Abstract
Background
Hypertension is a prevalent and complex disease that is increasingly recognized to be influenced by the gut microbiome and its metabolites. Understanding the relationship between gut microbial metabolites and blood pressure regulation could provide new therapeutic avenues.
Main body
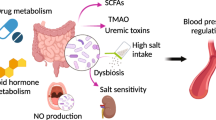
This review examines the role of key microbial metabolites—short-chain fatty acids, trimethylamine N-oxide, tryptophan derivatives, polyamines, bile acids, and phenylacetylglutamine—in blood pressure regulation. Short-chain fatty acids, produced through dietary fiber fermentation, can lower blood pressure by modulating immune responses and reducing inflammation. Elevated trimethylamine N-oxide levels are associated with increased cardiovascular risk and hypertension, influencing cholesterol metabolism and promoting atherosclerosis. Tryptophan derivatives interact with vascular and renal functions to modulate blood pressure. Polyamines affect blood pressure regulation through their impact on nitric oxide synthesis and vascular tone. Bile acids influence blood pressure via gut microbiota modulation and activation of metabolic receptors. Phenylacetylglutamine has been linked to hypertension through its effects on platelet hyperactivity and thrombosis. Therapeutic approaches targeting these metabolites, including probiotics, prebiotics, fecal microbiota transplantation, dietary interventions, and polyphenols, have shown varying degrees of success. Probiotics and prebiotics promote the growth of beneficial gut bacteria and may lower blood pressure. Dietary interventions, such as the Mediterranean diet, positively affect blood pressure and cardiovascular health by modulating the gut microbiota. Polyphenols, known for their antioxidant properties, are associated with blood pressure reductions and improved vascular function. Fecal microbiota transplantation shows promise in restoring gut microbial balance and improving metabolic health, potentially influencing blood pressure regulation.
Conclusion
The review highlights the significant role of gut microbial metabolites in regulating blood pressure, offering new avenues for hypertension management. Key metabolites, including short-chain fatty acids, trimethylamine N-oxide, and bile acids, play critical roles in blood pressure modulation. Therapeutic strategies targeting these metabolites, such as probiotics, prebiotics, and dietary interventions, hold promise, though further research is needed to fully understand their mechanisms and optimize their use. Advancing microbiota-based interventions through large-scale studies and exploring personalized therapies will be essential for developing effective treatments in hypertension management.
Similar content being viewed by others
Background
Hypertension, or high blood pressure, is a major public health concern globally. It is a significant risk factor for cardiovascular diseases and a leading cause of mortality and morbidity. The World Health Organization reports that approximately 1.28 billion people worldwide suffer from hypertension, with a significant number residing in low- and middle-income countries where awareness, treatment, and control are suboptimal (Available from 2023). Despite the availability of numerous antihypertensive drugs, many patients remain inadequately controlled, highlighting the need for novel therapeutic approaches (Ghatage et al. 2021). Recent research has illuminated the potential role of the gut microbiome in influencing blood pressure (BP) regulation.
There is a lot of data regarding the functions microorganisms play in human health and disease. Certain bacteria form symbiotic relationships with their human hosts that aid in physiological maintenance, whereas other bacteria are harmful and have a role in the development and pathogenesis of diseases (Fan and Pedersen 2021). The gut microbiota, comprising trillions of microorganisms, produces metabolites that can modulate host physiology (Fan and Pedersen 2021). These metabolites, including short-chain fatty acids (SCFAs) like acetate and butyrate, have been shown to exert BP-lowering effects through mechanisms such as vasodilation and anti-inflammatory actions (Wu et al. 2021; Xu and Marques 2022; Olalekan et al. 2024). Trimethylamine-N-oxide (TMAO), derived from dietary choline and carnitine, has been linked to increased cardiovascular risk, while tryptophan derivatives and polyamines have roles in modulating vascular function and inflammation. Bile acids and Phenylacetylglutamine (PAGln) also influence BP through their metabolic effects and interactions with host physiology.
Hypertension, characterized by elevated BP, remains a leading cause of mortality worldwide, with a significant portion of patients failing to achieve adequate control despite available therapeutic interventions. In this context, exploring novel interventions, such as targeting gut microbial metabolites, holds promise for advancing hypertension management (Xu and Marques 2022; Jama and Marques 2023). Studies have demonstrated the BP-lowering effects of certain gut microbial metabolites, such as acetate and butyrate, yet their full therapeutic potential remains underexplored (Jama and Marques 2023). Hence, this review aims to delve into the intricate relationships between these metabolites and hypertension, examining how they contribute to BP regulation and evaluating various therapeutic approaches. By doing so, the review seeks to highlight potential new strategies for enhancing hypertension management and improving patient outcomes, ultimately advancing our understanding of the gut microbiome’s role in cardiovascular health.
Gut microbiota composition and dysbiosis in hypertension
The human gut houses a diverse array of microorganisms, collectively known as the gut microbiota, which actively participate in various physiological processes. These microorganisms metabolize the host’s dietary intake, yielding an array of metabolic byproducts such as TMAO, tryptophan catabolites, and short-chain fatty acids (SCFAs) (Rahman et al. 2023). Alongside their influence on the development of the host immune system, gut microbiota profoundly impacts host metabolism by facilitating the breakdown of complex carbohydrates, providing protection against pathogenic bacteria, and contributing to the synthesis of vital compounds like vitamins, SCFAs, and bile acids (Turroni et al. 2020). This intricate interplay between gut microbial metabolism and host health underscores the significant role of the gut microbiota in shaping overall host physiology and metabolism, highlighting how arterial hypertension, a prevalent chronic disease, is closely intertwined with metabolites secreted by intestinal bacteria, particularly SCFAs, TMAO, Tryptophan and Indole Derivatives, Polyamines, Bile Acids and Phenylacetylglutamine as detailed in Table 1 (Agus et al. 2021; Liu et al. 2020; Suta et al. 2023; Tokarek et al. 2023).
Notably, dysbiosis, characterized by an imbalance in microbial composition, has emerged as a critical factor in various disease states (Brüssow 2020). The colonization of gut microbiota commences shortly after birth, with Actinobacteria, Proteobacteria, and Firmicutes being the primary phyla observed during early childhood (Caballero-Flores et al. 2023). Alterations in gut microbiota composition, or dysbiosis, have been implicated in hypertension, with changes in microbial metabolite production being a notable consequence (Avery et al. 2021). Studies have demonstrated that hypertension is associated with dysregulation of gut microbiota, particularly under conditions of high salt diet-induced hypertension (Zheng et al. 2023). Furthermore, differences in the structure and composition of gut microbiota have been observed in hypertensive individuals, with specific microbial profiles correlating with the severity of hypertension (Muralitharan et al. 2020; Xu et al. 2021).
In individuals with hypertension, elevated levels of bacteria such as Eubacteriumxylanophilum, Eisenbergiella, and LachnospiraceaeUCG001 have been observed, contrasting with a decrease in beneficial bacteria like Alistipes, Phascolarctobacterium, Bilophila, and Butyricimonas, which are more abundant in normotensive individuals (Li et al. 2023). Certain bacterial taxa were linked to various aspects of BP regulation. Alistipes finegoldii and Lactobacillus spp. were only present in individuals with normal BP variability, mean BP surges (MBPS), and nighttime dipping. Conversely, Prevotella spp. and Clostridium spp. were associated with extreme dipping and individuals in the highest quartiles of BP standard deviation and MBPS (Dinakis et al. 2022). Metabolic disorders linked to dysbiosis frequently involve changes in gut microbiota composition and function. Specific microbiota-derived metabolites, such as bile acids, SCFAs, TMAO, and tryptophan derivatives, play a role in their development (Agus et al. 2021).
The pathogenesis of hypertension involves multifaceted factors encompassing genetics, environment, hormones, hemodynamics, and inflammation. Emerging evidence underscores the significant role of the gut microbiome in hypertension development and pathogenesis. Interventions targeting the gut microbiota, such as the use of probiotics, hold promise as adjunctive therapies in managing hypertension, underscoring the potential for leveraging gut microbial modulation in the management of cardiovascular diseases.
Microbiota-derived short-chain fatty acids (SCFAs) in hypertension
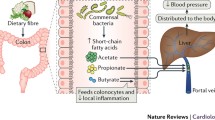
Microbiota-derived short-chain fatty acids (SCFAs), such as acetate, propionate, and butyrate, are essential metabolites produced in the gastrointestinal tract. They result from the bacterial fermentation of dietary fiber by microbes like Enterococcus, Bacteroidetes, Acidaminococcus, and Salmonella (Wu et al. 2021; Kaye et al. 2020). These SCFAs serve not only as energy sources for host cells but also as signaling molecules between the gut microbiota and extra-intestinal organs. They exert specific effects on cardiovascular health.
Butyrate, a short-chain fatty acid (SCFA) synthesized by gut bacteria such as Clostridium, Eubacterium, and Roseburia, is crucial in regulating blood pressure (BP) through various mechanisms. Butyrate induces vasodilation by activating G-protein-coupled receptors (GPR41 and GPR43) on vascular endothelial cells, which reduces vascular resistance and lowers BP (Onyszkiewicz et al. 2019). Additionally, butyrate exerts significant anti-inflammatory effects, mitigating systemic inflammation and oxidative stress—key contributors to hypertension development (Amiri et al. 2022). It also supports gut barrier integrity, preventing the translocation of pro-inflammatory substances into systemic circulation that could elevate BP (Xia et al. 2023). Furthermore, butyrate’s effects are modulated by its influence on serotonin (5-HT) release from enteroendocrine cells (EECs). Elevated 5-HT levels in the gut can enhance the gut-brain axis signaling, which may contribute to its BP-regulating effects by altering central nervous system responses (Cookson 2021).
Gut-derived acetate, produced by the fermentation of dietary fibers by specific gut bacteria such as Faecalibacterium and Roseburia, also plays a significant role in BP regulation. Acetate not only serves as a substrate for butyrate-producing bacteria, thereby indirectly influencing BP through butyrate production, but it also has direct effects on BP. Acetate can induce vasodilation and reduce vascular resistance, contributing to lower BP levels (Cookson 2021; Poll et al. 2020). Additionally, acetate increases the release of serotonin (5-HT) from EECs in the gut. This elevated 5-HT can further influence central BP regulation by modulating gut-brain axis signaling, which complements butyrate’s actions on BP (Cookson 2021). Acetate’s role in reducing mean arterial pressure (MAP) and heart rate (HR) is also significant; it directly decreases cardiac contractility and modulates the autonomic nervous system. This modulation is evidenced by the attenuation of HR reduction when β-1 adrenergic receptor antagonists and sympathomimetics are used, reflecting its influence on sympathetic tone (Poll et al. 2020). Overall, a balanced gut microbiota that produces both butyrate and acetate is essential for effective BP management and cardiovascular health.
Propionate, produced by bacteria including Veillonellaceae and Prevotella, exhibits anti-inflammatory effects and protects against cardiovascular diseases. It reverses the imbalance between regulatory and effector T cells caused by hypertension, promoting positive effects on cardiac remodeling (Bartolomaeus et al. 2019). Propionate, supplemented along with acetate has been show to play a vital role in regulating BP and cardiovascular health. It reduces cardiac hypertrophy, fibrosis, vascular dysfunction, and hypertension by lowering systemic inflammation and decreasing immune cell infiltration in the heart (Lin et al. 2022). Propionate activates G-protein-coupled receptors (GPR41 and GPR43) on cardiac fibroblasts, inhibiting myofibroblast formation and collagen production, thus preventing fibrosis and extracellular matrix disarray. Additionally, acetate also contributes to these beneficial effects, further improving heart function under pressure-overload stress (Lin et al. 2022).
SCFAs levels in circulation may be a critical determinant of their antihypertensive potency. In a cross-sectional study, hypertensive (HT) individuals had higher levels of short-chain fatty acids (SCFAs) in their feces but lower levels in their plasma compared to normotensive (NT) individuals (Calderón-Pérez et al. 2020). This suggests less efficient SCFA absorption in HT individuals, which may reduce the beneficial effects of SCFAs on BP regulation, such as vasodilation and anti-inflammatory properties. Inefficient SCFA absorption could thus contribute to higher BP in HT individuals, highlighting the role of SCFAs in maintaining cardiovascular health.
Microbiota-derived trimethylamine N-oxide (TMAO) in hypertension
SCFAs have garnered attention for their potential role in both the pathogenesis and treatment of hypertension. Additionally, TMAO has been recognized for its impact on hypertension. TMAO is a metabolite produced in the liver from trimethylamine (TMA), which is derived from gut bacterial metabolism of dietary substances such as choline, betaine, and L-carnitine (Yu et al. 2020). Emerging evidence suggests a close association between TMAO levels and hypertension pathogenesis (Mutengo et al. 2023; Zhen et al. 2023).
Elevated TMAO levels are implicated in several adverse cardiovascular outcomes, including endothelial dysfunction, atherosclerosis, and thrombosis. TMAO’s role in endothelial dysfunction is particularly significant, as it promotes inflammation, oxidative stress, and impaired vascular reactivity, all of which are critical factors in hypertension development (Mohan and George 2021; Shanmugham et al. 2023). Studies have shown that TMAO induces endothelial dysfunction through inflammation and oxidative stress, characterized by increased reactive oxygen species (ROS) production and upregulation of cytokines and adhesion molecules (Shanmugham et al. 2023). Excessive reactive oxygen species (ROS) activates calmodulin-dependent protein kinase II (CaMKII), increasing vasoconstriction, but the CaMKII inhibitor KN-93 prevents trimethylamine N-oxide (TMAO)-induced enhancement of this response and angiotensin II (Ang II)’s pressor effect (Jiang et al. 2021).
Further studies have elucidated the relationship between TMAO and hypertensive disorders during pregnancy. Higher plasma levels of betaine and an inverse relationship between the betaine/choline ratio and hypertensive disorders of pregnancy suggest that early pregnancy plasma betaine and choline levels can serve as predictive markers for these conditions (Xu et al. 2024). A meta-analysis has confirmed a dose-dependent association between circulating TMAO concentrations and hypertension risk in patients with pre-existing cardiovascular diseases, reinforcing the role of TMAO in increasing hypertension risk (Han et al. 2024).
Targeting TMAO holds promise for managing hypertension. Studies have indicated that reducing TMAO levels can improve endothelial function and reduce vascular reactivity. For example, therapeutic strategies such as the use of 3,3-dimethyl-1-butanol (DMB) to reduce TMAO levels have demonstrated significant reductions in right ventricle systolic pressure and pulmonary vascular muscularization in rat models of pulmonary hypertension (Huang et al. 2022). Additionally, Mendelian Randomization (MR) studies have provided causal evidence linking elevated TMAO levels with increased systolic BP, further suggesting that targeting TMAO production could be beneficial for BP reduction (Wang et al. 2022). Inhibition of TMAO generation in high salt diet-induced hypertension models has also shown potential by reducing neuroinflammation and oxidative stress in the hypothalamic paraventricular nucleus (Liu et al. 2022). Furthermore, other dietary factors such as high choline intake from supplements or certain foods, like egg yolks, have been shown to elevate TMAO levels, suggesting that dietary modifications could also influence TMAO-related hypertension management (Böckmann et al. 2022).
The significant role of TMAO in the pathogenesis of hypertension underscores its potential as a therapeutic target. While TMAO is associated with increased hypertension risk, therapeutic interventions targeting TMAO production, or its downstream effects could offer novel strategies for managing hypertension and associated cardiovascular diseases. Continued research is essential to fully elucidate the mechanisms underlying TMAO-induced hypertension and to develop effective preventive and therapeutic approaches.
Tryptophan and indole derivatives in hypertension
The role of tryptophan and its indole derivatives in the pathogenesis and treatment of hypertension is becoming increasingly intriguing, owing to their substantial impact on diverse biological processes and cardiovascular health. Tryptophan, an essential dietary amino acid, is metabolized into various compounds within the gut microbiota and tissue cells, affecting numerous physiological functions. Tryptophan metabolism produces several bioactive compounds, including Indole-3-aldehyde (3-IAId) and indole-3-propionic acid (IPA), which have shown significant cardiovascular effects.
In a study by Huang et al., 3-IAId demonstrated a protective role against aortic dissection (AD) in a murine model (Huang et al. 2023). The application of 3-IAId resulted in a significant decrease in aortic dissection and rupture rates, as well as mortality rates. This protective effect is attributed to its ability to inhibit the phenotype transition of vascular smooth muscle cells (VSMCs), reduce extracellular matrix degradation, decrease macrophage infiltration, and suppress inflammatory cytokine expression. These findings indicate that 3-IAId holds promise as an intervention strategy for preventing thoracic aortic dissection and potentially other vascular diseases, such as hypertension.
Gut microbiota-derived metabolites of tryptophan, such as indole-3-propionic acid (IPA), play a crucial role in cardiovascular health. Konopelski and Mogilnicka highlighted that IPA shares biological effects similar to its precursor tryptophan, including impacts on the cardiovascular system (Konopelski and Mogilnicka 2022). Indole-3-propionic acid (IPA) prevents oxidative stress injury, lipoperoxidation, and inhibits the synthesis of proinflammatory cytokines. It also impacts the energetic balance and cardiovascular health, although its synthesis may be influenced by atherosclerosis risk factors. Protective measures, like adopting a Mediterranean diet, have been shown to elevate plasma IPA concentrations, indicating that dietary interventions could improve cardiovascular health and potentially alleviate hypertension. However, Geddo et al. explored IPA’s effects on endothelial function and found that physiological concentrations of IPA reduced nitric oxide (NO) production in bovine aortic endothelial cells (Geddo et al. 2024). This reduction in NO, a crucial vasodilator, suggests that IPA could negatively affect vascular tone regulation, complicating its role in hypertension management. The contrasting results from these studies highlight its potential inhibitory effect on NO production which may reflect a dose-dependent response. Therefore, further investigation is needed to understand how varying IPA concentrations influence its cardiovascular effects and reconcile these findings with its overall role in hypertension management.
In conclusion, tryptophan and its indole derivatives play a multifaceted role in the pathogenesis and treatment of hypertension. Their influence on vascular function, immune and inflammatory responses, and interaction with gut microbiota highlight their potential as therapeutic targets. Further research into their specific mechanisms and effects will enhance our understanding and development of novel strategies for managing hypertension.
Gut-derived polyamines in hypertension
Polyamines, specifically spermidine and spermine, are positively charged aliphatic molecules essential for various cellular processes, including nucleic acid regulation, protein synthesis, oxidative balance, and cell proliferation. These molecules play a significant role in maintaining physiological homeostasis and mediating tissue injury. Disturbances in polyamine metabolism have been associated with several maladaptive changes, highlighting their importance in health and disease.
Polyamines are integral to several physiological functions due to their ability to regulate nucleic acids and proteins. They are involved in protein synthesis, nucleic acid interactions, oxidative stress management, and cell proliferation. Cellular levels of polyamines are meticulously controlled through mechanisms of import, export, de novo synthesis, and catabolism, with specific enzymes and enzymatic cascades dedicated to polyamine metabolism. The disruption of these pathways, whether through spontaneous mutations, genetic engineering, or experimentally induced injuries, leads to significant physiological disturbances. Studies have demonstrated the adverse effects of altered polyamine metabolism in both in vitro and in vivo models, emphasizing the critical role of polyamines in maintaining physiological balance and mediating injury responses (Zahedi et al. 2022).
Matsumoto et al. investigated the impact of inducing microbial polyamine production in the gut, particularly spermidine, on endothelial function crucial in hypertension and cardiovascular disease (Matsumoto et al. 2019). They found that consuming yogurt with Bifidobacterium animalis subsp. lactis (Bifal) and arginine (Arg) increased putrescine production, a spermidine precursor. Spermidine induces autophagy, reducing cardiovascular disease risk. The study showed that the Bifal + Arg yogurt group had improved endothelial function, suggesting potential in preventing or reducing atherosclerosis risk through enhanced microbial polyamine production and spermidine synthesis (Matsumoto et al. 2019).
Polyamines, crucial in hypertension, are primarily produced in the intestine by gut microbiota, involving complex biosynthetic pathways and specific transport systems. Notable bacteria like Enterococcus faecalis and Campylobacter jejuni are involved, synthesizing polyamines via various pathways. Studies show the synergistic effect of Bifidobacterium spp., E. faecalis, and Escherichia coli, with arginine supplementation enhancing polyamine production. B. animalis subsp. lactis supplementation increases intestinal polyamine levels (Yoon et al. 2023). Changes in polyamine levels, such as increased spermidine, can affect the composition and function of the gut microbiome in obese mice, resulting in reduced obesity rates. Spermidine exhibits a microbiota-dependent anti-obesity effect by promoting the expansion of Lachnospiraceae NK4A136, thereby enhancing gut barrier function (Ma et al. 2020b).
Spermine, a key polyamine, has garnered interest for its potential implications in hypertension and related conditions. While much research has focused on spermidine, recent studies have begun to highlight spermine’s role in hypertension. Liang et al. identified a significant reduction in spermine levels, alongside spermidine/spermine N1-acetyltransferase-1 (SAT1), in endothelial cells under ferroptosis-induced hypertension. This suggests a complex interplay between spermine levels and hypertension-related endothelial dysfunction (Liang et al. 2023). Spermine’s direct role in hypertension remains less established compared to spermidine. Wei et al. explored spermine’s regulatory effects on immune and signal transduction pathways in diabetic cardiomyopathy, yet did not find a direct connection to hypertension (Wei et al. 2022). Additionally, Sieckmann et al. observed that reduced spermine levels in kidney injury models, including hypertension, reflect broader impacts on kidney function rather than specific hypertensive effects (Sieckmann et al. 2023). Collectively, while spermine’s influence in hypertension is emerging, further research is needed to delineate its precise role and therapeutic potential.
Polyamines play a multifaceted role in the pathogenesis and treatment of hypertension. Their involvement in regulating oxidative balance, cell proliferation, and apoptosis underscores their importance in maintaining vascular health. Disruptions in polyamine metabolism contribute to vascular remodeling and oxidative stress, key factors in hypertension development. Targeting polyamine metabolic pathways offers a promising avenue for therapeutic intervention, potentially leading to novel treatments for hypertension and related vascular diseases. Further research is needed to fully elucidate the mechanisms by which polyamines influence hypertension and to develop effective polyamine-based therapies.
Gut-derived bile acids in hypertension
Bile acids play a crucial role in cardiovascular health, with levels increasing under pathological conditions. They impact cardiovascular health by activating various receptors, including the farnesoid X receptor (FXR), pregnane X receptor, vitamin D receptor, and G protein-coupled receptor Gpbar1 (TGR5) (Ishimwe et al. 2022; Li et al. 2020). Specifically, FXR, highly expressed in vascular smooth muscle cells and endothelial cells, modulates vasomotor function and vascular disease progression. Bile acids induce vasorelaxation, increase NO production, and regulate calcium influx in the vasculature. Moreover, FXR activation has implications in kidney health, influencing renal tubular cell survival and function. In salt-sensitive hypertension, bile acids may regulate BP through the epithelial Na + channel (ENaC) and BASIC (bile acid-sensitive ion channel). Activation of FXR leads to the upregulation of the angiotensin II type 2 receptor, which may contribute to the mitigation of salt-sensitive hypertension. Furthermore, bile acids are involved in regulating inflammation, which can impact hypertension. Activation of FXR and TGR5 by endogenous ligands and pharmacological agents presents potential therapeutic targets for addressing salt-sensitive hypertension (Ishimwe et al. 2022).
Taurine-conjugated bile acids, a subtype of bile acids, significantly impact cardiovascular health by enhancing vascular relaxation. They boost NO production and availability, essential for vascular health, through the upregulation of endothelial nitric oxide synthase (eNOS) expression and phosphorylation, improved NO bioavailability, and enhanced antioxidative defenses (Guizoni et al. 2020). Additionally, activation of FXR and TGR5 by taurine-conjugated bile acids promotes NO production by reducing asymmetric dimethylarginine (ADMA), an inhibitor of NO synthase, and mobilizing calcium ions.
Targeting bile acid metabolism and signaling pathways offers promising therapeutic strategies for managing hypertension. Modulating receptors like FXR and TGR5 to enhance NO production and improve vascular function could be effective. Therapies aimed at restoring healthy gut microbiota and bile acid profiles could alleviate salt-sensitive hypertension and improve overall cardiovascular health. Additionally, dietary interventions that promote a healthy gut microbiota and bile acid profile, such as a Mediterranean diet, could enhance cardiovascular health and mitigate hypertension (Guizoni et al. 2020).
Gut-derived phenylacetylglutamine in hypertension
Phenylacetylglutamine (PAGln), a gut-derived metabolite, has gained attention due to its association with heart failure (HF) severity and the progression of coronary artery disease (CAD). PAGln is formed through the conjugation of phenylacetic acid (PAA), a metabolite produced by gut bacteria from the amino acid phenylalanine, with glutamine (Krishnamoorthy et al. 2024). This conjugation typically occurs in the liver and kidneys. Genetic modification of certain microbes and studies with gnotobiotic mice revealed two pathways for PAA synthesis: phenylpyruvate oxidoreductase (PPFOR) and phenylpyruvate decarboxylase (PPDC). These enzymes are essential for bacterial PAA production via oxidative and non-oxidative decarboxylation of phenylpyruvate. Metagenomic analysis shows these pathways are more prevalent in gut microbiomes (Zhu et al. 2023).
PAGln influences cardiovascular health through several mechanisms. Elevated levels of PAGln have been associated with increased oxidative stress and inflammation in cardiovascular tissues. Specifically, PAGln can enhance the production of reactive oxygen species (ROS) and pro-inflammatory cytokines, which contribute to endothelial dysfunction and vascular inflammation (Fang et al. 2022). These mechanisms can contribute to the advancement of atherosclerosis and CAD by fostering the development of plaques and vascular lesions, which are also associated with hypertension. Elevated plasma PAGln levels have been linked to atrial fibrillation (AF) in HF by inducing thoracic aortic coarctation in mice. PAGln worsened ROS accumulation and increased levels of phosphorylated Phospholamban and CAMK II, indicating its involvement in promoting atrial fibrillation in heart failure mice through the activation of the CAMK II signaling pathway (Fu et al. 2024). A study demonstrated that PAGln directly influenced HF-related phenotypes, suggesting its potential as a therapeutic target for HF modulation (Romano et al. 2023).
Phenylacetylglutamine is a significant gut-derived metabolite that influences cardiovascular health through mechanisms involving oxidative stress, inflammation, and vascular remodeling. Dysbiosis plays a crucial role in altering PAGln levels, thereby affecting the pathogenesis of cardiovascular diseases. Ongoing research is essential to fully understand the mechanisms by which PAGln influences cardiovascular health and to develop effective treatments targeting this metabolite.
Studies on treatments targeting gut microbiome in the regulation of blood pressure
Conventional antihypertensive medications, such as ACE inhibitors, beta-blockers, and diuretics, are widely used to manage hypertension. Although these drugs are effective, they are often accompanied by side effects like dizziness, fatigue, and electrolyte imbalances (Karunarathna et al. 2024). As a result, there is growing interest in alternative treatments, including dietary supplements, herbal products, functional foods, and lifestyle modifications, which have shown promise in managing hypertension and other health conditions (Adeyanju et al. 2022; Oduyemi et al. 2023; Osonuga et al. 2022a, b, 2024). Among these alternatives, gut microbiota-based interventions are gaining attention due to their potential to offer fewer side effects and additional health benefits.
Microbiota-based interventions, such as probiotics, prebiotics, fecal microbiota transplantation (FMT), dietary modifications, and polyphenols, have been extensively studied for their potential in blood pressure regulation. Probiotics and prebiotics have shown promising yet diverse outcomes, along with tailored dietary interventions. Polyphenols and FMT have also been explored for their effects on BP.
Probiotics
Recent research on probiotics and blood pressure (BP) regulation, as summarized in Table 2, reveals mixed outcomes. One study reported that Bifidobacterium animalis subsp. lactis CECT 8145, particularly in its heat-killed form, led to a significant decrease in diastolic BP and improvements in waist circumference, although the results were specific to diastolic BP and not generalized by gender (Pedret et al. 2019). Another study found that probiotic-enriched orange juice improved peripheral BP and other metabolic markers but showed no significant changes in central BP (Papakonstantinou et al. 2024). In contrast, a study on multi-strain probiotics found improvements in subjective sleep quality but no significant impact on BP (Kerksick et al. 2024). These studies highlight the variable effects of probiotics on BP and underscore the need for further investigation.
Prebiotics
Prebiotics are non-digestible food components that promote the growth and activity of beneficial gut bacteria. A controlled open-label trial demonstrated that a specially designed diet combined with fecal microbiota transplantation improved blood glucose and BP levels by increasing beneficial bacteria like Bifidobacterium, although it had a limited duration and lacked long-term follow-up (Su et al. 2022). Another study comparing native inulin with maltodextrin found that prebiotic treatment led to significant reductions in diastolic BP and facilitated weight loss, though its effectiveness was reduced when used alongside metformin (Hiel et al. 2020). Additionally, a randomized controlled trial showed that oat bran supplementation resulted in significant reductions in both office and ambulatory BP, alongside decreased use of antihypertensive medications, by modulating gut microbiota (Xue et al. 2021). These findings, detailed in Table 3, underscore the potential of prebiotics in BP regulation and highlight the variability in their effectiveness.
Fecal microbiota transplantation
Fecal microbiota transplantation (FMT) involves transferring fecal microbiota from a healthy donor to a recipient’s gut to restore microbial balance. Clinical trials investigating FMT in blood pressure (BP) regulation have shown mixed results. One controlled open-label trial examined the effects of a specially designed diet and diet plus FMT on blood glucose and BP levels, revealing that both treatments increased beneficial bacteria like Bifidobacterium, which are associated with lower BP, and decreased harmful sulfate-reducing bacteria, suggesting improved BP regulation. However, the lack of a placebo control group was a significant limitation of this study (Su et al. 2022). Another randomized, double-masked placebo-controlled study investigated the impact of oral encapsulated fecal microbiome from healthy lean donors versus a saline placebo on BMI standard deviation scores over six weeks. This study suggested potential indirect benefits of FMT on BP regulation through reductions in abdominal adiposity and overall improvements in metabolic health, despite not finding a significant effect on weight loss (Leong et al. 2020).
Dietary intervention
Dietary interventions have demonstrated considerable potential in influencing blood pressure (BP) regulation and enhancing overall cardiovascular health. Table 4 outlines recent studies investigating various dietary approaches to BP management.
Polyphenols
Polyphenols are bioactive compounds found in plant-based foods, known for their antioxidant properties. Clinical trials investigating polyphenols in BP regulation by modulating gut microbiome have shown promising results as highlighted in Table 5. Specifically, these studies demonstrate that polyphenol-rich interventions can positively influence BP through mechanisms such as increasing beneficial gut bacteria, enhancing vascular function, and improving endothelial health.
Microbiota-based interventions offer various approaches for blood pressure regulation and improving cardiovascular health. While probiotics, prebiotics, and dietary modifications have shown promising results, polyphenols and FMT have produced mixed outcomes. Future research should focus on elucidating the mechanisms of these interventions and exploring their long-term effects to provide more definitive recommendations for clinical practice.
Conclusions
Hypertension is a complex, multifactorial disease influenced by genetic, environmental, and lifestyle factors. Recent research has highlighted the significant role of the gut microbiome and its metabolites in regulating BP. Key microbial metabolites, including short-chain fatty acids, trimethylamine N-oxide, tryptophan derivatives, polyamines, bile acids, and phenylacetylglutamine, have been identified as crucial players in BP regulation. Therapeutic approaches targeting these metabolites and their pathways offer promising avenues for hypertension management. Probiotics, prebiotics, dietary interventions, polyphenols, and fecal microbiota transplantation have shown varying degrees of success in modulating gut microbiota and influencing BP.
Despite the progress made in understanding the role of gut microbial metabolites in hypertension, several gaps in knowledge and areas for further research remain. Future research should focus on elucidating the precise mechanisms through which gut microbial metabolites influence BP. Understanding these pathways will help identify potential therapeutic targets. Large-scale, randomized controlled trials are needed to validate the efficacy of microbiota-based interventions in BP management. These studies should aim to determine the optimal strains and dosages of probiotics, prebiotics, and polyphenols for BP reduction. Among the identified metabolites, SCFAs and TMAO appear to have significant potential for therapeutic development. Future studies should prioritize these metabolites to explore their role in BP regulation and potential as therapeutic agents.
Research should investigate the potential for personalized gut microbiome interventions based on individual microbiota compositions. Personalized approaches could enhance the effectiveness of treatments and minimize adverse effects. Long-term studies are necessary to assess the sustainability of microbiota-based interventions and their impact on BP and overall cardiovascular health over time. Exploring the synergistic effects of combining different microbiota-based interventions, such as probiotics with dietary modifications, could provide more effective strategies for BP management. Establishing clear regulatory guidelines for the use of microbiota-based therapies in clinical practice will be crucial for their safe and effective implementation.
Data availability
All data in this study are included in this study.
Abbreviations
- BP:
-
Blood pressure
- SCFA:
-
Short-chain fatty acid
- TMAO:
-
Trimethylamine N-oxide
- TMA:
-
Trimethylamine
- 3-IAId:
-
Indole-3-Aldehyde
- IPA:
-
Indole-3-Propionic Acid
- NO:
-
Nitric Oxide
- MAP:
-
Mean arterial pressure
- HR:
-
Heart rate
- ROS:
-
Reactive oxygen species
- CaMKII:
-
Calmodulin-dependent protein kinase II
- Ang II:
-
Angiotensin II
- MR:
-
Mendelian randomization
- VSMCs:
-
Vascular smooth muscle cells
- FXR:
-
Farnesoid X receptor
- TGR5:
-
G protein-coupled receptor Gpbar1
- ENaC:
-
Epithelial Na + channel
- ADMA:
-
Asymmetric dimethylarginine
- HF:
-
Heart failure
- CAD:
-
Coronary artery disease
- PAGln:
-
Phenylacetylglutamine
- PAA:
-
Phenylacetic acid
- PPFOR:
-
Phenylpyruvate oxidoreductase
- PPDC:
-
Phenylpyruvate decarboxylase
- AF:
-
Atrial fibrillation
- FMT:
-
Fecal microbiota transplantation
- LDL:
-
Low-density lipoprotein
- MedDairy:
-
Mediterranean diet supplemented with dairy foods
- NAFLD:
-
Non-alcoholic fatty liver disease
- PDFF:
-
Proton density fat fraction
- BMI:
-
Body mass index
References
Adeyanju MM, Saheed IA, Oyelekan OI, Dele-Osibanjo TA, Adelegan AA, Raimi AJ et al (2022) Sesamum indicum diet prevents hyperlipidemia in experimental rats. Food Chem Mol Sci 4:100092
Agus A, Clément K, Sokol H (2021) Gut microbiota-derived metabolites as central regulators in metabolic disorders. Gut 70(6):1174–82
Amiri P, Hosseini SA, Ghaffari S, Tutunchi H, Ghaffari S, Mosharkesh E et al (2022) Role of butyrate, a gut microbiota derived metabolite, in cardiovascular diseases: a comprehensive narrative review. Front Pharmacol 12:837509
Avery EG, Bartolomaeus H, Maifeld A, Marko L, Wiig H, Wilck N et al (2021) The gut microbiome in hypertension. Circ Res 128(7):934–50
Bartolomaeus H, Balogh A, Yakoub M, Homann S, Markó L, Höges S et al (2019) Short-chain fatty acid propionate protects from hypertensive cardiovascular damage. Circulation 139(11):1407–21
Böckmann KA, Franz AR, Minarski M, Shunova A, Maiwald CA, Schwarz J et al (2022) Differential metabolism of choline supplements in adult volunteers. Eur J Nutr 61(1):219–230
Brüssow H (2020) Problems with the concept of gut microbiota dysbiosis. Microb Biotechnol 13(2):423–34
Caballero-Flores G, Pickard JM, Núñez G (2023) Microbiota-mediated colonization resistance: mechanisms and regulation. Nat Rev Microbiol 21(6):347–60
Calderón-Pérez L, Gosalbes MJ, Yuste S, Valls RM, Pedret A, Llauradó E et al (2020) Gut metagenomic and short chain fatty acids signature in hypertension: a cross-sectional study. Sci Rep 10(1):6436
Cheung CL, Ho DKC, Sing CW, Tsoi MF, Cheng VKF, Lee GKY et al (2017) Randomized controlled trial of the effect of phytosterols-enriched low-fat milk on lipid profile in Chinese. Sci Rep 7(1):41084
Choo JM, Murphy KJ, Wade AT, Wang Y, Bracci EL, Davis CR et al (2023) Interactions between Mediterranean diet supplemented with dairy foods and the gut microbiota influence cardiovascular health in an Australian population. Nutrients 15(16):3645
Chooi YC, Zhang QA, Magkos F, Ng M, Michael N, Wu X et al (2024) Effect of an Asian-adapted mediterranean diet and pentadecanoic acid on fatty liver disease: the TANGO randomized controlled trial. Am J Clin Nutr 119(3):788–99
Cookson TA (2021) Bacterial-induced blood pressure reduction: mechanisms for the treatment of hypertension via the gut. Front Cardiovasc Med 8:721393
Del Bo C, Bernardi S, Cherubini A, Porrini M, Gargari G, Hidalgo-Liberona N et al (2021) A polyphenol-rich dietary pattern improves intestinal permeability, evaluated as serum zonulin levels, in older subjects: the MaPLE randomised controlled trial. Clin Nutr 40(5):3006–18
Dinakis E, Nakai M, Gill P, Ribeiro R, Yiallourou S, Sata Y et al (2022) Association between the gut microbiome and their metabolites with human blood pressure variability. Hypertension 79(8):1690–701
Fan Y, Pedersen O (2021) Gut microbiota in human metabolic health and disease. Nat Rev Microbiol 19(1):55–71
Fang C, Zuo K, Jiao K, Zhu X, Fu Y, Zhong J et al (2022) PAGln, an atrial fibrillation-linked gut microbial metabolite, acts as a promoter of atrial myocyte injury. Biomolecules 12(8):1120
Fu H, Li D, Shuai W, Kong B, Wang X, Tang Y et al (2024) Effects of phenylacetylglutamine on the susceptibility of atrial fibrillation in overpressure-induced HF mice. Mol Cell Biol 44(4):149–63
Geddo F, Antoniotti S, Gallo MP, Querio G (2024) Indole-3-propionic acid, a gut microbiota-derived tryptophan metabolite, promotes endothelial dysfunction impairing purinergic-induced nitric oxide release in endothelial cells. Int J Mol Sci 25(6):3389
Ghatage T, Goyal SG, Dhar A, Bhat A (2021) Novel therapeutics for the treatment of hypertension and its associated complications: peptide- and nonpeptide-based strategies. Hypertens Res 44(7):740–55
Guizoni DM, Vettorazzi JF, Carneiro EM, Davel AP (2020) Modulation of endothelium-derived nitric oxide production and activity by taurine and taurine-conjugated bile acids. Nitric Oxide 94:48–53
Han JM, Guo L, Chen XH, Xie Q, Song XY, Ma YL (2024) Relationship between trimethylamine N-oxide and the risk of hypertension in patients with cardiovascular disease: a meta-analysis and dose-response relationship analysis. Medicine 103(1):e36784
Hiel S, Gianfrancesco MA, Rodriguez J, Portheault D, Leyrolle Q, Bindels LB et al (2020) Link between gut microbiota and health outcomes in inulin-treated obese patients: lessons from the Food4Gut multicenter randomized placebo-controlled trial. Clin Nutr 39(12):3618–28
Hill EB, Chen L, Bailey MT, Singh Khalsa A, Maltz R, Kelleher K et al (2022) Facilitating a high-quality dietary pattern induces shared microbial responses linking diet quality, blood pressure, and microbial sterol metabolism in caregiver-child dyads. Gut Microbes 14(1):2150502
Huang Y, Lin F, Tang R, Bao C, Zhou Q, Ye K et al (2022) Gut microbial metabolite trimethylamine N-oxide aggravates pulmonary hypertension. Am J Respir Cell Mol Biol 66:452–460
Huang SS, Liu R, Chang S, Li X, Weng X, Ge J (2023) Gut microbiota-derived tryptophan metabolite indole-3-aldehyde ameliorates aortic dissection. Nutrients 15(19):4150
Ishimwe JA, Dola T, Ertuglu LA, Kirabo A (2022) Bile acids and salt-sensitive hypertension: a role of the gut-liver axis. Am J Physiol-Heart Circ Physiol 322(4):H636-46
Istas G, Wood E, Le Sayec M, Rawlings C, Yoon J, Dandavate V et al (2019) Effects of aronia berry (poly) phenols on vascular function and gut microbiota: a double-blind randomized controlled trial in adult men. Am J Clin Nutr 110(2):316–29
Jama H, Marques FZ (2023) Gut microbial metabolites lower blood pressure in patients with hypertension. Nat Cardiovasc Res 2(1):18–9
Jiang S, Shui Y, Cui Y, Tang C, Wang X, Qiu X et al (2021) Gut microbiota dependent trimethylamine N-oxide aggravates angiotensin II–induced hypertension. Redox Biol 25(46):102115
Karunarathna I, Kusumarathna K, Jayathilaka P, Withanage C (2024) Comprehensive management of hypertension: strategies, guidelines, and emerging therapies. Uva Clin Lab Retrieved Compr Manag Hypertens Strateg Guidel Emerg Ther [Internet]. [cited 2024 Jul 5]; Available from: https://www.researchgate.net/profile/Indunil-Karunarathna/publication/380374676_Comprehensive_Management_of_Hypertension_Strategies_Guidelines_and_Emerging_Therapies/links/6639dc847091b94e93f5e30f/Comprehensive-Management-of-Hypertension-Strategies-Guidelines-and-Emerging-Therapies.pdf
Kaye DM, Shihata WA, Jama HA, Tsyganov K, Ziemann M, Kiriazis H et al (2020) Deficiency of prebiotic fiber and insufficient signaling through gut metabolite-sensing receptors leads to cardiovascular disease. Circulation 141(17):1393–403
Kerksick CM, Moon JM, Walden KE, Hagele AM, Allen LE, Gaige CJ et al (2024) Multi-strain probiotic improves subjective sleep quality with no impact on body composition, hemodynamics, and physical activity. Benef Microbes 15(2):179–94
Konopelski P, Mogilnicka I (2022) Biological effects of indole-3-propionic acid, a gut microbiota-derived metabolite, and its precursor tryptophan in mammals’ health and disease. Int J Mol Sci 23(3):1222
Krishnamoorthy NK, Kalyan M, Hediyal TA, Anand N, Kendaganna PH, Pendyala G et al (2024) Role of the gut bacteria-derived metabolite phenylacetylglutamine in health and diseases. ACS Omega. 9(3):3164–72
Le Sayec M, Xu Y, Laiola M, Gallego FA, Katsikioti D, Durbidge C et al (2022) The effects of Aronia berry (poly) phenol supplementation on arterial function and the gut microbiome in middle aged men and women: results from a randomized controlled trial. Clin Nutr 41(11):2549–61
Leong KS, Jayasinghe TN, Wilson BC, Derraik JG, Albert BB, Chiavaroli V et al (2020) Effects of fecal microbiome transfer in adolescents with obesity: the gut bugs randomized controlled trial. JAMA Netw Open 3(12):e2030415–e2030415
Li C, Yang J, Wang Y, Qi Y, Yang W, Li Y (2020) Farnesoid X receptor agonists as therapeutic target for cardiometabolic diseases. Front Pharmacol 11:1247
Li Y, Fu R, Li R, Zeng J, Liu T, Li X et al (2023) Causality of gut microbiome and hypertension: a bidirectional mendelian randomization study. Front Cardiovasc Med 10:1167346
Liang C, Zhu D, Xia W, Hong Z, Wang QS, Sun Y et al (2023) Inhibition of YAP by lenvatinib in endothelial cells increases blood pressure through ferroptosis. Biochim Biophys Acta BBA-Mol Basis Dis 1869(1):166586
Lin CJ, Cheng YC, Chen HC, Chao YK, Nicholson MW, Yen ECL et al (2022) Commensal gut microbiota-derived acetate and propionate enhance heart adaptation in response to cardiac pressure overload in mice. Theranostics 12(17):7319–34
Liu Y, Hou Y, Wang G, Zheng X, Hao H (2020) Gut microbial metabolites of aromatic amino acids as signals in host–microbe interplay. Trends Endocrinol Metab 31(11):818–34
Liu G, Cheng J, Zhang T, Shao Y, Chen X, Han L et al (2022) Inhibition of microbiota-dependent trimethylamine N-oxide production ameliorates high salt diet-induced sympathetic excitation and hypertension in rats by attenuating central neuroinflammation and oxidative stress. Front Pharmacol. 13:856914
Ma L, Ni Y, Wang Z, Tu W, Ni L, Zhuge F et al (2020) Spermidine improves gut barrier integrity and gut microbiota function in diet-induced obese mice. Gut Microbes 12(1):1832857
Ma L, Ni Y, Wang Z, Tu W, Ni L, Zhuge F et al (2020) Spermidine improves gut barrier integrity and gut microbiota function in diet-induced obese mice. Gut Microbes 12(1):1832857
Matsumoto M, Kitada Y, Naito Y (2019) Endothelial function is improved by inducing microbial polyamine production in the gut: a randomized placebo-controlled trial. Nutrients 11(5):1188
Mohan VK, George M (2021) A review of the contribution of gut-dependent microbiota derived marker, trimethylamine N-oxide (TMAO), in coronary artery disease. Curr Res Nutr Food Sci J 9(3):712–21
Muralitharan RR, Jama HA, Xie L, Peh A, Snelson M, Marques FZ (2020) Microbial peer pressure: the role of the gut microbiota in hypertension and its complications. Hypertension 76(6):1674–87
Mutengo KH, Masenga SK, Mweemba A, Mutale W, Kirabo A (2023) Gut microbiota dependant trimethylamine N-oxide and hypertension. Front Physiol 14:1075641
Oduyemi OI, Faderera BS, Adejoke OB, Albert O, Abiodun EEN, Oluyemisi AB, et al. (2023) Comparative Effects of Phyllantus Amarus on the Serum Electrolyte Level of Hypertensive and Normotensive Male Wistar Rats [cited 2024 Aug 25]; Available from: https://e-journal.unair.ac.id/CCJ/article/download/42782/24271
Olalekan SO, Bakare OO, Osonuga IO, Faponle AS, Adegbesan BO, Ezima EN (2024) Gut microbiota-derived metabolites: implications for metabolic syndrome and therapeutic interventions. Egypt J Intern Med 36(1):72
Onyszkiewicz M, Gawrys-Kopczynska M, Konopelski P, Aleksandrowicz M, Sawicka A, Koźniewska E et al (2019) Butyric acid, a gut bacteria metabolite, lowers arterial blood pressure via colon-vagus nerve signaling and GPR41/43 receptors. Pflüg Arch - Eur J Physiol 471(11):1441–53
Osonuga I, Olukade B, OgunladeSamuel Olalekan A, Olalekan S (2022) The effect of vibratory massage hammer on superoxide dismutase (sod) and catalase in undergradrate students of Olabisi Onabanjo University. J Res Rev Sci. https://doi.org/10.36108/jrrslasu/2202.90.0140
Osonuga IO, Oyesola OA, Olukade BA, Okebule BO, Ogunlade AA, Olalekan SO (2022) Effect of electrical acupuncture (foot massage) on blood pressure in presumably healthy students of Olabisi Onabanjo University, Ogun state, south-west Nigeria. Niger J Sci Res. 21(1):57–60
Osonuga IO, Olalekan SO, Olukade BA, Adedokun TA (2024) Plain water intake in the prevention and management of type 2 diabetes mellitus (T2DM)–A study of biomarkers associated with insulin resistance in a black African population. Sci Afr. 23:e02114
Papakonstantinou E, Zacharodimos N, Georgiopoulos G, Athanasaki C, Bothou DL, Tsitsou S et al (2024) Two-month consumption of orange juice enriched with vitamin D3 and probiotics decreases body weight, insulin resistance, blood lipids, and arterial blood pressure in high-cardiometabolic-risk patients on a westernized type diet: results from a randomized clinical trial. Nutrients 16(9):1331
Pedret A, Valls RM, Calderón-Pérez L, Llauradó E, Companys J, Pla-Pagà L et al (2019) Effects of daily consumption of the probiotic Bifidobacterium animalis subsp. lactis CECT 8145 on anthropometric adiposity biomarkers in abdominally obese subjects: a randomized controlled trial. Int J Obes 43(9):1863–8
Poll BG, Cheema MU, Pluznick JL (2020) Gut microbial metabolites and blood pressure regulation: focus on SCFAs and TMAO. Physiology 35(4):275–84
Rahman S, O’connor AL, Becker SL, Patel RK, Martindale RG, Tsikitis VL (2023) Gut microbial metabolites and its impact on human health. Ann Gastroenterol 36(4):360
Romano KA, Nemet I, Prasad Saha P, Haghikia A, Li XS, Mohan ML et al (2023) Gut microbiota-generated phenylacetylglutamine and heart failure. Circ Heart Fail 16(1):e009972
Shanmugham M, Bellanger S, Leo CH (2023) Gut-derived metabolite, trimethylamine-N-oxide (TMAO) in cardio-metabolic diseases: detection, mechanism, and potential therapeutics. Pharmaceuticals 16(4):504
Sieckmann T, Schley G, Ögel N, Kelterborn S, Boivin FJ, Fähling M et al (2023) Strikingly conserved gene expression changes of polyamine regulating enzymes among various forms of acute and chronic kidney injury. Kidney Int 104(1):90–107
Su L, Hong Z, Zhou T, Jian Y, Xu M, Zhang X et al (2022) Health improvements of type 2 diabetic patients through diet and diet plus fecal microbiota transplantation. Sci Rep 12(1):1152
Suta S, Ophakas S, Manosan T, Honwichit O, Charoensiddhi S, Surawit A et al (2023) Influence of prolonged whole egg supplementation on insulin-like growth factor 1 and short-chain fatty acids product: implications for human health and gut microbiota. Nutrients 15(22):4804
Tokarek J, Budny E, Saar M, Kućmierz J, Młynarska E, Rysz J et al (2023) Does the composition of gut microbiota affect hypertension? Molecular mechanisms involved in increasing blood pressure. Int J Mol Sci 24(2):1377
Turroni F, Milani C, Duranti S, Lugli GA, Bernasconi S, Margolles A et al (2020) The infant gut microbiome as a microbial organ influencing host well-being. Ital J Pediatr 46(1):16
Wang H, Luo Q, Ding X, Chen L, Zhang Z (2022) Trimethylamine N-oxide and its precursors in relation to blood pressure: a mendelian randomization study. Front Cardiovasc Med 9:922441
Wei C, Sun M, Liang X, Che B, Wang N, Shi L et al (2022) Spermine regulates immune and signal transduction dysfunction in diabetic cardiomyopathy. Front Endocrinol 12:740493
World Health Organization (2023) Hypertension [Internet] [cited 2024 Jul 5]. Available from: https://www.who.int/news-room/fact-sheets/detail/hypertension
Wood E, Hein S, Mesnage R, Fernandes F, Abhayaratne N, Xu Y et al (2023) Wild blueberry (poly) phenols can improve vascular function and cognitive performance in healthy older individuals: a double-blind randomized controlled trial. Am J Clin Nutr 117(6):1306–19
Wu Y, Xu H, Tu X, Gao Z (2021) The role of short-chain fatty acids of gut microbiota origin in hypertension. Front Microbiol 12:730809
Xia H, Guo J, Shen J, Jiang S, Han S, Li L (2023) Butyrate ameliorated the intestinal barrier dysfunction and attenuated acute pancreatitis in mice fed with ketogenic diet. Life Sci 334:122188
Xu C, Marques FZ (2022) How dietary fibre, acting via the gut microbiome, lowers blood pressure. Curr Hypertens Rep 24(11):509–21
Xu DJ, Wang KC, Yuan LB, Li HF, Xu YY, Wei LY et al (2021) Compositional and functional alterations of gut microbiota in patients with stroke. Nutr Metab Cardiovasc Dis 31(12):3434–48
Xu H, Feng P, Sun Y, Wu D, Wang D, Wu L et al (2024) Plasma trimethylamine N-oxide metabolites in the second trimester predict the risk of hypertensive disorders of pregnancy: a nested case-control study. Hypertens Res off J Jpn Soc Hypertens 47(3):778–789
Xue Y, Cui L, Qi J, Ojo O, Du X, Liu Y et al (2021) The effect of dietary fiber (oat bran) supplement on blood pressure in patients with essential hypertension: a randomized controlled trial. Nutr Metab Cardiovasc Dis 31(8):2458–70
Yoon JH, Do JS, Velankanni P, Lee CG, Kwon HK (2023) Gut microbial metabolites on host immune responses in health and disease. Immune Netw 23(1):e6
Yu Z, Zhang L, Jiang X, Xue C, Chi N, Zhang T et al (2020) Effects of dietary choline, betaine, and L-carnitine on the generation of trimethylamine-N-oxide in healthy mice. J Food Sci 85(7):2207–15
Zahedi K, Barone S, Soleimani M (2022) Polyamines and their metabolism: from the maintenance of physiological homeostasis to the mediation of disease. Med Sci 10(3):38
Zhen J, Zhou Z, He M, Han HX, Lv EH, Wen PB et al (2023) The gut microbial metabolite trimethylamine N-oxide and cardiovascular diseases. Front Endocrinol 14:1085041
Zheng T, Wu Y, Xiao GK, Jin TZ, Yang T (2023) The process of hypertension induced by high-salt diet: association with interactions between intestinal mucosal microbiota, and chronic low-grade inflammation, end-organ damage. Front Microbiol 14:1123843
Zhu Y, Dwidar M, Nemet I, Buffa JA, Sangwan N, Li XS et al (2023) Two distinct gut microbial pathways contribute to meta-organismal production of phenylacetylglutamine with links to cardiovascular disease. Cell Host Microbe 31(1):18
Acknowledgements
None
Funding
Not applicable.
Author information
Authors and Affiliations
Contributions
SOO conceived and designed the study. SOO wrote the initial version of the manuscript. OOB, ASP, PGO reviewed the initial version of the manuscript. SOO, OOB, ASP, PGO wrote the final draft of the manuscript. All authors have read and approved the final version of the manuscript.
Corresponding author
Ethics declarations
Ethical approval
Not applicable.
Consent for publication
Not applicable.
Competing interests
The authors declare no known competing interests.
Additional information
Publisher's Note
Springer Nature remains neutral with regard to jurisdictional claims in published maps and institutional affiliations.
Rights and permissions
Open Access This article is licensed under a Creative Commons Attribution 4.0 International License, which permits use, sharing, adaptation, distribution and reproduction in any medium or format, as long as you give appropriate credit to the original author(s) and the source, provide a link to the Creative Commons licence, and indicate if changes were made. The images or other third party material in this article are included in the article's Creative Commons licence, unless indicated otherwise in a credit line to the material. If material is not included in the article's Creative Commons licence and your intended use is not permitted by statutory regulation or exceeds the permitted use, you will need to obtain permission directly from the copyright holder. To view a copy of this licence, visit http://creativecommons.org/licenses/by/4.0/.
About this article
Cite this article
Olalekan, S.O., Bakare, O.O., Faponle, A.S. et al. A review of gut microbial metabolites and therapeutic approaches in hypertension. Bull Natl Res Cent 48, 95 (2024). https://doi.org/10.1186/s42269-024-01252-9
Received:
Accepted:
Published:
DOI: https://doi.org/10.1186/s42269-024-01252-9