Abstract
Background
Polycystic ovarian syndrome (PCOS) is recognized as the most common endocrine disorder among women of reproductive age and the most common cause of infertility. Given the importance of the subject and the inconsistency of the results of the primary studies, the present study aimed at estimating the pooled effect of vitamin D on the hormonal profile of women with PCOS using systematic review and meta-analysis.
Main body
A systematic literature review was performed in PubMed, Scopus, Embase, Web of Science (WoS), Cochrane, ClinicalTrials.gov databases, and Google Scholar motor engine using related Medical Subject Headings (MeSH) and Free Text words with no time limit to April 2022. Heterogeneity among studies was quantified using I2 index. After eliminating duplicates and irrelevant studies, ultimately, 19 articles with a sample size of 450 in the intervention group and 450 in the control group were included in the meta-analysis. As a result of the combination of studies, mean the standardized difference (SMD) before and after the intervention was obtained 0.241 ± 0.098 for dehydroepiandrosterone sulfate (DHEAS), 0.330 ± 0.092 for sex hormone-binding globulin (SHBG), 0.707 ± 0.171 for testosterone, 0.614 ± 0.199 for luteinizing hormone (LH), 0.220 ± 0.119 for follicle-stimulating hormone (FSH), 0.655 ± 0.505 for anti-Müllerian hormone (AMH), and 0.369 ± 0.109 for Free Androgen Index (FAI) in the intervention group compared to the control group. The results indicated that 8-week interventions had a greater positive effect than 12-week interventions.
Conclusion
The results of the current meta-analysis revealed a significant positive effect of vitamin D on the hormonal profile of women with PCOS, which should be considered by obstetricians and midwives.
Similar content being viewed by others
Introduction
Polycystic ovarian syndrome (PCOS) is a common endocrine and metabolic disorder among women of childbearing age [1, 2]. PCOS is characterized by a set of disorders, including androgen elevation (clinical and biochemical symptoms), lack of ovulation or oligo-anovulation, and the appearance of small and abundant cysts on ultrasound scans of ovaries [3]. Several underlying factors, such as diet, environmental factors, physical activity, genetic factors, and neuroendocrine factors, are determinants in the development of PCOS at different periods of life [4]. Compared to healthy individuals, people with PCOS typically exhibit lower levels of follicle-stimulating hormone (FSH), sex hormone-binding globulin (SHBG), and high-density lipoprotein (HDL), as well as higher levels of luteinizing hormone (LH), testosterone, insulin, cholesterol, and triglycerides in serum tests [5,6,7].
The prevalence of PCOS varies between 2.2 and 26% across different countries [8]. PCOS is associated with adverse complications, including infertility, gestational hypertension, preeclampsia, gestational diabetes, cardiovascular complications, diabetes, cancer, hypertension, and psychological complications [9]. The incidence of these symptoms and complications can lead to disorders in various aspects of the quality of life [10,11,12,13].
Lifestyle modification interventions are considered the first-line treatment for women with PCOS. Additionally, medications such as metformin, oral contraceptive pills, and anti-androgens are prescribed by specialists [14]. Various non-pharmacological therapies (NPT), including exercise and physical activity, diet, acupressure, and dietary supplements have been studied and have reported improving effects on hormone levels, metabolic indices, and anthropometric measures [15].
Serum vitamin D status is one of the factors associated with PCOS [16]. Vitamin D deficiency is more common among people with PCOS and obesity since the accumulation of vitamin D in adipose tissue reduces its availability [17, 18]. The relationship between inadequate serum vitamin D levels and endocrine disorders has been reported in many studies [18, 19]. Vitamin D receptors are expressed in skeletal tissue, parathyroid glands, and ovaries, mediating the biological function of vitamin D [20, 21].
Several primary studies have been conducted on the effect of vitamin D on the hormonal profile of women with PCOS with conflicting results [7, 22, 23]. The only relevant meta-analysis study, conducted by Pergialiotis et al. [24], had several limitations, including the search for articles being limited to 2016, the lack of searching some important databases (Embase and Web of Science (WoS)), the lack of investigating some items of the hormonal profile, the limited number of studies included, the lack of examining publication bias, and the lack of evaluating the impact of potential factors, such as the year of publication, sample size, total dose of vitamin D, mean age, Body Mass Index (BMI), baseline vitamin D level, score of Joanna Briggs Institute (JBI), number of weeks of intervention, and frequency of taking vitamin D. Given the importance of the subject, inconsistencies of the results of primary studies, and limitations of the aforementioned meta-analysis, the present study aimed to estimate the pooled effect of vitamin D on the hormonal profile of women with PCOS using systematic review and meta-analysis.
Materials and method
The present systematic review and meta-analysis were carried out according to the PRISMA 2020 protocol (http://www.prisma-statement.org/), including identification, screening, eligibility, and included [25]. No time limit to April 2022. All steps of identification, selection, and study quality assessment, as well as data extraction were independently done by two researchers (M.K. and F.R.). Any disagreement between the two researchers was resolved through consultation with a third researcher (M.R.).
Identification of studies
A systematic literature review was conducted in the international databases of PubMed, Scopus, Embase, Web of Science (WoS), Cochrane, and ClinicalTrials.gov to identify relevant publications. The searches included the combinations of the Medical Subject Headings (MeSH) for PubMed/Emtree (Elsevier’s authoritative life science thesaurus) for Embase and Free Text words. No time and language limitations were regarded for the search to retrieve as comprehensive as possible relevant studies. Finally, the Google Scholar motor engine and references of all eligible articles were manually reviewed to maximize the comprehensiveness of the search. For instance, the PubMed search strategy was defined as follows:
((((((((((((((((((((((((((((((((“Vitamin D”[MeSH Terms]) OR (Ergocalciferols[MeSH Terms])) OR (“Vitamin D”[Title/Abstract])) OR (Ergocalciferol*[Title/Abstract])) OR (Calciferols[Title/Abstract])) OR (“Vitamin D 2”[Title/Abstract])) OR (“Vitamin D2”[Title/Abstract])) OR (“D2, Vitamin”[Title/Abstract])) OR (Cholecalciferols[Title/Abstract])) OR (“Vitamin D3”[Title/Abstract])) OR (“Vitamin D 3”[Title/Abstract])) OR (“(3 Beta,5Z,7E)-9,10-Secocholesta-5,7,10(19)-trien-3-ol”[Title/Abstract])) OR (Calciol[Title/Abstract])) OR (“Hydroxyvitamins D”[Title/Abstract])) OR (Hydroxycholecalciferol[Title/Abstract])) OR (“25-Hydroxyvitamin D 3”[Title/Abstract])) OR (“25 Hydroxyvitamin D 3”[Title/Abstract])) OR (“25-Hydroxycholecalciferol Monohydrate”[Title/Abstract])) OR (“25 Hydroxycholecalciferol Monohydrate”[Title/Abstract])) OR (“Monohydrate, 25-Hydroxycholecalciferol”[Title/Abstract])) OR (“25-Hydroxyvitamin D3”[Title/Abstract])) OR (“25 Hydroxyvitamin D3”[Title/Abstract])) OR (Calcidiol[Title/Abstract])) OR (“25-Hydroxycholecalciferol”[Title/Abstract])) OR (“25 Hydroxycholecalciferol”[Title/Abstract])) OR (“Calcifediol, (3 beta,5E,7E)-Isomer”[Title/Abstract])) OR (“Calcifediol Anhydrous”[Title/Abstract])) OR (“Anhydrous, Calcifediol”[Title/Abstract])) OR (Dedrogyl[Title/Abstract])) OR (Hidroferol[Title/Abstract])) OR (“Calcifediol, (3 alpha,5Z,7E)-Isomer”[Title/Abstract])) OR (Calderol[Title/Abstract])) AND (((((((((((((((((“Polycystic Ovary Syndrome”[MeSH Terms]) OR (“Polycystic Ovary Syndrome”[Title/Abstract])) OR (PCOS[Title/Abstract])) OR (PCO[Title/Abstract])) OR (“Ovary Syndrome, Polycystic”[Title/Abstract])) OR (“Syndrome, Polycystic Ovary”[Title/Abstract])) OR (“Stein-Leventhal Syndrome”[Title/Abstract])) OR (“Stein Leventhal Syndrome”[Title/Abstract])) OR (“Syndrome, Stein-Leventhal”[Title/Abstract])) OR (“Sclerocystic Ovarian Degeneration”[Title/Abstract])) OR (“Ovarian Degeneration, Sclerocystic”[Title/Abstract])) OR (“Sclerocystic Ovary Syndrome”[Title/Abstract])) OR (“Polycystic Ovarian Syndrome”[Title/Abstract])) OR (“Ovarian Syndrome, Polycystic”[Title/Abstract])) OR (“Sclerocystic Ovaries”[Title/Abstract])) OR (“Ovary, Sclerocystic”[Title/Abstract])) OR (“Sclerocystic Ovary”[Title/Abstract])).
Inclusion criteria
The inclusion criteria were original articles, interventional studies, and studies that investigated the effect of vitamin D on the hormonal profile of women with PCOS.
Exclusion criteria
The exclusion criteria encompassed a range of study types, including irrelevant studies, cross-sectional studies, qualitative studies, case series, case reports, letters to the editor, papers presented at conferences, secondary studies, dissertations, animal studies, studies with overlapping data, and those that lacked sufficient data, specifically the failure to report the mean and standard deviation before and after the intervention in both the placebo and intervention groups.
Selection process of studies
All studies obtained from different databases were imported into EndNote X8 software. After excluding the duplicates, the title and abstract of the articles were thoroughly screened to eliminate irrelevant studies. In the following, the full text of the remaining articles was carefully inspected for eligibility. Researchers extracted the articles without knowing the names of authors, institutes, and journals. The quality assessment of all studies included in the systematic review and meta-analysis was done.
Quality assessment of the studies
The quality assessment of the studies was done using the Joanna Briggs Institute (JBI) checklist, known as a standard checklist for the quality assessment of studies [26]. This checklist consists of 13 different items, including randomization, allocation, the similarity of treatment groups in the beginning, the blindness of the participants, the blindness of doers, the blindness of the evaluators of the results, similar treatment in groups except intervention, follow up, participant analysis, outcomes, reliability of the method of measuring results, appropriate statistical analysis, and trial design appropriate. Each item is answered as “yes” for pointed, “no” for not pointed, and “not applicable” for not reported. The total score range based on the JBI items is between 0 and 13. Studies with a score of 1–4 were considered as “low quality”, 5–8 as “medium quality”, and 9–13 as “high quality” [27].
Data extraction
The data were manually collected from all articles included in the systematic review and meta-analysis using a pre-prepared checklist. The items of this checklist consisted of the name of the first author, country, year of publication, sample size, intervention duration, study design, total dose, baseline BMI, baseline vitamin D, and JBI score.
Statistical analysis
The present study estimated the effect of vitamin D on the hormonal profile of women with PCOS. In this regard, the mean and SD of placebo and intervention groups in each study were used to combine the results of different studies. Heterogeneity among studies was quantified using I2 index and I2 < 50% was considered for “low heterogeneity” and I2 > 50% for “high heterogeneity”. The fixed effects model was used in low heterogeneity conditions and the random effects model was applied in high heterogeneity conditions [28]. Egger’s regression intercept was employed to investigate publication bias. Further, the meta-regression was used to investigate the relationship between SMD before and after the intervention in the intervention and placebo groups and the year of publication, sample size, total dose of vitamin D, mean age, BMI, baseline vitamin D level, and JBI score. The data were analyzed using Comprehensive Meta-Analysis (Version 2) software and P-value less than 0.05 was considered statistically meaningf.ull.
Results
The summary of how articles included in the meta-analysis
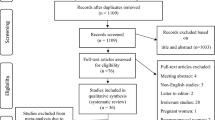
The initial systematic literature search retrieved 1650 articles using defined search strategies. After excluding 799 duplicates in different databases, 825 irrelevant studies were eliminated by screening the title and abstract. Then, the full text of the remaining 26 studies was inspected carefully and 7 studies were removed, due to not meeting the eligibility criteria. After quality assessment, 19 eligible studies were finally included in the meta-analysis. Figure 1 displays the PRISMA 2020 flow diagram.
General characteristics of the eligible studies
The total sample size was 450 in the intervention group and 450 in the placebo group. The oldest study was carried out in 2009 and the most recent study in 2022. In this regard, a large number of studies were conducted in Iran with 11 articles. The highest quality assessment score based on the JBI checklist was related to the study of Dastorani et al. (2018) with a score of 13 [16]. In addition, all the articles included in the study were of medium or high quality based on the JBI checklist. The study design of the majority of the studies (n = 13) were randomized, double-blind, and placebo-controlled trials. Table 1 indicates the characteristics of articles included in the study.
Generally, 9 studies examined the effect of vitamin D on the level of DHEAS, 9 studies investigated the effect of intervention on the level of SHBG, 13 studies considered the effect of intervention on the level of testosterone, 7 studies evaluated the effect of intervention on the level of LH, 6 studies assessed the effect of intervention on the FSH level, 3 studies examined the effect of intervention on AMH level, and 6 studies investigated the effect of intervention on Free Androgen Index (FAI). We reported vitamin D dosage in the included studies weekly. The total dose intake ranged from 11,200 to 72,000 IU weekly. Table 2 displays the data on the effect of the intervention on the hormonal profile.
Meta-analysis
Since the result of I2 test demonstrated a high significant heterogeneity among studies for Testosterone, LH, and anti-Müllerian hormone (AMH) (I2 testosterone = 75.57, I2 LH = 63.31, and I2 AMH = 83.77), the random effects model was employed to combine the effect size of the studies. However, given that the results indicated low heterogeneity (I2 = 0.00) among studies for DHEAS, SHBG, FSH, and FAI, the fixed effects model was used.
As a result of the combination of studies, SMD before and after the intervention was obtained 0.241 ± 0.098 for DHEAS (Fig. 2), 0.330 ± 0.092 for SHBG (Fig. 3), 0.707 ± 0.171 for testosterone (Fig. 4), 0.614 ± 0.199 for LH (Fig. 5), 0.220 ± 0.119 for FSH (Fig. 6), 0.655 ± 0.505 for AMH (Fig. 7), and 0.369 ± 0.109 for FAI (Fig. 8) in the intervention group compared to the placebo group. The forest plot demonstrates the estimated SMD ± 95% CI of each study and the pooled SMD ± 95% CI of all included studies before and after the intervention in the intervention group compared to the placebo group. The 95% CI is indicated by the horizontal line of each square (Fig. 8).
According to Egger’s regression intercept, there was no publication bias at the 0.05 level in the studies (P DHEAS = 0.445, P SHBG = 0.928, P testosterone = 0.050, P LH = 0.735, P FSH = 0.146, P AMH = 90 and P FAI = 0.405).
Meta-regression
As shown in Table 3, the effect of potential factors, such as year of publication, sample size, total dose of vitamin D, mean age, baseline BMI, baseline vitamin D level, and JBI score on the hormonal profile of women with PCOS was investigated using meta-regression. The results revealed that the effect of the intervention on AMH level decreases with increasing sample size and the total dose of consumption (P < 0.05). Further, the impact of the intervention on testosterone levels is enhanced by increasing the total dose of consumption (P < 0.05). The effect of the intervention on the level of DHEAS and LH decreases by increasing the level of baseline vitamin D (P < 0.05). Furthermore, the effect of the intervention on LH level increases with age (P < 0.05). The effect of the year of publication, BMI, and JBI score on the hormonal profile was not significant (P ˃ 0.05).
Sub-group analysis
The subgroup analysis was reported in terms of the number of weeks of intervention (8-week and 12-week) and the frequency of consumption (once a day, twice a day, three times a day, and once a week) (Table 4). Based on the results, 8-week interventions had a more positive effect on the hormonal profile of women with PCOS compared to 12-week interventions. The consumption of vitamin D three times a day had the greatest effect on DHEAS, Testosterone, LH, FSH, and FAI, twice a day on SHBG, and once a day on AMH.
Discussion
The present study aimed to estimate the effect of vitamin D intake on the hormonal profile of women with PCOS using systematic review and meta-analysis. After combining the data collected from 19 articles, the standardized mean difference before and after the intervention was obtained for DHEAS, SHBG, testosterone, LH, FSH, AMH, and FAI in the intervention group compared to the control group, indicating a positive effect of vitamin D intake on the hormonal profile of women with PCOS.
Insulin resistance seems to be one of the predominant features of PCOS, along with the dysfunction of the hypothalamic-pituitary axis, leading to hormonal changes, including an increase in the LH/FSH ratio and circulating androgens [42]. Further, hyperinsulinemia influences the production of SHBG and its circulating level, resulting in the enhancement of serum testosterone levels [43]. The most common clinical manifestations of hormonal changes among women with PCOS include menstrual disorders, such as amenorrhea and oligomenorrhea, hirsutism, type II diabetes, obesity, cardiovascular disease (CVDs), persistent acne, hyperhidrosis, dysfunctional uterine bleeding (DUB), and development of metabolic syndrome [44, 45]. Vitamin D plays an important role in bone homeostasis and may increase insulin receptor expression by increasing insulin production and glucose metabolism, thereby reducing insulin resistance, which is a predominant feature of PCOS, and improving the hormonal profile [46].
SHGB is a glycoprotein that binds to the sex hormones, androgens, and estrogen. Other steroid hormones, including progesterone, cortisol, and other corticosteroids, also bind to it by transcortin. SHGB regulates the access of target cells to sex steroids by binding to and carrying them in the blood [47]. SHBG often elevates in patients with hypogonadism, hyperthyroidism, liver cirrhosis, anorexia nervosa, and those taking oral contraceptive pills and antiepileptic drugs. Decreased SHBG concentrations are associated with hypothyroidism, PCOS, obesity, hirsutism, high androgens, hair loss, acne, Cushing’s disease, and acromegaly [48]. Based on the results of the present study, vitamin D supplementation increases SHGB levels, and 8-week interventions with taking vitamin D twice daily are more effective.
DHEAS is a steroid hormone found in both men and women. DHEAS plays an important role in the production of the male sex hormone testosterone and the female sex hormone estrogen. DHEAS is mainly produced by the adrenal glands and smaller amounts made by the testes in men and the ovaries in women [49]. DHEAS levels are typically higher in women with PCOS compared to healthy women [50]. The results of the present study illustrated that taking vitamin D supplementation reduces DHEAS levels. Although DHEAS levels typically increase with age [51], the meta-regression results of the present study revealed no significant relationship between mean age and the effect of vitamin D on DHEAS levels.
The results of the present study demonstrated that taking vitamin D supplementation reduces testosterone levels. This finding is consistent with the systematic review and meta-analysis conducted by Azadi-Yazdi et al. (2017), which reported that vitamin D supplementation reduces total testosterone in women with PCOS [52]. However, the results of the Azadi-Yazdi et al. study should be interpreted with caution, as they only considered the standardized mean difference (SMD) before and after the intervention in the intervention group, without comparing it to the placebo group. In contrast, the current study estimated the SMD before and after the intervention in both the placebo and intervention groups. Additionally, the number of studies included in the meta-analysis on the effect of vitamin D supplementation on testosterone in women with PCOS is larger in the present study (13 articles) compared to the Azadi-Yazdi et al. study (6 articles). Therefore, the results of the current study provide stronger evidence of the effect of vitamin D supplementation in reducing testosterone levels in women with PCOS. However, due to high heterogeneity in some sub-group analyses on testosterone and LH levels, the interpretation should be considered cautiously [27].
Women with PCOS have elevated serum LH levels and decreased FSH levels. The increased LH stimulates theca cells in the ovary, which in turn stimulates the production of androgens [42]. Based on the results of the present study, although taking vitamin D supplementation reduces LH levels, it does not have a significant effect on FSH levels, which is consistent with the results of the meta-analysis by Pergialiotis et al. (2017) [24].
FAI is a ratio used to assess the abnormal androgen status in humans. FAI is calculated as the total testosterone level divided by SHBG level and then, multiplied by 100. FAI has no unit. Woman’s androgens are often measured when there is a concern about elevated serum levels, which may be associated with conditions such as hirsutism or PCOS. The standard values for FAI in women range from 7 to 10. FAI is typically increased in women with PCOS. The results of the current study indicated that taking vitamin D supplementation significantly reduces FAI levels and that interventions performed three times a day are more effective.
The study results of Pergialiotis et al. (2017) rejected the effect of vitamin D on the hormonal profile of women with PCOS and reported its effect as mild [24]. The difference between the results of the Pergialiotis et al. study and the findings of the present study can be attributed to the difference in the number of articles included in the meta-analysis, the total sample size of studies, and the effect of potential factors, such as total vitamin D dose, mean age, BMI, baseline vitamin D level, number of weeks of intervention, and frequency of taking vitamin D per day.
Overall, it can be concluded that taking vitamin D supplementation has a positive impact on SHBG, DHEAS, testosterone, LH, and FAI, and has no significant effect on FSH and AMH levels. However, these results should be interpreted with caution, as the number of studies conducted on some hormonal profile items, such as FSH, FAI, and AMH, was limited. Therefore, it is suggested to perform more primary studies with larger sample sizes to examine all items of the hormonal profile.
Limitations
The present study and the studies included in the meta-analysis come with several limitations:
First, the included studies did not follow a consistent reporting format, which can affect the quality of the data synthesis. Some studies may have used non-random sampling methods, which can introduce selection bias. Second, the included studies had varying study designs, which can contribute to the heterogeneity of the results. Third, the limited number of studies for certain hormonal profile items, such as FSH, FAI, and AMH, restricted the ability to perform robust subgroup analyses. Fourth, the inability to access the full-text versions of conference abstracts may have led to the exclusion of potentially relevant studies. Moreover, the meta-analysis exhibited relatively high heterogeneity among studies on some hormonal profile items, such as testosterone, LH, and AMH. To address this, the researchers performed subgroup analyses, which were able to reduce the heterogeneity to some extent. However, there was still high heterogeneity among some subgroups, likely due to factors such as sample size, demographic characteristics, and study methodology. These limitations should be taken into consideration when interpreting the findings of the present study and when designing future research in this area.
Conclusion
The results of the present systematic review and meta-analysis, based on the available randomized clinical trials, indicate a significant positive effect of vitamin D supplementation on certain hormonal profile items in women with PCOS. Specifically, vitamin D supplementation was found to have a beneficial impact on SHBG, DHEAS, testosterone, LH, and FAI. Furthermore, the findings suggest that 8-week interventions had a more positive effect compared to 12-week interventions. Additionally, the impact of vitamin D on the hormonal profile of women with PCOS was influenced by potential factors such as dose, age, BMI, baseline vitamin D level, and frequency of vitamin D intake per day. Given these findings, the results of the present study should be considered and incorporated by obstetricians and midwives in the management and care of women with PCOS.
Availability of data and materials
Datasets are available through the corresponding author upon reasonable request.
Abbreviations
- SID:
-
Scientific Information Database
- WoS:
-
Web of Science
- MeSH:
-
Medical Subject Headings
- PRISMA:
-
Preferred Reporting Items for Systematic Reviews and Meta-Analysis
- JBI:
-
Joanna Briggs Institute
- FAI:
-
Free Androgen Index
- SHBG:
-
Sex hormone-binding globulin
- FSH:
-
Follicle-stimulating hormone
- LH:
-
Luteinizing hormone
- DHEAS:
-
Dehydroepiandrosterone sulfate
- AMH:
-
Anti-Müllerian hormone
- BMI:
-
Body Mass Index
References
Lizneva D, Suturina L, Walker W, Brakta S, Gavrilova-Jordan L, Azziz R (2016) Criteria, prevalence, and phenotypes of polycystic ovary syndrome. Fertil Steril 106(1):6–15
Wolf WM, Wattick RA, Kinkade ON, Olfert MD (2018) Geographical prevalence of polycystic ovary syndrome as determined by region and race/ethnicity. Int J Environ Res Public Health 15(11):2589
Azziz R, Carmina E, Chen Z, Dunaif A, Laven JS, Legro RS et al (2016) Polycystic ovary syndrome. Nat Rev Dis Primers 2(1):1–18
Witchel SF, Oberfield SE, Peña AS (2019) Polycystic ovary syndrome: pathophysiology, presentation, and treatment with emphasis on adolescent girls. Journal of the Endocrine Society 3(8):1545–1573
Jafari-Sfidvajani S, Ahangari R, Hozoori M, Mozaffari-Khosravi H, Fallahzadeh H, Nadjarzadeh A (2018) The effect of vitamin D supplementation in combination with low-calorie diet on anthropometric indices and androgen hormones in women with polycystic ovary syndrome: a double-blind, randomized, placebo-controlled trial. J Endocrinol Invest 41(5):597–607
Javed Z, Papageorgiou M, Deshmukh H, Kilpatrick ES, Mann V, Corless L et al (2019) A randomized, controlled trial of vitamin D supplementation on cardiovascular risk factors, hormones, and liver markers in women with polycystic ovary syndrome. Nutrients 11(1):188
Maktabi M, Chamani M, Asemi Z (2017) The effects of vitamin D supplementation on metabolic status of patients with polycystic ovary syndrome: a randomized, double-blind, placebo-controlled trial. Horm Metab Res 49(07):493–498
Sayehmiri F, Kiani F, Maleki F, Ahmadi M, Shohani M (2014) Prevalence of polycystic ovary syndrome in Iranian women: a systematic review and meta-analysis. The Iranian J Obstet, Gynecol Infer 17(115):11–21
Palomba S, Santagni S, Falbo A, La Sala GB (2015) Complications and challenges associated with polycystic ovary syndrome: current perspectives. Int J Women’s Health 7:745
Greenwood EA, Pasch LA, Cedars MI, Legro RS, Huddleston HG, Network HDRM et al (2018) Association among depression, symptom experience, and quality of life in polycystic ovary syndrome. Am J Obstet Gynecol. 219(3):279
Sidra S, Tariq MH, Farrukh MJ, Mohsin M (2019) Evaluation of clinical manifestations, health risks, and quality of life among women with polycystic ovary syndrome. PLoS ONE 14(10):e0223329
Taghavi SA, Bazarganipour F, Hugh-Jones S, Hosseini N (2015) Health-related quality of life in Iranian women with polycystic ovary syndrome: a qualitative study. BMC Womens Health 15(1):1–8
Teede HJ, Misso ML, Costello MF, Dokras A, Laven J, Moran L et al (2018) Recommendations from the international evidence-based guideline for the assessment and management of polycystic ovary syndrome. Hum Reprod 33(9):1602–1618
Ibáñez L, Oberfield SE, Witchel S, Auchus RJ, Chang RJ, Codner E et al (2017) An international consortium update: pathophysiology, diagnosis, and treatment of polycystic ovarian syndrome in adolescence. Horm Res Paediat 88:371–395
Pundir J, Charles D, Sabatini L, Hiam D, Jitpiriyaroj S, Teede H et al (2019) Overview of systematic reviews of non-pharmacological interventions in women with polycystic ovary syndrome. Hum Reprod Update 25(2):243–256
Dastorani M, Aghadavod E, Mirhosseini N, Foroozanfard F, Zadeh Modarres S, Amiri Siavashani M et al (2018) The effects of vitamin D supplementation on metabolic profiles and gene expression of insulin and lipid metabolism in infertile polycystic ovary syndrome candidates for in vitro fertilization. Reprod Biol Endocrinol 16(1):1–7
Lagunova Z, Porojnicu AC, Lindberg F, Hexeberg S, Moan J (2009) The dependency of vitamin D status on body mass index, gender, age and season. Anticancer Res 29(9):3713–3720
Mahmoudi T, Gourabi H, Ashrafi M, Yazdi RS, Ezabadi Z (2010) Calciotropic hormones, insulin resistance, and the polycystic ovary syndrome. Fertil Steril 93(4):1208–1214
Rodríguez-Rodríguez E, Navia-Lombán B, López-Sobaler A, Ortega R (2010) Associations between abdominal fat and body mass index on vitamin D status in a group of Spanish schoolchildren. Eur J Clin Nutr 64(5):461–467
Ding C, Gao D, Wilding J, Trayhurn P, Bing C (2012) Vitamin D signalling in adipose tissue. Br J Nutr 108(11):1915–1923
Wehr E, Trummer O, Giuliani A, Gruber H-J, Pieber TR, Obermayer-Pietsch B (2011) Vitamin D-associated polymorphisms are related to insulin resistance and vitamin D deficiency in polycystic ovary syndrome. Eur J Endocrinol 164(5):741
Jamilian M, Foroozanfard F, Rahmani E, Talebi M, Bahmani F, Asemi Z (2017) Effect of two different doses of vitamin D supplementation on metabolic profiles of insulin-resistant patients with polycystic ovary syndrome. Nutrients 9(12):1280
Razavi M, Jamilian M, Karamali M, Bahmani F, Aghadavod E, Asemi Z (2016) The effects of vitamin DK-calcium co-supplementation on endocrine, inflammation, and oxidative stress biomarkers in vitamin D-deficient women with polycystic ovary syndrome: a randomized, double-blind, placebo-controlled trial. Horm Metab Res 48(07):446–451
Pergialiotis V, Karampetsou N, Panagopoulos P, Trakakis E, Papantoniou N (2017) The effect of Vitamin D supplementation on hormonal and glycaemic profile of patients with PCOS: A meta-analysis of randomised trials. Int J Clin Pract 71(6):e12957
Page MJ, McKenzie JE, Bossuyt PM, Boutron I, Hoffmann TC, Mulrow CD et al (2021) The PRISMA 2020 statement: an updated guideline for reporting systematic reviews. Syst Rev 10(1):1–11
Tufanaru C, Munn Z, Aromataris E, Campbell J, Hopp L (2017) Chapter 3: Systematic reviews of effectiveness. In: Aromataris E, Munn Z, editors. Joanna Briggs Institute Reviewer's Manual The Joanna Briggs Institute, 2017
Ghavami T, Kazeminia M, Rajati F (2022) The effect of lavender on stress in individuals: A systematic review and meta-analysis. Complement Ther Med. 68:102832
Yang J, Hu J, Zhu C (2021) Obesity aggravates COVID-19: a systematic review and meta-analysis. J Med Virol 93(1):257–261
Maktabi M, Jamilian M, Asemi Z (2018) Magnesium-zinc-calcium-vitamin D co-supplementation improves hormonal profiles, biomarkers of inflammation and oxidative stress in women with polycystic ovary syndrome: a randomized, double-blind, placebo-controlled trial. Biol Trace Elem Res 182(1):21–28
Ostadmohammadi V, Jamilian M, Bahmani F, Asemi Z (2019) Vitamin D and probiotic co-supplementation affects mental health, hormonal, inflammatory and oxidative stress parameters in women with polycystic ovary syndrome. J Ovar Res 12(1):1–8
Dravecká I, Figurova J, Javorský M, Petrikova J, Valkova M, Lazurova I (2016) The effect of alfacalcidiol and metformin on phenotype manifestations in women with polycystic ovary syndrome-a preliminary study. Physiol Res 65(5):815
Gupta T, Rawat M, Gupta N, Arora S (2017) Study of effect of vitamin D supplementation on the clinical, hormonal and metabolic profile of the PCOS women. J Obstet Gynecol of India 67(5):349–355
Kadoura S, Alhalabi M, Nattouf AH (2019) Effect of calcium and vitamin D supplements as an adjuvant therapy to metformin on menstrual cycle abnormalities hormonal profile, and IGF-1 system in polycystic ovary syndrome patients: a randomize, placebo-controlled clinical trial. Adv in Pharmacol Sci. 2019:9680390
Yahya AA, Abdulridha MK, Al-Rubuyae BJ, Al-Atar HA (2019) The effect of vitamin D and co-enzyme Q10 replacement therapy on hormonal profile and ovulation status in women with clomiphene citrate resistant polycystic ovary syndrome. J Pharm Sci Res 11(1):208–215
Sert ZS, Yılmaz SA, Seçilmiş Ö, Abuşoğlu S, Ünlü A, Çelik Ç (1971) Effect of calcium and vitamin D supplementation on the clinical hormonal and metabolic profile in non-obese women with polycystic ovary syndrome. Ir J Med Sci 2022(191):2657–2662
Rashidi B, Haghollahi F, Shariat M, Zayerii F (2009) The effects of calcium-vitamin D and metformin on polycystic ovary syndrome: a pilot study. Taiwan J Obstet Gynecol 48(2):142–147
Bonakdaran S, Khorasani ZM, Davachi B, Khorasani JM (2012) The effects of calcitriol on improvement of insulin resistance, ovulation and comparison with metformin therapy in PCOS patients: a randomized placebo-controlled clinical trial. Iran J Reprod Med 10(5):465
Garg G, Kachhawa G, Ramot R, Khadgawat R, Tandon N, Sreenivas V et al (2015) Effect of vitamin D supplementation on insulin kinetics and cardiovascular risk factors in polycystic ovarian syndrome: a pilot study. Endocr Connect 4(2):108–116
Irani M, Seifer DB, Grazi RV, Julka N, Bhatt D, Kalgi B et al (2015) Vitamin D supplementation decreases TGF-β1 bioavailability in PCOS: a randomized placebo-controlled trial. J Clin Endocrinol Metab 100(11):4307–4314
Raja-Khan N, Shah J, Stetter CM, Lott ME, Kunselman AR, Dodson WC et al (2014) High-dose vitamin D supplementation and measures of insulin sensitivity in polycystic ovary syndrome: a randomized, controlled pilot trial. Fertil Steril 101(6):1740–1746
Hosseini SA, Kazemi N, Shadmehri S, Jalili S, Ahmadi M (2019) The effect of resistance training in water and land with vitamin D supplementation on anti-Mullerian hormone in women with polycystic ovary syndrome. Women’s Health Bulletin 6(2):1–6
Qi X, Pang Y, Qiao J (2016) The role of anti-Müllerian hormone in the pathogenesis and pathophysiological characteristics of polycystic ovary syndrome. Eur J Obstet Gynecol Reprod Biol 199:82–87
Pugeat M, Crave JC, Tourniaire J, Forest MG (1996) Clinical utility of sex hormone-binding globulin measurement. Horm Res Paediat 45(3–5):148–155
Bozdag G, Mumusoglu S, Zengin D, Karabulut E, Yildiz BO (2016) The prevalence and phenotypic features of polycystic ovary syndrome: a systematic review and meta-analysis. Hum Reprod 31(12):2841–2855
El Hayek S, Bitar L, Hamdar LH, Mirza FG, Daoud G (2016) Poly cystic ovarian syndrome: an updated overview. Front Physiol 7:124
He C, Lin Z, Robb SW, Ezeamama AE (2015) Serum vitamin D levels and polycystic ovary syndrome: a systematic review and meta-analysis. Nutrients 7(6):4555–4577
Dias JP, Piggott DA, Sun J, Wehbeh L, Garza J, Abraham A et al (2022) SHBG, Bone mineral density and physical function among injection drug users with and without HIV and HCV. J Clin Endocrinol Metab. 107:e2971–e2981
Xing C, Zhang J, Zhao H, He B (2022) Effect of sex hormone-binding globulin on polycystic ovary syndrome: mechanisms, manifestations, genetics, and treatment. Int J Women’s Health 14:91
Cannarella R, Condorelli RA, Dall’Oglio F, La Vignera S, Mongioì LM, Micali G et al (2020) Increased DHEAS and decreased total testosterone serum levels in a subset of men with early-onset androgenetic alopecia: does a male PCOS-equivalent exist? Int J Endocrinol. 2020:1942126
Güdücü N, Kutay SS, Görmüş U, Kavak ZN, Dünder İ (2015) High DHEAS/free testosterone ratio is related to better metabolic parameters in women with PCOS. Gynecol Endocrinol 31(6):495–500
Sorwell KG, Urbanski HF (2010) Dehydroepiandrosterone and age-related cognitive decline. Age 32(1):61–67
Azadi-Yazdi M, Nadjarzadeh A, Khosravi-Boroujeni H, Salehi-Abargouei A (2017) The effect of vitamin D supplementation on the androgenic profile in patients with polycystic ovary syndrome: a systematic review and meta-analysis of clinical trials. Horm Metab Res 49(03):174–179
Acknowledgements
This study is the result of research project (IR.KUMS.REC.1401.086) approved by the Kermanshah University of Medical Sciences. We would like to thank the esteemed officials of that center for accepting the financial expenses of this study. We also thank the officials of the Systematic Review and Meta-Analysis Center (SYRMAN) for their guidance and advice in conducting this research.
Funding
This study is supported by Deputy for Research and Technology, Kermanshah University of Medical Sciences (IR) (IR.KUMS.REC.1401.086). This deputy has no role in the study process.
Author information
Authors and Affiliations
Contributions
M.K and M.R contributed to the design. M.K, M.R, and F.R participated in most of the study steps. M.K and M.R prepared the manuscript. M.K and R.R assisted in designing the study and helped in the interpretation of the study. All authors have read and approved the content of the manuscript.
Corresponding author
Ethics declarations
Ethics approval and consent to participate
Ethics approval was received from the ethics committee of the deputy of research and technology, Kermanshah University of Medical Sciences (IR.KUMS.REC.1401.086).
Consent for publication
Not applicable.
Competing interests
The authors declare that they have no competing interests.
Additional information
Publisher's Note
Springer Nature remains neutral with regard to jurisdictional claims in published maps and institutional affiliations.
Rights and permissions
Open Access This article is licensed under a Creative Commons Attribution 4.0 International License, which permits use, sharing, adaptation, distribution and reproduction in any medium or format, as long as you give appropriate credit to the original author(s) and the source, provide a link to the Creative Commons licence, and indicate if changes were made. The images or other third party material in this article are included in the article's Creative Commons licence, unless indicated otherwise in a credit line to the material. If material is not included in the article's Creative Commons licence and your intended use is not permitted by statutory regulation or exceeds the permitted use, you will need to obtain permission directly from the copyright holder. To view a copy of this licence, visit http://creativecommons.org/licenses/by/4.0/.
About this article
Cite this article
Kazeminia, M., Rajati, F., Rasulehvandi, R. et al. The effect of vitamin D on the hormonal profile of women with polycystic ovarian syndrome: a systematic review and meta-analysis. Middle East Fertil Soc J 29, 45 (2024). https://doi.org/10.1186/s43043-024-00201-w
Received:
Accepted:
Published:
DOI: https://doi.org/10.1186/s43043-024-00201-w