Abstract
Antarctic krill (Euphausia superba) oil is attracting more interest for its nutritional as well as functional potentials. Nevertheless, its potential as new and innovative food component remains largely unexplored. This review aims to outline the chemical composition, extraction methods, and health advantages of krill oil, offering insights for its utilization and provides evidence why it is now on the spotlight. Krill oil presents a distinctive fat profile, rich in lipid classes, with phospholipids (PLs) comprising a significant portion (38.93—79.99%) with high levels of eicosapentaenoic acid (EPA) and docosahexaenoic acid (DHA). Additionally, it includes several minor bioactive components like astaxanthin, tocopherols, sterols, flavonoids, and vitamin A. Various extraction technics, including solvent and solvent-free extraction, enzyme-assisted pretreatment extraction, super/subcritical fluid extraction, significantly influence both output as well as standard of the resulting product. Furthermore, the oil had been linked to a number of health advantages, including prevention of cardiovascular disease (CVD), anti-inflammatory effects, support for women's physiology, anticancer activities, as well as neuroprotection, among others. Despite the commercial availability of krill oil products as dietary supplement, there is a scarcity of studies exploring the underlying molecular mechanisms responsible for its various biological activities. Despite this, apply krill oil as an innovative food ingredient has not been thoroughly investigated. This review consolidates information on the chemical composition, extraction techniques, possible health advantages, as well as existing uses as applications, aiming to offer insights for its complete exploitation. In addition, it attempts to unravel the fundamental molecular mechanisms that being investigated to deeply understand how krill oil produces various biological effects.
Graphical Abstract

Similar content being viewed by others
Introduction
Krill, scientifically known as Euphausia superba, is a tiny crustacean found in the seas of the Antarctic Ocean, its also holds significant ecological importance as a primary food source for many fish species (Zhou et al., 2021). Although quantifying krill biomass poses challenges, estimates suggest approximately 379 million metric tons. In an effort to preserve the marine ecosystem, the agency of Antarctic Marine Living has implemented the catch maximum per season which is < 619,000 tons (Zeb et al., 2021). However, the actual catch less than 250,000 tons, which is below the allowed limits, likely due to challenges in preserving krill due to its delicate nature (Fuxing et al., 2017). Krill composition has a water content of 77.9—83.1%, lipids from 0.5 to 3.6%, protein from 11.9 to 15.4%, chitin at 2%, along with carbohydrates, and 3% ash (Xie et al., 2017).
Krill oil (KO) finds its way in the aquaculture sectors and as a dietary supplement to enhance health. This is attributed to its nutritional profile, that is high in omega-3 fatty acids as triacylglycerols (TAGs) also phospholipids (PLs), astaxanthin, and vitamins (A and E) (Cicero and Colletti, 2015).
Krill has a lipid content of 0.5–3.6% (Xie et al., 2019), notably phospholipids (PLs) that account for 30 to 65% of the total. Unlike fish oils (FO), which primarily occur as TAGs, the oil has a significant portion of phosphatidylcholine. With roughly 40% of its overall fatty acids are due to EPA (C20:5) and DHA (C22:6) (Ramprasath et al., 2013). The C20:5 and C22:6 in KO exhibit various beneficial pharmacological properties, hence serve a major role in the management of several chronic conditions like CVD as well as inflammatory diseases (Cicero et al., 2012; Costanzo et al., 2016). Moreover, they contribute to cancer prevention and improve the gut health (Saravanan et al., 2010). Research findings indicate that C20:5 and C22:6 obtained from KO shown better bioaccessibility when compared to different n-3 PUFA sources and varieties (Rossmeisl et al., 2012).
Krill oil received approval from the U.S. Food and Drug Administration (FDA) in 2008, designated as Generally Recognized as Safe (GRAS) status. It was also granted approval as a novel food by the European Food Safety Authority (EFSA) in 2009 and was granted authorization in China in 2014. Additionally, EFSA approved the use of KO for pregnant women in 2014.
This write up offers a comprehensive account of krill oil with regard to its chemical composition, bioavailability, health benefits, mechanisms of action, extraction methods (both traditional and unconventional), and existing applications. Additionally, it explores the future prospects of krill oil as nutraceutical and why it has captured the spotlight.
Composition of Antarctic krill oil (Euphausia superba)
Lipid class composition
Unlike typical edible oils, which predominantly comprising mostly TAGs (over 95%) (Shahidi and Abad, 2019; Shahidi et al., 2020), krill oil consists of a broader range of lipid classes. Phospholipids constitute the primary class, followed by TAGs, DAGs, MAGs, FFAs, and other constituents (Ahmmed et al., 2020; Phleger et al., 2002). Various factors affect the composition of each lipid portion in krill oil. These include year-to-year environmental changes, seasonal fluctuations, the maturity level of KO, and storage conditions, transportation means, and preparation techniques (Xie et al., 2019). For instance, dehydrating krill through hot air yields higher amounts of free fatty acids (Gang et al., 2019). Moreover, several studies have employed different analytical methods to analyze the composition of krill oil, thus comparison of the results may not always be straightforward (Han and Liu, 2019).
Numerous studies have documented varying lipid compositions in different krill samples. Krill oils typically exhibit a substantial content of PLs, varying from 39.89 to 80.69%, which can vary based on the sample type as well as the analytical method employed (Table 1). Discrepancies in lipid compositions observed in different years and regions may be due to fluctuations in feeding behavior (Phleger et al., 2002). Clarke (1980) noted that oil extracted from krill ovarian tissues had higher phospholipid levels compared to that from muscle tissues, similar to that observed in fish (Takama et al., 1994). Thus, females tended to possess higher PL levels than males due to the presence of developing ovaries (Kołakowska, 1991). Moreover, the extracted KO's PL content was greatly impacted by the extraction solvent selection, with mix of ethanol and isopropanol extraction resulting in elevated levels of phospholipids compared to solvents like hexane and acetone (Xie et al., 2017).
Table 2 provides an overview of the phospholipid (PL) composition of Antarctic krill oil, indicating that phosphatidylcholine (PC) constitutes the majority, 44.58 to 99.80%, followed by phosphatidylethanolamine (PE) at 0.20 to 24.74%. Additionally, lysophosphatidylcholine (LPC) is also present in significant proportions, possibly attributed to PC hydrolysis due to either incorrect storage or preparation of KO (Lim et al., 2015). Other PLs types such as lysophosphatidylethanolamine, phosphatidylinositol, phosphatidylserine, cardiolipin, sphingomyelin, phosphatidic acid, and phosphatidylglycerol have also been observed in smaller amounts, typically not surpassing 15%, in certain studies (Fricke et al., 1984; Kołakowska, 1991). PLs, particularly PC, have long been used as food additives and nutritional supplements, mainly derived from sources such as egg yolk, plant oils, and milk products (Chen et al., 2023). The abundance of PC in KO provides a promising marine source for supplying PLs.
Lipid fraction (TAGs and PLs)
As already noted, KO composition consists primarily phospholipids (PLs) followed by triacylglycerols (Abad and Shahidi, 2023; Ahmmed et al., 2020). These components significantly contribute to in absorption, and metabolism (Zhang et al., 2021).
More than 64 molecular species of triacylglycerols have been identified in krill oil, with carbon number (CN) 42 to 60 and with one to eleven double bonds. Among these, the primary prevalent TAG species are 16:0/16:0/18:1, 14:0/16:0/18:1, 16:0/18:1/18:1, and 16:0/16:1/16:1 (Castro-Gómez et al., 2015), constituting relative proportions of 4.8, 8, 5.5, and 6% of all TAG species found, in that order. This observation is consistent with the primary fatty acid profiles of TAG that contain 14:0, 16:0, 16:1, and 18:1. While the TAG fraction in krill oil contains relatively low levels of C20:5 and C22:6, the majority of residues are EPA found in the sn-1,3 positions, whereas C22:6 residues are located mainly in the sn-2 position (Fuller et al., 2020). This distribution pattern mirrors that observed in FO (Akanbi et al., 2013; Standal et al., 2009). Moreover, another study found that about 21% of n-3 PUFAs were in TAGs fraction of krill oil, notably found in the sn-2 location (Araujo et al., 2014).
Choline-containing PLs are dominant in krill oil. Using HPLC–ESI–MS, Winther et al. (2011) 69 PL species in krill oil have choline-head groups, comprising 60 PC species and nine LPC species. Notably, PC (16:0/22:6), PC (16:0/18:1), also PC (16:0/20:5) were the species with the highest abundance based on relative intensity, consistent with findings reported by Castro-Gómez et al. (2015) as well as Le Grandois et al. (2009). Although differing numbers of PC and LPC species were reported, it was discovered that about seven species of PC have omega-3 fatty acid in both two positions (sn-1 and sn-2). These species included PC, (18:4/22:6), (18:4/20:5), (20:5/22:6) (20:5/20:5), (20:5/23:5), (20:5/22:5), as well as (22:6/22:6) (Winther et al., 2011), highlighting the prevalence of omega-3 fatty acid within PC molecules. Furthermore, NMR analysis (Hupfeld, 2018) indicated that in contrast to the first position (sn-1), the majority of omega-3 fatty acids in krill phospholipids were located in the sn-2 positions.
Fatty acid composition
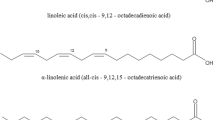
Krill oil is rich in PUFAs, including C20:5 and C22:6, together with high amounts of C14:0, C16:1, C16:0, C18:1, and C20:1(Sun et al., 2018). In addition, n-3 PUFAs, in particular C20:5 and C22:6 derived from dietary lipids, are recognized for their vital role in health (Marventano et al., 2015). While other marine oil including fish known for their high C20:5 and C22:6 content and have traditionally been used as supplements of n-3 PUFAs, similar composition could be offered by KO. Table 3 provides a comparative overview of the composition of fatty acid in KO (Xie et al., 2018) along with other marine oils including algal oil (DHASCO) (Abuzaytoun and Shahidi, 2006), cod liver oil (Dalheim et al., 2021), as well as tuna and menhaden oils (Codex Standard 329–2017, WHO, Food and Agriculture Organization of the United Nations, 2017). Furthermore, KO has similar levels of C20:5 and C22:6 as other marine oils, although a significant portion of these fatty acids in KO are linked to PLs rather than TAGs found in other oil like fish.
The levels of C20:5 and C22:6 in krill oil are similar to what been found in FO (Table 3), but occurring mainly in the PLs rather than TAGs. Clarke (1980) found that the PLs portion of KO exhibited substantially greater proportions of PUFAs, as well as n-3 PUFAs, along with lower concentrations of monounsaturated and saturated fatty acids. Specifically, 31.13% of C20:5 and 14.87% of C22:6 been identified in PL portion, compared to about 3.17% of C20:5 and 1.5% of C22:6 in TAG portion. Those findings were supported by several additional research (Cicero and Colletti, 2015; Laidlaw et al., 2014), which indicate that KO with elevated PL levels have higher amount of C20:5 and C22:6 (Sun et al., 2019). The latest studies have indicated that omega-3 fatty acid that located in PLs demonstrate notably enhanced bioavailability compared to other omega-3 that located in TAGs (Jiang et al., 2020). Consequently, krill oil may offer superior bioavailability of C20:5 and C22:6 compared to FO.
Minor components
Astaxanthin
Astaxanthin, consider as a primary carotenoid present in certain marine organisms as well as algae, exhibits potent antioxidant properties as well as significant biological advantages (Table 4) (Ambati et al., 2014). Miki (1991) highlighted astaxanthin's antioxidant potency that is ten times stronger than zeaxanthin, lutein, canthaxanthin, and β-carotene; furthermore, hundred times superior to alpha-tocopherol. Moreover, existence of astaxanthin responsable the deep red color in KO (Zeng et al., 2024). The astaxanthin content in krill oilKO varies from 4 to 500 mg/100 g, also is affected by factors such as extraction techniques and analytical methods (Ali-Nehari et al., 2012; Sun et al., 2017a; Tandyet al., 2009). Extraction by acetone solvent has been shown to yield krill oil with higher levels of astaxanthin (Ahmadkelayeh and Hawboldt, 2020).
In KO astaxanthin predominantly exists as form of fatty acid esters. Foss et al. (1987) reported that astaxanthin diesters (51%), monoesters (43%), as well as free astaxanthin (6%), which coincides with findings of other studies (Lambertsen and Braekkan, 1971; Yamaguchi et al., 1983) and similar to that of other shellfish. Subsequently, research has identified C14:0, C16:0, C16:1, C18:1, C20:0, C20:5, as well as C22:6 as primary fatty acids present as astaxanthin esters form (Cao et al., 2023). Furthermore, astaxanthin was found to exist as three astaxanthin isomers in KO, namely all-trans, 9-cis, and 13-cis astaxanthin, with the all-trans isomer being most abundant.
Sterols
KO have a considerable proportion of sterols, ranging from 2.3 to 3.9% of total lipids, mainly as cholesterol and desmosterol (Table 1) (Huenerlage et al., 2016). Cholesterol constitutes approximately 81.31—82.57% of total sterols at concentrations ranging from 1895 to 3196 mg/100 g (Colletti et al., 2021). These concentrations exceed those found in certain fish oils like tuna oil (204 mg/100 g) and hoki oil (515 mg/100 g) (Huenerlage et al., 2016), also those present in egg yolk (1181 mg/100 g) (Albalat et al., 2016). Since diet that including high cholesterol is associated with CVD (Nissinen et al., 2008), concerns have been expressed regarding the consumption of krill oil. Bruheim et al. (2017) suggested that employing one solvent for extraction like ethanol may end to reduced cholesterol levels in KO in contrast to ethanol–water mixtures. Nonetheless, there is a need to explore novel extraction techniques to further mitigate cholesterol amounts in KO.
Desmosterol, recognized as the forerunner to cholesterol which constitutes 1.70—18.63% of all sterols (Fricke et al., 1984). Additionally, Phleger et al. (2002), identified several other sterols in KO like brassica-sterol brassica-sterol (0.5—1.7%), 24-nordehydrocholesterol (0.1—1.7%), 24-methylenecholesterol (0.1—0.4%), transdehydrocholesterol (1.1—1.5%), and stanols (0.1—0.2%). Minor discrepancies in sterol composition may arise from variations in krill diet over different years, as crustaceans rely heavily on dietary sources or phytosterol dealkylation for sterol acquisition, rather than de novo synthesis (Xie et al., 2018).
Vitamins
Vitamin E encompasses all tocopherols and tocotrienols, comprising four pairs of homologues (α-, β-, γ-, δ-), each possessing antioxidant properties and biological advantages, with α-tocopherol being the most potent (Valk and Hornstra, 2000). Similar to many other marine organisms (Ackman and Cormier, 1967), α-tocopherol predominates in krill oil, ranging from 14.74 to 63.0 mg/100 (Xie et al., 2017; Tilseth, 2010). Some studies have also identified γ-tocopherol vary in concentration from 0.25 to 3.67 mg/100; however, traces of δ-tocopherol from 0 to 0.65 mg/100 g (Sun et al., 2018; Xie et al., 2017). Typically, in KO over 90% of tocopherols exist as α-tocopherol. Tocopherols in KO may enhance antioxidant capacity also potentially synergize with some bioactive constituents.
Vitamin A, crucial for human nutrition, is essential for both immune function as well as the management of certain infectious diseases (Mayo-Wilson et al., 2011). According to wet weight basis, frozen krill normally contains 0.11 mg/100 g of vitamin A, (Suzuki and Shibata, 1990). a nutrient that is fat-soluble and may concentrate in the oil during the lipid extraction process. Xie et al. (2017) reported vitamin A contents of 16.40—28.55 mg/100 g of krill oil, with variations attributed to the extraction solvents used. Tilseth (2010) noted that oil extracted from cookedd krill had a vitamin A content of about 18 mg/100 g. Krill oil has a higher content of vitamin A than some FO, like menhaden oil (0.1–0.6 mg/100 g) as well as tuna oil (11.09 mg/100 g), but less than the 99.76 mg/100 g found in hoki oil.
Flavonoids
Flavonoids exhibit various biological activities, including antioxidant, antibacterial, immunomodulatory, antitumor, also anti-inflammatory properties (Ullah et al., 2020). While fruits, vegetables, and grains are primary sources of flavonoids (Merken and Beecher, 2000; Shahidi and Yeo, 2018), krill oil contains a novel flavonoid, 6,8-di-C-glucosyl luteolin. Sampalis (2013) patented a KO extract containing approximately 40% phospholipids (PLs) and about 7 mg/100 mL of flavonoids. This extract demonstrated efficacy in protecting the skin against harmful ultraviolet B (UVB) radiation also in improving dyslexia and abnormal motor function. According to certain research (Omar et al., 2011), flavonoids' ability to function as antioxidants is increased when they are C-glycosylated at particular locations as well as their antidiabetic properties (Matsuda et al., 2003). However, information about the characteristics of flavonoids found in KO are currently unavailable.
Minerals
Whole krill possesses significant levels of minerals that are necessary for bone health, including magnesium, calcium, and phosphorus 360, 1322 1140 mg/100 g respectively, that fulfill the recommended amount for adults (Colletti et al., 2021). Even though processing of krill may lead to loss of some minerals, using the Sampalis (2011) patented process, a krill lipid extract enriched with multiple minerals including potassium, calcium, selenium, and zinc may be obtained. Moreover, KO also have a great amount of fluoride which is 2,400 mg/kg (Soevik and Braekkan, 1979). However, fluoride is recognized as a global health concern (Barbier et al., 2010). While fluoride in krill predominantly accumulates in the exoskeleton, there is a potential chance that it gets released in deceased krill. Hence, careful consideration of fluoride transfer is essential during krill oil extraction to prevent excessive fluoride levels in the oil. Typically, removing the exoskeleton from krill prior to extract the oil yields KO with low concentration of fluoride < 0.5 mg/kg, whereas extract by use whole body of krill exhibit a great concentration of fluoride from 3—5 mg/kg (Bruheim et al., 2016; Jansson et al., 2018).
Extraction methods
Krill oil extraction uses dried material as well as fresh krill (Katevas et al., 2014; Ronen et al., 2017). High concentrations of active proteolytic enzymes in krill allow for quick autolysis following catch. Therefore, it is imperative to commence on-board processing as soon as krill are captured in order to extract oil from fresh krill (Beaudoin et al., 2004). The krill biomass serves as a more suitable material for on-shore krill oil extraction, particularly in regions lacking onboard or offshore processing capabilities (Yoshitomi et al., 2003). Various extraction techniques, such as solvent extraction, mechanical pressing (nonsolvent extraction), enzyme-assisted extraction, and super/subcritical fluid extraction, are well-documented for krill oil extraction (see Table 5). Every approach has pros and disadvantages of its own, which are discussed below.
Traditional extraction methods
Solvent extraction
Solvent extraction, a traditional method in oil production (Abad and Shahidi, 2017, 2021), remains prevalent for krill oil production. Since one type of solvent cannot effectively extract all of the lipids from krill because of presence of different lipid classes with differing polarities, Xie et al. (2017) found that alcohols like ethanol as well as isopropanol could extract substantial volumes of PLs from krill meal but yield KO has less minor components. Conversely, acetone efficiently extracts minor components but fails to fully extract PLs. Hexane is widely used in oil extraction from seeds and is cost-effective with high extraction efficiency (Abad and Shahidi, 2020a, 2020b); as such, it shows moderate capabilities to extract PLs as well as minor components (Li et al., 2013). Combining solvents such a polar with a nonpolar can help balance the extraction efficiencies of PLs as well as minor component (Ronen et al., 2017). Although the Folch method (Folch et al., 1957) is widely applied to extract lipids from animal tissues with high lipid (Bruheim et al., 2016), its commercial feasibility is limited due to the solvent’s toxicity such as chloroform as well as methanol.
Presently, the most used technique for extracting KO involves a two-step process using ethanol and acetone (Beaudoin et al., 2004) which yields better lipid extraction (2.62%) compared to a single solvent extraction (acetone, 2.15%). Alternatively, a simpler one-step strategy, using a mixture of ethanol and acetone (1:1, v/v), can also achieve high lipid yields (Gigliotti et al., 2011). Furthermore, Yin et al. (2015) found that combining solvent extraction with extrusion pretreatment enhances lipid extraction efficiency from krill. Defatted krill remains a valuable resource for protein recovery (Chen et al., 2009), including enzymatic hydrolysis for producing peptides (Zhao et al., 2013) or by fermentation (Sun and Mao, 2016). While solvent extraction is cost-effective and scalable, it necessitates large quantities of solvents, thus posing potential environmental concerns. Moreover, the process takes a lot of time also labor-intensive.
Mechanical pressing (solvent-free extraction)
Solvent-free extraction, unlike solvent-based methods, does not rely on organic solvents for extracting KO. Mechanical pressing, which is known as classic solvent-free extraction technique, is commonly used for oilseeds with great content of oil including sesame oil 49 to 58% and sunflower oil 40 to 43% (Khan and Hanna, 1983). Although less efficient compared to solvent extraction, mechanical pressing is often employed to remove the majority of the oil before recovering the remaining oil via solvent extraction. Fresh krill is not inherently suited for conventional mechanical pressing due to its relatively and 17.24% in krill meal (Yin et al., 2015) using this method, fresh or thawed material should be ground as slurry in fluid medium, facilitating lipid release during subsequent mechanical disruption procedures, followed by oil recovery using centrifugation (Larsen et al., 2007). However, the resultant slurry during grinding could lead to emulsification because of nature of phospholipids, hence complicating the removal of the fat off the mixture. To address this concern, Katevas et al. (2014) introduced an alternative method, which includes cooking, drain, and centrifuging. The approach allows simultaneous extraction of PLs-enriched KO as well as neutral lipid-enriched KO. It is worth noting that the initial cooking step take place at 90 °C with no agitation to prevent emulsification. Additionally, it is preferable to process krill when fresh, because ice crystals that grow as a result of freezing might harm krill tissues, resulting in emulsification during processing and yielding low-quality products.
Solvent-free extraction offers the advantage of providing a safer and more environmentally friendly process compared to solvent extraction methods. However, it presents significant drawbacks such as the need for investing in equipment purchase and high energy requirements. Additionally, the high operating temperatures involved may lead to product oxidation. Furthermore, solvent-free extraction may not efficiently extract all oil that exist in krill, as indicated by Katevas et al. (2014) who reported a yield of only 2.1%. Consequently, some krill manufacturers opt to use mechanical separation consider as very first step to extract a portion of oil while at the same time producing krill meal. Thereafter, some techniques like solvent extraction or supercritical fluid extraction are employed to extract oil from the remaining krill meal (Tilseth et al., 2015).
Other extraction techniques
Enzymatic extraction
Enzyme pretreatment represents an efficient method for releasing bound compounds and increasing lipid yield during the extraction process (Dom´ınguez et al., 1994). By using specific enzymes, the extractability of oil can be improved. Moreover, the gentle nature of this process guarantees better-quality meal and oil. These advantages render enzyme pretreatment an attractive option for KO extraction.
Oil has been extracted from raw krill using proteases, as demonstrated by Bruheim et al. (2016). The typical process involves disintegrating the krill into small particles, followed by the addition of water then heating. Subsequently, enzymes are added to hydrolyze resultant material, after which the enzymes are deactivated. The solids, primarily the exoskeleton, are removed and then PL-protein complex is separated then dried. KO is then extracted from this complex (Bruheim et al., 2016). It is worth noting that the remove the exoskeleton from material can lead to a reduction in fluoride content in the resulting products. Lee (2014) patented an alternative enzyme assisted extraction method by using ultra high-pressure reactor ranging from 10 to 300 MPa to liquefy krill and assure effective interaction with enzyme (proteases). Following undergoing enzymatic processing for a duration of 4 to 24 h, the krill that had turned into liquid was thereafter subjected to filtration in order to separate the resulting filtrate from the solid residue known as sludge via centrifugation. moreover, the astaxanthin-enriched oils were separated from sludge using another solvent such as ethanol.
This method's main benefit is gentle operating conditions, that enable the extraction of high-quality protein and oil from krill at the same time. Additionally, the enzymatic hydrolysis process facilitates the recovery useful byproduct like krill peptides. These peptides are gaining attention and recognized as bioactive compounds in functional foods and nutraceuticals, that have positive effects on health and low the risk of disease. Nevertheless, importantly, the longer hydrolysis time restricts the enzymes' potential for large-scale industrial applications, Furthermore, the high coast compared to other extraction method.
Supercritical fluid extraction
Lipid extraction via supercritical extraction has attracted a lot of attention recently for its solvent-free nature, environmental friendliness, and gentle operating conditions. Among supercritical solvents, supercritical carbon dioxide (SC-CO2) is preferred for its chemical inertness, safety, non-toxicity, and moderate critical properties (Friedrich and Pryde, 1984). Despite its advantages, SC-CO2 is not optimal for extracting all krill lipids, particularly phospholipids (PLs) (Yamaguchi et al., 1986). Nevertheless, extracting lipids using SC-CO2 yields good quality as well as more thermally stable proteins from krill compared to the traditional solvent extraction methods. The addition of ethanol at 5 to 20% in SC-CO2 could enhance PL solubility, thereby improving lipid recovery. However, because ethanol is liquid at ambient temperature, use it not be ideal. on the other hand, for commercial of extraction by supercritical fluid remains limited because of the restricted processing capacity and expensive high-pressure equipment (Bruheim et al., 2018).
Although it works at lower pressure and temperature levels than supercritical extraction, subcritical fluid extraction has many of the same benefits. Liu et al. (2015) reported that propane as well as butane are primary subcritical fluids used in extraction due to their colorless nature also easy removal from the extracted products. Extracting krill oil using subcritical butane at 30 °C and 0.3—0.8 MPa conditions (Xie et al., 2017) yielded similar oil quantity and quality as hexane but in a faster process with less solvent usage. A study by Sun et al. (2018) showed that KO extracted with subcritical butane contained great levels of tocopherols also astaxanthin while maintaining lower oxidation level compared to solvent extraction methods. However, similar to supercritical fluid extraction is also not yet cost-effective for routine applications.
Health benefits of Antarctic krill oil
Krill oil contains a number of nutrients and bioactives like C20:5, C22:6, PLs, astaxanthin, vitamin A, also tocopherols (vitamin E), all of which contribute to human health support. Multiple research studies have examined the potential health benefits of Antarctic krill oil, encompassing cardiovascular disease prevention, anti-inflammatory activities, potential anti-cancer properties, effects on diabetes and obesity, neuroprotection, and benefits for women's physiology. These findings are summarized in Table 6.
Cardiovascular health
CVD is recognized as a significant worldwide health challenge and a leading cause of mortality among adults and the elderly. Research by Harris et al. (1988), also Rizos et al. (2012) has indicated that incorporating n-3PUFAs into the diet can help mitigate CVD risks. Fish oil consumption, for having a high n-3 PUFA content, is widely acknowledged for its positive impact on CVD prevention. Studies are presently underway to investigate any possible connection between the use of KO and CVD prevention.
Elevation of triacylglycerols (TAG), total cholesterol (TC), also low-density lipoprotein cholesterol (LDL-C) usually linked to increased risk of CVD disease and are commonly considered as CVD risk indicators. Papakonstantinou et al. (2013) have demonstrated this association. Several studies (Batetta et al., 2009; Hals et al., 2017; Sun et al., 2017b; Zhu et al., 2008) utilizing animal models have assessed the impact KO on CVD risk factor in both tissues and blood. Through eight weeks feeding trial, where mice were supplemented with 1.25, 2.50, or 5.0% KO in their diet, significant reductions in hepatic TAG and TC levels were observed, along with a decrease in serum TAG levels in mice fed with diet contains lots of fat (Tandy et al., 2009). Additionally, in the diet that has a highest dosage of 5%, rise in serum adiponectin level was noted in mice fed with krill oil, supporting its anti-atherogenic properties (Lu et al., 2008).
In another investigation, revealed that KO supplementation (5% in the diet) caused the drop in serum LDL-C after twelve weeks (0.45 mol/L) and TC (reaching 2.50 mol/L) levels in mice fed with diet contains lots of fat, compared to control group (0.65 mol/L, 3.70 mol/L, respectively). Additionally, Zhu et al. (2008) also Batetta et al. (2009) showed that KO can lower TAGs, TC, as well as LDL levels in mice with metabolic dysfunction induced by diet contains lots of fat. Similar findings observed in a separate study, using cynomolgus monkeys as a model Hals et al. (2017), where KO effectively improved various CVD risk factors, including HDL-C, LDL, TC, TAG, apolipoprotein, as well as A1, apolipoprotein B100 in dyslipidemic nonhuman primates with diabetes type 2.
Further support for the preventive effects of KO against CVD has emerged from human clinical trials conducted by, Cicero et al. (2016), also Rundblad et al. (2017). Bunea et al. (2004) examined the relationship between the consumption of KO and level of lipid in blood in 120 hyperlipidemic patients with moderately very high level of TC as well as high TAGs. Patients receiving 1- 3 g/day of KO for 3 months exhibited significantly elevated levels of HDL also decreased levels of glucose in blood, TC, LDL, as well as TAG compared to others were given placebo. In a similar vein, Berge et al. (2014) noted reduced risk of CVD in 300 adults with extremely high or high fasting serum TAG levels after consuming KO capsules. An about 10 percentage reduction in serum TAG level (relative to the placebo group) was observed in subjects administered krill oil at doses ranging from 0.5 – 4.0 g/day for 3 months. Similar improvements in lipid profiles following KO treatment were observed in overweight subjects as well as healthy individuals with fasting serum TAG levels range from 1.3 to 4.0 mmol/l (Rundblad et al., 2017).
Anti-inflammatory properties
Chronic inflammation is strongly linked to numerous illnesses, including inflammatory bowel disease, asthma, psoriasis, and rheumatoid arthritis (Barnes and Karin, 1997). Additionally, systemic inflammation might be contributed to development of exacerbated like atherosclerosis, obesity, cachexia, osteoporosis, as well as anorexia (Gan, 2004; Monteiro and Azevedo, 2010). Hence, it is crucial to focus on controlling inflammation for overall health improvement. The anti-inflammatory properties of KO have been validated through in vivo as well as vitro studies, as detailed in Table 6.
In laboratory experiments, it has been demonstrated that krill oil can markedly reduce the tumor necrosis factor α (TNF-α). This reduction is achieved by blocking the attachment of lipopolysaccharide (LPS) to toll-like receptor 4 (TLR4) (Bonaterra et al., 2017) in LPS induced inflammatory human acute monocytic leukemia cell line THP-1, in a manner that depends on the dosage of KO used. At a concentration of 49 μg/ml in the medium, KO totally prevented the irrevocable of LPS to TLR4 and decreased TNF-α production by 75%. Similarly, Batetta et al. (2009) observed reduced TNF-α release in LPS-treated peritoneal macrophages from obese Zucker rats, given KO supplements in their diets compared to control. The n-3PUFAs induced modifications in the endocannabinoid (EC) system which affects these anti-inflammatories.
The endocannabinoids (ECs) derived from n-3PUFAs have been shown to possess anti-inflammatory properties (Calder, 2009). Additionally, KO effectively reduced the mRNA expression levels of pro-inflammatory cytokines such as interleukin-8 (IL-8) and TNF-α in inflammatory cells exposed to LF82 bacteria or cytomix. Treatment with about 250 (mg/L) of krill oil in the medium also inhibited bacterial adhesion/invasion in epithelial cells as well as promoted wound healing. Those findings provide support for the health benefits associated with KO, particularly in the process of mitigating epithelial restitution and enhancing intestinal barrier integrity (Costanzo et al., 2016).
The main focus of in vivo investigations has been on investigating the anti-inflammatory effects of krill oil on conditions such as arthritis or colitis in both humans and mice, as summarized in Table 6. One study investigated the anti-inflammatory properties of KO using an experimental model of collagen-induced arthritis in mice (DBA/1) (Ierna et al., 2010). They found that administering KO at a daily dosage approximately 0.45 g of C20:5 and C22:6/100 g of diet for 8 weeks (in mice) improved arthritis pathology. This improvement was evidenced by cartilage erosion, synovial membrane thickening, and reductions in cell influx. Moreover, krill oil demonstrated potential in mitigating inflammation in a rat model of colitis. Rats supplemented with KO at amount of 4.9% in diet for one month exhibited preserved colon length and favorable changes in prostaglandin (PG) and interleukin (IL) levels associated with inflammation.
The plasma level of CRP notably increases during inflammatory states and serves as a marker for multiple forms of inflammation (Young et al., 1991). Deutsch (2007) observed that everyday intake of 300 mg of KO over 2 weeks led to a significant decrease in CRP concentration and relief of arthritic symptoms in patients with chronic inflammatory conditions. Similarly, another study reported that supplementing with 500 mg of KO two time a day for one month resulted in a significant reduction in high-sensitivity CRP levels in plasma, decreased from 2.15 to 0.43 mg/L in overweight subjects (Cicero et al., 2016).
Anti-cancer
Cancer has emerged as a primary cause of death worldwide in both developed and developing nations (Jemal et al., 2011) The worldwide prevalence of cancer is steadily increasing due to factors such as population growth, aging, as well as lifestyle habits, including smoking, also food rich in fat, sugar, and salt (Torre et al., 2015) While chemotherapy as well as radiotherapy known as crucial in cancer treatment to slow down disease progression or inducing apoptosis to halt tumor formation, they are often come with undesirable consequences like diarrhea, myelosuppression, mucositis, as well as dermatitis (Jayathilake et al., 2016). There is an increasing interest in exploring the potential of dietary factors to modulate apoptosis as a means of anticancer therapy (Block et al., 1992). Although there are relatively few experimental studies examining the in vivo anticancer effect of KO in the literature, several in vitro studies have evaluated its impact on the growth of certain cancer cell lines (Xie et al., 2019).
Colon cancer ranks as the second most common cause of cancer related deaths the United States (Jemal et al., 2005). Research indicates that KO exhibits dose- duration of treatment effects on the growth of colon cancer cells, specifically SW480 cells (Jemal et al., 2005). Application of KO at dose of 20 μg/ml in Dulbecco’s modified Eagle’s medium for two days led to a 29.9% suppression of SW480 cell growth (Zhu et al., 2008). Jayathilake et al. (2016) investigated impact of KO free fatty acid (FFA) extracts on cell proliferation and apoptosis in three human colon adenocarcinoma cell lines. Treatment use 0.12 μL of FFA from KO in 100 μl of DMEM for two inhibited the proliferation of HCT-15 as well as SW-480 cells. Additionally, The FFA extract elicited markedly elevated levels of apoptosis in all three colon cell lines (Su et al., 2018) compared to control. Furthermore, the anticancer activity of FFA that extracted from KO was validated in human osteosarcoma cells, where the inhibitory effect of 1.89 μM of FFA from KO comparable to 0.5 to 1.0 μM of doxorubicin which is commonly used anticancer drug (Su et al., 2018).
Zheng et al. (2017) conducted research to isolate and identify the trans (E) -configuration of certain FAs detected in KO, such as C20:5 and C22:6. They discovered that these FAs exhibited significantly stronger inhibitory effects on the growth of various cancer cell lines (including K562, PC-3, HL60, MCF-7, and U937) compared to C20:5 and C22:6 from FO. Additionally, astaxanthins as well as tocopherols have shown anti-cancer effects (Constantinou et al., 2008; Rao et al., 2013). It is plausible that the combined effects of the bioactive compounds in KO contribute to its potent anti-cancer capabilities. However, further in vivo studies are necessary to elucidate the underlying molecular mechanisms and validate these anti-cancer effects.
Anti-diabetic and anti-obesity effects
Imbalanced intake of energy could disrupt the endocannabinoid (EC) system, leading to excessive accumulation of visceral fat and reduced release of adiponectin, thereby the chances of type 2 diabetes and obesity. Anandamide (AEA) and 2-arachidonoylglycerol (2-AG) are the primary ECs studied, known for their roles in regulating fat as well as glucose metabolism (Di Marzo, 2008). Obese people's tissues have been found to contain elevated quantities of 2-AG and AEA (Batetta et al., 2009; Di Marzo et al., 2010). Diets supplemented with KO have been shown to reduce the concentration of AEA as well as 2-AG in various tissues, including the kidneys, heart, as well as adipose tissues in high fat-fed C57BL/6 mice (Piscitelli et al., 2011). Furthermore, krill oil diets decreased body weight gain in obese mice (Sun et al., 2017b; Yang et al., 2016) also hyperlipidemic rats (Zhu et al., 2008). The n-3PUFAs diminished the biosynthesis of arachidonic acid and its integration into phospholipids, possibly decreasing the quantity available of biosynthetic precursors for anandamide as well as 2-arachidonoylglycerol (Matias et al., 2008). Effect of the anti-obesity associated with KO are likely due to its high content of n-3 PUFAs. Maki et al. (2009) also, observed that one month of KO supplementation at amount of 2 g/day led to increased the plasma level of C20:5 and C22:6 in obese as well as overweight individuals.
Insulin resistance caused by fat is a prevalent issue. Ivanova et al. (2015) demonstrated that consuming a diet supplemented with KO, has about 600 mg of n-3PUFAs daily for one month, resulted in reduced the fasting blood glucose levels also improved the glucose tolerance in obese rabbits (New Zealand with rabbits). Similarly, healthy subjects experienced a decrease in fasting blood glucose after consuming about 4 g per day of KO for two months, indicating its potential as an anti-diabetic agent (Rundblad et al., 2017) suggested that n-3PUFAs from KO had improved insulin sensitivity as well as secretion and altered the expression level of key enzymes involved in β-oxidation and lipogenesis in muscles as well as liver (Ivanova et al., 2015).
Neuroprotective effects
Alzheimer’s disease (AD) is a neurological condition that progresses over time, frequently seen in the elderly (Francis et al., 1999). It manifests as a gradual decline in cognitive function, often accompanied by behavioral changes like wandering, aggression as well as depression, significantly affecting both patients and their caregivers' quality of life. Various studies in animal and human models have explored the neuroprotective properties of KO (Table 6).
Using the Aversive Light Stimulus Avoidance Test (ALSAT), the Unavoidable ALSAT, as well as the Forced Swimming Test (FST), rats were treated with 1.25 g/100 g of food containing KO for around two months exhibited a positive impact on memory processes and learning. Additionally, rats supplemented with krill oil showed elevated expression levels of mRNA for brain-derived neurotrophic factor (Bdnf), a gene linked to neuronal growth also differentiation in the hippocampus. These results are consistent with those of Tome-Carneiro (2018). Moreover, Cheong et al. (2017) noted a correlation between KO consumption and alterations in the proteome of aged mice's brain tissues. They observed that giving elderly mice KO at doses ranging from 150 to 600 mg/kg daily for seven weeks dramatically changed the expression levels of 28 different proteins in their brain regions. Notably, the group receiving KO showed a significant increase in the expression levels of Celsr3 as well as Ppp1r1b mRNA, that linked to working memory, brain development, and learning acquisition. KO was found to enhance oxidative stress biomarkers in serum like malondialdehyde (MDA) as well as superoxide dismutase (SOD). Furthermore, Li et al. (2018) demonstrated that KO has a beneficial impact on Alzheimer's disease in animal model using senescence-accelerated prone mouse strain 8 mice, providing evidence for its preventive properties. Over three months, using supplement have 1% of KO in the diet effectively improved the memory capabilities and learning while alleviating nervousness in SAMP8 old mice, as determined through the open field test as well as Morris water. The accumulation of β-amyloid (Aβ) is implicated in cognitive decline as well as AD pathology. Moreover, KO mitigated the accumulation of Aβ in the hippocampus, along with reducing oxidative stress in the brain.
The decline in estrogen levels among aging women may heighten risk of AD (Janicki and schupf, 2010). Research conducted on ovariectomized rats have revealed significantly reduced levels of serotonin, insulin growth factor, estrogen, and dopamine, alongside alterations in the gene expression of amyloid precursor protein, glycogen synthase-3beta, Bdnf, as well as selective AD indicator-1, all of which are associated with AD in rats. Mansour et al. (2017) observed that supplementation with KO at 200 mg/kg per day for 8 weeks resulted in the normalization of all these parameters in ovariectomized rats, suggesting the effects of KO in inhibition of AD development and neurodegeneration in old women.
Human studies have also provided evidence supporting the favorable impacts of KO on cognitive function. Oxyhemoglobin, which is mostly linked to cerebral blood flow, acts as a measure of regional brain function activation during cognitive tasks (Hibino et al., 2013). The P300 event-related potential is a cognitive component utilized to objectively evaluate neuroelectrical activity-linked cognitive behaviors as well as activities (Alvarenga et al., 2005; Hansenne, 2000). Konagai et al. (2013) assessed the effects of dietary KO on cognitive function in more than 45 healthy elderly males during calculation and memory tasks by monitoring oxyhemoglobin variations as well as P300 event-related potential components in the cerebral cortex. Following the administration of KO about 1.98 g/day for three months, subjects exhibited important alterations in oxyhemoglobin concentration during working memory tasks as well as reduced differential value of P300 latency during calculation tasks compared to the control. These findings demonstrate the positive effects of KO in enhancing cognitive function among the elderly.
Women’s physiology
Premenstrual syndrome (PMS) is a cyclic disorder commonly experienced by young as well as middle aged women during the luteal phase of menstruation, characterized by psychological, emotional, also behavioral symptoms (Dickerson et al., 2003; Stevinson and Ernst, 2001). While the exact cause of PMS is unclear, around 75% of women encounter some PMS symptoms (Barnhart et al., 1995) during their reproductive years. Various dietary supplements, such as multivitamin/mineral supplements, vitamins (A, E, and B6), and minerals (magnesium and calcium) have been suggested for alleviating certain PMS symptoms (Bendich, 2000; Dickerson et al., 2003). Additionally, it has been noted thatn-3 PUFAs (Sohrabi et al., 2013) may help reduce both psychiatric as well as somatic of PMS.
Krill oil as source of n-3 PUFAs as well as vitamins (E and A), has demonstrated beneficial effects in managing both emotional and physical symptoms associated with PMS. Individuals who took KO soft gels for three menstrual cycles reported decreased usage of pain relievers for menstrual pain as well as lower scores on the self assessment questionnaire for PMS, based on the American College of Obstetricians and Gynecologists (ACOG) diagnostic criteria for PMS, which ranged from zero to no symptoms to ten for unbearable. Moreover, KO exhibited greater efficacy in managing PMS and dysmenorrhea compared to FO (Sampalis et al., 2003). This superior performance is attributed to the unique profile of krill oil which includes a combination of phospholipids, n-3 PUFAs as well as antioxidative substances. The n-3 PUFAs linked with PLs in KO are believed to offer higher bioavailability than TAGs in FO, thus potentially playing a more active role in regulating emotional symptoms (Sampalis et al., 2003; Schuchardt et al., 2011). Nevertheless, additional inquiries are required to elucidate the underlying mechanisms involved.
Postmenopausal women commonly encounter cerebrovascular dysfunction as a result of estrogen deficiency (Serock et al., 2008). Key regulatory components like KCa channels, KATP channels, as well as Na+/Ca2+ exchanger 1 (NCX1) are crucial in maintaining cerebral blood flow autoregulation; however, they are susceptible to disruption in cases of ovarian dysfunction. In a study involving ovariectomized rats, the administration of KO (providing 182 mg EPA + 118 mg DHA sourced from KO) for a duration of 2 weeks was found to beneficially regulate the expression of NCX1 mRNA, KCa channels, as well as KATP channels in the basilar artery, leading to an enhancement in cerebral blood circulation. The results indicate that KO may serve as a beneficial supplement for women who gone through menopause (Sakai et al., 2014).
Effect on depression
The impact of KO supplementation on cognitive function as well as depression like behaviors was assessed through both preclinical and clinical studies. One of the initial investigations in this area involved a one and half month trial conducted on rats which received either krill oil at a dosage of 0.2 g/rat per day, imipramine at 20 mg/kg per day (utilized as a reference drug for antidepressant effects), or a placebo. Following the treatment period, cognitive abilities were evaluated using the ALSAT, while the potential antidepressant effects were assessed through FST and the Unavoidable Aversive Light Stimulus Test (UALST). The rats treated with krill oil demonstrated a notable ability to differentiate between active as well as inactive levers in the ALSAT test from the initial day of training. Furthermore, rats receiving KO and imipramine showed high improvements in behavioral aspects, including reduced level in the UALST test from the third day onwards as well as decreased immobility time in the FST test. Moreover, study examined the expression of Bdnf (Zadeh-Ardabili et al., 2019), which was observed to be elevated in the hippocampus of rats treated with KO.
Zadeh-Ardabili et al. (2019) conducted a study involving mice subjected to treatments with FO, KO, vitamin B12, imipramine, or over two weeks, beginning after seven days of exposure to the Chronic Unpredictable Stress (CUS) paradigm overnight procedure. During the CUS procedures, mice were exposed to stress overnight using 10W LED light at a frequency of 15 Hz for 12 h over 21 days. The potential therapeutic benefits of the treatments on depression were assessed using the tail suspension test (TST) as well as FST. After the animals were sacrificed, oxidation markers were assessed in the brain tissue. Both KO and FO were high decreased immobility factors as well as increased climbing and swimming time, similar to the effects observed with imipramine. Moreover, both KO and FO reduced levels of MDA and hydrogen peroxide, decreased catalase activity, increased glutathione peroxidase levels, and increased superoxide dismutase activities as well as glutathione levels in hippocampal tissue (Mendoza et al., 2018).
In another pre-clinical study, Mendoza et al. (2018) examined the impact of krill oil on restraint stress in mice following reduced mobility. The study investigated the effects of KO on the response to restraint stress in mice after experiencing limited mobility. Following 14 days of handling and acclimation, the mice were immobilized for one month, and then behavioral test took place for seven days. Over the course of the one month study, mice orally received either PBS, nicotine derivative cotinine about 5 mg, or combination of cotinine with 140 mg/kg of KO. Although cotinine by itself reduced the loss of memory deficiencies and the behaviors associated with anxiety and depression, cotinine in combination with KO proved to be more beneficial. This underscores the role of KO in mechanisms related to depression (van der Wurff et al., 2016). Subsequently, these authors conducted, over the course of one year employed a double-blind, randomized, and controlled methodology to investigate the impact of KO supplementation on the learning and cognitive function of teenagers, mental well-being, also visual processing. The study involved 260 adolescents aged 13 to 15 years, divided into two cohorts. The first cohort initially received 400 mg/day of C20:5 and C22:6 or a placebo, with dose increased to 800 mg of C20:5 and C22:6 per day after 12 weeks. The second cohort started directly with 800 mg of C20:5 and C22:6 per day (Zheng et al., 2017). The efficacy of these treatments was evaluated through omega-3 index finger-prick blood measurements, using the Centre for Epidemiologic Studies Depression Scale, and the Rosenberg Self-Esteem questionnaire. The authors did not find any evidence supporting the effectiveness of KO in reducing depressive feeling (van der Wurff et al., 2020).
Exercise and bodily performance
Krill oil enhances exercise performance and reduces oxidative stress and inflammation, leading to the initiation of several clinical trials. One of the initial studies involved a small double blind trial conducted on 16 members of Polish National Rowing Team. Participants were divided into 2 groups, first one had received 1 g of KO per day for one and half month, while the other received a placebo. Various parameters were assessed before, after 1 min, and after 24 h of exercise, with the latter representing maximum effort after rowing 2000 m. Exercise increased levels of certain markers including superoxide dismutase, TNF-α, and TBARS, which indicate lipid oxidation. While there were generally no significant differences between the control and KO groups in most parameters, during the recovery period, TBARS levels kept rising in the control, while the KO group displayed notably lower levels of lipid oxidation (Da Boit et al., 2015). Thus, krill oil supplementation helped reducing exercise-induced free radical mediated injuries.
The effects of KO on exercise performance as well as post effort immune function were investigated in small randomized clinical trial involving 37 athletes (Average age of 25.8 years). Participants, in two groups, first one has receiving about 2 g/day of KO for one and half month. On the other hand, second group receiving a placebo. A cycling time test was conducted before and after the supplementation period, during which blood samples were collected for five time test as follow: prior to supplementation, immediately post-exercise, after 1 h, after 3 h, as well as at rest. The results showed that after one and half month of supplementation, athletes who received krill oil showed significant increases in peripheral blood mononuclear cell IL-2 production and natural killer cell cytotoxic activity 3 h post exercise (Georges et al., 2018). Based on these findings, additional research examined the potential of KO to increase body mass. In vitro experiments utilized C2C12 rat myoblasts (skeletal muscle) treated with KO, PC derived from soy, or control. Only KO was capable of stimulating the mTOR pathway. Subsequently, a double-blind, placebo controlled clinical trial was conducted on resistance-trained athletes who received 3 g per day of KO or placebo over two months resistance trained program. The findings showed no significant differences in complete blood count, comprehensive metabolic panel, and urine analysis between the two groups. Nevertheless, KO supplementation resulted in an increase in lean body mass by approximately 2.2% compared to the baseline (Barenie et al., 2022).
In another study, a specific mixed formulation called ESPO-572®, consisting of 75% PCSO-524® (mussel oil) at 600 mg/day and 25% KO for 26 days, was found to alleviate exercise-induced muscle damage as well as cytokine-induced tissue degradation in untrained men who underwent a running test As choline is associated with maintaining muscle function and exercise performance, a reduction in choline level may occur after high resistance or high intensity exercise. To investigate whether krill oil could offer any protective effect against this choline loss, Storsve et al. (2020) conducted a study involving 47 triathletes randomly divided into 2 groups. First one received 4 g per day of KO called SuperbaBoost™ for 40 days leading up to the race, while the other group received a placebo. Blood test results showed high decrease in choline levels after the race; however, athletes in KO group had higher choline levels compared to those in placebo group. Thus, krill oil supplementation may help mitigating the negative effects on exercise performance, particularly during high-resistance activities by preventing choline decline (Ibrahim et al., 2016).
Comparison with fish oil (bioavailability and bioaccessibility)
When KO and FO supplements compared, it has been found that KO had more pronounced effects in managing cognitive function (Konagai et al., 2013), PMS (Sampalis et al., 2003), and hyperlipidemia (Bunea et al., 2004). Several studies carried out by Ulven et al. (2011), Rossmeisl et al. (2012), Ramprasath et al. (2013), and Laidlaw et al. (2014) attributed the superior performance of KO to its higher bioavailability of EPA and DHA in phospholipid form. However, these studies failed to administer identical doses of EPA and DHA from KO and FO as well as the existing differences arising from other components present, thus additional work is needed to verify these findings. In this connection, problems noted may be exemplified by the work of Ramprasath et al. (2013) who found that KO can elevate plasma n-3 PUFA concentrations more effectively than fish oil, but used daily amount of EPA and DHA of 777 mg for KO group and about 664 mg for FO group. In addition, Ulven et al. (2011) used dosages of EPA and DHA of around 543 mg for KO group with ratio of EPA (1.74) also 864 mg for FO with ratio o EPA/DHA (1.12). As already mentioned, most studies did not account for the minor components present in two olis (Bunea et al., 2004; Konagai et al., 2013; Sampalis et al., 2003). Meanwhile, Köhler et al. (2015) reported that EPA and DHA in krill meal had a lower bioavailability compared to KO, but similar to FO. Thus, EPA and DHA in phospholipid form alone may not fully explain the superior performance of KO.
The exact mechanism of superior effect of KO compared to FO effect remains elusive. This may arise from the fact that KO contain high concentration of various biological active components such as astaxanthin, tocopherols, and vitamin A, hence the effects may be multifactorial. Therefore, more investigation is required to comprehend the underlying mechanisms and to use better controlled human trials to determine the performance as well as the efficacy of KO and FO after prolonged administration (Tillander et al., 2014).
Applications and future perspectives of KO
Production and use of KO has emerged as a highly appealing within the food industry. Recognized as unique food ingredient, KO shows promise for applications in food, pharmaceutical, and nutraceuticals because of its wide range of health advantages.
Presently, KO products can be easily obtained as supplements in various forms including capsules, soft gels, tablets, and gummies. There are leading global producers of krill oil, with their products enjoying popularity in European and American health markets. Patents and patent applications for krill oil highlight its potential in preventing inflammation, CVD, PMS, cognitive diseases, and enhancing brain function. Some KO products are formulated with additional beneficial additives including various carotenoids, conjugated linoleic acid, and vitamin D, in order to offer enhanced benefits (Rockway, 2006; Derohanes et al., 2018). For instance, a combination of KO, vitamin D, and probiotic Lactobacillus reuteri is proposed to alleviate gut inflammation by promoting epithelial restitution and modulation the gut microbiota (Costanzo et al., 2018). Additionally, intranasal administration of KO along with cotinine (a product found in tobacco) exhibits potential in treating depressive symptoms associated with recurrent associative trama memories in patients with posttraumatic stress disorder (Alvarez-Ricartes et al., 2018).
While numerous studies have explored the various functionalities as well as commercial applications of KO only a limited number have detailed molecular mechanisms involved in its diverse activities. For instance, Xie et al. (2018) observed significant differences in antioxidant activity among krill oils with distinct chemical compositions. Nevertheless, the majority of studies that examined the health advantages in KO (Table 6) do not provide detailed information abut the composition of KO which used in these studies. Therefore, future research must clarify the relationship between the content and health benefits of KO in order to facilitate the development of more specialized and a variety of useful KO products with specific health benefits. This would accelerate broader application of KO by taking advantage of its multicomponent feature.
Conclusion
Krill oil derived from Antarctic krill (Euphausia superba) garnered growing interest due to its unique benefits. This contribution explored the chemical composition, health benefits, extraction methods as well as some of current application of KO. As noted, KO is abundant in EBA, DHA, tocopherols, vitamin A, astaxanthin, and flavonoids, all of which offer significant health benefits. Notably, a substantial portion of EPA and DHA in KO exists in the phospholipid (PL) form, prompting extensive research comparing its benefits with those of fish oil. Four primary extraction technologies are employed in KO production, namely solvent extraction, solvent-free extraction, enzyme-assisted pretreatment extraction, as well as super/subcritical fluid extraction, each with its own advantages as well as limitations.
At present, commercially available KO products are utilized as supplements. Numerous researches inclusive in vivo as well as in vitro experiments, have indicated that KO offers a range of benefits, including CVD, anti-inflammatory, anti-cancer, anti-obesity and antidiabetic effects, neuroprotective effects, benefits for women’s physiology, and effect on depression. In spite of that, the precise mechanisms underlying these effects require further investigation. It is widely acknowledged that the functionalities of product are closely linked to its chemical composition. Hence, further research is imperative to help understanding of the intricate relationship between the chemical composition and functional properties of KO. This enhanced knowledge would not only enable the refinement of extraction techniques but also empower the creation of a wider range of KO products to address the requirements of specific markets and health objectives.
Availability of data and materials
N/A.
References
Abad, A., & Shahidi, F. Importance of Minor components to the Oxidative Stability of camelina, chia, and sophia seed oils. In Proceedings of the International Society for Nutraceuticals and Functional Foods (ISNFF), Gunsan, South Korea, 22–25 October 2017.
Abad, A., & Shahidi, F. Stabilization of an algal oil with tocopherols and phospholipids from krill oil. In: Proceedings of the International Society for Nutraceuticals and Functional Foods (ISNFF), Honolulu, HI, USA, 10-13 December 2023.
Abad, A., & Shahidi, F. (2020a). A robust stripping method for the removal of minor components from edible oils. Food Production, Processing and Nutrition, 2(1), 1–9.
Abad, A., & Shahidi, F. (2020b). Compositional characteristics and oxidative stability of chia seed oil (Salvia hispanica L). Food Production, Processing and Nutrition, 2(9), 1–8.
Abad, A., & Shahidi, F. (2021). Fatty acid, triacylglycerol and minor component profiles affect oxidative stability of camelina and sophia seed oils. Food Bioscience, 40, 100849.
Abuzaytoun, R., & Shahidi, F. (2006). Oxidative stability of algal oils as affected by their minor components. Journal of Agricultural and Food Chemistry, 54(21), 8253–8260.
Ackman, R. G., & Cormier, M. G. (1967). α-Tocopherol in some Atlantic fish and shellfish with particular reference to live-holding without food. Journal of the Fisheries Research Board of Canada, 24(2), 357–373.
Ahmadkelayeh, S., & Hawboldt, K. (2020). Extraction of lipids and astaxanthin from crustacean by-products: A review on supercritical CO2 extraction. Trends in Food Science & Technology, 103, 94–108.
Ahmmed, M. K., Ahmmed, F., Tian, H., Carne, A., & Bekhit, A. E. D. (2020). Marine omega-3 (n-3) phospholipids: A comprehensive review of their properties, sources, bioavailability, and relation to brain health. Comprehensive Reviews in Food Science and Food Safety, 19(1), 64–123.
Akanbi, T. O., & Barrow, C. J. (2018). Compositional information useful for authentication of krill oil and the detection of adulterants. Food Analytical Methods, 11, 178–187.
Akanbi, T. O., Adcock, J. L., & Barrow, C. J. (2013). Selective concentration of EPA and DHA using Thermomyces lanuginosus lipase is due to fatty acid selectivity and not regioselectivity. Food Chemistry, 138(1), 615–620.
Albalat, A., Nadler, L. E., Foo, N., Dick, J. R., Watts, A. J., Philp, H., … & Monroig, O. (2016). Lipid composition of oil extracted from wasted Norway lobster (Nephrops norvegicus) heads and comparison with oil extracted from Antarctic krill (Euphasia superba). Marine Drugs, 14(12), 219.
Ali-Nehari, A., Kim, S.-B., Lee, Y.-B., & Chun, B.-S. (2012). Digestive enzymes characterization of krill (Euphausia superba) residues deoiled by supercritical carbon dioxide and organic solvents. Journal of Industrial and Engineering Chemistry, 18(4), 1314–1319.
Alvarenga, K. F., Duarte, J. L., Silva, D. P., Agostinhopesse, R. S., Negrato, C. A., & Costa, O. A. (2005). Cognitive P300 potential in subjects with diabetes mellitus. Brazilian Journal of Otorhinolaryngology, 71(2), 202–207.
Alvarez-Ricartes, N., Oliveros-Matus, P., Mendoza, C., Perez-Urrutia, N., Echeverria, F., Iarkov, A., & Echeverria, V. (2018). Intranasal cotinine plus krill oil facilitates fear extinction, decreases depressive-like behavior, and increases hippocampal calcineurin A levels in mice. Molecular Neurobiology, 55(10), 7949–7960.
Ambati, R. R., Phang, S. M., Ravi, S., & Aswathanarayana, R. G. (2014). Astaxanthin: Sources, extraction, stability, biological activities, and its commercial applications—A review. Marine Drugs, 12(1), 128–152.
Andraka, J. M., Sharma, N., & Marchalant, Y. (2020). Can krill oil be of use for counteracting neuroinflammatory processes induced by high fat diet and aging? Neuroscience Research, 157, 1–14.
Araujo, P., Zhu, H., Breivik, J. F., Hjelle, J. I., & Zeng, Y. (2014). Determination and structural elucidation of triacylglycerols in krill oil by chromatographic techniques. Lipids, 49(2), 163–172.
Barbier, O., Arreola-Mendoza, L., & Del Razo, L. M. (2010). Molecular mechanisms of fluoride toxicity. Chemico-Biological Interactions, 188(2), 319–333.
Barenie, M. J., Freemas, J. A., Baranauskas, M. N., Goss, C. S., Freeman, MS, K. L., Chen, MS, X., … & Mickleborough, T. D. (2022). Effectiveness of a combined New Zealand green-lipped mussel and Antarctic krill oil supplement on markers of exercise-induced muscle damage and inflammation in untrained men. Journal of Dietary Supplements, 19(2), 184-211
Barnes, P. J., & Karin, M. (1997). Nuclear factor-κB—a pivotal transcription factor in chronic inflammatory diseases. The New England Journal of Medicine, 336(15), 1066–1071.
Barnhart, K. T., Freeman, E. W., & Sondheimer, S. J. (1995). A clinician’s guide to the premenstrual syndrome. Medical Clinics of North America, 79(6), 1457–1472.
Batetta, B., Griinari, M., Carta, G., Murru, E., Ligresti, A., Cordeddu, L., & Banni, S. (2009). Endocannabinoids may mediate the ability of (n-3) fatty acids to reduce ectopic fat and inflammatory mediators in obese Zucker rats. Journal of Nutrition, 139(8), 1495–1501.
Beaudoin, A., & Martin, G. (2004). Method of extracting lipids from marine and aquatic animal tissues. US Patent 6800299 B1.
Bendich, A. (2000). The potential for dietary supplements to reduce premenstrual syndrome (PMS) symptoms. Journal of the American College of Nutrition, 19(1), 3–12.
Berge, K., Musa-Veloso, K., Harwood, M., Hoem, N., & Burri, L. (2014). Krill oil supplementation lowers serum triglycerides without increasing low-density lipoprotein cholesterol in adults with borderline high or high triglyceride levels. Nutrition Research, 34(2), 126–133.
Block, G., Patterson, B., & Subar, A. (1992). Fruit, vegetables, and cancer prevention: A review of the epidemiological evidence. Nutrition and Cancer, 18(1), 1–29.
Bonaterra, A. G., Driscoll, D., Schwarzbach, H., & Kinscherf, R. (2017). Krill oil-in-water emulsion protects against lipopolysaccharide-induced proinflammatory activation of macrophages in vitro. Marine Drugs, 15(3), 74.
Bruheim, I., Griinari, M., Ervik, J. R., Remoy, S. R., Remoy, E., & Cameron, J. (2016). Method for processing crustaceans to produce low fluoride/low trimethyl amine products thereof. US Patent 20160345616 Al.
Bruheim, I., & Cameron, J. (2017). Flowable concentrated phospholipid krill oil composition. US Patent 20170020928 Al.
Bruheim, I., Tilseth, S., & Mancinelli, D. (2018). Bio effective krill oil compositions. US Patent 9889163 B2.
Bunea, R., El Farrah, K., & Deutsch, L. (2004). Evaluation of the effects of Neptune krill oil on the clinical course of hyperlipidemia. Alternative Medicine Review, 9(4), 420–428.
Burri, L., Hoem, N., Monakhova, Y. B., & Diehl, B. W. (2016). Fingerprinting krill oil by 31 P, 1 H and 13 C NMR spectroscopies. Journal of the American Oil Chemists’ Society, 93, 1037–1049.
Calder, P. C. (2009). Polyunsaturated fatty acids and inflammatory processes: New twists in an old tale. Biochimie, 91(6), 791–795.
Cao, Y., Yang, L., Qiao, X., Xue, C., & Xu, J. (2023). Dietary astaxanthin: An excellent carotenoid with multiple health benefits. Critical Reviews in Food Science and Nutrition, 63(18), 3019–3045.
Castro-Gómez, M. P., Holgado, F., Rodríguez-Alcalá, L. M., Montero, O., & Fontecha, J. (2015). Comprehensive study of the lipid classes of krill oil by fractionation and identification of triacylglycerols, diacylglycerols, and phospholipid molecular species by using UPLC/QToF-MS. Food Analytical Methods, 8(10), 2568–2580.
Chen, Y. C., Tou, J. C., & Jaczynski, J. (2009). Amino acid and mineral composition of protein and other components and their recovery yields from whole Antarctic krill (Euphausia superba) using isoelectric solubilization/precipitation. Journal of Food Science, 74(2), H31–H39.
Chen, D. W., Wan, P., Yao, J., Yang, X., & Liu, J. (2023). Egg yolk phospholipids as an ideal precursor of fatty note odorants for chicken meat and fried foods: a review. Food Chemistry, 407, 135177.
Cheong, L.-Z., Sun, T., Li, Y., Zhou, J., Lu, C., Li, Y., & Su, X. (2017). Dietary krill oil enhances neurocognitive functions and modulates proteomic changes in brain tissues of D-galactose induced aging mice. Food & Function, 8(5), 2038–2045.
Cicero, A. F., & Colletti, A. (2015). Krill oil: Evidence of a new source of polyunsaturated fatty acids with high bioavailability. Clinical Lipidology, 10(1), 1–4.
Cicero, A. F., Reggi, A., Parini, A., & Borghi, C. (2012). State of the art paper Application of polyunsaturated fatty acids in internal medicine: Beyond the established cardiovascular effects. Archives of Medical Science, 8(5), 784–793.
Cicero, A. F., Rosticci, M., Morbini, M., Cagnati, M., Grandi, E., Parini, A., & Borghi, C. (2016). Lipid-lowering and anti-inflammatory effects of omega 3 ethyl esters and krill oil: A randomized, cross-over, clinical trial. Archives of Medical Science, 12(3), 507–512.
Clarke, A. (1980). The biochemical composition of krill, Euphausia superba Dana, from South Georgia. Journal of Experimental Marine Biology and Ecology, 43(3), 221–236.
Colletti, A., Cravotto, G., Citi, V., Martelli, A., Testai, L., & Cicero, A. F. (2021). Advances in technologies for highly active omega-3 fatty acids from krill oil: Clinical applications. Marine Drugs, 19(6), 306.
Constantinou, C., Papas, A., & Constantinou, A. I. (2008). Vitamin E and cancer: An insight into the anticancer activities of vitamin E isomers and analogs. International Journal of Cancer, 123(4), 739–752.
Costanzo, M., Cesi, V., Prete, E., Negroni, A., Palone, F., Cucchiara, S., & Stronati, L. (2016). Krill oil reduces intestinal inflammation by improving epithelial integrity and impairing adherent-invasive Escherichia coli pathogenicity. Digestive and Liver Disease, 48(1), 34–42.
Costanzo, M., Cesi, V., Palone, F., Pierdomenico, M., Colantoni, E., Leter, B., & Stronati, L. (2018). Krill oil, vitamin D and Lactobacillus reuteri cooperate to reduce gut inflammation. Beneficial Microbes, 9(3), 389–399.
Da Boit, M., Mastalurova, I., Brazaite, G., McGovern, N., Thompson, K., & Gray, S. R. (2015). The effect of krill oil supplementation on exercise performance and markers of immune function. PLoS ONE, 10(9), e0139174.
Dalheim, L., Svenning, J. B., & Olsen, R. L. (2021). In vitro intestinal digestion of lipids from the marine diatom Porosira glacialis compared to commercial LC n-3 PUFA products. PLoS ONE, 16(6), e0252125.
Derohanes, E., Rayl, K., Siwek, M., & Williams, J. (2018). Co-Q10, krill oil and vitamin D. US Patent 20180084813 Al.
Deutsch, L. (2007). Evaluation of the effect of Neptune krill oil on chronic inflammation and arthritic symptoms. Journal of the American College of Nutrition, 26(1), 39–48.
Di Marzo, V. (2008). The endocannabinoid system in obesity and type 2 diabetes. Diabetologia, 51(8), 1356.
Di Marzo, V., Griinari, M., Carta, G., Murru, E., Ligresti, A., Cordeddu, L., & Banni, S. (2010). Dietary krill oil increases docosahexaenoic acid and reduces 2-arachidonoylglycerol but not N-acylethanolamine levels in the brain of obese Zucker rats. International Dairy Journal, 20(4), 231–235.
Dickerson, L. M., Mazyck, P. J., & Hunter, M. H. (2003). Premenstrual syndrome. American Family Physician, 67(8), 1743–1752.
Domínguez, H., Núñez, M. J., & Lema, J. M. (1994). Enzymatic pretreatment to enhance oil extraction from fruits and oilseeds: A review. Food Chemistry, 49(3), 271–286.
Folch, J., Lees, M., & Stanley, G. S. (1957). A simple method for the isolation and purification of total lipides from animal tissues. Journal of Biological Chemistry, 226(1), 497–509.
Foss, P., Renstrøm, B., & Liaaen-Jensen, S. (1987). Natural occurrence of enantiomeric and Meso astaxanthin 7∗-crustaceans including zooplankton. Comparative Biochemistry and Physiology Part b: Comparative Biochemistry, 86(2), 313–314.
Francis, P. T., Palmer, A. M., Snape, M., & Wilcock, G. K. (1999). The cholinergic hypothesis of Alzheimer’s disease: A review of progress. Journal of Neurology, Neurosurgery & Psychiatry, 66(2), 137.
Fricke, H., Gercken, G., Schreiber, W., & Oehlenschläger, J. (1984). Lipid, sterol and fatty acid composition of Antarctic krill (Euphausia superba Dana). Lipids, 19(11), 821–827.
Friedrich, J. P., & Pryde, E. H. (1984). Supercritical CO2 extraction of lipid-bearing materials and characterization of the products. Journal of the American Oil Chemists’ Society, 61(2), 223–228.
Fuller, I. D., Cumming, A. H., Card, A., Burgess, E. J., Barrow, C. J., Perry, N. B., & Killeen, D. P. (2020). Free fatty acids in commercial krill oils: Concentrations, compositions, and implications for oxidative stability. Journal of the American Oil Chemists’ Society, 97(8), 889–900.
Fuxing, W., Lu, D., Yanyun, Z., & Zhengwang, Z. (2017). Impacts of global climate change on birds and marine mammals in Antarctica. Advances in Polar Science, 28(1), 1–12.
Gan, W. Q. (2004). Association between chronic obstructive pulmonary disease and systemic inflammation: A systematic review and a meta-analysis. Thorax, 59(7), 574–580.
Gang, K. Q., Wu, Z. X., Zhou, D. Y., Zhao, Q., Zhou, X., Lv, D. D., & Shahidi, F. (2019). Effects of hot air drying process on lipid quality of whelks Neptunea arthritica cumingi Crosse and Neverita didyma. Journal of Food Science and Technology, 56, 4166–4176.
Georges, J., Sharp, M. H., Lowery, R. P., Wilson, J. M., Purpura, M., Hornberger, T. A., & Jäger, R. (2018). The effects of krill oil on mTOR signaling and resistance exercise: a pilot study. Journal of Nutrition and Metabolism, 7625981, 1–7.
Gigliotti, J. C., Davenport, M. P., Beamer, S. K., Tou, J. C., & Jaczynski, J. (2011). Extraction and characterisation of lipids from Antarctic krill (Euphausia superba). Food Chemistry, 125(3), 1028–1036.
Grimstad, T., Bjørndal, B., Cacabelos, D., Aasprong, O. G., Janssen, E. A. M., Omdal, R., & Berge, R. K. (2012). Dietary supplementation of krill oil attenuates inflammation and oxidative stress in experimental ulcerative colitis in rats. Scandinavian Journal of Gastroenterology, 47(1), 49–58.
Hals, P.-A., Wang, X., & Xiao, Y.-F. (2017). Effects of a purified krill oil phospholipid rich in long-chain omega-3 fatty acids on cardiovascular disease risk factors in non-human primates with naturally occurring diabetes type-2 and dyslipidemia. Lipids in Health and Disease, 16(1), 1–11.
Han, X., & Liu, D. (2019). Detection and analysis of 17 steroid hormones by ultra-high-performance liquid chromatography-electrospray ionization mass spectrometry (UHPLC-MS) in different sex and maturity stages of Antarctic krill (Euphausia superba Dana). PLoS ONE, 14(3), e0213398.
Hansenne, M. (2000). The p300 cognitive event-related potential. II. Individual variability and clinical application in psychopathology. Neurophysiologie Clinique-Clinical Neurophysiology, 30(4), 211–231.
Harris, W. S., Connor, W. E., Alam, N., & Illingworth, D. R. (1988). Reduction of postprandial triglyceridemia in humans by dietary n-3 fatty acids. Journal of Lipid Research, 29(11), 1451–1460.
Hibino, S., Mase, M., Shirataki, T., Nagano, Y., Fukagawa, K., Abe, A., & Kabasawa, H. (2013). Oxyhemoglobin changes during cognitive rehabilitation after traumatic brain injury using near infrared spectroscopy. Neurologia Medico-Chirurgica, 53(5), 299–303.
Huenerlage, K., Graeve, M., & Buchholz, F. (2016). Lipid composition and trophic relationships of krill species in a high Arctic fjord. Polar Biology, 39, 1803–1817.
Hupfeld, S. (2018). U.S. Patent No. 10,080,803. U.S. Patent and Trademark Office.
Ibrahim, S. H., Hirsova, P., Malhi, H., & Gores, G. J. (2016). Animal models of nonalcoholic steatohepatitis: Eat, delete, and inflame. Digestive Diseases and Sciences, 61, 1325–1336.
Ierna, M., Kerr, A., Scales, H., Berge, K., & Griinari, M. (2010). Supplementation of diet with krill oil protects against experimental rheumatoid arthritis. BMC Musculoskeletal Disorders, 11(1), 1–11.
Ivanova, Z., Bjørndal, B., Grigorova, N., Roussenov, A., Vachkova, E., Berge, K., & Georgiev, I. P. (2015). Effect of fish and krill oil supplementation on glucose tolerance in rabbits with experimentally induced obesity. European Journal of Nutrition, 54(7), 1055–1067.
Janicki, S. C., & Schupf, N. (2010). Hormonal influences on cognition and risk for Alzheimer’s disease. Current Neurology and Neuroscience Reports, 10(5), 359–366.
Jansson, S. T. K., Ervik, J. R., & Grimsmo, L. (2018). Separating crustacean polar phospholipid compositions without emulsification. US Patent 9907321 B2.
Jayathilake, A. G., Senior, P. V., & Su, X. Q. (2016). Krill oil extract suppresses cell growth and induces apoptosis of human colorectal cancer cells. BMC Complementary and Alternative Medicine, 16(1), 328.
Jayathilake, A. G., Veale, M. F., Luwor, R. B., Nurgali, K., & Su, X. Q. (2020). Krill oil extract inhibits the migration of human colorectal cancer cells and down-regulates EGFR signalling and PD-L1 expression. BMC Complementary Medicine and Therapies, 20, 1–15.
Jemal, A., Murray, T., Ward, E., Samuels, A., Tiwari, R. C., Ghafoor, A., & Thun, M. J. (2005). Cancer statistics, 2005. CA: A Cancer Journal for Clinicians, 55(1), 10–30.
Jemal, A., Bray, F., Center, M. M., Ferlay, J., Ward, E., & Forman, D. (2011). Global cancer statistics. CA: A Cancer Journal for Clinicians, 61(2), 69–90.
Jiang, S., Zhang, T. T., Cong, P. X., Xu, J., Xue, C. H., Jiang, X. M., & Wang, Y. M. (2020). Comparative study on the digestion and absorption characteristics of n-3 LCPUFA-enriched phospholipids in the form of liposomes and emulsions. Food Research International, 137, 109428.
Katevas, D. S., Toro Guerra, R. R., & Chiong Lay, M. (2014). Solvent-free process for obtaining phospholipids and neutral enriched krill oils. US Patent 8865236 B2.
Khan, L. M., & Hanna, M. A. (1983). Expression of oil from oilseeds—A review. Journal of Agricultural Engineering Research, 28(6), 495–503.
Kim, J. H., Meng, H. W., He, M. T., Choi, J. M., Lee, D., & Cho, E. J. (2020). Krill oil attenuates cognitive impairment by the regulation of oxidative stress and neuronal apoptosis in an amyloid β-induced Alzheimer’s disease mouse model. Molecules, 25(17), 3942.
Köhler, A., Sarkkinen, E., Tapola, N., Niskanen, T., & Bruheim, I. (2015). Bioavailability of fatty acids from krill oil, krill meal and fish oil in healthy subjects–a randomized, single-dose, cross-over trial. Lipids in Health and Disease, 14(1), 1–10.
Kołakowska, A. (1991). The influence of sex and maturity stage of krill (Euphausia superba Dana) upon the content and composition of its lipid. Polish Polar Research, 12(1), 73–78.
Konagai, C., Yanagimoto, K., Hayamizu, K., Han, L., Tsuji, T., & Koga, Y. (2013). Effects of krill oil containing n-3 polyunsaturated fatty acids in phospholipid form on human brain function: A randomized controlled trial in healthy elderly volunteers. Clinical Interventions in Aging, 8, 1247–1257.
Laidlaw, M., Cockerline, C. A., & Rowe, W. J. (2014). A randomized clinical trial to determine the efficacy of manufacturers’ recommended doses of omega-3 fatty acids from different sources in facilitating cardiovascular disease risk reduction. Lipids in Health and Disease, 13(1), 99.
Lambertsen, G., & Braekkan, O. R. (1971). Method of analysis of astaxanthin and its occurrence in some marine products. Journal of the Science of Food and Agriculture, 22(2), 99–101.
Larsen, P. M., Fey, S. J., Breuning, J., & Ludvigsen, B. A. (2007). Method for the Extraction of Lipid Fractions from Krill. WO Patent, 2007080514, A2.
Laslett, L. L., Antony, B., Wluka, A. E., Hill, C., March, L., Keen, H. I., & Jones, G. (2020). KARAOKE: Krill oil versus placebo in the treatment of knee osteoarthritis: Protocol for a randomised controlled trial. Trials, 21, 1–14.
Le Grandois, J., Marchioni, E., Zhao, M., Giuffrida, F., Ennahar, S., & Bindler, F. (2009). Investigation of natural phosphatidylcholine sources: Separation and identification by liquid chromatography-electrospray ionization-tandem mass spectrometry (LC-ES-MS2) of molecular species. Journal of Agricultural and Food Chemistry, 57(14), 6014–6020.
Lee, S. (2014). Krill oil and method for manufacturing the same. US Patent 8624046 B2.
Li, D.-M., Zhou, D.-Y., Zhu, B.-W., Chi, Y.-L., Sun, L.-M., Dong, X.-P., & Murata, Y. (2013). Effects of krill oil intake on plasma cholesterol and glucose levels in rats fed a high-cholesterol diet. Journal of the Science of Food and Agriculture, 93(11), 2669–2675.
Li, Q., Wu, F., Wen, M., Yanagita, T., Xue, C., Zhang, T., & Wang, Y. (2018). The protective effect of Antarctic krill oil on cognitive function by inhibiting oxidative stress in the brain of senescence-accelerated prone mouse strain 8 (SAMP8) mice. Journal of Food Science, 83(2), 543–551.
Lim, C. W., Kim, B. H., Kim, I. H., & Lee, M. W. (2015). Modeling and optimization of phospholipase A 1-catalyzed hydrolysis of phosphatidylcholine using response surface methodology for lysophosphatidylcholine production. Biotechnology Progress, 31(1), 35–41.
Liu, H. M., Wang, F. Y., Li, H. Y., Wang, X. D., & Qin, G. Y. (2015). Subcritical butane and propane extraction of oil from rice bran. BioResources, 10(3), 4652–4662.
Liu, S., Hu, W., Fang, Y., Cai, Y., Zhang, J., Liu, J., & Ding, Y. (2019). Extraction of oil from wet Antarctic krill (Euphausia superba) using a subcritical dimethyl ether method. RSC Advances, 9(59), 34274–34282.
Lu, J.-Y., Huang, K.-C., Chang, L.-C., Huang, Y.-S., Chi, Y.-C., Su, T.-C., & Yang, W.-S. (2008). Adiponectin: A biomarker of obesity-induced insulin resistance in adipose tissue and beyond. Journal of Biomedical Science, 15(5), 565–576.
Maki, K. C., Reeves, M. S., Farmer, M., Griinari, M., Berge, K., Vik, H., & Rains, T. M. (2009). Krill oil supplementation increases plasma concentrations of eicosapentaenoic and docosahexaenoic acids in overweight and obese men and women. Nutrition Research, 29(9), 609–615.
Mansour, S. Z., Moustafa, E. M., Hassan, A. A., & Thabet, N. M. (2017). Protective role of krill oil against estrogen deficiency induced neurodegeneration in ovariectomized rats. Indian Journal of Experimental Biology, 55(05), 279–285.
Marventano, S., Kolacz, P., Castellano, S., Galvano, F., Buscemi, S., Mistretta, A., & Grosso, G. (2015). A review of recent evidence in human studies of n-3 and n-6 PUFA intake on cardiovascular disease, cancer, and depressive disorders: Does the ratio really matter. International Journal of Food Sciences and Nutrition, 66(6), 611–622.
Matias, I., Carta, G., Murru, E., Petrosino, S., Banni, S., & Di Marzo, V. (2008). Effect of polyunsaturated fatty acids on endocannabinoid and N-acyl-ethanolamine levels in mouse adipocytes. Biochimica et Biophysica Acta (BBA)-Molecular and Cell Biology of Lipids, 1781(1), 52–60.
Matsuda, H., Wang, T., Managi, H., & Yoshikawa, M. (2003). Structural requirements of flavonoids for inhibition of protein glycation and radical scavenging activities. Bioorganic & Medicinal Chemistry, 11(24), 5317–5323.
Mayo-Wilson, E., Imdad, A., Herzer, K., Yakoob, M. Y., & Bhutta, Z. A. (2011). Vitamin A supplements for preventing mortality, illness, and blindness in children aged under 5: Systematic review and meta-analysis. British Medical Journal, 343:d5094, 1–19.
Mendoza, C., Perez-Urrutia, N., Alvarez-Ricartes, N., Barreto, G. E., Pérez-Ordás, R., Iarkov, A., & Echeverria, V. (2018). Cotinine plus krill oil decreased depressive behavior, and increased astrocytes survival in the hippocampus of mice subjected to restraint stress. Frontiers in Neuroscience, 12, 952.
Merken, H. M., & Beecher, G. R. (2000). Measurement of food flavonoids by high-performance liquid chromatography: A review. Journal of Agricultural and Food Chemistry, 48(3), 577–599.
Miki, W. (1991). Biological functions and activities of animal carotenoids. Pure and Applied Chemistry, 63(1), 141–146.
Monteiro, R., & Azevedo, I. (2010). Chronic inflammation in obesity and the metabolic syndrome. Mediators of Inflammation, 2010, 1–10.
Nissinen, M. J., Gylling, H., & Miettinen, T. A. (2008). Responses of surrogate markers of cholesterol absorption and synthesis to changes in cholesterol metabolism during various amounts of fat and cholesterol feeding among healthy men. British Journal of Nutrition, 99(2), 370–378.
Omar, M. H., Mullen, W., & Crozier, A. (2011). Identification of proanthocyanidin dimers and trimers, flavone C-glycosides, and antioxidants in Ficus deltoidea, a Malaysian herbal tea. Journal of Agricultural and Food Chemistry, 59(4), 1363–1369.
Papakonstantinou, E., Lambadiari, V., Dimitriadis, G., & Zampelas, A. (2013). Metabolic syndrome and cardiometabolic risk factors. Current Vascular Pharmacology, 11(6), 858–879.
Phleger, C. F., Nelson, M. M., Mooney, B. D., & Nichols, P. D. (2002). Interannual and between species comparison of the lipids, fatty acids and sterols of Antarctic krill from the US AMLR Elephant Island survey area. Comparative Biochemistry and Physiology Part b: Biochemistry and Molecular Biology, 131(4), 733–747.
Piscitelli, F., Carta, G., Bisogno, T., Murru, E., Cordeddu, L., Berge, K., & Di Marzo, V. (2011). Effect of dietary krill oil supplementation on the endocannabinoidome of metabolically relevant tissues from high-fat-fed mice. Nutrition & Metabolism, 8(1), 51.
Prommetta, K., Attamangkune, S., & Ruangpanit, Y. (2020). Krill meal enhances antioxidant levels and n-3 fatty acid content of egg yolk from laying hens fed a low-pigment diet. The Journal of Poultry Science, 57(3), 192–199.
Ramprasath, V. R., Eyal, I., Zchut, S., & Jones, P. J. (2013). Enhanced increase of omega-3 index in healthy individuals with response to 4-week n-3 fatty acid supplementation from krill oil versus fish oil. Lipids in Health and Disease, 12(1), 1–11.
Rao, A. R., Sindhuja, H. N., Dharmesh, S. M., Sankar, K. U., Sarada, R., & Ravishankar, G. A. (2013). Effective inhibition of skin cancer, tyrosinase, and antioxidative properties by astaxanthin and astaxanthin esters from the green alga Haematococcus pluvialis. Journal of Agricultural and Food Chemistry, 61(16), 3842–3851.
Rizos, E. C., Ntzani, E. E., Bika, E., Kostapanos, M. S., & Elisaf, M. S. (2012). Association between omega-3 fatty acid supplementation and risk of major cardiovascular disease events: A systematic review and meta-analysis. Journal of the American Medical Association, 308(10), 1024–1033.
Rockway, S. (2006). Compositions including krill extracts and conjugated linoleic acid and methods of using same. US Patent 20060078625 Al.
Ronen, L., Ran, N., & Ben-Dror, G. (2017). Krill oil preparations with optimal mineral and metal composition, low impurities and low and stable TMA levels. US Patent 20170354694 Al.
Rossmeisl, M., Macek Jilkova, Z., Kuda, O., Jelenik, T., Medrikova, D., Stankova, B., & Kopecky, J. (2012). Metabolic effects of n-3 PUFA as phospholipids are superior to triglycerides in mice fed a high-fat diet: Possible role of endocannabinoids. PLoS ONE, 7(6), e38834.
Rundblad, A., Holven, K. B., Bruheim, I., Andreassen, R., Myhrstad, M. C., & Ulven, S. M. (2017). Effects of krill oil and lean and fatty fish on lipoprotein subclasses and low molecular-weight metabolites: A randomized controlled trial. Scripta Scientifica Pharmaceutica, 4(1), 1–7.
Rundblad, A., Holven, K. B., Bruheim, I., Myhrstad, M. C., & Ulven, S. M. (2018). Effects of krill oil and lean and fatty fish on cardiovascular risk markers: A randomised controlled trial. Journal of Nutritional Science, 7, e3.
Saito, M., Ishida, N., Yamada, H., Ibi, M., & Hirose, M. (2020). 8-HEPE-concentrated materials from Pacific krill improve plasma cholesterol levels and hepatic steatosis in high cholesterol diet-fed low-density lipoprotein (LDL) receptor-deficient mice. Biological and Pharmaceutical Bulletin, 43(6), 919–924.
Sakai, Y., Hashimoto, M., Enkhjargal, B., Mitsuishi, H., Nobe, H., Horie, I., & Yanagimoto, K. (2014). Effects of krill-derived phospholipid-enriched n-3 fatty acids on Ca2+ regulation system in cerebral arteries from ovariectomized rats. Life Sciences, 100(1), 18–24.
Sampalis, F., Bunea, R., Pelland, M. F., Kowalski, O., Duguet, N., & Dupuis, S. (2003). Evaluation of the effects of Neptune Krill OilTM on the management of premenstrual syndrome and dysmenorrhea. Alternative Medicine Review, 8(2), 171–179.
Sampalis, T. (2011). Krill extracts for treatment of cardiovascular diseases. US Patent 8057825 B2.
Sampalis, F. (2013). Natural marine source phospholipids comprising flavonoids, polyunsaturated fatty acids and their applications. AU Patent 2013205516 A1.
Saravanan, P., Davidson, N. C., Schmidt, E. B., & Calder, P. C. (2010). Cardiovascular effects of marine omega-3 fatty acids. The Lancet, 376(9740), 540–550.
Schuchardt, J. P., Schneider, I., Meyer, H., Neubronner, J., von Schacky, C., & Hahn, A. (2011). Incorporation of EPA and DHA into plasma phospholipids in response to different omega-3 fatty acid formulations – a comparative bioavailability study of fish oil vs. krill oil. Lipids in Health and Disease, 10(1), 145.
Serock, M. R., Wells, A. K., & Khalil, R. A. (2008). Modulators of vascular sex hormone receptors and their effects in estrogen-deficiency states associated with menopause. Recent Patents on Cardiovascular Drug Discovery, 3(3), 165–186.
Shahidi, F., & Abad, A. (2019). Lipid-Derived Flavours and Off-Flavours in Food. In Encyclopedia of Food Chemistry, 2, 182–192.
Shahidi, F., & Yeo, J. (2018). Bioactivities of phenolics by focusing on suppression of chronic diseases: A review. International Journal of Molecular Sciences, 19(6), 1573.
Shahidi, F., Ambigaipalan, P., Abad, A., & Pegg, R. B. (2020). Food and bioactive encapsulation. In: Handbook of food preservation. CRC Press. pp. 529–596
Siriwardhana, N., Kalupahana, N. S., & Moustaid-Moussa, N. (2012). Health benefits of n-3 polyunsaturated fatty acids: Eicosapentaenoic acid and docosahexaenoic acid. Advances in Food and Nutrition Research, 65, 211–222.
Soevik, T., & Braekkan, O. R. (1979). Fluoride in Antarctic krill (Euphausia superba) and Atlantic krill (Meganyctiphanes norvegica). Journal of the Fisheries Research Board of Canada, 36(11), 1414–1416.
Sohrabi, N., Kashanian, M., Ghafoori, S. S., & Malakouti, S. K. (2013). Evaluation of the effect of omega-3 fatty acids in the treatment of premenstrual syndrome: “a pilot trial.” Complementary Therapies in Medicine, 21(3), 141–146.
Standal, I. B., Axelson, D. E., & Aursand, M. (2009). Differentiation of fish oils according to species by 13C-NMR regiospecific analyses of triacyglycerols. Journal of the American Oil Chemists’ Society, 86(5), 401–407.
Stevinson, C., & Ernst, E. (2001). Complementary/alternative therapies for premenstrual syndrome: A systematic review of randomized controlled trials. American Journal of Obstetrics and Gynecology, 185(1), 227–235.
Storsve, A. B., Johnsen, L., Nyborg, C., Melau, J., Hisdal, J., & Burri, L. (2020). Effects of krill oil and race distance on serum choline and choline metabolites in triathletes: A field study. Frontiers in Nutrition, 7, 133.
Su, X., Tanalgo, P., Bustos, M., & Dass, C. R. (2018). The effect of krill oil and n-3 polyunsaturated fatty acids on human osteosarcoma cell proliferation and migration. Current Drug Targets, 19(5), 479–486.
Sun, J., & Mao, X. (2016). An environmental friendly process for Antarctic krill (Euphausia superba) utilization using fermentation technology. Journal of Cleaner Production, 127, 618–623.
Sun, D., Cao, C., Li, B., Chen, H., Cao, P., Li, J., & Liu, Y. (2017a). Study on combined heat pump drying with freeze-drying of Antarctic krill and its effects on the lipids. Journal of Food Process Engineering, 40(6), 12577.
Sun, D., Zhang, L., Chen, H., Feng, R., Cao, P., & Liu, Y. (2017b). Effects of Antarctic krill oil on lipid and glucose metabolism in C57BL/6J mice fed with high-fat diet. Lipids in Health and Disease, 16(1), 218.
Sun, D., Cao, C., Li, B., Chen, H., Li, J., Cao, P., & Liu, Y. (2018). Antarctic krill lipid extracted by subcritical n-butane and comparison with supercritical CO2 and conventional solvent extraction. LWT - Food Science and Technology, 94, 1–7.
Sun, W., Shi, B., Xue, C., & Jiang, X. (2019). The comparison of krill oil extracted through ethanol–hexane method and subcritical method. Food Science & Nutrition, 7(2), 700–710.
Suzuki, T., & Shibata, N. (1990). The utilization of Antarctic krill for human food. Food Reviews International, 6(1), 119–147.
Takama, K., Suzuki, T., Yoshida, K., Arai, H., & Anma, H. (1994). Lipid content and fatty acid composition of phospholipids in white-flesh fish species. Fisheries Science, 60(2), 177–184.
Tandy, S., Chung, R. W. S., Wat, E., Kamili, A., Berge, K., Griinari, M., & Cohn, J. S. (2009). Dietary krill oil supplementation reduces hepatic steatosis, glycemia, and hypercholesterolemia in high-fat-fed mice. Journal of Agricultural and Food Chemistry, 57(19), 9339–9345.
Tillander, V., Bjørndal, B., Burri, L., Bohov, P., Skorve, J., Berge, R. K., & Alexson, S. E. H. (2014). Fish oil and krill oil supplementations differentially regulate lipid catabolic and synthetic pathways in mice. Nutrition & Metabolism, 11(1), 1–17.
Tilseth, S., & Høstmark, Ø (2015). New method for making krill meal. US Patent 20150050403 A1.
Tilseth, S. (2010). Low viscosity phospholipid compositions. U.S. Patent Application No. 12/711,553.
Tomé-Carneiro, J., Crespo Carmen, M., Burgos-Ramos, E., Tomas-Zapico, C., García-Serrano, A., Castro-Gómez, & Visioli, F. (2018). Buttermilk and krill oil phospholipids improve hippocampal insulin resistance and synaptic signaling in aged rats. Molecular Neurobiology, 55(9), 7285–7296.
Torre, L. A., Bray, F., Siegel, R. L., Ferlay, J., Lortet-Tieulent, J., & Jemal, A. (2015). Global cancer statistics, 2012. CA: A Cancer Journal for Clinicians, 65(2), 87–108.
Ullah, A., Munir, S., Badshah, S. L., Khan, N., Ghani, L., Poulson, B. G., & Jaremko, M. (2020). Important flavonoids and their role as a therapeutic agent. Molecules, 25(22), 5243.
Ulven, S. M., Kirkhus, B., Lamglait, A., Basu, S., Elind, E., Haider, T., & Pedersen, J. I. (2011). Metabolic effects of krill oil are essentially similar to those of fish oil but at lower dose of EPA and DHA, in healthy volunteers. Lipids, 46(1), 37–46.
Valk, & Hornstra, G. (2000). Relationship between vitamin E requirement and polyunsaturated fatty acid intake in man: A review. International Journal for Vitamin and Nutrition Research, 70(2), 31–42.
Van der Wurff, I. S. M., Von Schacky, C., Berge, K., Kirschner, P. A., & de Groot, R. H. M. (2016). A protocol for a randomised controlled trial investigating the effect of increasing Omega-3 index with krill oil supplementation on learning, cognition, behaviour and visual processing in typically developing adolescents. British Medical Journal Open, 6(7), e011790.
Van der Wurff, I. S. M., Von Schacky, C., Bergeland, T., Leontjevas, R., Zeegers, M. P., Kirschner, P. A., & de Groot, R. H. M. (2020). Effect of one year krill oil supplementation on depressive symptoms and self-esteem of Dutch adolescents: A randomized controlled trial. Prostaglandins, Leukotrienes and Essential Fatty Acids, 163, 102208.
Vigerust, N. F., Bjørndal, B., Bohov, P., Brattelid, T., Svardal, A., & Berge, R. K. (2013). Krill oil versus fish oil in modulation of inflammation and lipid metabolism in mice transgenic for TNF-α. European Journal of Nutrition, 52(4), 1315–1325.
Wang, L., Yang, F., Rong, Y., Yuan, Y., Ding, Y., Shi, W., & Wang, Z. (2019). Effects of different proteases enzymatic extraction on the lipid yield and quality of Antarctic krill oil. Food Science & Nutrition, 7(7), 2224–2230.
WHO, Food and Agriculture Organization of the United Nations. Codex Standard for Fish Oils. Codex Alimentarius Commission; Codex Standard 329–2017; WHO: Geneva, Switzerland; Food and Agriculture Organization of the United Nations: Quebec, QC, Canada, 2017.
Winther, B., Hoem, N., Berge, K., & Reubsaet, L. (2011). Elucidation of phosphatidylcholine composition in krill oil extracted from Euphausia superba. Lipids, 46(1), 25–36.
Wu, Y., Mou, B., Song, S., Tan, C. P., Lai, O. M., Shen, C., & Cheong, L. Z. (2020). Curcumin-loaded liposomes prepared from bovine milk and krill phospholipids: Effects of chemical composition on storage stability, in-vitro digestibility and anti-hyperglycemic properties. Food Research International, 136, 109301.
Xie, D., Jin, J., Sun, J., Liang, L., Wang, X., Zhang, W., & Jin, Q. (2017). Comparison of solvents for extraction of krill oil from krill meal: Lipid yield, phospholipids content, fatty acids composition and minor components. Food Chemistry, 233, 434–441.
Xie, D., Mu, H., Tang, T., Wang, X., Wei, W., Jin, J., & Jin, Q. (2018). Production of three types of krill oils from krill meal by a three-step solvent extraction procedure. Food Chemistry, 248, 279–286.
Xie, D., Gong, M., Wei, W., Jin, J., Wang, X., Wang, X., & Jin, Q. (2019). Antarctic krill (Euphausia superba) oil: A comprehensive review of chemical composition, extraction technologies, health benefits, and current applications. Comprehensive Reviews in Food Science and Food Safety, 18(2), 514–534.
Xie, D., He, F., Wang, X., Wang, X., Jin, Q., & Jin, J. (2021). Diverse krill lipid fractions differentially reduce LPS-induced inflammatory markers in RAW264. 7 macrophages in vitro. Foods, 10(11), 2887.
Yamaguchi, K., Miki, W., Toriu, N., Kondo, Y., Murakami, M., Konosu, S., & Fujta, T. (1983). The composition of carotenoid pigments in the Antarctic krill (Euphausia superba). Nippon Suisan Gakkaishi, 49(9), 1411–1415.
Yamaguchi, K., Murakami, M., Nakano, H., Konosu, S., Kokura, T., Yamamoto, H., & Hata, K. (1986). Supercritical carbon dioxide extraction of oils from Antarctic krill. Journal of Agricultural and Food Chemistry, 34(5), 904–907.
Yang, G., Lee, J., Lee, S., Kwak, D., Choe, W., Kang, I., & Ha, J. (2016). Krill oil supplementation improves dyslipidemia and lowers body weight in mice fed a high-fat diet through activation of AMP-activated protein kinase. Journal of Medicinal Food, 19(12), 1120–1129.
Yin, F.-W., Liu, X.-Y., Fan, X.-R., Zhou, D.-Y., Xu, W.-S., Zhu, B.-W., & Murata, Y.-Y. (2015). Extrusion of Antarctic krill (Euphausia superba) meal and its effect on oil extraction. International Journal of Food Science & Technology, 50(3), 633–639.
Yoshitomi, B., & Shigematsu, Y. (2003). Process for making dried powdery and granular krill. US Patent, 20030113432, A1.
Young, B., Gleeson, M., & Cripps, A. W. (1991). C-reactive protein: A critical review. Pathology, 23(2), 118–124.
Zadeh-Ardabili, P. M., Rad, S. K., Rad, S. K., & Movafagh, A. (2019). Antidepressant–like effects of fish, krill oils and Vit B12 against exposure to stress environment in mice models: Current status and pilot study. Scientific Reports, 9(1), 19953.
Zeb, L., Teng, X. N., Shafiq, M., Wang, S. C., Xiu, Z. L., & Su, Z. G. (2021). Three-liquid-phase salting-out extraction of eicosapentaenoic acid (EPA) and docosahexaenoic acid (DHA)-rich oils from Euphausia superba. Engineering in Life Sciences, 21(10), 666–682.
Zeng, X. B., Yin, F. W., Zhao, G. H., Guo, C., Li, D. Y., Liu, H. L., … & Zhou, D. Y. (2024). Mechanism of color change in Antarctic krill oil during storage. Food Chemistry, 444(3), 138583.
Zhang, Y., Zhang, T., Liang, Y., Jiang, L., & Sui, X. (2021). Dietary bioactive lipids: A review on absorption, metabolism, and health properties. Journal of Agricultural and Food Chemistry, 69(32), 8929–8943.
Zhao, L., Yin, B., Liu, Q., & Cao, R. (2013). Purification of antimicrobial peptide from Antarctic krill (Euphausia superba) and its function mechanism. Journal of Ocean University of China, 12(3), 484–490.
Zheng, W., Wang, X., Cao, W., Yang, B., Mu, Y., Dong, Y., & Xiu, Z. (2017). E-configuration structures of EPA and DHA derived from Euphausia superba and their significant inhibitive effects on growth of human cancer cell lines in vitro. Prostaglandins, Leukotrienes and Essential Fatty Acids, 117, 47–53.
Zhou, L., Wu, X., Yang, F., Zhang, M., Huang, R., & Liu, J. (2021). Characterization of molecular species and anti-inflammatory activity of purified phospholipids from Antarctic krill oil. Marine Drugs, 19(3), 124.
Zhu, J.-J., Shi, J.-H., Qian, W.-B., Cai, Z.-Z., & Li, D. (2008). Effects of krill oil on serum lipids of hyperlipidemic rats and human SW480 cells. Lipids in Health and Disease, 7(1), 30–36.
Acknowledgements
Fereidoon Shahidi thanks the NSERC of Canada for Grant. Abrehem abad thank Libyan Ministry of Higher Education & Scientific Research provided a scholarship as well as thnk Food and Drug Control Center- Libya.
Funding
The author FS thank the Natural Science and Engineering Research Council (NSERC) of Canada for support in the form of a Discovery Grant (RGPIN-2016–04468). The author AA achnowledges a scholarship from the Libyan Ministry of Higher Education and Scientific Research as well as Food and Drug Control Center – Libya.
Author information
Authors and Affiliations
Contributions
Conceptualization, AA and FS; AA wrote the original draft; Preparation of the final version and editing, FS; supervision, FS; Both authors have read and agreed to the publication of the manuscript.
Corresponding author
Ethics declarations
Ethics a pproval and consent to participate
Not applicaple.
Consent for publication
Authors have read and agreed to the submission of the manuscript.
Competing interests
Dr. Fereidoon Shahidi is the EiC of Food Production, Processing and Nutrition and he was not involved in the journal's review of, or decisions related to this manuscript.
Additional information
Publisher's Note
Springer Nature remains neutral with regard to jurisdictional claims in published maps and institutional affiliations.
Rights and permissions
Open Access This article is licensed under a Creative Commons Attribution 4.0 International License, which permits use, sharing, adaptation, distribution and reproduction in any medium or format, as long as you give appropriate credit to the original author(s) and the source, provide a link to the Creative Commons licence, and indicate if changes were made. The images or other third party material in this article are included in the article's Creative Commons licence, unless indicated otherwise in a credit line to the material. If material is not included in the article's Creative Commons licence and your intended use is not permitted by statutory regulation or exceeds the permitted use, you will need to obtain permission directly from the copyright holder. To view a copy of this licence, visit http://creativecommons.org/licenses/by/4.0/.
About this article
Cite this article
Shahidi, F., Abad, A. Why is Antactic krill (Euphasia superba) oil on the spotlight? A review. Food Prod Process and Nutr 6, 88 (2024). https://doi.org/10.1186/s43014-024-00260-6
Received:
Accepted:
Published:
DOI: https://doi.org/10.1186/s43014-024-00260-6