Abstract
Background
β-Thalassemia is an inherited haematological blood disorder in the HBB gene, and variations in this HBB gene lead to the absence/deficiency of the Beta chain synthesis of haemoglobin leading to severe anaemia. Klinefelter syndrome is a chromosomal abnormality that affects physical and cognitive development in males. Affected individuals are taller, show gynaecomastia and behavioural problems and have small testes that do not produce much testosterone. We describe a boy with β-Thalassemia major referred for chromosomal analysis due to delayed puberty and short stature. This is a second case reported in the literature that gives information on two different contradicting genetic disorders in a single individual but novel case as he exhibits additional short-stature phenotype.
Case presentation
A 17-year-old boy with short stature and gonadal dysfunction was referred for chromosomal analysis. He needed blood transfusion every 4 weeks. The GTG banding for chromosomal analysis and standard PCR for variant detection of HBB gene, Bi-directional Sanger sequencing of the PCR products and multiplex PCR for Y microdeletion of the AZF a, b and c regions on the Y chromosome were performed. The cytogenetic analysis revealed a karyotype of 47,XXY. The HBB gene detected two heterozygous variants forming a pathogenic compound heterozygous condition. The multiplex PCR revealed that the AZF a, b and c regions were intact and were not deleted.
Conclusion
To our knowledge, this is the second case of a patient with β-Thalassemia associated with Klinefelter syndrome but a novel case with short-stature phenotype association instead of tall stature. The possible association of these two disorders and the unusual phenotypic presentation are discussed. This study highlights the possibility of ruling out Thalassemia while evaluating patients with short stature and delayed puberty.
Similar content being viewed by others
Background
Klinefelter syndrome (KS) is the most common sex chromosomal abnormality affecting approximately 1 in 500–700 males. It is usually associated with the occurrence of an extra X chromosome [1]. In addition, it is an endocrine disorder associated with tall stature, gynaecomastia, hypogonadism, small testes, impaired spermatogenesis and androgen deficiency.
β-Thalassemia is a genetic blood disorder that reduces the production of haemoglobin. It shows an autosomal recessive inheritance pattern. A person with two copies of the abnormal gene has the disease, while those with only one copy are carriers. Carriers do not have the disease condition but can pass the defective gene to their offspring. The homozygous variations at the haemoglobin Beta locus (HBB) gene on chromosome 11p15.4 cause this disorder. There are more than 300 variations reported in this gene. The clinical symptoms include failure to thrive, jaundice, enlarged spleen, liver and heart and misshapen of bones. Few adults show growth retardation, delayed puberty and frequent blood transfusions. In some cases, excess blood transfusions may result in iron overload in the body resulting in liver, heart and hormonal problems.
Usually, the association of these two different genetic disorders is rare, with a few exceptions reported in the literature, e.g., a case of Klinefelter syndrome with Thalassemia intermedia [2], a case of mosaic Klinefelter syndrome associated with Thalassemia [3]; recently, there is a case of hereditary xerocytosis due to variations in PIEZO1 gene associated with heterozygous pyruvate kinase deficiency and β-Thalassemia trait [4]. Also, there is a case of Thalassemia and Klinefelter syndrome but without short-stature phenotype [5]. This study reports a boy with β-Thalassemia major associated with Klinefelter syndrome with short stature. Ours is the second report in the literature showing the association of these two genetic disorders to the best of our knowledge.
Case presentation
A 17-year-old boy who is second born to non-consanguineous parents was referred for short stature, gonadal dysfunction, small testes, and he was on transfusion for anaemia every 4 weeks. He had undergone splenectomy 4 years ago. His height was in the 3rd centile and not in the puberty age. His haemoglobin concentration was decreased about 10.5 g/dl (normal range is 13–16); ESR showed a mild increase, the WBC count was normal, but RBC was decreased; the hormonal profiles were done for serum oestradiol which was within the range (19 pg/ml) (normal range 10–40), and serum testosterone was decreased (237.18 ng/dL) (normal range 300–800), but serum FSH (41mIU/ml) (normal range 1.5–12) and serum LH (31.50 mIU/ml) (1.2–8) were increased. The hormonal profile was suggestive of primary hypogonadism. His iron overload was maintained by chelating therapy. The HBA2 level was 4.5%. The institutional ethical consent was taken from the patient as per the guidelines.
Cytogenetic analyses
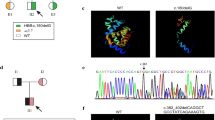
Chromosomal analysis was carried out on peripheral blood lymphocytes by GTG banding, revealing a karyotype of 47,XXY (Fig. 1A).
HBB mutation analysis
The DNA from the peripheral blood was isolated (Qiagen DNeasy extraction kit) and subjected to PCR (Qiagen Fast Cycling PCR kit) using multiple primer sets covering the HBB gene. The PCR products were bi-directionally sequenced by the Sanger method on ABI PRISM 3730XL Genetic analyser. The chromatograms showed two heterozygous variants. One is in exon 1, showing an IVS1-5 (G > C) heterozygous variation (Fig. 1B) and the other in exon 3 showing Poly A (T > C) variant (Fig. 1C). Thus, a pathogenic compound heterozygous condition was identified in the HBB gene.
Y microdeletion detections (AZF genes)
The DNA was subjected to multiplex PCR with seven sets of STR primers specific for two AZFa, AZFb and AZFc regions, along with internal control ZFX/Y genes. Two multiplex PCRs were set up; 1: AZFc1 (sY254), AZFa2 (sY86), AZFb1 (sY127) ZFX/Y. 2: AZFa1 (sY134), AZFc2 (sY255), ZFX/Y. The amplified products were analysed by agarose gel electrophoreses. All the AZF genes in this patient were intact, and there were no deletions.
Discussion
Thalassemia is among the most common genetic disorders occurring worldwide, resulting in the defective synthesis of haemoglobin. Treatment can help, but this condition cannot be cured as there will be no or insufficient blood production in such patients; they require regular blood transfusions. The patients are usually stunted in growth with hepatosplenomegaly and other features.
KS is usually associated with gynaecomastia, tall stature and infertility. This condition is generally identified in men's prepubertal stage due to the non-development of secondary sexual characters. But, rarely, the chromosomal analysis would be done in childhood since the manifestation of the secondary sexual characters would be shown in the pubertal stage.
In our study, the patient was anaemic and was under transfusion and suspected of Thalassemia with additional features of short stature and gonadal dysfunction. He was referred for short-stature evaluation, and a chromosomal analysis was done. Although haemoglobin electrophoresis confirmed Thalassemia, the mutation was unknown. Hence, mutation detection confirmed compound heterozygous variants in the HBB gene. Also, as the secondary sexual characters were underdeveloped, the AZF gene deletions were checked. The chromosomal analysis revealed 47,XXY, and the AZF gene on Y chromosome showed no deletions. There are reports of Thalassemia with delayed puberty and growth retardation in literature. And as mentioned, the features of KS are tall stature and infertility. In this case, although delayed puberty is a feature associated with both Thalassemia and KS, the short-stature phenotype in the patient is a feature that is not found in KS. Still, the stunted growth may be due to blood transfusion.
KS is associated with the development of tumours of the seminiferous tubules and breast cancer [6]. There have also been few reports of the coexistence of haematological malignancies and KS [6]. Autoimmune diseases, diabetes mellitus and hypothyroidism, are also common in KS. Hence, testosterone replacement therapy could be started early to minimize androgen deficiency's physical and psychological effects [7].
In our case, the short stature is probably caused due to Thalassemia, and although KS is present, the tall stature is masked.
We recommend that chromosomal analysis be offered as the first-tier test to all the Thalassemia male children to rule out any sex chromosomal abnormalities. This would help the patients in early diagnosis of suspected malignancies. Furthermore, it also helps in counselling the patient and further referring to the Endocrinologist for any tumours.
In conclusion, this is the second case in the literature with the coexistence of two different genetic disorders exhibited in a single individual. Although the first case reports both Thalassemia and Klinefelter syndrome associations, their case had no short stature. The diagnosis at an early age could help better manage genetic disorders.
Availability of data materials
The datasets used and/or analysed during the current study are available from the corresponding author on reasonable request.
Abbreviations
- β-Thalassemia:
-
Beta Thalassemia
- KS:
-
Klinefelter syndrome
- HBB gene:
-
Haemoglobin Beta locus
- ESR:
-
Erythrocyte sedimentation rate
- WBC:
-
White blood cells
- RBC:
-
Red blood cells
- FSH:
-
Follicle-stimulating hormone
- LH:
-
Luteinizing hormone
- GTG:
-
G-banding using trypsin and Giemsa
- DNA:
-
Deoxyribonucleic acid
- PCR:
-
Polymerase chain reaction
- AZF:
-
Azoospermia factor
References
Jacobs PA (1979) Recurrence risks for chromosome abnormalities. Birth Defects 15:71–80
Vlachaki E, Katzos G, Koussi A, Tsatra I, Perifanis V, Athanasioun M (2005) A patient with Klinefelter’s syndrome and thalassemia intermedia. J Pediatr Endocrinol Metab 18:413–415
Crawfurd MD (1961) Chromosomal mosaicism in a case of Klinefelter’s syndrome associated with thalassaemia. Ann Hum Genet 25:153–158
Fermo E, Vercellati C, Marcello AP, Zaninoni A, Wijk R, Mirra N, Curcio C, Cortelezzi A, Zanella A, Barcellini W, Bianchi P (2017) Hereditary Xerocytosis due to mutations in PIEZO1 gene associated with heterozygous pyruvate kinase deficiency and beta-thalassemia trait in two unrelated families. Case Rep Hematol. https://doi.org/10.1155/2017/2769570
Venkatanarasu A, Boddula R, Chinte C, Santosh B, Tickoo V (2020) Klinefelters syndrome and thalassemia major- a rare association. Int J Adv Res 8:955–958
Keung YK, Buss D, Chauvenet A, Pettenati M (2002) Hematologic malignancies and Klinefelter syndrome: a chance association? Cancer Genet Cytogenet 139:9–13
Visootsak J, Aylstock Μ, Graham JM Jr (2001) Klinefelter syndrome and its variants: an update and review for the primary paediatrician. Clin Pediatr 40:639651
Acknowledgements
The authors acknowledge the patient family.
Funding
None.
Author information
Authors and Affiliations
Contributions
SGC had performed molecular genetic testing of the Beta Thalassemia gene. MJM is involved in the multiplex PCR of Y microdeletions of the AZF gene. RMR has done the chromosomal analysis. TRS had done the clinical workup of the patient. URD conceptualized and wrote the manuscript. All authors read and approved the final manuscript.
Corresponding author
Ethics declarations
Ethical approval and consent to participate
The ethical approval is taken by the Institutional ethical committee. The written consent was taken from the patient's father for publication.
Consent for publication
The institute consent form also includes the consent to publish.
Competing interests
The authors declare that they have no competing interests.
Additional information
Publisher's Note
Springer Nature remains neutral with regard to jurisdictional claims in published maps and institutional affiliations.
Rights and permissions
Open Access This article is licensed under a Creative Commons Attribution 4.0 International License, which permits use, sharing, adaptation, distribution and reproduction in any medium or format, as long as you give appropriate credit to the original author(s) and the source, provide a link to the Creative Commons licence, and indicate if changes were made. The images or other third party material in this article are included in the article's Creative Commons licence, unless indicated otherwise in a credit line to the material. If material is not included in the article's Creative Commons licence and your intended use is not permitted by statutory regulation or exceeds the permitted use, you will need to obtain permission directly from the copyright holder. To view a copy of this licence, visit http://creativecommons.org/licenses/by/4.0/.
About this article
Cite this article
Billapati, S., Sowmya, G.C., Tapadia, R.S. et al. Beta Thalassemia and Klinefelter syndrome: a rare occurrence. Egypt J Med Hum Genet 23, 87 (2022). https://doi.org/10.1186/s43042-022-00300-1
Received:
Accepted:
Published:
DOI: https://doi.org/10.1186/s43042-022-00300-1