Abstract
Background
Metabolic tumor volume (MTV) and total lesion glycolysis (TLG) are volumetric parameters derived from 18F-FDG PET/CT, suggested to have a prognostic value in cancer patients. Our study aimed to test whether these volumetric parameters of the primary tumor and whole-body tumor burden (WBTB) can predict overall survival (OS) in non-small cell lung cancer (NSCLC) patients.
Materials and methods
Thirty biopsy-proven NSCLC patients who had not begun anti-tumor therapy were included in this prospective study. A baseline 18F-FDG PET/CT study was acquired. Scans were interpreted visually and semi-quantitatively by drawing a 3D volume of interest (VOI) over the primary tumor and all positive lesions to calculate metabolic, volumetric parameters, and WBTB. The PET parameters were used to stratify patients into high- and low-risk categories. The overall survival was estimated from the date of scanning until the date of death or last follow-up.
Results
At a median follow-up of 22.73 months, the mean OS was shorter among patients with higher tu MTV and tu TLG and high WBTB. High WB TLG was independently associated with the risk of death (p < 0.025). Other parameters, e.g., SUVmax, SUVpeak, and SUVmean, were not predictive of outcomes in these patients. Conclusion: In patients with NSCLC, tu MTV, tu TLG, and WBTB determined on initial staging 18F-FDG PET/CT seems to be a strong, independent imaging biomarker to predict OS, superior to the clinical assessment of the primary tumor. The WB TLG was found to be the best predictor of OS.
Similar content being viewed by others
Explore related subjects
Discover the latest articles, news and stories from top researchers in related subjects.Background
Lung cancer has the highest mortality rate globally (Sung et al. 2021). More than 85% of lung cancer cases are non-small cell lung cancer (NSCLC), and adenocarcinoma is the most prevalent histological subtype (Bousquet Mur et al. 2020). The primary curative treatment for NSCLC is surgical excision, which is generally regarded as the best treatment option for patients in the early stages. Unfortunately, many NSCLC patients have missed the chance for surgery or are not candidates for radiation, targeted therapy, or immunotherapy (Shea et al. 2016). Therefore, conventional cytotoxic chemotherapy continues to be the mainstay of care for people with advanced NSCLC (Shea et al. 2016).
Currently, tumor staging is the primary factor used to determine NSCLC patients’ prognosis (Detterbeck 2018; Zappa and Mousa 2016). However, patients with the same TNM stage and identical treatment regimens have quite different tumor biology and survival rates, indicating that staging alone cannot provide sufficient clinical information (Woodard et al. 2016; Xie et al. 2018). Thus, it is necessary to identify additional discriminative prognostic markers that will improve stratification, guide tailored suitable therapy, and provide more precise forecasts of treatment outcomes and survival (Chang et al. 2019).
The guidelines include the use of 18F-FDG PET/CT for the diagnosis, staging, restaging, and evaluation of treatment response in lung cancer (Liu et al. 2016). FDG PET/CT functional imaging is preferred to morphological imaging, such as CT because tumor load is easier and quicker to measure (Eude et al. 2022). While maximum standardized uptake value (SUVmax) reflects the metabolic status of the tumor, metabolic tumor volume (MTV) and total lesion glycolysis (TLG) are parameters that reflect both metabolic and volumetric status (Ventura et al. 2020). High MTV and TLG levels in lung cancer have been suggested to be prognostic factors for disease-free survival (DFS) and overall survival (OS) (Im et al. 2015). PET/CT whole-body tumor burden (WBTB), as a measure for overall burden of cancer, has been shown to bear a strong correlation with prognosis. Software developments and the growing accessibility of positron-emitting radiopharmaceuticals have allowed for considerable advancements in WBTB determination over the past ten years. However, it is still difficult to accurately estimate tumor burden, particularly when the metastatic disease is extensive (Santos et al. 2022).
Patient selection
Between May 2019 and June 2022, 63 patients with pathologically or cytologically confirmed diagnoses of primary NSCLC were referred for whole-body 18F-FDG PET/CT scans at the nuclear medicine unit of Assiut University in Egypt. Thirty individuals were eligible to participate in our study after the exclusion of 12 patients who had begun anti-tumor therapy and 21 patients who had finished their therapy regimens and were referred for the detection of residual/ recurrent disease. All included patients were PET/CT staged according to the National Comprehensive Cancer Network guidelines. Patients with (squamous cell carcinoma, adenocarcinoma, large cell carcinoma, and poorly differentiated) NSCLC were eligible for inclusion, while, SCLC, sarcoma, neuroendocrine carcinoma, and those patients who had undergone neoadjuvant therapy before PET/CT examination, died from other diseases, or lost follow-up were excluded.
Clinical patient characteristics including gender, age, smoking status, histological type and pathological tumor grade, surgery status, and chemotherapy regimen were included. The median duration of follow-up was 17.23 months (ranging from 4 to 36 months).
Acquisition of PET/CT
FDG data were acquired using a whole-body PET/CT scanner (Biograph, Horizon 16; Siemens, Germany). For each patient, plasma glucose levels were measured (mean Blood glucose was 6.2 ± 1.5 mmol/l) and all patients fasted for at least 6 h before scanning. PET data were acquired 60–90 min after intravenous administration of 18F-FDG (mean Dose was 259 ± 59.94 MBq). The scan range started at the mid-thighs and proceeded to the head. A whole-body unenhanced CT scan was performed using the following parameters: 140 kV, 150 mA, 0.8 s/rotation, 22.5 mm/s table speed, and slice thickness of 3.75 mm. Data from the CT scans were reconstructed from a 512 × 512 matrix to a 128 × 128 matrix to satisfactorily match the PET data and allow image fusion. The PET image data sets were reconstructed using an iterative algorithm (the ordered subsets expectation maximization).
Image analysis
Two nuclear medicine physicians (with four and fourteen years of post-residency experience) examined each patient's tumor foci. The junior nuclear medicine specialist first evaluated the images, which were then corrected by the senior consultant, who then verified the final report. The primary lesion was identified by visual observation in Syngo.via Workstation and the region of interest (ROI) was outlined around the lesion by surrounding the entire lesion with a threshold of 40% SUVmax. The relevant semi-quantitative metabolic parameters for quantifying 18F-FDG uptake values were subsequently derived from the software by automatically segmenting the lesion volumetrically in the cross-sectional, coronal, and sagittal planes. The maximum standardized uptake value normalized to body mass (SUVmax), using a SUVmax of 5 g/ml threshold level to view the PET images. SUVmax, SUVmean, and MTV were obtained from 3D isocontour at 40% of the maximal pixel value. TLG was calculated according to the following formula: TLG = SUVmean × MTV.
The whole-body metabolic tumor volume (WB MTV) and whole-body total lesion glycolysis (WB TLG) estimated from 18F-FDG PET images, including primary tumors, nodes, and metastases using free LIFEx software version 7.1 (www.lifexsoft.org). Tumor volume delineation is refined using two criteria in combination, including: absolute SUV threshold (SUV > 2.5) and percent SUVmax threshold (41%). Then, manually removing the brain, heart, and other areas with physiological uptake. Manual adjustments were made to regions that are close to or overlap high physiological uptake regions, such as lesions in the brain or near the urinary bladder. The WB MTV and WB TLG values were automatically updated during these operations. All regions were displayed using axial, sagittal, and coronal slices and using a maximum-intensity-projection (MIP) representation. The results are exported in an Excel file and in the form of an image booklet displaying the regions. Figures 1 and 2 are representative of two patients being treated for small and large tumor volumes. Whole-body MTV was obtained by the sum of all lesions considered related to cancer and the WB TLG was obtained as the sum of the TLG values of all lesions.
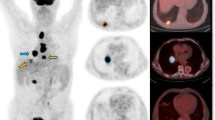
Representative 18F-FDG PET-CT MIP (maximum intensity projection) and axial images of a patient with low whole-body tumor burden (WBTB). A 62-year-old male patient with pathologically proven right middle lobe NSCLC, adenocarcinoma. FDG uptake was increased abnormally corresponding to the right lung primary lesion, another focal FDG uptake is noted at abdominal LN. Corresponding SUVmax, SUVpeak, SUVmean, tu MTV, tu TLG, WB MTV, and WB TLG were 3.48, 2.71, 2.04, 5.23, 10.56, 30.8, 56
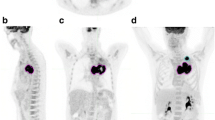
Representative 18F-FDG PET/CT MIP and coronal images for a patient with a high whole-body tumor burden (WBTB). A 61-year- old male patient with pathologically proven right lower lobe NSCLC, adenocarcinoma. FDG uptake was abnormally increased at the primary tumor mass as well as multiple metastatic nodal, osseous brain, and muscular deposits, corresponding SUVmax, SUVpeak, SUVmean, tu MTV, tu TLG, WB MTV, and WB TLG 4.9, 4.2, 2.9, 33, 96.03, 317.53, 931.88, respectively
Overall survival (OS) was defined as the time from the date of initial PET/CT to the date of death. Several recent studies defined overall survival as the time from enrollment in the study (initial PET/CT imaging) (Dolan et al. 2020; Peng et al. 2022; Rocha et al. 2021). The patients last known to be alive, were censored at the date of the end of the study (August 2022).
Statistical analysis
Data were analyzed using the IBM SPSS Statistics (Version 26.0. Armonk, NY: IBM Corp). Descriptive data were shown as number and percentage values in categorical data, and mean values in continuous data. Survival receiver–operating characteristic curve analysis (ROC curve) was used to achieve the maximal area under the curve (AUC) and the optimal cutoff value for each of PET/CT-derived metabolic parameters. The PET/CT metabolic parameters were divided into high-risk and low-risk groups according to the cutoff value derived from the survival ROC. Univariate analysis of prognostic factors for OS was achieved using the Kaplan–Meier method, and the log-rank test was used to evaluate the significance of the differences between the survival curves, the Cox proportional hazards model that included significant univariate variables was used to determine independent prognostic factors for OS in multivariate survival analyses. Risk of death was estimated based on hazard ratios and the 95% confidence interval and was recorded. P < 0.05 was accepted for statistical significance in all analyses.
Results
Clinical and pathological characteristics
During the study time frame, Thirty NSCLC patients who had no prior anti-tumor therapy and planned to start first-line chemotherapy (CTH) were included. Table 1 lists the clinical features of the patients. The mean age was 63.6 ± 10.82 years, with a majority of men (73.3%). Fifteen patients (50%) had adenocarcinoma, 5 patients (16.7%) had squamous cell carcinoma, 2 patients (6.7%) had large cell carcinoma, and 8 patients (26.6%) were NSCLC of a type that was not further specified. All patients were treated with CTH alone (83.3%) while (16.7%) received CTH after surgical resection.
To employ metabolic and volumetric parameters of FDG PET, we dichotomized the variable with a calculated cutoff point. The cutoff point chosen based on ROC curve analysis with area under curve were: 11.3 (0.688), 9.55 (0.730),8.05 (0.724), 30.9 (0.845), 190 (0.849), 141.98 (0.757), and 832.4 (0.783) SUVmax, SUVpeak, SUVmean, tu MTV, tu TLG, WB MTV, and WB TLG, respectively. The results of ROC analysis of PET prognostic markers are presented on Table 2 and Fig. 3
Kaplan–Meier survival analysis
The probability of survival above or below the calculated cutoff points of different variables was calculated. The results of univariate survival analysis are presented in Table 3. High tu MTV and tu TLG as well as WB MTV and WB TLG were associated with significantly shorter OS. Mean OS for MTV ≥ 30.9 mL versus MTV < 30.9 mL was 16.56 months (95% CI: 14.532–30.31) versus 34 months (95% CI: 27.022–37.121) (P = 0.006). Mean OS for TLG ≥ 190 was 17.31 months (95% CI: 11.088–23.5) versus 33.83 months for TLG < 190 g (95% CI: 29.77–37.89) (P = 0.008). For WB MTV the mean OS was 21.38 months for values ≥ 141.98 (95% CI 13.82–28.94) versus 34 months for values < 141.98 (95% CI: 30.234–37.76) (P = 0.011). Mean OS for WB TLG ≥ 832.4 g was 20.17 months (95% CI: 12.35–27.98) vs 34 months for WB TLG < 832.40 g (95% CI 30.23–37.76) (P = 0.007). See Fig. 4 for Kaplan–Meier curves based on SUVmax, SUVpeak, SUVmean, tu MTV, tu TLG, WB MTV and WB TLG.
Kaplan–Meier curves of overall survival (OS) stratified according to dichotomized PET/CT parameters. a SUVmax, b SUVpeak, c SUVmean, d tu MTV and e tu TLG f WB MTV g WB TLG in all patients (n = 30). The blue lines indicate the group with values less than the cutoff values and the red lines are the group with values equal to or greater than the cutoff values
Mean OS for high SUVmax was shorter than for low SUVmax, but the difference was not significant. Mean OS for SUVmax < 11.3 versus SUVmax ≥11.3 was 32.07 months (95% CI: 27.02-37.12) versus 22.42 months (95% CI: 14.53- 30.31) (P = 0.054). Mean OS for SUVpeak < 9.55 versus SUVpeak ≥ 9.55 was 32.07 months (95% CI: 27.022-37.12) versus 17.05 months (95% CI 10.31–23.8) (P= 0.018). Mean OS for SUVmean < 8.05 versus SUVmean ≥ 8.05 was 31.18 months (95% CI 26.21-36.13) versus 12.73 months (95% CI: 6.58-18.) (P= 0.005). (Table 3).
Regarding clinical and pathological factors, patients with squamous cell pathological subtype had lower mean OS (P = 0.002). Otherwise, no other clinical factors of age, sex, smoking history were predictors of OS (P value = 0.4, 0.3, and 0.2, respectively).
Cox univariate and multivariate analyses
The previous cutoffs were used to divide the study population into 2 distinct prognostic groups for Kaplan–Meier and Cox survival analysis. Multivariate Cox regression analysis showed that tu MTV (hazard ratio of 1.017, 95% CI:1.003–1.031, p = 0.019), tu TLG (hazard ratio of 1.002, 95% CI:1.000–1.004, P = 0.023) and WB TLG (hazard ratio of 1.001, 95% CI:1.000–1.002, p = 0.005) were significant in multivariate analysis for OS.
WB TLG was confirmed as an independent predictor. High WB TLG patients had higher risk of death than low WB TLG patients, with an adjusted hazard ratio of 1.001 (95% CI:1.000–1.002, P = 0.025). Parameters that independently affect OS in the COX regression analysis are shown in Table 4
Discussion
NSCLC management is a primary concern, therefore prognostic assessment is critical to realizing the potential of personalized treatment. A wide range of information sources are available of which the noninvasive type plays a fundamental role in reducing the patients' burden (Lambin et al. 2015). As a noninvasive imaging modality, the 18F-FDG PET/CT, which reflects tumor metabolic activity, such as cell viability and proliferative activity, is becoming more and more significant in studies of initial diagnosis, accurate staging, evaluation of treatment response, and prognosis of lung cancer (Li et al. 2019).
In this prospective study, we hypothesized that the metabolic parameters derived from 18F-FDG PET/CT may provide more accurate quantitative imaging features and thus might be of value in predicting OS in patients with NSCLC.
In PET/CT imaging, the SUV is the most widely used semi-quantitative measure. The individuals' physical characteristics, the blood glucose level, the uptake period, the image acquisition and reconstruction, the lesion size, and other variables all have an impact on SUV (Lee et al. 2012).
SUVmax, SUVmean, and SUVpeak, represent the maximum, average, and peak SUV of the region of interest, respectively. All three of these parameters were measured and examined in our study in order to determine which was the most valuable. We found that higher levels of SUVpeak and SUVmean were associated with shorter OS which was not true for SUVmax. Our findings further support the hypothesis that SUVmax could not be a reliable predictor of OS, emphasizing the significance of volumetric parameters. Given that SUVmax simply represents a single-pixel value of peak metabolic activity, hence, has less impact on patient’s outcome, Huang and colleagues suggested that SUVmax may not be the most reliable predictor (Huang et al. 2014).
Increasing evidence from studies on different tumor types shows that MTV and TLG are more accurate prognostic indicators than do SUV metrics (Hyun et al. 2014; Liao et al. 2012).
Several automated techniques are used to segment regions of interest in PET/CT scans, including gradient-based threshold (adaptive iterative algorithm, AIA), fixed SUV threshold (e.g., SUV > 2.5), percentage threshold of SUVmax (e.g., > 42%), and background-related threshold (AT 40%) approaches. The fixed threshold method was not used in our study since it ignores the background activity as reported in earlier studies (Soret et al. 2007). Percentage threshold can be performed rapidly and consistently, with less inter-observer variability (Wang et al. 2017). In our study we used percentage threshold method for delineation of MTV. Our results reported a significantly worse OS and higher risk of death in patients with higher tu MTV and tu TLG. These results are in line with MTV's established predictive value in previous studies (Popinat et al. 2019; Seban et al. 2020; Jreige et al. 2019).
The definition of "high tumor burden" is still up for debate. Its definition and use as a prognostic factor in the context of lung cancer could assist in the development of specialized treatment regimens that would not only enhance patient quality of life and treatment outcomes but also lower the occurrence of negative adverse effects (Reck and Rabe 2017).
Although earlier studies have employed the MTV methodology (with a threshold value of 41% of SUVmax in each lesion), more advanced methods might yield more precise measurement of WB MTV values. Chardin et al. reported that WB MTV, as calculated by threshold value method, offers a reliable and reproducible estimate of whole-body tumor burden (Chardin et al. 2020).
The optimal cutoff value of WB MTV in our study was 141.98 ml. Due to the lack of current standard 18F-FDG PET/CT parameters cutoff definitions, external validation of the results may be challenging. The WB MTV cutoff values published in research to date have varied, while utilizing comparable methods to compute MTV. These values range from 17.8 to 143.2 ml (Hashimoto et al. 2020; Dall'Olio et al. 2021; Monaco et al. 2021; Yamaguchi et al. 2020). Pu et al. validated WB MTV through analysis of a quite large sample of NSCLC patients of all clinical stages reporting outcomes comparable to ours (Pu et al. 2018). Consequently, Pellegrino and colleagues additionally found that WB MTV was a reliable predictor of OS at all TNM stages (Pellegrino et al. 2019).
In addition to WB MTV, we also assessed the other volumetric value WB TLG. Supposedly, TLG could be more promising since it combines volumetric and metabolic information. Our results found that WB TLG is an independent predictor of OS. Similar results were reported by a previous study by Vanhove whose results showed that only WB TLG is of prognostic value in NSCLC for both OS and progression free survival (PFS) (Vanhove et al. 2018).
In a recent study by Oliveira et al. 52 NSCLC patients who performed 18F-FDG PET/CT for staging in who were followed for a median of 11.0 months, they found that The WB TLG/tu TLG ratio is an independent prognostic indicator of OS in advanced-stage NSCLC (Oliveira et al. 2022).
A multi-institutional investigation confirms that SCC histology is independently correlated with lower overall survival and local, regional, and distant recurrence (Baine et al. 2018). This comes in agreement with our results that showed a significantly shorter OS survival of SCC. Future research is required to evaluate whether early-stage NSCLC therapy paradigms should vary based on histology.
The Cox multivariate analysis revealed that the degree of histological differentiation had no statistically significant effect on OS. Possible explanation of this result is that OS is frequently impacted by numerous other factors, such as type of therapy, tumor recurrence or metastasis. Additionally, the histological differentiation of the tumor was not determined in this study for one-third of the patients. Therefore, we were only working with 20 cases whose degree of difference was known. The statistical analysis result may be biased by a quite small sample size.
In conclusion, we have demonstrated that, in patients with NSCLC, the whole-body metabolic tumor burden determined on a baseline 18F-FDG PET/CT performed for staging seems to be a strong, independent imaging biomarker to predict OS, superior to the clinical assessment of the primary tumor itself. The most accurate predictor of OS in our patients was the WB TLG. Efforts should be made to unify its definition and to further explore its potential as a prognostic factor for patients with metastatic NSCLC.
Our study was limited by the small number of study group and short follow-up period. This may be attributed to COVID-19 pandemic. According to a survey conducted online by the International Atomic Energy Agency on a variety of nuclear medicine services and completed by 434 doctors from 72 different countries, nuclear medicine services have dramatically declined, by over 50% for diagnostic tests and as much as 45% for radionuclide therapy (Freudenberg et al. 2020).
Other limitations include the heterogeneity of the study population considering the various NSCLC subtypes, various metastatic statuses, and different previous therapies.
Our benefits were a single-center research design, patient preparation, waiting period, and FDG doses at the stage preceding FDG PET/CT were uniform, the same FDG PET /CT scanner for all patients.
Availability of data and materials
All data used in this study can be available on request.
Abbreviations
- OS:
-
Overall survival
- NSCLC:
-
Non-small cell lung cancer
- tu MTV:
-
Metabolic tumor volume of the primary tumor
- tu TLG:
-
Total lesion glycolysis of the primary tumor
- WBTB:
-
Whole-body tumor burden
- WB MTV:
-
Whole-body metabolic tumor volume
- WB TLG:
-
Whole-body total lesion glycolysis
References
Baine MJ, Verma V, Schonewolf CA, Lin C, Simone CB 2nd (2018) Histology significantly affects recurrence and survival following SBRT for early stage non-small cell lung cancer. Lung Cancer 118:20–26
Bousquet Mur E, Bernardo S, Papon L, Mancini M, Fabbrizio E, Goussard M et al (2020) Notch inhibition overcomes resistance to tyrosine kinase inhibitors in EGFR-driven lung adenocarcinoma. J Clin Invest 130(2):612–624
Chang H, Lee SJ, Lim J, Lee JS, Kim YJ, Lee WW (2019) Prognostic significance of metabolic parameters measured by (18)F-FDG PET/CT in limited-stage small-cell lung carcinoma. J Cancer Res Clin Oncol 145(5):1361–1367
Chardin D, Paquet M, Schiappa R, Darcourt J, Bailleux C, Poudenx M et al (2020) Baseline metabolic tumor volume as a strong predictive and prognostic biomarker in patients with non-small cell lung cancer treated with PD1 inhibitors: a prospective study. J Immunother Cancer 8(2):e000645
Dall’Olio FG, Calabro D, Conci N, Argalia G, Marchese PV, Fabbri F et al (2021) Baseline total metabolic tumour volume on 2-deoxy-2-[18F]fluoro-d-glucose positron emission tomography-computed tomography as a promising biomarker in patients with advanced non-small cell lung cancer treated with first-line pembrolizumab. Eur J Cancer 150:99–107
Detterbeck FC (2018) The eighth edition TNM stage classification for lung cancer: what does it mean on main street? J Thorac Cardiovasc Surg 155(1):356–359
Dolan RD, Maclay JD, Abbass T, Colville D, Buali F, MacLeod N et al (2020) The relationship between (18)F-FDG-PETCT-derived tumour metabolic activity, nutritional risk, body composition, systemic inflammation and survival in patients with lung cancer. Sci Rep 10(1):20819
Eude F, Guisier F, Salaun M, Thiberville L, Pressat-Laffouilhere T, Vera P et al (2022) Prognostic value of total tumour volume, adding necrosis to metabolic tumour volume, in advanced or metastatic non-small cell lung cancer treated with first-line pembrolizumab. Ann Nucl Med 36(3):224–234
Freudenberg LS, Paez D, Giammarile F, Cerci J, Modiselle M, Pascual TNB et al (2020) Global impact of COVID-19 on nuclear medicine departments: an international survey in april 2020. J Nucl Med 61(9):1278–1283
Hashimoto K, Kaira K, Yamaguchi O, Mouri A, Shiono A, Miura Y et al (2020) Potential of FDG-PET as prognostic significance after anti-PD-1 antibody against patients with previously treated non-small cell lung cancer. J Clin Med 9(3):725
Huang W, Fan M, Liu B, Fu Z, Zhou T, Zhang Z et al (2014) Value of metabolic tumor volume on repeated 18F-FDG PET/CT for early prediction of survival in locally advanced non-small cell lung cancer treated with concurrent chemoradiotherapy. J Nucl Med 55(10):1584–1590
Hyun SH, Ahn HK, Kim H, Ahn MJ, Park K, Ahn YC et al (2014) Volume-based assessment by (18)F-FDG PET/CT predicts survival in patients with stage III non-small-cell lung cancer. Eur J Nucl Med Mol Imaging 41(1):50–58
Im HJ, Pak K, Cheon GJ, Kang KW, Kim SJ, Kim IJ et al (2015) Prognostic value of volumetric parameters of (18)F-FDG PET in non-small-cell lung cancer: a meta-analysis. Eur J Nucl Med Mol Imaging 42(2):241–251
Jreige M, Letovanec I, Chaba K, Renaud S, Rusakiewicz S, Cristina V et al (2019) (18)F-FDG PET metabolic-to-morphological volume ratio predicts PD-L1 tumour expression and response to PD-1 blockade in non-small-cell lung cancer. Eur J Nucl Med Mol Imaging 46(9):1859–1868
Lambin P, Zindler J, Vanneste B, van de Voorde L, Jacobs M, Eekers D et al (2015) Modern clinical research: how rapid learning health care and cohort multiple randomised clinical trials complement traditional evidence based medicine. Acta Oncol 54(9):1289–1300
Lee PBJ, Lavori PW, Weerasuriya DK, Quon A, Le QT et al (2012) Metabolic tumor volume is an independent prognostic factor in patients treated definitively for non–small-cell lung cancer. Clin Lung Cancer 13(1):52–58
Li X, Wang D, Yu L (2019) Prognostic and predictive values of metabolic parameters of (18)F-FDG PET/CT in patients with non-small cell lung cancer treated with chemotherapy. Mol Imaging 18:108735
Liao S, Penney BC, Wroblewski K, Zhang H, Simon CA, Kampalath R et al (2012) Prognostic value of metabolic tumor burden on 18F-FDG PET in nonsurgical patients with non-small cell lung cancer. Eur J Nucl Med Mol Imaging 39(1):27–38
Liu J, Dong M, Sun X, Li W, Xing L, Yu J (2016) Prognostic value of 18F-FDG PET/CT in surgical non-small cell lung cancer: a meta-analysis. PLoS ONE 11(1):e0146195
Monaco L, Gemelli M, Gotuzzo I, Bauckneht M, Crivellaro C, Genova C et al (2021) Metabolic parameters as biomarkers of response to immunotherapy and prognosis in non-small cell lung cancer (NSCLC): a real world experience. Cancers (basel) 13(7):1634
Oliveira FR, de Oliveira SA, de Lima MD, Toro IF, de Souza TF, Amorim BJ, Barbeiro AS, Etchebehere E (2022) The ratio between the whole-body and primary tumor burden, measured on 18F-FDG PET/CT studies, as a prognostic indicator in advanced non-small cell lung cancer. Radiol Bras Set 54(5):289–294
Pellegrino S, Fonti R, Mazziotti E, Piccin L, Mozzillo E, Damiano V et al (2019) Total metabolic tumor volume by 18F-FDG PET/CT for the prediction of outcome in patients with non-small cell lung cancer. Ann Nucl Med 33(12):937–944
Peng SM, Ren JJ, Yu N, Xu JY, Chen GC, Li X et al (2022) The prognostic value of the Naples prognostic score for patients with non-small-cell lung cancer. Sci Rep 12(1):5782
Popinat G, Cousse S, Goldfarb L, Becker S, Gardin I, Salaun M et al (2019) Sub-cutaneous fat mass measured on multislice computed tomography of pretreatment PET/CT is a prognostic factor of stage IV non-small cell lung cancer treated by nivolumab. Oncoimmunology 8(5):e1580128
Pu Y, Zhang JX, Liu H, Appelbaum D, Meng J, Penney BC (2018) Developing and validating a novel metabolic tumor volume risk stratification system for supplementing non-small cell lung cancer staging. Eur J Nucl Med Mol Imaging 45(12):2079–2092
Reck M, Rabe KF (2017) Precision diagnosis and treatment for advanced non-small-cell lung cancer. N Engl J Med 377(9):849–861
Rocha ALG, da Conceicao MAM, da Cunha Sequeira Mano FXP, Martins HC, Costa G, Dos Santos Oliveiros Paiva BCB et al (2021) Metabolic active tumour volume quantified on [(18)F]FDG PET/CT further stratifies TNM stage IV non-small cell lung cancer patients. J Cancer Res Clin Oncol 147(12):3601–3611
Santos DF, Takahashi ME, Camacho M, de Lima MdCL, Amorim BJ, Rohren EM et al (2022) Whole-body tumor burden in PET/CT expert review. Clin Transl Imaging. https://doi.org/10.1007/s40336-022-00517-5
Seban RD, Assie JB, Giroux-Leprieur E, Massiani MA, Soussan M, Bonardel G et al (2020) Association of the metabolic score using baseline FDG-PET/CT and dNLR with immunotherapy outcomes in advanced NSCLC patients treated with first-line pembrolizumab. Cancers (basel) 12(8):2234
Shea M, Costa DB, Rangachari D (2016) Management of advanced non-small cell lung cancers with known mutations or rearrangements: latest evidence and treatment approaches. Ther Adv Respir Dis 10(2):113–129
Soret M, Bacharach SL, Buvat I (2007) Partial-volume effect in PET tumor imaging. J Nucl Med 48(6):932–945
Sung H, Ferlay J, Siegel RL, Laversanne M, Soerjomataram I, Jemal A et al (2021) Global cancer statistics 2020: GLOBOCAN estimates of incidence and mortality worldwide for 36 cancers in 185 countries. CA Cancer J Clin 71(3):209–249
Vanhove K, Mesotten L, Heylen M, Derwael R, Louis E, Adriaensens P et al (2018) Prognostic value of total lesion glycolysis and metabolic active tumor volume in non-small cell lung cancer. Cancer Treat Res Commun 15:7–12
Ventura L, Scarlattei M, Gnetti L, Silini EM, Rossi M, Tiseo M et al (2020) Prognostic value of [(18)F]FDG PET/CT parameters in surgically resected primary lung adenocarcinoma: a single-center experience. Tumori J 106:212–222
Wang XY, Zhao YF, Liu Y, Yang YK, Zhu Z, Wu N (2017) Comparison of different automated lesion delineation methods for metabolic tumor volume of 18F-FDG PET/CT in patients with stage I lung adenocarcinoma. Medicine (Baltimore) 96(51):e9365
Woodard GA, Jones KD, Jablons DM (2016) Lung cancer staging and prognosis. Cancer Treat Res 170:47–75
Xie D, Allen MS, Marks R, Jiang G, Sun Z, Nichols F et al (2018) Nomogram prediction of overall survival for patients with non-small-cell lung cancer incorporating pretreatment peripheral blood markers. Eur J Cardiothorac Surg 53(6):1214–1222
Yamaguchi O, Kaira K, Hashimoto K, Mouri A, Shiono A, Miura Y et al (2020) Tumor metabolic volume by (18)F-FDG-PET as a prognostic predictor of first-line pembrolizumab for NSCLC patients with PD-L1 >/= 50. Sci Rep 10(1):14990
Zappa C, Mousa SA (2016) Non-small cell lung cancer: current treatment and future advances. Transl Lung Cancer Res 5(3):288–300
Funding
There is no source of funding.
Author information
Authors and Affiliations
Contributions
HA Mahmoud contributed to study concept and design, data analysis, interpretation of the study results, and writing the manuscript. NM, AMZ, and TZM contributed to study concept and design. HE contributed in the interpretation of study results and substantial revision of the manuscript. All authors read and approved the final manuscript.
Corresponding author
Ethics declarations
Ethics approval and consent to participate
The study was approved by medical ethics committee, Faculty of medicine, Assiut University, IRB NO: 17200461. All methods were carried out in accordance with the ethical standards as laid down in the 1964 Declaration of Helsinki and its later amendments.
Consent for publication
Not applicable.
Competing interests
The authors declare that they have no competing interests.
Additional information
Publisher's Note
Springer Nature remains neutral with regard to jurisdictional claims in published maps and institutional affiliations.
Rights and permissions
Open Access This article is licensed under a Creative Commons Attribution 4.0 International License, which permits use, sharing, adaptation, distribution and reproduction in any medium or format, as long as you give appropriate credit to the original author(s) and the source, provide a link to the Creative Commons licence, and indicate if changes were made. The images or other third party material in this article are included in the article's Creative Commons licence, unless indicated otherwise in a credit line to the material. If material is not included in the article's Creative Commons licence and your intended use is not permitted by statutory regulation or exceeds the permitted use, you will need to obtain permission directly from the copyright holder. To view a copy of this licence, visit http://creativecommons.org/licenses/by/4.0/.
About this article
Cite this article
Mahmoud, H.A., Oteify, W., Elkhayat, H. et al. Volumetric parameters of the primary tumor and whole-body tumor burden derived from baseline 18F-FDG PET/CT can predict overall survival in non-small cell lung cancer patients: initial results from a single institution. European J Hybrid Imaging 6, 37 (2022). https://doi.org/10.1186/s41824-022-00158-x
Received:
Accepted:
Published:
DOI: https://doi.org/10.1186/s41824-022-00158-x