Abstract
Background
Arterial tortuosity syndrome (ATS) (OMIM #208050) is a very rare autosomal recessive connective tissue disease characterized by elongation, tortuosity, and predisposition of aneurysms formation in medium and large-caliber arteries, vascular dissection, and ischemic events. To date, approximately 100 patients have been reported carrying some of the fewer than 35 causal mutations in the SLC2A10 gene.
Case presentation
Here we present the clinical and molecular characterization of two new Spanish pediatric ATS patients from two unrelated families in the same city in a short period of time. Due to the knowledge of the pathology through the first case this pathology was suspected from birth in the second case, requesting the directed genetic study.
Conclusion
In addition to arterial tortuosity and connective tissue features, sequencing analysis revealed the homozygous and heterozygous Frameshift Deletion. Confirm diagnosis in the first few years of life is the most critical for possible life-threatening events and to offer adequate genetic counseling.
Similar content being viewed by others
Background
Arterial tortuosity syndrome (ATS) (OMIM #208050) is a rare autosomal recessive (AR) connective tissue disease characterized by elongation, tortuosity, and predisposition of aneurysms in medium and large-caliber arteries, vascular dissection, and ischemic events. Approximately 100 ATS patients have been described, and more than 35 causal mutations (80 families) have been identified in the SLC2A10 gene. Associated with pulmonary artery stenosis and / or aorta with or without dysmorphic features, skeletal, ophthalmic abnormalities, and hypotonia in addition to hyperextensible skin, joint laxity, and other connective tissue features. This syndrome is caused by loss-of-function mutations in the SLC2A10 (Solute Carrier Family 2 Member 10) gene, locus 20q.13.12 cytoband (Coucke et al. 2006; Ritelli et al. 2014; Faiyaz-Ul-Haque et al. 2009; Morris 2015; Beyens et al. 2018; Kocova et al. 2018), encoding the facilitative glucose transporter GLUT10. The diagnosis of ATS is established in a proband with generalized arterial tortuosity and biallelic (homozygous or compound heterozygous) pathogenic variants in SLC2A10 identified on molecular genetic testing.
Case presentation
Patient 1 (family A)
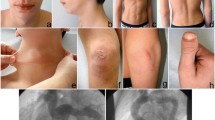
His perinatal period and psychomotor development were normal. The following features were observed on examination at 2 months hyperlaxity, elongated face, high palate, beaked nose, high-arched palate, microretrognathia, elongated philtrum, and hyperextensible skin and moderate generalized hypotonia and was referred to neuropediatrics. An electromyogram and electroneurogram was performed at 7 months and revealed signs of denervation on deltoid and biceps muscles. On examination at 2 years of age was referred for cardiological evaluation for possible colagen disease due to elongation of the aortic arch, detected on a plain chest radiograph. Echocardiography excluded heart disease and revealed tortuosity and elongation of aortic arch, supra-aortic trunks and compressing the inferior vena cava. A heart magnetic resonance angiography (MRA) revealed a severe elongation and tortuous aorta and brachiocephalic vessels with supra-aortic trunks with crooked hypoplasia distal. The aorta heads back framing heart, crossing right side zigzag at the level of the diaphragm compressing the inferior vena cava. Striking elongation of celiac trunk, superior mesenteric and renal as vertebral, subclavian, axillary and brachial arteries and cerebral vascular loops. Both parents (non-consanguinity) and the brother of the index case, asymptomatic, also presented the pathogenic variant in heterozygosity. On magnetic resonance angiography (MRA) control at 6 years findings were similar than previous MRA study with elongations and tortuous arteries both of supra-aortic trunks (Fig. 1a) and of the Willis polygon, without apreciate signs of arteriovascular malformations (AVM) or aneurysms (Fig. 1b).
Patient 1. MR angiography at 6 years of age. a 3D TOF acquisition of supra-aortic trunks, without contrast injection. Severely elongated and tortuous vessels. b 3D TOF acquisition of polygon of Willis vessels, without contrast injection. Multiple vascular elongations and loops in Willis polygon, without observing signs of AVM or aneurysms
He was monitored clinically without intervention and grew well at 6 years of age.
The skeletal signs includedmild scoliosis (T7-L2-D7), patella hyperlaxity, genu recurvatum. Aspirin was added for stroke prevention.
Molecular genetic study: Targeted exome [TNXB, ADAMTS2, CHST14, B4GALT7, PLOD1, ZNF469, COL6A1, BMP1, COL5A1, SERPING1, PPIB, COL1A2, COL3A1, COL5A2, COL5A3, ALDH18A1, ATP7A, COL1A1, COL6A3, CRTAP, FBN1, FKBP10, FKBP14, FLNA, KIF22, P3H1, PLOD2, PLOD3, SERPINH1, SLC2A10, SLC39A13, SP7; 32 genes associated with joint hypermobility], next generation sequencing (NGS), (ExoNIM®, NIMGenetics, Madrid) was studied. It was detected a pathogenic variant in homozygosity in SLC2A10 gene related to STA. This variant is a deletion of a nucleotide c.1334delG (NM_030777.3) in exon 3 of SLC2A10 gene (GAGATA^CGAGgAAGAGCCTTC), generates a stop codon and is therefore considered a loss of function mutation. This change of place to the substitution of a glycine by a glutamic acid at position 455 of the protein, causing a change in the reading pattern (frameshift) that generates a premature stop codon (p.Gly445Glufs*40). This variant has been previosly identified in homozygosity in four individuals with ATS from a consaguineous family of Italian origin (Coucke et al. 2006). This variant has not been identified in a homozygous state in population control.
Both parents were asymptomatic carriers of this variant in heterozygous. The brother of index case was carrier of this pathogenic variant in heterozygous.
Patient 2 (Family B) was a pre-term male neonate presented with a heart murmur along with, joint hypermobility, and abnormal facies at birth. Characteristic facial features: elongated face, high palate, beaked nose, high-arched palate, microretrognathia, elongated philtrum, large and sparsely folded ears, and other connective tissue features (hyperextensible skin, joint laxity, moderate generalized hypotonia, redundant skin on extremities, especially wrists and ankles, long arms and fingers. The weight at birth was 2.77 kg, which was in the 75th–90th centile (35 + 4 GA). After birth an echocardiography performed due to fetal echocardiography revealed pulmonary stenosis and tricuspidal regurgitation and revealed mild right ventricle dilatation and mild tricuspidal regurgitation and showed a very tortuous aortic arch with abnormal takeoff of the neck vessels. Collagen disease was suspected and molecular studies was performed.
Alterations in suction-deglution at 3 months was observed. Assessed by rehabilitation treatment and early intervention service. Videoelectroencephalogram and brainstem auditory evoked responses were normal.
In patient 2 (Family B) genetic study, clinical exome was made in order to identify variants related to signs or symptoms present in the patient in genes that may have an association with their genotype, identified a series of variants with a probable genotype–phenotype association in this case (see Table 1).
Patient 2 presented two variants in SLC2A10 gene: c.685C > T and c.1334del confirmed clinical diagnosis of STA. Both parents non-consanguinity, asymptomatic, also were carrier of one of these variants in heterozygosity. The rest of detected variants did not justified clinical symptons.
At 3 month of age, a heart magnetic resonance angiography (MRA) demonstrated severe arterial tortuosity of the aorta and its branches and pulmonary artery and its branches. Elongated and dilated ascending aorta. No aneurysms or stenosis was found.
Discussion
The clinical and molecular data for 40 newly identified ATS families including 50 affected individuals and a clinical review of 52 patients described in the literature was published by Beyens et al. (2018) in 2019. After this study, individual cases of pediatric and adult patients have been published.
Despite these manifestations, ATS is often misdiagnosed as other heritable connective tissue diseases (HCTDs), including Loeys Dietz syndrome (LDS), Marfan syndrome (MFS), and Ehlers–Danlos syndrome (EDS).
The diagnostic suspicion of physicians in one of the different clinical fields of interest for this HCTDs. In a specialized center, the careful clinical evaluation and the assessment of the reported findings is sufficient to confirm the diagnosis in the majority of the cases. Only in pediatric patients and in patients without a complete or with a borderline phenotype the diagnosis is doubtful and requires a second evaluation in older age or targeted molecular testing for the exclusion of overlapping disorders.
When specific signs of the different HCTDs are found, the initial diagnosis is addressed accordingly. When the presence of other (MFS, LDS, ATS) is suspected, additional investigations for the assessment of vascular, skeletal, ophthalmologic, and bone health should be performed.
When ATS is suspected: facial dysmorphinsms (elongated face, hypertelorism, cleft palate and/or bifid uvula, and micro/retrognathia), keratoconus, abdominal hernias, joint hypermobility and instability, and other skeletal anomalies, pulmonary artery stenosis, acute respiratory insufficiency and/or cardiac failure in early childhood), and the suspicion of a vascular disorder persists, extensive vascular imaging (i.e., whole body or brain angio-MRI plus thoracic and abdominal angio-CT, or heart, abdominal aorta, and limb vessels) is mandatory. For patients without cardiovascular complications, is difficult to suspect for the cutaneous, articular and skeletal signs; in these cases, cardiovascular evaluation and SLC2A10 molecular analysis allow the distinction between these disorders.
When MFS is suspected (Marfanoid habitus, ectopia lentis, aortic root, dilatation/aneurysm, Ghent criteria) heart ultrasound for mitral valve prolapse and aortic root ectasia, ocular evaluation for ectopia lentis and/or myopia, and spine MRI for dural ectasia must be performed (Colombi et al. 2015).
ATS usually begins in early childhood, with variable clinical manifestations, depending on the arterial territory involved. Early diagnosis and exhaustive follow-up through imaging tests and screening for associated abnormalities will try to avoid complications by offering a better long-term prognosis. Due to the knowledge of the pathology through the first case (Palanca Arias et al. 2020), this pathology was suspected from birth, requesting the directed genetic study. Confirm diagnosis in the first few years of life is the most critical for possible life-threatening events.
Although invasive therapy is often required for severe pulmonary artery stenosis, aortic arch abnormalities do not need intervention in most cases of ATS. Antiplatelet therapy for stroke prevention to reduce clot formation and possible embolic phenomenon was described (Naunheim et al. 2011).
Further works in the comprehension of disease presentation and complications onset, particularly in pediatric age, and on molecular diagnosis is mandatory to distinguish ATS from other CTDs with strictly overlapping clinical signs and with a high rate complications.
In patient 1, the presence of this variant in heterozygosity in the parents (non-consanguinity) and the absence of homozygosity in the healthy sibling with the clinical signs of the index case supports the causal relationship of the variant with the phenotype. The same occurs in patient 2. In this case, parents were carrier of one of the variants in heterozygosity.
The genetic study allowed an early diagnosis helping parents to plan their future offspring, offering them different reproductive options (prenatal and/or preimplantation diagnosis). Their offspring will have a 50% probability of being asyptomatic carrier, like their parents, 25% of developing ATS and 25% of not inheriting any of the variants from their parents.
In case that brothers/sisters of index cases and brothers/sisters of each of the parents were carriers of a variant in heterozygosity it should be made genetic study, sequencing SLC2A10 gene to their reproductive partners (family pedigree chart; Fig. 2).
Conclusions
Hereby we report the clinical and molecular characterization of two novel ATS pediatric patients from two unrelated families in the same city in a short period of time. Due to the knowledge of the pathology through the first case this pathology was suspected from birth in the second case, requesting the directed genetic study.
Overall, three ATS unrelated families are known in Spain so far (Rodríguez-Capitán et al. 2020).
In these two families the advances in genetic diagnostic techniques, NGS, helps to establish a genotype–phenotype relationship, offering adequate genetic counseling to parents and first-degree relatives.
The majority of the reported ATS patients are infants or children in whom colageno disease was suspected and molecular studies was performed.
Early diagnosis in the first few years of life through echocardiography and imaging tests is the most critical for possible life-threatening events and early recognition of complications.
Availability of data and materials
The data used to support the findings of this study are included within the article.
Abbreviations
- ATS:
-
Arterial tortuosity syndrome
- MRA:
-
Magnetic resonance angiography
- AVM:
-
Arteriovascular malformations
- HCTDs:
-
Heritable connective tissue diseases
- LDS:
-
Loeys Dietz syndrome
- MFS:
-
Marfan syndrome
- EDS:
-
Ehlers–Danlos syndrome
References
Beyens A, Albuisson J, Boel A, Al-Essa M, Al-Manea W, Bonnet D et al (2018) Arterial tortuosity syndrome: 40 new families and literature review. Genet Med 20(10):1236–1245
Colombi M, Dordoni C, Chiarelli N, Ritelli M (2015) Differential diagnosis and diagnostic flow chart of joint hypermobility syndrome/ehlers-danlos syndrome hypermobility type compared to other heritable connective tissue disorders. Am J Med Genet C Semin Med Genet 169C(1):6–22
Coucke PJ, Willaert A, Wessels MW, Callewaert B, Zoppi N, De Backer J et al (2006) Mutations in the facilitative glucosa transporter GLUT10 alter angionenesis and cause arterial tortuosity síndrome. Nat Genet 38(4):452–457
Faiyaz-Ul-Haque M, Zaidi SH, Al-Sanna N, Alswaid A, Momenah T, Kaya N et al (2009) A novel missense and a recurrent mutation in SLC2A10 gene of patients affected with arterial tortuosity syndrome. Atherosclerosis 203(2):466–471
Kocova M, Kacarska R, Kuzevska-Maneva K, Prijic S, Lazareska M, Dordoni C, Ritelli M, Colombi M (2018) Clinical variability in two Macedonian families with arterial tortuosity syndrome. Balkan J Med Genet 21(1):47–52
Morris SA (2015) Arterial tortuosity in genetic arteriopathies. Curr Opin Cardiol 30(6):587–593
Naunheim MR, Walcott BP, Nahed BV, MacRae CA, Levinson JR, Ogilvy CS (2011) Arterial tortuosity syndrome with multiple intracranial aneurysms: a case report. Arch Neurol 68(3):369–371
Palanca Arias D, Ayerza Casas A, Gutiérrez Alonso C, Jiménez ML (2020) Arterial tortuosity syndrome in a paediatric patient. An Pediatr (Barc) 92(2):111–112
Ritelli M, Chiarelli N, Dordoni C, Reffo E, Venturini M, Quinzani S et al (2014) Arterial Tortuosity Syndrome: homozygosity for two novel and one recurrent SLC2A10 missense mutations in three families with severe cardiopulmonary complications in infancy and a literature review. BMC Med Genet 15:122
Rodríguez-Capitán J, Macías-Benítez M, Conejo-Muñoz L, Cordero-Aguilar A, López-Salguero R, Pérez-Villardón B (2020) Arterial tortuosity syndrome: a late and unexpected diagnosis and description of a novel likely pathogenic mutation. Rev Esp Cardiol (Engl Ed) 73(6):504–506
Acknowledgments
Not applicable.
Funding
Not applicable.
Author information
Authors and Affiliations
Contributions
DP and SI conceived of the presented idea. DP wrote the manuscript with support from AA. LJ, ML contributed to the design and implementation of the research, to the analysis of the results and to the writing of the manuscript. MC and VE authors contributed to the final version of the manuscript. VE designed the figure. SI contributed to the study and genetic analysis. CG contributed to the study and image analysis. All authors read and approved the final manuscript.
Corresponding author
Ethics declarations
Ethics approval and consent to participate
Not applicable.
Consent for publication
We have a copy of our institutional consent to send at any stage. All data generated or analyzed during this study are included in this published article [and its Additional files].
Competing interests
The authors declare that they have no competing interests.
Additional information
Publisher's Note
Springer Nature remains neutral with regard to jurisdictional claims in published maps and institutional affiliations.
Rights and permissions
Open Access This article is licensed under a Creative Commons Attribution 4.0 International License, which permits use, sharing, adaptation, distribution and reproduction in any medium or format, as long as you give appropriate credit to the original author(s) and the source, provide a link to the Creative Commons licence, and indicate if changes were made. The images or other third party material in this article are included in the article's Creative Commons licence, unless indicated otherwise in a credit line to the material. If material is not included in the article's Creative Commons licence and your intended use is not permitted by statutory regulation or exceeds the permitted use, you will need to obtain permission directly from the copyright holder. To view a copy of this licence, visit http://creativecommons.org/licenses/by/4.0/.
About this article
Cite this article
Palanca Arias, D., Ayerza Casas, A., Clavero Adell, M. et al. Arterial tortuosity syndrome (variants in SLC2A10 gene) in two pediatric patients in the same city of Spain: a case report. Bull Natl Res Cent 46, 247 (2022). https://doi.org/10.1186/s42269-022-00938-2
Received:
Accepted:
Published:
DOI: https://doi.org/10.1186/s42269-022-00938-2