cyclotron facilities (RCNP and RIKEN) as starting materials, we purified 211At and subsequently synthesized [211At]PSMA-5 with equivalent radiochemical yields and consistent quality. These results demonstrate that the investigational drug can be produced reproducibly, independent of the cyclotron facility. This establishes a robust and flexible production and supply system that not only ensures stable manufacturing regardless of 211At production site but also provides resilience against unexpected cyclotron downtime or technical issues. Indeed, when a production interruption occurred at the RCNP cyclotron shortly before the scheduled patient administration, we seamlessly switched to 211At supplied by RIKEN, allowing manufacturing to proceed as planned and the investigational drug to be successfully administered.
At the Institute for Radiation Sciences, University of Osaka, water has conventionally been used for recovering 211At from cold trap to maintain flexibility in experimental conditions. Following this non-optimized approach, the recovery rate in the present study was only about 33%, insufficient to obtain the radioactivity required for clinical administration. In contrast, when sodium hydrogen carbonate (7%), also used as a reaction solvent, was employed, the recovery rate increased to approximately 90%. It has been reported that heating alcohol solution of 211At causes significant vaporization, whereas the addition of carbonates such as K₂CO₃ or NaHCO₃ suppresses this vaporization (WO2021225147; Kondo et al. 2025). These findings suggest that the improved recovery observed here may be attributed to changes in the chemical form of 211At in the presence of carbonate ion. To accommodate the automated synthesis system, a universal glass vial was adopted as the reaction vessel, with a total reaction volume to 1 mL. Although sufficient recovery was achieved with ≥ 500 µL of sodium hydrogen carbonate (7%), the volume was adjusted to 700 µL to account for the system’s dead volume. The concentration of precursor and KI were also scaled up proportionally to those used in the manual synthesis.
All components of automated synthesis unit of COSMiC-Mini VTRSC2 for [211At]PSMA-5 production, including the 10-way flow switching valve, tubing, and reaction vessel, were sterilized and used as disposable items. The washing process, using two 1 mL doses of sodium hydrogen carbonate (7%), each was loaded into a syringe injected and then replaced under conditions that minimized radiation exposure and contamination. However, since the first wash introduced into the reaction vessel had negligible impact on residual [211At]PSMA-5 recovery (no fluctuation in the RI sensor value in the reaction vessel), streamlining the procedure to a single wash for the line and HLB cartridge alone could enable full automation of the process. In this study, [211At]PSMA-5 retained on the HLB cartridge was recovered using WFI and ethanol. Preliminary tests showed that [211At]PSMA-5 elutes in small quantities even with WFI alone, and that passing WFI through the HLB cartridge before ethanol improves recovery rate, finding incorporated into the automated protocol.
Radiochemical purity was evaluated using radio-TLC, following the same procedure applied to [211At]PSMA-5 synthesized for preclinical studies (Watabe et al. 2024). This method was chosen over HPLC because unreacted 211At may not elute in HPLC analysis, potentially leading to overestimation of radiochemical purity. In the radio-TLC analysis, the impurity is presumed to be AtI or AtI2 produced by the reaction with KI, however this has not been identified. In addition, LC-MS analysis performed under scaled-down HPLC conditions (as used for chemical impurities testing) confirmed the presence of a peak with the principal radioactive peak. The consistent retention time offset of 4.0–4.1 min between the radioactive peak of [211At]PSMA-5 and the UV-absorbing precursor peak in the HPLC conditions for chemical impurities with the radioactivity detector (Gavi nova, raytest) connected after the UV detector (Fig. 5-a and c) enable identification of the labeled compound without requiring routine LC-MS verification (once every 12 months).
HPLC analysis was used to quantify any chemical impurities. In addition to PSMA-5 precursor, several expected derivatives could theoretically form, namely hydroxylated (OH-PSMA-5), iodinated (I-PSMA-5), and de-boronated (nonB-PSMA-5) analogs. These derivatives were synthesized at Peptide Institute and previously identified by their retention times in HPLC analysis. The relative retention times of these compounds (normalized to PSMA-5 precursor) were 1.05, 1.2, and 1.4, respectively. In the chromatogram (Fig. 5-a), only OH-PSMA-5 (10.923 min) was quantifiable in addition to PSMA-5 precursor, indicating the absence of significant de-boronation form or halogen exchange side reactions. Furthermore, we confirmed that the R.T. of [211At]PSMA-5 and I-PSMA-5 were comparable (both with a relative retention time of 1.4 against the PSMA-5 precursor). While the protocol for qualitative analysis of the target compound labeling 211At, which is not available stable isotopes, is subject to debate, these results suggest that the homologous iodine-labeled compound can be used as a standard reference and may similarly be widely applicable to 211At compounds.
Conclusion
In this study, we successfully synthesized [211At]PSMA-5 in quantities and quality suitable for clinical administration using an automated synthesizer. An investigator-initiated clinical trial is currently ongoing to evaluate the safety and preliminary efficacy of this Investigational drug in patients with castration-resistant prostate cancer (Watabe et al. 2025a, b). The establishment of an automated and reproducible production process for 211At therapeutic drug, as demonstrated here, is expected to play a pivotal role in advancing the development and clinical application of next-generation α-therapeutic agents.
Data availability
The datasets used and/or analyzed in the current study are available from the corresponding author upon reasonable request.
Abbreviations
- EOS:
-
End of synthesis
- HPLC:
-
High-performance liquid chromatography
- TLC:
-
Thin layer chromatography
- ICP-MS:
-
Inductively coupled plasma mass spectrometry
- LC-MS:
-
Liquid chromatography-mass spectrometry
- RT:
-
Retention time
- Rf:
-
Retention factor
References
Afshar-Oromieh A, Haberkorn U, Eder M, Eisenhut M, Zechmann CM. [68Ga]Gallium-labelled PSMA ligand as superior PET tracer for the diagnosis of prostate cancer: comparison with 18F-FECH. Eur J Nucl Med Mol Imaging. 2012;39:1085–6. https://doi.org/10.1007/s00259-012-2069-0.
Emmett L, Crumbaker M, Ho B, Willowson K, Eu P, Ratnayake L, et al. Results of a prospective phase 2 pilot trial of 177Lu-PSMA-617 therapy for metastatic castration-resistant prostate cancer. J Nucl Med. 2019;60:917–22.
Hofman MS, Violet J, Hicks RJ, Ferdinandus J, Thang SP, Akhurst T, et al. [177Lu]-PSMA-617 radionuclide treatment in patients with metastatic castration-resistant prostate cancer (LuPSMA trial): a single-arm, phase 2 study. Lancet Oncol. 2018;19:825–33. https://doi.org/10.1016/s1470-2045(18)30198-0.
https://www.nishina.riken.jp/researcher/APR/APR050/pdf/262.pdf
Kondo Y, Joho T, Sasaki S, Mochizuki K, Hasegawa N, Ukon N. Production of 211At and automated radiosynthesis of [211At]MABG via electrophilic Astatodesilylation. EJNMMI Radiopharm Chem. 2025;10:52. https://doi.org/10.1186/s41181-025-00376-1.
Kratochwil C, Bruchertseifer F, Rathke H, Bronzel M, Apostolidis C, Weichert W, et al. Targeted α-therapy of mCRPC with 225Ac-PSMA-617: dosimetry estimate and empiric dose finding. J Nucl Med. 2017;58:1624–31.
Kratochwil C, Bruchertseifer F, Giesel FL, Weis M, Verburg FA, Mottaghy F, et al. 225Ac-PSMA-617 for PSMA-targeted α-radiation therapy of metastatic castration-resistant prostate cancer. J Nucl Med. 2018;59:529–34.
Morgenstern A, Apostolidis C, Bruchertseifer F. Supply and clinical application of Actinium-225 and Bismuth-213. Semin Nucl Med. 2020;50:119–23.
Naka S, Ooe K, Shirakami Y, Kurimoto K, Sakai T, Takahashi K, et al. Production of [211At]NaAt solution under GMP compliance for investigator-initiated clinical trial. EJNMMI Radiopharm Chem. 2024;9:29. https://doi.org/10.1186/s41181-024-00257-z.
Parida GK, Panda RA, Bishnoi K, Agrawal K. Efficacy and safety of Actinium-225 prostate-Specific membrane antigen radioligand therapy in metastatic prostate cancer: A systematic review and metanalysis. Med Princ Pract. 2023;32:178–91.
Sartor O, de Bono J, Chi KN, Fizazi K, Herrmann K, Rahbar K, et al. Lutetium-177–PSMA-617 for metastatic Castration-Resistant prostate cancer. N Engl J Med. 2021;385:1091–103. https://doi.org/10.1056/nejmoa2107322.
Sato N, Yano S, Toyoshima A, Haba H, Komori Y, Shibata S, et al. Development of a production technology of 211At at the RIKEN AVF cyclotron: (i) production of 211At in the 209Bi(α,2n)211At reaction. RIKEN Accel Prog Rep. 2017;50:262.
Silver DA, Pellicer I, Fair WR, Heston WD, Cordon-Cardo C. Prostate-specific membrane antigen expression in normal and malignant human tissues. Clin Cancer Res. 1997;3:81–5.
Watabe T, Kaneda-Nakashima K, Shirakami Y, Kadonaga Y, Ooe K, Wang Y, et al. Targeted α-therapy using Astatine (211At)-labeled PSMA1, 5, and 6: a preclinical evaluation as a novel compound. Eur J Nucl Med Mol Imaging. 2023;50:849–58. https://doi.org/10.1007/s00259-022-06016-z.
Watabe T, Kaneda-Nakashima K, Kadonaga Y, Ooe K, Sampunta T, Hirose N, et al. Preclinical evaluation of biodistribution and toxicity of [211At]PSMA-5 in mice and primates for the targeted alpha therapy against prostate cancer. Int J Mol Sci. 2024;25:5667.
Watabe T, Hatano K, Naka S, Sasaki H, Kamiya T, Shirakami Y, et al. First-in-human SPECT/CT imaging of [211At]PSMA-5: targeted alpha therapy in a patient with refractory prostate cancer. Eur J Nucl Med Mol Imaging. 2025a;52:2253–5. https://doi.org/10.1007/s00259-024-07017-w.
Watabe T, Naka S, Shirakami Y, Kaneda K, Murakami M, Toyoshima A et al. Development of PSMA-Targeted alpha therapy using [211At]PSMA-5. Semin nucl med. 2025b:S0001-2998(25)00120-5.
Zalutsky MR, Pruszynski M. Astatine-211: production and availability. Curr Radiopharm. 2011;4:177–85. https://doi.org/10.2174/1874471011104030177.
Acknowledgements
We would like to thank all members of Nuclear Medicine and Tracer Kinetics and hot-lab staff in the hospital for their support during the study. We would also like to thank Xiaojie Yin, Nozomi Sato, Akihiro Nambu, Yousuke Kanayama, and Yudai Shigekawa for technical assistance with the production of 211At at RIKEN RI Beam Factory.
Funding
This study was funded by an AMED translational research grant (Seeds-F) (Grant Number: JP23ym0126091).
Author information
Authors and Affiliations
Contributions
SN conducted the study, performed data analysis, and wrote the manuscript. YS, KO, KK, TS, AT, MM, YK, HH, JC, FG, KI and NT assisted with the study. TW supervised the study. All authors contributed to the writing of the manuscript and approved the final version.
Corresponding author
Ethics declarations
Ethics approval and consent to participate
Not applicable.
Consent for publication
Not applicable.
Competing interests
The authors declare that they have no competing interests.
Additional information
Publisher’s note
Springer Nature remains neutral with regard to jurisdictional claims in published maps and institutional affiliations.
Supplementary Information
Below is the link to the electronic supplementary material.
Rights and permissions
Open Access This article is licensed under a Creative Commons Attribution-NonCommercial-NoDerivatives 4.0 International License, which permits any non-commercial use, sharing, distribution and reproduction in any medium or format, as long as you give appropriate credit to the original author(s) and the source, provide a link to the Creative Commons licence, and indicate if you modified the licensed material. You do not have permission under this licence to share adapted material derived from this article or parts of it. The images or other third party material in this article are included in the article’s Creative Commons licence, unless indicated otherwise in a credit line to the material. If material is not included in the article’s Creative Commons licence and your intended use is not permitted by statutory regulation or exceeds the permitted use, you will need to obtain permission directly from the copyright holder. To view a copy of this licence, visit http://creativecommons.org/licenses/by-nc-nd/4.0/.
About this article
Cite this article
Naka, S., Watabe, T., Shirakami, Y. et al. Scale-up production of [211At]PSMA-5 using automated synthesizer for Investigator-Initiated clinical trial. EJNMMI radiopharm. chem. 11, 6 (2026). https://doi.org/10.1186/s41181-025-00416-w
Received:
Accepted:
Published:
Version of record:
DOI: https://doi.org/10.1186/s41181-025-00416-w