Abstract
Background
Multidrug-resistant tuberculosis (MDR-TB) presents persistent global health challenges, characterized by low treatment success rates among patients enrolled for treatment. The World Health Organization recommends decentralization to improve outcomes. This study aims to assess predictors of mortality among MDR-TB patients after decentralization of services in Tanzania. This was a retrospective cohort study involving all MDR-TB patients enrolled in treatment in all 31 regions in Tanzania from 2017 to 2019. The overall mortality rate among MDR-TB patients was calculated using the incidence rate. Additionally, independent factors of MDR-TB mortality were determined using multivariable cox proportional hazards models.
Results
The study followed 985 patients for a total of 12,929 months. During this time, it found that approximately 12 out of every 1000 patients died each month. Specifically, the death rates were about 18 out of 1000 patients at 6 months, 8 out of 1000 at 12 months, and 7 out of 1000 at 24 months. Patients who had both MDR-TB and HIV, as well as those who were malnourished, had a lower chance of surviving at 6, 12, and 24 months. Malnourished patients had almost three times the risk of dying [adjusted hazard ratio (aHR) 2.96, with a 95% confidence interval (CI) of 2.10–4.19], while those with HIV had nearly double the risk [aHR 1.91, with a 95% CI of 1.37–2.65].
Conclusion
In summary, our study on MDR-TB patient outcomes in Tanzania between 2017 and 2019 reveals a pattern of high mortality rates within the first 6 months of treatment. Furthermore, malnutrition and HIV co-infection were found to be significant predictors of mortality. To decrease mortality, it is crucial to closely monitor patients during the initial 6 months of treatment, especially those who are malnourished or co-infected with HIV, and ensure they receive appropriate and timely care. Additionally, further investigation is needed to find out what may be contributing to possible rise in mortality rate.
Similar content being viewed by others
Background
Multidrug-resistant tuberculosis (MDR-TB), is defined as a form of Tuberculosis (TB) that is resistant to at least isoniazid and rifampicin the two most effective TB drugs. This poses a pressing public health issue, as indicated by the increase in the global number of new TB and MDR-TB cases reported in 2022 compared to the preceding 2 years (WHO) World Health Organization 2023).
In 2022, the World Health Organization (WHO) reported a global total of 176,586 cases of MDR-TB that were detected among bacteriologically confirmed pulmonary TB cases who were tested for resistance. Additionally, only 63% of cohort of patients who began treatment in 2020 had successful outcomes, with a significant portion of unsuccessful outcome contributed by fatalities (WHO) World Health Organization 2023; 2024).
Tanzania remains among the list of 30 countries globally with a high burden of TB and TB/HIV (i.e., condition where a person is infected with both TB and HIV at the same time), yet it has a low burden of MDR-TB (WHO) World Health Organization 2023; 2021). In 2019, the prevalence of MDR-TB among new and retreatment patients in the country was reported to be 0.97% and 13% respectively (Ministry of Health Tanzania 2020). While these statistics suggest a lower prevalence of MDR-TB compared to other high-burden countries, there has been a continuous increase in number of patients from 167 in 2017 to 534 in 2019. Moreover, the most recent data from a group of patients who began treatment in 2019 indicates a decline in the rate of treatment success, dropping from 83% in 2017 to 73% in 2019. This decline has been linked to increase in patient mortality (Tanzania 2023; Nkiligi et al. 2023).
The World Health Organization (WHO) has taken steps to improve MDR-TB patient treatment outcome which includes updating management guidelines and recommending decentralization of services (WHO 2019, 2022, 2011). Experience from studies conducted in sub-Saharan Africa have generally favored decentralization as it shows that decentralizing MDR-TB services has improved access to care, with more patients being tested, diagnosed and enrolled in treatment. Furthermore, it has been observed to be more cost-effective, particularly in settings with limited resources, resulting in higher rates of treatment success and improved patient retention in care (Achayo 2022).
In line with WHO recommendations, Tanzania began the decentralization of MDR-TB services in 2016 while also introducing new treatment guidelines (Ministry of Health Tanzania 2020). This decentralization efforts involved increasing treatment facilities to provide care to MDR-TB patients across the country, thus increasing accessibility. Moreover, capacitating the regions with molecular test to facilitate detection of drug resistance. Despite these commendable measures, treatment outcome of MDR-TB is still not satisfying hence the need for further investigation to comprehend the factors contributing to the observed trend.
This study therefore aimed at assessing predictors of mortality among MDR-TB patients after decentralization of services in Tanzania. Correct identification of predictors of mortality serves as a cornerstone for formulating strategies to further enhance MDR-TB patient care and prevent premature deaths attributed to drug-resistant tuberculosis.
Specific objectives
-
1.
To determine mortality rates of MDR-TB patients after decentralization of services in Tanzania from 2017 to 2019
-
2.
To identify predictors of mortality of MDR-TB patients after decentralization of services in Tanzania from 2017 to 2019
Methods
Study design
A retrospective cohort study was conducted involving MDR-TB patients who had been enrolled in second-line treatment from January 2017 to December 2019. The follow-up period was between June 2017 and December 2021.
Study area
This study included all 31 regions of Tanzania. As of 2021, 304 out of 8446 total health facilities in the country provide management of MDR-TB patients. Furthermore, there are 1752TB diagnostic centers throughout the country that comprises microscopes, line probe assay (LPA) and GeneXpert (i.e., advanced diagnostic tool for rapid and accurate diagnosis of TB, including drug-resistant strains).
Study participants
Study participants included patients of any age with confirmed resistance to rifampicin and/or isoniazid on drug susceptibility testing who were initiated on the standard MDR-TB treatment regimen from January 2017 to December 2019.
Inclusion and exclusion criteria
Inclusion
The inclusion criteria were all MDR-TB patients who were enrolled on second line treatment available in the NTLP data from January 2017 to December 2019.
Exclusion
Exclusion criteria were inconsistent treatment dates/missing dates to treatment initiation, extensively drug-resistant TB (XDR-TB) and duplicates.
Variable
Dependent variable
The dependent variable in this study was MDR-TB mortality. This was a binary outcome where we had a group of those patients who died and another group patient who were alive which included patients who were cured, completed treatment, lost to follow up and those failed treatment.
Independent variables
Independent variables included demographic characteristics and clinical factors. Demographic characteristics included sex and age. Clinical factors included previous history of TB, nutritional status, HIV status, resistance pattern, regime, and drug adverse event.
Power of the study
Power of the study was calculated using the formula for estimation of power for cohort studies as shown below (Sahai and Khurshid 1996)
The notations for the formulae are:
Δ = difference of risk of disease between exposed group and non-exposed group.
κ = ratio of sample size: non-exposed group/exposed group.
p1 = risk of disease among the exposed group.
p2 = risk of disease among the non-exposed group.
p = (p1*n1 + p2*n2)/(n1 + n2).
q = 1 − p.
n1 = available sample size among the exposed group.
We used the following assumptions;
-
A total of estimated 985 MDR-TB cases were enrolled in treatment from 2017 to 2019
-
We took comorbidity as a variable for comparisons
-
MDR-TB patient without comorbidity was used as un exposed group where there were 583 cases reported
-
MDR-TB patient with comorbidity was used as exposed group where there were 402 cases reported
-
Calculating K, we obtained 0.7
-
n1 = 402, n2 = 583
-
So, in this study our outcome of interest was mortality, hence risk in exposed group is 22.9% and risk among un exposed group is 10.5%
-
Power was calculated using open epi software version 3.01 which can be assessed at https://www.openepi.com/Menu/OE_Menu.htm.
-
Power of the study was 100%
Operational definitions
Centralized setting: Defined as inpatient treatment and care provided solely by centres or teams specialized in drug-resistant TB for the duration of the intensive phase of therapy or until culture or smear conversion.
Cured: Treatment completed as recommended by the national policy without evidence of failure and three or more consecutive cultures taken at least 30 days apart are negative after the intensive phase.
Decentralized setting: Defined as care provided in the local community where the patient lives, by non-specialized or peripheral health centres, by community health workers or nurses, non-specialized doctors, community volunteers or treatment supporters.
Extensive drug resistance Tuberculosis: Resistance to any fluoroquinolone and to at least one of three second-line injectable drugs (capreomycin, kanamycin and amikacin) in addition to multidrug resistance.
Lost to follow up: Treatment interrupted for 2 consecutive months or more.
Malnutrition: Refers to deficiencies or excess in nutrient intake which consist of both undernutrition and overweight/obesity (i.e., Body Mass Index (BMI) below 18.5 or above 24.9).
Multidrug resistance Tuberculosis: Resistance to at least both isoniazid and rifampicin.
Normal nutrition status: Refers to a BMI within a healthy range between 18.5 and 24.9
Polyresistance: Resistance to more than one first-line anti-TB drug, other than both isoniazid and rifampicin.
Rifampicin Resistance: Resistance to rifampicin detected using phenotypic or genotypic methods, with or without resistance to other anti-TB drugs.
TB/HIV: Refers to the co-infection of tuberculosis and HIV.
Treatment completed: Treatment completed as recommended by the national policy without evidence of failure BUT no record that three or more consecutive cultures taken at least 30 days apart are negative after the intensive phase.
Treatment failed: Treatment terminated or need for permanent regimen change of at least two anti-TB drugs because of: lack of conversion by the end of the intensive phase or bacteriological reversion in the continuation phase after conversion to negative or evidence of additional acquired resistance to fluoroquinolones or second-line injectable drugs or adverse drug reactions (ADRs).
Treatment outcome: Refers to the final result of a patient's treatment for tuberculosis infection.
Treatment success: Cured + treatment completed.
Dataset description and data abstraction procedure
Data was obtained from NTLP database, information usually recorded on case-based system through available Electronic Tuberculosis and Leprosy System (ETL). This electronic system acts as surveillance tool for monitoring performance of different NTLP program indicators. The system also monitors progress on treatment of TB, MDR-TB and Leprosy patients from when registered throughout to treatment completion.
Data collection starts at the primary health facilities where patients go for treatment after presenting with symptoms. Normally, clinicians make a diagnosis by matching patient’s symptoms and signs to form a preliminary diagnosis. Then, a sputum sample is requested for gene expert testing to confirm the presence of Mycobacterium tuberculosis and drug resistance. Patients with confirmed diagnoses are then connected to TB clinics for treatment initiation by trained Directly Observed Treatment (DOT) clinicians and nurses. In TB clinics, MDR-TB patients identified, their information is recorded in the normal TB register (TB 03), as well as the MDR-TB register, both of which are accessible at the facility. Subsequently, the notification is sent to the District TB and Leprosy Coordinator (DTLC). The DTLC or trained facility staffs registers the patient in the electronic TB register (ETL) and transfers them to the MDR-TB register. As soon as the patient is registered in the system, this information is immediately reflected at all levels (i.e., national and sub-national level). To ensure that patient information is both accurate and complete, the ETL system mandates that all fields be filled out before proceeding to the next stage of registration. Additionally, regional quarterly data review meetings are convened to further compliment accuracy and completeness.
Data was extracted from the ETL system in form of an MS Excel.
Data management and analysis
Data extracted from ETL in form of MS Excel was then cleaned and analysed using Stata 15.0.
The demographic and clinical variables were presented using frequencies/proportions for all categorical data, while measures of central tendency and dispersion were used for continuous variables.
The overall mortality rate among MDR-TB patients was calculated using the incidence rate. Additionally, mortality rates at different follow-up times i.e., 6 months, 12 months, and 24 months were estimated (The 6-month period provides insights into the initial response to treatment, while the 12-month period allows for a more comprehensive evaluation of treatment outcomes. The 24-month follow-up is essential for assessing the long-term outcomes of MDR-TB treatment). The mortality rate was also analysed based on the demographic and clinical characteristics of the MDR-TB patients. To visualize regional-specific mortality rates, the Quantum Geographic Information System (QGIS) software version 3.26.3 was used to create a map.
To examine independent predictors of MDR-TB mortality, multivariable cox proportional hazards models was utilized after verification of proportional hazard assumptions. All eight explanatory variables met the proportional hazard assumption after running a Schoenfeld’s test. Bivariate analysis was conducted for all eight variables, which were later included in the multivariable model to adjust for confounding. The likelihood ratio test was used to get the overall p value for each variable. The adjusted hazard ratio and its 95% confidence interval were presented. Factors that exhibited statistical significance (P value ≤ 0.05) was used to predict death in MDR-TB patients.
Results
Study participants included patients of any age with confirmed resistance to rifampicin and/or isoniazid on drug susceptibility testing who were initiated on the standard MDR-TB treatment regimen from January 2017 to December 2019.
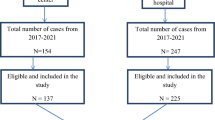
From January 2017 to December 2019, 1,001 records were found in the NTLP database. Among these, 16 (1.6%) were omitted from the study analysis because they missed the treatment initiation date, were XDR-TB patients, were duplicated or were not evaluated at the end of treatment. The final analysis included 985(98.4%) patients (Fig. 1). Patients who have been included in the study and those who have been excluded from it share similar demographic and clinical characteristics.
Baseline demographic and clinical characteristics
A total of 985 cases were included in the analysis, with male patients accounting for the majority at 647 (65.7%). The majority of patients, 726 (73.7%), were between the ages of 15–49 years with a mean age of 40 (SD = 15.3) years. Furthermore, 594 (60.3%) of the cases were new patients, and 778 (79.1%) had rifampicin resistance (RR) as their only form of resistance (Table 1).
Mortality rate of MDR TB patients
The study followed 985 patients for 12,929 person-months and found a crude mortality rate of 11.83 per 1000 person-months (95% Confidence Interval (CI), 10.10–13.87). At the 6-month follow-up point, we observed a relatively high mortality rate of 17.74 (95% CI 14.54–21.64) in comparison to the mortality rates at other follow-up times (Table 2).
The regional specific mortality rate from 2017 to 2019 showed that the Dodoma region was the leading region with a high mortality rate of 28 deaths per 1000 person-months (95% CI 13.68–60.20). Conversely, the lowest mortality rate was observed in the Mbeya region, with only 6 deaths per 1000 person-months (95% CI 1.54–24.65). However, during the period analysed, Pemba Island and three regions i.e. Songwe, Rukwa, and Katavi did not report any deaths of MDR-TB patients (Fig. 2).
Survival probability of MDR TB patients
HIV-positive patients had lower survival probabilities at 6, 12, and 24 months compared to HIV-negative patients. Malnourished MDR-TB patients also had lower survival probabilities compared to those with normal nutrition status at the same time intervals (Figs. 3 and 4).
Predictors of mortality among MDR-TB patients
During the follow-up period, MDR-TB patients who are malnourished had 2.96 hazard of dying from MDR-TB compared to patients with normal nutrition status [aHR 2.96 (95% CI 2.10–4.19)]. Additionally, MDR-TB HIV co-infected patients had 1.91 hazards of dying from MDR-TB compared to MDR-TB patients who are HIV negative [aHR 1.91 (95% CI 1.37–2.65)] (Table 3).
Discussion
The study revealed that, mortality rate was higher during the first 6 months of treatment, then progressively decreased until treatment was completed. Additionally, the study also identified that malnutrition and HIV coinfection were significant predictors of mortality.
In the current study it was observed that during intensive phase of treatment, mortality rate was considerably greater compared to the evaluation done at 12 and 24 months. Our findings were comparable to other studies done in Africa which also reported high death rate in the first six month of treatment (Matambo et al. 2021; Muluneh et al. 2021; Kassa et al. 2020). In other settings, the death rate is determined by capacity of health care facilities, social economic status and presence of comorbidity by the time of treatment initiation (Kassa et al. 2020). Since most African countries have similar social and economic statuses, these factors may be the same in our situation. However, additional investigation is necessary to ascertain whether this pattern is influenced by patient-related factors or problems within the healthcare system.
Moreover, our study found that among enrolled MDR-TB patients for treatment, mortality rate increases with age with patients aged 50 years and older having the greatest mortality rate. This finding is consistent with previous study conducted in Tanzania that found that the older the individual, the higher the mortality rate (Myemba et al. 2021). Contrary to our finding, a study conducted in China found that mortality rates were higher in younger age groups and decreased gradually with increasing age (Wang et al. 2020). The conflicting findings in China may be attributed to the study being carried out at a single location, suggesting that the results may only be applicable to that specific site and not generalizable. Moreover, our study revealed a higher rate of mortality in male individuals and those who had previous history of TB treatment, a trend that is consistent with similar studies conducted in Tanzania and China. Additionally, the study showed that HIV positive patients had a mortality rate twice as high as those without HIV, a finding that was also reflected in studies carried out in Tanzania and China, where higher mortality rates were observed in HIV positive patients and those with other underlying health conditions, respectively (Myemba et al. 2021; Wang et al. 2020).
The results of this study revealed that patient baseline nutrition status of patients is a significant factor in predicting mortality among individuals with MDR-TB. It has been demonstrated that malnourished MDR-TB patients at the time of diagnosis have higher relapse rates and shorter survival times during treatment (Wagnew et al. 2023; Sayem et al. 2020; Ockenga et al. 2023). The study shows that, individuals with malnutrition had almost thrice-as-high risk of death when compared to those with normal nutrition status. This result was in line with previous studies that also demonstrated a high correlation between mortality and undernutrition among MDR-TB patients (Kassa et al. 2020; Wagnew et al. 2023; Alemu et al. 2021). Our study showed that, MDR-TB patients who are HIV coinfected to have almost twice the risk of mortality as compared to those who are HIV negative. In previously conducted study, risk of death in MDR-TB HIV coinfected patients ranged from 1.34 to 5.70 fold in MRD-TB HIV coinfected (Matambo et al. 2021; Muluneh et al. 2021; Alemu et al. 2021; Ketema et al. 2020; Habimana et al. 2021). One possible reason for the increased risk of mortality in this group of patients could be the immunosuppression caused by both conditions.
This study utilized secondary data, which has its limitations. Several variables, such as Cluster of Differentiation 4 (CD4) counts, history of alcohol use, and cigarette smoking, which were among other predictors of mortality in MDR-TB patients were not present in the database hence potentially introducing confounding biases that were not addressed or quantified in this analysis. Despite this drawback, the study offers fresh perspectives that will help researchers better understand the risk factors that affect patient mortality.
Conclusions
In summary, our study on MDR-TB patient outcomes in Tanzania between 2017 and 2019 reveals a pattern of high mortality rates within the first 6 months of treatment. Furthermore, malnutrition and HIV co-infection were found to be significant predictors of mortality. To decrease mortality, it is crucial to closely monitor patients during the initial 6 months of treatment, especially those who are malnourished or co-infected with HIV, and ensure they receive appropriate and timely care. Lastly, further investigation is needed to find out what may be contributing to possible rise in mortality rate.
Availability of data and materials
Data generated during and/or analysed during the current study are available from the corresponding authors upon reasonable request.
Abbreviations
- aHR:
-
Adjusted hazard ratio
- ART:
-
Anti-retroviral therapy
- CDC:
-
Centers for disease control and prevention
- CD4:
-
Cluster of differentiation 4
- CI:
-
Confidence interval
- DOT:
-
Direct observed treatment
- DR:
-
Drug resistance
- DTLC:
-
District TB and leprosy coordinator
- ETL:
-
Electronic TB and leprosy system
- HIV:
-
Human immunodeficiency virus
- MDR-TB:
-
Multidrug-resistance tuberculosis
- MUHAS:
-
Muhimbili University of Health and Allied Science
- NTLP:
-
National tuberculosis and leprosy program
- QGIS:
-
Quantum geographic information system
- RR:
-
Rifampicin resistance
- SD:
-
Standard deviation
- TB:
-
Tuberculosis
- TB/HIV:
-
Tuberculosis and human immunodeficiency virus
- TFELTP:
-
Tanzania field epidemiology and laboratory training program
- WHO:
-
World Health Organization
- XDR_TB:
-
Extensive drug resistance tuberculosis
References
Achayo M (2022) The effect of decentralized services on multidrug-resistant tuberculosis care in Sub-Saharan Africa: a systematic review. https://doi.org/10.21203/rs.3.rs-2077078/v1
Alemu A, Bitew ZW, Worku T, Gamtesa DF, Alebel A (2021) Predictors of mortality in patients with drug-resistant tuberculosis: a systematic review and meta-analysis. PLoS ONE 16(6):1–24. https://doi.org/10.1371/journal.pone.0253848
Habimana DS et al (2021) Predictors of rifampicin-resistant tuberculosis mortality among hiv-coinfected patients in Rwanda. Am J Trop Med Hyg 105(1):47–53. https://doi.org/10.4269/ajtmh.20-1361
Kassa GM et al (2020) Predictors of mortality among multidrug-resistant tuberculosis patients in central Ethiopia; a retrospective follow-up study. Epidemiol Infect. https://doi.org/10.1017/S0950268820002514
Ketema DB, Alene M, Assemie MA, Yismaw L, Merid MW (2020) Risk factors for mortality among drug-resistant tuberculosis patients registered for drug-resistant treatment in Amhara region, Ethiopia: a historical cohort study. Arch Public Heal 78(1):1–9. https://doi.org/10.1186/s13690-020-00448-5
Matambo R et al (2021) Predictors of mortality and treatment success of multi-drug resistant and rifampicin resistant tuberculosis in Zimbabwe: a retrospective cohort analysis of patients initiated on treatment during 2010 to 2015. Pan Afr Med J 39:1–16. https://doi.org/10.11604/pamj.2021.39.128.27726
Ministry of Health Tanzania. Report of the United Republic of Tanzania National Tuberculosis and Leprosy Programme External Review. Dodoma, 2020. [Online]. Available: https://ntlp.go.tz/site/assets/files/1075/joint_external_program_review_report_2020_final.pdf
Muluneh MA, Zeru AB, Derseh BT, Molla Kebede A (2021) Survival status and predictors of mortality among multidrug-resistant tuberculosis patients in Saint Peter’s Specialized hospital, Addis Ababa, Ethiopia. Can J Infect Dis Med Microbiol. https://doi.org/10.1155/2021/6696199
Myemba DT et al (2021) Clinical characteristics and treatment outcomes of patients with MDR tuberculosis in Dar es Salaam region, Tanzania. JAC-Antimicrob Resist. https://doi.org/10.1093/jacamr/dlaa108
Nkiligi E et al. (2023) National tuberculosis and leprosy programme epidemiological review in the United Republic of Tanzania. Ministry of Health [Online]. Available: https://ntlp.go.tz/site/assets/files/1196/epi-review_jan2023_-_united_republic_of_tanzania_-_final_report.pdf
Ockenga J et al (2023) Tuberculosis and malnutrition: The European perspective. Clin Nutr 42(4):486–492. https://doi.org/10.1016/j.clnu.2023.01.016
Sahai H, Khurshid A (1996) Formulae and tables for the determination of sample sizes and power in clinical trials for testing differences in proportions for the two-sample design: a review. Stat Med 15(1):1–21. https://doi.org/10.1002/(sici)1097-0258(19960115)15:1%3c1::aid-sim134%3e3.3.co;2-5
Sayem MA, Hossain MG, Ahmed T, Hossain K, Saud ZA (2020) Effect of nutritional support on treatment of multi-drug resistant tuberculosis in Rajshahi Division, Bangladesh. J Tuberc Res 08(04):223–236. https://doi.org/10.4236/jtr.2020.84020
Tanzania National Tuberculosis and leprosy Programme. Report of the Tanzania Mid-Term Review of the National Tuberculosis and Leprosy Programme Strategic Plan VI, 1 Table of Contents. February 2023, p. 40, 2023, [Online]. Available: https://ntlp.go.tz/site/assets/files/1228/report_of_tz_mtr_of_ntlp_sp_vi_2.pdf
Wagnew F, Alene KA, Kelly M, Gray D (2023) The effect of undernutrition on sputum culture conversion and treatment outcomes among people with multidrug-resistant tuberculosis: a systematic review and meta-analysis. Int J Infect Dis 127:93–105. https://doi.org/10.1016/j.ijid.2022.11.043
Wang J et al (2020) Survival of patients with multidrug-resistant tuberculosis in central China: a retrospective cohort study. Epidemiol Infect. https://doi.org/10.1017/S0950268820000485
WHO consolidated guidelines on drug-resistant tuberculosis treatment. Geneva: World Health Organization (2019). Licence: CC BY-NC-SA 3.0 IGO
WHO consolidated guidelines on tuberculosis. Module 4: treatment - drug-resistant tuberculosis treatment, 2022 update. Geneva: World Health Organization; 2022. Licence: CC BY-NC-SA 3.0 IGO
World Health Organization (WHO) (2011). Guidelines for the programmatic management of drug-resistance tuberculosis. World Heal. Organ., pp. 1–44, [Online]. Available:https://iris.who.int/bitstream/handle/10665/44597/9789241501583_eng.pdf?sequence=1
World Health Organization (WHO) Global Tuberculosis Report (2023), vol. 4, no. 1. World Health Organization, 2023. [Online]. Available: https://www.who.int/teams/global-tuberculosis-programme/tb-reports/global-tuberculosis-report-2023
World Health Organization (WHO), Drug-resistant TB: treatment enrolment, coverage and outcomes. Accessed: May 15, 2024. [Online]. Available: https://www.who.int/teams/global-tuberculosis-programme/tb-reports/global-tuberculosis-report-2023/tb-diagnosis---treatment/drug-resistant-tb-treatment
World Health Organization (WHO). WHO global lists of high burden countries for TB, multidrug/rifampicin-resistant TB (MDR/RR-TB) and TB/HIV, 2021–2025, pp. 1–13, 2021, [Online]. Available: https://www.who.int/tb/publications/global_report/high_tb_burdencountrylists2016-2020.pdf
Acknowledgements
We acknowledge Muhimbili University of Health and Allied Science, Tanzania Ministry of Health and Centers for Disease Control and Prevention (CDC) through Tanzania Field Epidemiology and Laboratory Training Program (TFELTP) for their support while conducting this study.
Funding
This work is supported by the United States President’s Emergency Plan for AIDS Relief (PEPFAR) through the Centre of Excellence in Health Monitoring and Evaluation, Mzumbe University under the U.S Centers for Disease Control and Prevention (CDC), Project Cooperative Agreement No: NU2GGH002292. Additionally, they provide support in the training of residents in Tanzania's Field Epidemiology and Laboratory Training Program.
Author information
Authors and Affiliations
Contributions
All authors have read and approved the manuscript and take responsibility for the integrity of the data and the accuracy of the data analysis. GM, AH, WM, GK conceptualized and designed the study; GM collected data; GM, AH, WM, GK performed data cleaning and analysis; GM, AH, WM, VM, RB, EN, IL, RK, GK drafted, reviewed and edited the manuscript. All authors read and approved the final manuscript.
Corresponding author
Ethics declarations
Ethics approval and consent to participate
Muhimbili University of Health and Allied Science (MUHAS) approved the study protocol with approval number MUHAS-REC-12-2022-1468. Permission to use MDR-TB data was obtained from the Program Manager of the National TB and Leprosy Programme (NTLP) in the Ministry of Health. Unique number was used in place of names to maintain confidentiality.
Consent for publication
Not applicable.
Competing interests
All authors declare that they have no commercial or other associations that may pose a conflict of interest.
Additional information
Publisher's Note
Springer Nature remains neutral with regard to jurisdictional claims in published maps and institutional affiliations.
Rights and permissions
Open Access This article is licensed under a Creative Commons Attribution 4.0 International License, which permits use, sharing, adaptation, distribution and reproduction in any medium or format, as long as you give appropriate credit to the original author(s) and the source, provide a link to the Creative Commons licence, and indicate if changes were made. The images or other third party material in this article are included in the article's Creative Commons licence, unless indicated otherwise in a credit line to the material. If material is not included in the article's Creative Commons licence and your intended use is not permitted by statutory regulation or exceeds the permitted use, you will need to obtain permission directly from the copyright holder. To view a copy of this licence, visit http://creativecommons.org/licenses/by/4.0/.
About this article
Cite this article
Mrema, G., Hussein, A., Magoge, W. et al. Predictors of mortality among multidrug-resistant tuberculosis patients after decentralization of services in Tanzania from 2017 to 2019: retrospective cohort study. Bull Natl Res Cent 48, 79 (2024). https://doi.org/10.1186/s42269-024-01235-w
Received:
Accepted:
Published:
DOI: https://doi.org/10.1186/s42269-024-01235-w