Abstract
Objective
Recent studies have indicated a potential association between giant cell arteritis (GCA) and diabetes mellitus, encompassing both type 1 diabetes (T1D) and type 2 diabetes (T2D). However, the exact nature of this relationship requires further investigation to be fully elucidated.
Methods
Genetic links between T1D/T2D and GCA were explored using data from genome-wide association studies available to the public, focusing on populations of European ancestry. We applied a bidirectional mendelian randomization (MR) approach to assess the potential association between these diseases. Confirmatory analyses, including additional datasets and a comprehensive meta-analysis, were utilized. The inverse-variance-weighted (IVW) method was applied to pinpoint heterogeneity and pleiotropy, while subsequent sensitivity analyses aimed to trace the origins of any heterogeneity.
Results
Initial analysis demonstrated a correlation between T1D and an elevated likelihood of developing GCA (IVW odds ratio = 1.33, with a 95% confidence interval of 1.22–1.46, and a P-value of 9.42E−10). The causal association was verified through four validation datasets and meta-analysis (all P-value < 0.001). However, the reverse MR analysis was unable to detect any genetic basis for the increased risk of T1D due to GCA. Furthermore, we could not establish any causal links between T2D and GCA.
Conclusion
T1D patients may have a higher risk of developing GCA, whereas an inverse causal relationship was not evident. Furthermore, no causal relationship was detected between T2D and GCA. These insights shed light on the possible pathological mechanisms underlying GCA and may influence the future clinical handling of both T1D and GCA.
Similar content being viewed by others
Background
Giant cell arteritis (GCA) is a type of vascular inflammation characterized by granulomatous swelling within the medium to large arteries, notably the temporal artery, and predominantly affects individuals over the age of 50 [1]. This condition manifests through symptoms such as persistent headaches, pain in the jaw when chewing, and tenderness of the scalp, in addition to increased levels of inflammatory markers like C-reactive protein and the erythrocyte sedimentation rate [2]. While Temporal artery biopsy (TAB) remains the standard for diagnosing GCA, less invasive techniques such as Doppler ultrasound, PET-CT, and MRI are progressively being adopted in diagnostics [3,4,5]. GCA has been traditionally treated using substantial doses of glucocorticoids (GCs), which come with a spectrum of potential side effects. Tocilizumab, an inhibitor to the interleukin-6 (IL-6) receptor, is a promising alternative that may lower the reliance on glucocorticoids [6, 7].
Diabetes mellitus (DM) refers to a group of chronic conditions marked by inadequate regulation of blood glucose levels due to insufficient insulin production and/or inefficient insulin action. If left unmanaged, the chronic hyperglycemic state arising as a result can cause various complications affecting the cardiovascular, nervous, and renal systems [8]. Type 1 diabetes (T1D) is characterized by an autoimmune attack on the insulin-secreting beta cells in the pancreas, and typically develops in younger populations [9]. On the other hand, type 2 diabetes (T2D) usually arises from insulin resistance and is more common among adults and the elderly [10]. Both types of diabetes pose significant health risks if not properly managed, including cardiovascular complications, kidney disease, and nerve damage.
The correlation between GCA and blood glucose levels is ambiguous. Population-based cohort studies have shown an inverse correlation between the incidence of GCA and increased levels of fasting blood glucose [11]. Moreover, several studies have reported lower glycated hemoglobin (HbA1c) levels and prevalence of diabetes among individuals diagnosed with GCA [12, 13]. However, Esen et al. [14] demonstrated increased baseline glucose levels in GCA patients compared to the general population, although no differences were observed in HbA1c levels. In a case report of a male T1D patient, acute upper respiratory infection was followed by the development of biopsy-proven GCA [15], which underscores the complex interactions between autoimmune diseases and infections. The interrelation between T2D and GCA is similarly multifaceted, with some studies suggesting a potential protective effect of T2D against developing GCA [16], Abel et al. [17] found that T2D increased the risk of GCA by 100% among the beneficiaries of Medicare. In addition, high-dose (GC) treatment of GCA increases the body mass index (BMI), which in turn can elevate the risk of T2D [18,19,20].
Case studies and observational analyses are often restricted by various factors, such as potential confounding influences, limited participant numbers, and variations across population ethnicities, which hinders the ability to firmly establish causative links. Consequently, the nature of the association between DM and GCA—whether causative, coincidental, or a one-way or two-way relationship—remains to be clarified. Mendelian randomization (MR) leverages genetic variants as instrumental variables to sidestep limitations inherent in observational studies, and therefore can offer more definitive insights. MR utilizes the single nucleotide polymorphisms (SNPs) sourced from extensive genome-wide association studies (GWAS) to elucidate the relationship between an exposure and its outcome, while minimizing the confounding effects [21,22,23]. As SNPs assort independently according to Mendelian inheritance during gamete formation, MR analyses are generally less susceptible to reverse causation and lingering bias [24]. The aim of this study was to examine a possible causal link between DM and GCA using bi-directional MR analyses based on data collated in GWAS.
Materials and methods
Study design
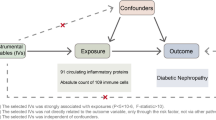
Several key hypotheses were made: (1) Appropriateness of IVs: the selected instrumental variables (IVs) are appropriate because they have strong correlations with exposure (T1D/T2D or GCA). These IVs are well-documented in existing research for their strong association with exposure (T1D/T2D or GCA), making them suitable for the analysis. (2) Independence from confounders: the IVs used are not influenced by confounding variables in the T1D/T2D and GCA relationship. The IVs known to be independent of confounding factors were selected to ensure a clear causal pathway. (3) Exogeneity of IVs regarding outcome: the IVs affect outcome (GCA or T1D/T2D) through mechanisms unrelated to exposure (T1D/T2D or GCA). Causality was investigated with diabetes as the influencing factor and GCA as the outcome, followed by an inverse assessment with GCA as the influencer and diabetes as the outcome (Fig. 1).
Overview of the MR analysis. (A) T1D/T2D-related SNPs were used as instrumental variables (IVs) to investigate the potential causal effect of T1D/T2D on GCA. (B) GCA-related SNPs were used as IVs to investigate the potential causal effect of GCA on T1D/T2D. Solid lines: instrumental variables (SNPs) that are linked to the exposure and can solely affect the outcome through exposure. Dotted lines: instrumental variables (SNPs) that are unrelated to any confounding factors and are between the exposure and the outcome. The confounding factors included exposure to environmental toxins, radiation, infectious agents, chemical substances, smoking, and prior pregnancy
Data sources
The inclusion criteria for data were as follows: (1) The data must be from articles published within the last three years. (2) The included data should represent either the largest or one of the largest sample sizes currently available. (3) The number of SNPs in the data must reach 107. The exclusion criteria for data were as follows: (1) Data from articles published a long time ago. (2) Data with a relatively small sample size. (3) Data with a small number of SNPs. (4) Data for which the original datasets are not accessible. The data for T1D was obtained from five GWASs. The discovery T1D GWAS data was sourced from FinnGen Release 10 [25], using the phenocode “T1D_STRICT2” for identification. In addition, four validation T1D datasets (GCST90014023 [26], GCST90018925 [27], GCST90013891 [28], and GCST90013941 [28]), along with one T2D dataset (GCST90018926 [27]), were accessed from the IEU OpenGWAS database, located at https://gwas.mrcieu.ac.uk/. Furthermore, aggregate GCA genetic insights were also derived from FinnGen Release 10, earmarked with the phenocode “M13_GIANTCELL”. All GWAS data were retrieved by December 31, 2023. Comprehensive GWAS datasets pertaining to T1D, T2D, and GCA are delineated in Table 1.
Selection of IVs
Genome-wide association significance for the SNPs was set as P value < 5 × 10–8, the independence of all selected SNPs was within 10,000 kb, and linkage disequilibrium threshold r2 was less than 0.001 when T1D/T2D (GCST90014023, GCST90018925, and GCST90018926) was taken as the exposure. When T1D (T1D_STRICT2, GCST90013891, and GCST90013941) was the exposure, the significance threshold for the SNPs was P value < 1 × 10–6, the independence of all selected SNPs was within 1000 kb, and linkage disequilibrium threshold r2 was 0.01. In scenarios where GCA served as the exposure, SNP significance was defined by a P value of less than 1 × 10–5, LD coefficient cap set at r2 < 0.01, and LD search span restricted to 10,000 kb. The F-statistic of each SNP was ascertained to confirm robustness, requiring an F-value above 10. The SNP dataset was further refined to exclude SNPs that were palindromic or could not be unambiguously interpreted. To guard against possible pleiotropic effects, IVs tied to confounding factors or recognized GCA risk factors, like exposure to other autoimmune disorders, were screened out using the PhenoScanner V2 tool (available at http://www.phenoscanner.medschl.cam.ac.uk/).
MR analyses
The primary methodology adopted for MR analysis was the inverse variance weighted (IVW) method, which can mitigate pleiotropic variations and produce dependable outcomes. To further evaluate causation under varying conditions, MR-Egger regression was employed alongside the calculation of a weighted median [29,30,31]. The findings were articulated as odds ratios (ORs) along with their 95% confidence intervals (CIs). A p value threshold of less than 0.05 was determined to denote statistical significance. The statistical computations were performed using the TwoSampleMR package (version 0.5.7) of R version 4.3.1 [32].
Sensitivity analysis
The MR-Egger intercept was employed to probe for horizontal pleiotropy, where the IVs might influence the outcome through several distinct channels. The presence of horizontal pleiotropy was visualized using scatter plots. Heterogeneity among the IVs was quantified using Cochran’s Q test, with P values below 0.05 indicating significant variance. Funnel plots were used to visualize heterogeneity and validate the stability of the results. The results were further verified through the leave-one-out sensitivity analysis, which involves sequential omission of each SNP. Moreover, the MR pleiotropy residual sum and outlier test was applied to scrutinize the direct influence of the IVs on the outcomes, and to evaluate any residual horizontal pleiotropy.
Results
Causal relationships between T1D and GCA
We use the rigorously screened T1D dataset (T1D_STRICT2) as the exposure data for the discovery stage. Based on the IVW approach, T1D was associated with a significant increase in the risk of GCA (OR = 1.33, 95% CI 1.22–1.46, P = 9.42E−10) (Table 2). Therefore, we selected the GWAS data of four European T1D datasets (GCST90013891, GCST90013941, GCST90014023, and GCST90018925) with a current sample size of over 400000 as the exposure data for the validation phase, and observed that T1D increased the risk of GCA development (IVW: all P-value < 0.05) (Table 3, Supplementary Figs. 1–4). Additionally, a meta-analysis of the IVW method was conducted on these four datasets, which also revealed a significant increase in the GCA risk due to T1D (P < 0.0001; Fig. 2). We then performed reverse MR analysis to explore the effect of GCA on five T1D GWAS data. However, there was no indication of a reverse causal link with the IVW technique (Table 2 and Supplementary Table 1). Furthermore, the MR-Egger intercept test (scatter plots shown in Fig. 3A, Supplementary Figs. 1A, 2A, 3A and 4A), sensitivity analysis (forest plots shown in Fig. 3B, Supplementary Figs. 1B, 2B, 3B and 4B), leave-one-out analysis (Fig. 3C, Supplementary Figs. 1C, 2C, 3C and 4C), and Cochran’s Q statistics (funnel plots shown in Fig. 3D, Supplementary Figs. 1D, 2D, 3D and 4D) did not indicate any significant heterogeneity in the impact of T1D on GCA risk. We also did not detect any directional pleiotropy (Table 2 and Table 3), and the robustness of the data was confirmed by the leave-one-out analyses. The analysis revealed a lack of significant heterogeneity or directional pleiotropy when evaluating the influence of GCA on T1D, as demonstrated by the MR-Egger intercept, Cochran’s Q test, and further corroborated by leave-one-out and sensitivity analyses (Supplementary Table 1 and Figs. 5–9).
Causal links between T2D and GCA
To elucidate the potential causal links between T2D and GCA, a bidirectional MR approach was employed. As indicated in Table 1, the analysis did not establish any causal link between T2D and GCA. Furthermore, the sensitivity tests, including the MR-Egger intercept and Cochran’s Q statistics, along with the leave-one-out method, consistently found no notable heterogeneity or directional pleiotropy (Supplementary Figs. 10 and 11).
Discussion
This study is possibly the first to apply a bidirectional MR framework to uncover a causative link between DM and GCA. Our results confirm a significant correlation between genetically predicted T1D and a greater risk of GCA. However, reverse MR analysis did not indicate that a genetic predisposition to GCA increased the risk of developing T1D. In addition, no causal relationship was detected between T2D and GCA.
GCA, an inflammatory disease of large and medium-sized blood vessels, is characterized by granulomatous inflammation affecting, and mainly occurs in the elderly [1]. In addition to specific symptoms like headaches, jaw claudication, limb claudication and vision loss, patients frequently experience non-specific manifestations such as fever and weight loss [2]. Previous studies have reported abnormalities in blood glucose and HbAc1 levels in patients with GCA [11, 12, 14]. However, there are no documented cases of GCA patients developing T1D. This is not surprising given that GCA is more common in individuals older than 50 years [1], while T1D primarily manifests in adolescence due to a genetic predisposition [9]. Nevertheless, T1D and GCA share a few susceptibility genetic variations, such as PTPN22 1858C/T polymorphism and STAT4 rs7574865, which provide indirect evidence of an association between the two diseases [33, 34]. Furthermore, several inflammatory factors are elevated during T1D and GCA, such as interleukin 21 [35]. There is however one reported case of late GCA onset in a 62-year-old patient with T1D, who developed biopsy-confirmed GCA after suffering an acute upper respiratory infection. Serological tests revealed concomitant Streptococcus pneumoniae infection. The clinical signs of vasculitis disappeared in a month without any immunosuppressive therapy, and no relapse was noted during the ensuing 12 months [18]. The natural course of GCA is still unknown, and the respiratory infection may have contributed to its development. In fact, some recent studies have demonstrated an indirect link between infection and GCA development [36, 37]. However, the aforementioned individual case reports do not clearly establish a causal relationship between T1D and GCA. Furthermore, there is a lack of large-scale and comprehensive genetic studies investigating the relationship between the two conditions. In this study, we used the latest five GWAS datasets for both T1D and GCA, and applied rigorous selection criteria to T1D as the exposure variable. Subsequently, four sets of T1D GWAS data were used for validation, and a meta-analysis was also conducted. Therefore, our findings regarding the causal relationship between T1D and GCA are reliable and highly representative, and consistently indicate that T1D can increase the risk of developing GCA. Conversely, there is no genetic evidence of GCA influencing the development of T1D.
T2D, a common chronic metabolic disorder in the elderly population, is often caused by the development of insulin resistance, which gradually impairs the ability of pancreatic beta cells to secrete sufficient levels of insulin. Several studies have suggested a potential protective influence of T2D against GCA [19, 38, 39], and the proposed mechanisms include aberrant cytokine secretion, weakened T cell response, and decreased dendritic cell activity due to high antigen glycosylation [19, 38, 39]. Furthermore, a new study reported lower incidence of biopsy-positive GCA in T2D patients, along with less prevalence of T2D in subjects with positive TAB as opposed to those with a negative TAB [19]. However, another study conducted on a single-center large cohort showed that T2D patients older than 68 years had a higher chance of being diagnosed with GCA [20]. A possible explanation is that the cell-mediated immune dysfunction in T2D and GCA exhibit similar abnormalities. GCA patients often experience a reduction in the circulating regulatory T cells (Tregs), which has not been observed in T2D patients [40, 41]. Therefore, the presence of functional Tregs in T2D patients may increase the risk of GCA. The primary treatment for GCA entails high doses of orally administered GCs [42]. However, prolonged GC exposure can lead to severe adverse events, notably T2D [18,19,20]. The average daily dose of oral GC has been linked to hyperglycemia, glucose intolerance, and the initiation of new T2D. Furthermore, up to 50% of GCA patients experience worsening of underlying T2D in response to GC treatment, although it may not always correspond with the average daily GC dose [43,44,45,46]. However, these observational studies and individual case reports do not clearly establish a causal relationship between T2D and GCA. The complex relationship between these two conditions needs to be validated through large-scale, extensive, and comprehensive genetic studies. We used the most recent GWAS datasets for T2D and GCA for the present MR analysis. The GWAS for T2D included 38,841 cases and 451,248 controls from the IEU OpenGWAS database, and the GWAS for GCA had 1066 cases and 399,355 controls from the FinnGen database. Our results indicated lack of any causal connections between T2D and GCA.
The present study is the first to employ a 2-sample bidirectional MR approach to dissect the possible bidirectional causality between GCA and T1D/T2D. Currently, most of the research on the relationship between T1D/T2D and GCA consists of observational studies or individual case reports. Reverse causation, confounding, and inaccurate non-differentially assessed exposures are less common with MR analysis compared to observational studies or individual case reports. We also employed several MR framework methodologies to conduct sensitivity analyses on these causal links, which verified the consistency of point estimates both before and after outlier removal, and successfully reduced heterogeneity. Furthermore, we employed an extensive summary-level available GWAS dataset to obtain all the relevant genetic data.
There are several limitations in this study that ought to be considered. First, the modest number of SNPs included as IVs decreased the total statistical power, even though there was minimal possibility of moderate IV bias affecting our findings (all F-statistics were larger than 10). Therefore, additional subjects need to be included in future MR analyses. Second, the publicly available GWAS data for GCA are currently limited to populations of European ancestry. The lack of GWAS datasets for GCAin the Asian, Black, or Middle Eastern populations limits our ability to study the causal relationship between T1D/T2D and GCA in these populations. Therefore, caution should be exercised when extrapolating the findings of this study to non-European populations. To ensure broader applicability and understand the potential differences in genetic predispositions, further studies are needed that include diverse populations. This will help validate the findings in a more comprehensive manner and explore any potential variations in the causal relationship between T1D/T2D and GCA across different ethnic groups. Third, MR studies are essentially statistical constructs, which overlook the intricate interactions between genetic and environmental factors in autoimmune illnesses like GCA and DM. Therefore, the causal factors identified through this approach should be applied with caution in clinical settings. Given the steady increase in both the number and size of GWASs, future studies will likely involve larger samples and more comprehensive patient data.
Conclusion
This study pioneers the use of MR to dissect the bidirectional causality between DM and GCA. The evidence we have gathered indicates an increased risk of GCA in patients with T1D, but not a reciprocal causal effect. Furthermore, our study did not establish any causal links between T2D and GCA. These outcomes underscore the need for a more comprehensive investigation into the mechanisms underlying the causal association between DM and the occurrence of GCA.
Availability of data and materials
This study utilized publicly available datasets, which were obtained from the IEU OpenGWAS database (https://gwas.mrcieu.ac.uk/) and the FinnGen consortium (https://www.finngen.fi/).
Abbreviations
- GCA:
-
Giant cell arteritis
- TAB:
-
Temporal artery biopsy
- DM:
-
Diabetes mellitus
- T1D:
-
Type 1 diabetes
- T2D:
-
Type 2 diabetes
- GC:
-
Glucocorticoid
- MR:
-
Mendelian randomization
- IVs:
-
Instrumental variables
- SNPs:
-
Single nucleotide polymorphisms
- GWAS:
-
Genome-wide association studies
- IVW:
-
Inverse variance weighted
- ORs:
-
Odds ratios
- CIs:
-
Confidence intervals
References
Farina N, Tomelleri A, Campochiaro C, Dagna L (2023) Giant cell arteritis: update on clinical manifestations, diagnosis, and management. Eur J Intern Med 107:17–26. https://doi.org/10.1016/j.ejim.2022.10.025
Bilton EJ, Mollan SP (2023) Giant cell arteritis: reviewing the advancing diagnostics and management. Eye 37(12):2365–2373. https://doi.org/10.1038/s41433-023-02433-y
Narvaez J, Estrada P, Vidal-Montal P, Nolla JM (2023) Performance of the new 2022 ACR/EULAR classification criteria for giant cell arteritis in clinical practice in relation to its clinical phenotypes. Autoimmun Rev 22(10):103413. https://doi.org/10.1016/j.autrev.2023.103413
Moreel L, Betrains A, Doumen M, Molenberghs G, Vanderschueren S, Blockmans D (2023) Diagnostic yield of combined cranial and large vessel PET/CT, ultrasound and MRI in giant cell arteritis: a systematic review and meta-analysis. Autoimmun Rev 22(7):103355. https://doi.org/10.1016/j.autrev.2023.103355
Owen CE, Yates M, Liew DFL, Poon AMT, Keen HI, Hill CL, Mackie SL (2023) Imaging of giant cell arteritis - recent advances. Best Pract Res Clin Rheumatol 37:101827. https://doi.org/10.1016/j.berh.2023.101827
Ford JA, Gewurz D, Gewurz-Singer O (2023) Tocilizumab in giant cell arteritis: an update for the clinician. Curr Opin Rheumatol 35(3):135–140. https://doi.org/10.1097/BOR.0000000000000937
Springer JM, Kermani TA (2023) Recent advances in the treatment of giant cell arteritis. Best Pract Res Clin Rheumatol 37:101830. https://doi.org/10.1016/j.berh.2023.101830
Zhao X, An X, Yang C, Sun W, Ji H, Lian F (2023) The crucial role and mechanism of insulin resistance in metabolic disease. Front Endocrinol 14:1149239. https://doi.org/10.3389/fendo.2023.1149239
Siddiqui K, Nawaz SS (2023) Exploration of immune targets for type 1 diabetes and latent autoimmune disease immunotherapy. Immunotargets Ther 12:91–103. https://doi.org/10.2147/ITT.S417917
Berbudi A, Rahmadika N, Tjahjadi AI, Ruslami R (2020) Type 2 diabetes and its impact on the immune system. Curr Diabetes Rev 16(5):442–449. https://doi.org/10.2174/1573399815666191024085838
Wadstrom K, Jacobsson L, Mohammad AJ, Warrington KJ, Matteson EL, Turesson C (2021) Comment on: negative associations for fasting blood glucose, cholesterol and triglyceride levels with the development of giant cell arteritis: reply. Rheumatology 60(7):e262–e263. https://doi.org/10.1093/rheumatology/keab143
Mukhtyar C, Myers H, Jones C, Dhatariya K (2020) The relationship between glycated haemoglobin levels and the risk of giant cell arteritis - a case-control study. Rheumatol Adv Pract 4(2):rkaa018. https://doi.org/10.1093/rap/rkaa018
Ungprasert P, Upala S, Sanguankeo A, Warrington KJ (2016) Patients with giant cell arteritis have a lower prevalence of diabetes mellitus: a systematic review and meta-analysis. Mod Rheumatol 26(3):410–414. https://doi.org/10.3109/14397595.2015.1081722
Esen I, Arends S, Dalsgaard Nielsen B, Therkildsen P, Hansen I, van ‘t Ende A, Heeringa P, Boots A, Hauge E, Brouwer E, van Sleen Y (2023) Metabolic features and glucocorticoid-induced comorbidities in patients with giant cell arteritis and polymyalgia rheumatica in a Dutch and Danish cohort. RMD Open 9(1):e002640. https://doi.org/10.1136/rmdopen-2022-002640
Maekawa M, Iwadate T, Watanabe K, Yamamoto R, Imaizumi T, Yamakawa T (2019) Spontaneous remission of giant cell arteritis: possible association with a preceding acute respiratory infection and seropositivity to Chlamydia pneumoniae antibodies. Nagoya J Med Sci 81(1):151–158. https://doi.org/10.18999/nagjms.81.1.151
Matthews JL, Gilbert DN, Farris BK, Siatkowski RM (2012) Prevalence of diabetes mellitus in biopsy-positive giant cell arteritis. J Neuroophthalmol 32(3):202–206. https://doi.org/10.1097/WNO.0b013e31825103cb
Abel AS, Yashkin AP, Sloan FA, Lee MS (2015) Effect of diabetes mellitus on giant cell arteritis. J Neuroophthalmol 35(2):134–138. https://doi.org/10.1097/WNO.0000000000000218
Lai LYH, Harris E, West RM, Mackie SL (2018) Association between glucocorticoid therapy and incidence of diabetes mellitus in polymyalgia rheumatica and giant cell arteritis: a systematic review and meta-analysis. RMD Open 4(1):e000521. https://doi.org/10.1136/rmdopen-2017-000521
Savas M, Muka T, Wester VL, van den Akker ELT, Visser JA, Braunstahl GJ, Slagter SN, Wolffenbuttel BHR, Franco OH, van Rossum EFC (2017) Associations between systemic and local corticosteroid use with metabolic syndrome and body mass index. J Clin Endocrinol Metab 102(10):3765–3774. https://doi.org/10.1210/jc.2017-01133
Wu J, Mackie SL, Pujades-Rodriguez M (2020) Glucocorticoid dose-dependent risk of type 2 diabetes in six immune-mediated inflammatory diseases: a population-based cohort analysis. BMJ Open Diabetes Res Care 8(1):e001220. https://doi.org/10.1136/bmjdrc-2020-001220
Smith GD, Ebrahim S (2003) ‘Mendelian randomization’: can genetic epidemiology contribute to understanding environmental determinants of disease? Int J Epidemiol 32(1):1–22. https://doi.org/10.1093/ije/dyg070
Smith GD, Ebrahim S (2004) Mendelian randomization: prospects, potentials, and limitations. Int J Epidemiol 33(1):30–42. https://doi.org/10.1093/ije/dyh132
Emdin CA, Khera AV, Kathiresan S (2017) Mendelian randomization. JAMA 318(19):1925–1926. https://doi.org/10.1001/jama.2017.17219
Zheng J, Baird D, Borges MC, Bowden J, Hemani G, Haycock P, Evans DM, Smith GD (2017) Recent developments in mendelian randomization studies. Curr Epidemiol Rep 4(4):330–345. https://doi.org/10.1007/s40471-017-0128-6
Kurki MI, Karjalainen J, Palta P, Sipilä TP, Kristiansson K, Donner K, Reeve MP, Laivuori H, Aavikko M, Kaunisto MA, Loukola A, Lahtela E, Mattsson H, Laiho P, Della Briotta Parolo P, Lehisto A, Kanai M, Mars N, Rämö J, Kiiskinen T, Heyne HO, Veerapen K, Rüeger S, Lemmelä S, Zhou W, Ruotsalainen S, Pärn K, Hiekkalinna T, Koskelainen S, Paajanen T, Llorens V, Gracia-Tabuenca J, Siirtola H, Reis K, Elnahas AG, Aalto-Setälä K, Alasoo K, Arvas M, Auro K, Biswas S, Bizaki-Vallaskangas A, Carpen O, Chen C-Y, Dada OA, Ding Z, Ehm MG, Eklund K, Färkkilä M, Finucane H, Ganna A, Ghazal A, Graham RR, Green E, Hakanen A, Hautalahti M, Hedman Å, Hiltunen M, Hinttala R, Hovatta I, Hu X, Huertas-Vazquez A, Huilaja L, Hunkapiller J, Jacob H, Jensen J-N, Joensuu H, John S, Julkunen V, Jung M, Junttila J, Kaarniranta K, Kähönen M, Kajanne RM, Kallio L, Kälviäinen R, Kaprio J, Kerimov N, Kettunen J, Kilpeläinen E, Kilpi T, Klinger K, Kosma V-M, Kuopio T, Kurra V, Laisk T, Laukkanen J, Lawless N, Liu A, Longerich S, Mägi R, Mäkelä J, Mäkitie A, Malarstig A, Mannermaa A, Maranville J, Matakidou A, Meretoja T, Mozaffari SV, Niemi MEK, Niemi M, Niiranen T, O’Donnell CJ, Obeidat Me, Okafo G, Ollila HM, Palomäki A, Palotie T, Partanen J, Paul DS, Pelkonen M, Pendergrass RK, Petrovski S, Pitkäranta A, Platt A, Pulford D, Punkka E, Pussinen P, Raghavan N, Rahimov F, Rajpal D, Renaud NA, Riley-Gillis B, Rodosthenous R, Saarentaus E, Salminen A, Salminen E, Salomaa V, Schleutker J, Serpi R, Shen H-y, Siegel R, Silander K, Siltanen S, Soini S, Soininen H, Sul JH, Tachmazidou I, Tasanen K, Tienari P, Toppila-Salmi S, Tukiainen T, Tuomi T, Turunen JA, Ulirsch JC, Vaura F, Virolainen P, Waring J, Waterworth D, Yang R, Nelis M, Reigo A, Metspalu A, Milani L, Esko T, Fox C, Havulinna AS, Perola M, Ripatti S, Jalanko A, Laitinen T, Mäkelä T, Plenge R, McCarthy M, Runz H, Daly MJ, Palotie A (2022) FinnGen: unique genetic insights from combining isolated population and national health register data. medRxiv. https://doi.org/10.1101/2022.03.03.22271360
Chiou J, Geusz RJ, Okino ML, Han JY, Miller M, Melton R, Beebe E, Benaglio P, Huang S, Korgaonkar K, Heller S, Kleger A, Preissl S, Gorkin DU, Sander M, Gaulton KJ (2021) Interpreting type 1 diabetes risk with genetics and single-cell epigenomics. Nature 594(7863):398–402. https://doi.org/10.1038/s41586-021-03552-w
Sakaue S, Kanai M, Tanigawa Y, Karjalainen J, Kurki M, Koshiba S, Narita A, Konuma T, Yamamoto K, Akiyama M, Ishigaki K, Suzuki A, Suzuki K, Obara W, Yamaji K, Takahashi K, Asai S, Takahashi Y, Suzuki T, Shinozaki N, Yamaguchi H, Minami S, Murayama S, Yoshimori K, Nagayama S, Obata D, Higashiyama M, Masumoto A, Koretsune Y, FinnGen IK, Terao C, Yamauchi T, Komuro I, Kadowaki T, Tamiya G, Yamamoto M, Nakamura Y, Kubo M, Murakami Y, Yamamoto K, Kamatani Y, Palotie A, Rivas MA, Daly MJ, Matsuda K, Okada Y (2021) A cross-population atlas of genetic associations for 220 human phenotypes. Nat Genet 53(10):1415–1424. https://doi.org/10.1038/s41588-021-00931-x
Mbatchou J, Barnard L, Backman J, Marcketta A, Kosmicki JA, Ziyatdinov A, Benner C, O’Dushlaine C, Barber M, Boutkov B, Habegger L, Ferreira M, Baras A, Reid J, Abecasis G, Maxwell E, Marchini J (2021) Computationally efficient whole-genome regression for quantitative and binary traits. Nat Genet 53(7):1097–1103. https://doi.org/10.1038/s41588-021-00870-7
Bowden J, Davey Smith G, Haycock PC, Burgess S (2016) Consistent estimation in mendelian randomization with some invalid instruments using a weighted median estimator. Genet Epidemiol 40(4):304–314. https://doi.org/10.1002/gepi.21965
Burgess S, Thompson SG (2017) Interpreting findings from Mendelian randomization using the MR-Egger method. Eur J Epidemiol 32(5):377–389. https://doi.org/10.1007/s10654-017-0255-x
Bowden J, Davey Smith G, Burgess S (2015) Mendelian randomization with invalid instruments: effect estimation and bias detection through Egger regression. Int J Epidemiol 44(2):512–525. https://doi.org/10.1093/ije/dyv080
Hemani G, Zheng J, Elsworth B, Wade KH, Haberland V, Baird D, Laurin C, Burgess S, Bowden J, Langdon R, Tan VY, Yarmolinsky J, Shihab HA, Timpson NJ, Evans DM, Relton C, Martin RM, Davey Smith G, Gaunt TR, Haycock PC (2018) The MR-base platform supports systematic causal inference across the human phenome. Elife 7:e34408. https://doi.org/10.7554/eLife.34408
Liang YL, Wu H, Shen X, Li PQ, Yang XQ, Liang L, Tian WH, Zhang LF, Xie XD (2012) Association of STAT4 rs7574865 polymorphism with autoimmune diseases: a meta-analysis. Mol Biol Rep 39(9):8873–8882. https://doi.org/10.1007/s11033-012-1754-1
Tizaoui K, Kim SH, Jeong GH, Kronbichler A, Lee KS, Lee KH, Shin JI (2019) Association of PTPN22 1858C/T polymorphism with autoimmune diseases: a systematic review and bayesian approach. J Clin Med 8(3):347. https://doi.org/10.3390/jcm8030347
Ren HM, Lukacher AE, Rahman ZSM, Olsen NJ (2021) New developments implicating IL-21 in autoimmune disease. J Autoimmun 122:102689. https://doi.org/10.1016/j.jaut.2021.102689
Salvarani C, Pipitone N, Versari A, Hunder GG (2012) Clinical features of polymyalgia rheumatica and giant cell arteritis. Nat Rev Rheumatol 8(9):509–521. https://doi.org/10.1038/nrrheum.2012.97
Rhee RL, Grayson PC, Merkel PA, Tomasson G (2017) Infections and the risk of incident giant cell arteritis: a population-based, case-control study. Ann Rheum Dis 76(6):1031–1035. https://doi.org/10.1136/annrheumdis-2016-210152
Cid MC, Font C, Oristrell J, de la Sierra A, Coll-Vinent B, Lopez-Soto A, Vilaseca J, Urbano-Marquez A, Grau JM (1998) Association between strong inflammatory response and low risk of developing visual loss and other cranial ischemic complications in giant cell (temporal) arteritis. Arthritis Rheum 41(1):26–32. https://doi.org/10.1002/1529-0131(199801)41:1%3c26::AID-ART4%3e3.0.CO;2-0
Gonzalez-Gay MA, Pineiro A, Gomez-Gigirey A, Garcia-Porrua C, Pego-Reigosa R, Dierssen-Sotos T, Llorca J (2004) Influence of traditional risk factors of atherosclerosis in the development of severe ischemic complications in giant cell arteritis. Medicine 83(6):342–347. https://doi.org/10.1097/01.md.0000145369.25558.b5
Chen X, Wu Y, Wang L (2013) Fat-resident Tregs: an emerging guard protecting from obesity-associated metabolic disorders. Obes Rev 14(7):568–578. https://doi.org/10.1111/obr.12033
Samson M, Audia S, Fraszczak J, Trad M, Ornetti P, Lakomy D, Ciudad M, Leguy V, Berthier S, Vinit J, Manckoundia P, Maillefert JF, Besancenot JF, Aho-Glele S, Olsson NO, Lorcerie B, Guillevin L, Mouthon L, Saas P, Bateman A, Martin L, Janikashvili N, Larmonier N, Bonnotte B (2012) Th1 and Th17 lymphocytes expressing CD161 are implicated in giant cell arteritis and polymyalgia rheumatica pathogenesis. Arthritis Rheum 64(11):3788–3798. https://doi.org/10.1002/art.34647
Buttgereit F, Matteson EL, Dejaco C, Dasgupta B (2018) Prevention of glucocorticoid morbidity in giant cell arteritis. Rheumatology 57(suppl_2):ii11–ii21. https://doi.org/10.1093/rheumatology/kex459
Wilson JC, Sarsour K, Collinson N, Tuckwell K, Musselman D, Klearman M, Napalkov P, Jick SS, Stone JH, Meier CR (2017) Serious adverse effects associated with glucocorticoid therapy in patients with giant cell arteritis (GCA): a nested case-control analysis. Semin Arthritis Rheum 46(6):819–827. https://doi.org/10.1016/j.semarthrit.2016.11.006
Gale S, Wilson JC, Chia J, Trinh H, Tuckwell K, Collinson N, Dimonaco S, Jick S, Meier C, Mohan SV, Sarsour K (2018) Risk associated with cumulative oral glucocorticoid use in patients with giant cell arteritis in real-world databases from the USA and UK. Rheumatol Ther 5(2):327–340. https://doi.org/10.1007/s40744-018-0112-8
Faurschou M, Ahlstrom MG, Lindhardsen J, Obel N, Baslund B (2017) Risk of diabetes mellitus among patients diagnosed with giant cell arteritis or granulomatosis with polyangiitis: comparison with the general population. J Rheumatol 44(1):78–83. https://doi.org/10.3899/jrheum.160797
Best JH, Kong AM, Unizony S, Tran O, Michalska M (2019) Risk of potential glucocorticoid-related adverse events in patients with giant cell arteritis: results from a USA-based electronic health records database. Rheumatol Ther 6(4):599–610. https://doi.org/10.1007/s40744-019-00180-9
Acknowledgements
We appreciated all the genetics consortiums for making the GWAS summary data publicly available.
Funding
This work was supported by funding from STI2030-Major Projects (2021ZD0200600, 2021ZD0200603), the National Key Research and Development Program (2022YFC2009600) (2022YFC2009602), the “Beijing Major Epidemic Prevention and Control Key Specialty Construction Project” (2022), and the National Natural Science Foundation of China Grants 82270427.
Author information
Authors and Affiliations
Contributions
Conceptualization, S.C. and X.Z.; methodology, S.C.; software, S.C.; validation, L.H., X.M.; formal analysis, R.N.; investigation, Y.W.; resources, R.N.; data curation, H.L.; writing—original draft preparation, S.C.; writing—review and editing, L.P. and H.Y.; visualization, S.C.; supervision, X.Z.; project administration, L.H.; funding acquisition, X.Z. and H.Y. All authors have read and agreed to the published version of the manuscript.
Corresponding authors
Ethics declarations
Ethics approval and consent to participate
Not applicable.
Consent for publication
All authors read and approved the final manuscript. And agree to the publication of the manuscript.
Competing interests
The authors declare no competing interests.
Additional information
Publisher's Note
Springer Nature remains neutral with regard to jurisdictional claims in published maps and institutional affiliations.
Supplementary Information
Rights and permissions
Open Access This article is licensed under a Creative Commons Attribution 4.0 International License, which permits use, sharing, adaptation, distribution and reproduction in any medium or format, as long as you give appropriate credit to the original author(s) and the source, provide a link to the Creative Commons licence, and indicate if changes were made. The images or other third party material in this article are included in the article's Creative Commons licence, unless indicated otherwise in a credit line to the material. If material is not included in the article's Creative Commons licence and your intended use is not permitted by statutory regulation or exceeds the permitted use, you will need to obtain permission directly from the copyright holder. To view a copy of this licence, visit http://creativecommons.org/licenses/by/4.0/.
About this article
Cite this article
Chen, S., Zeng, X., Ma, X. et al. Association between diabetes mellitus and giant cell arteritis: a bidirectional 2-sample mendelian randomization study. Egypt J Med Hum Genet 25, 92 (2024). https://doi.org/10.1186/s43042-024-00561-y
Received:
Accepted:
Published:
DOI: https://doi.org/10.1186/s43042-024-00561-y