Abstract
Background
Metabolic-associated fatty liver disease (MAFLD) is a public health issue that can result in liver cirrhosis and its complications.
Aim of work
Assess the dietary pattern, physical activity, and sleep quality of MAFLD patients.
Methods
Cross-sectional study of 77 MAFLD patients being present in the outpatient clinic of Endemic Medicine Department at Cairo University Hospital. An interview questionnaire including sociodemographic, medical history, validated food frequency questionnaire (FFQ), Pittsburg Sleep Quality Index (PSQI), and international physical activity questionnaire (IPAQ) were utilized to collect data.
Results
Among patients, 36%, 34%, and 30% had liver steatosis grades I (mild), II (moderate), and III (severe) respectively. Waist circumference, body mass index (BMI), daily caloric and carbohydrate intake, impaired sleep quality, and low physical activity were significantly linked with steatosis grades. The independent significant predictors for MAFLD severity were waist circumference (P = 0.011, OR 1.119), poor sleep quality (P = 0.038, OR 3.871), habitual sleep efficiency (OR 3.402, 95%CI 1.403–8.252), daytime dysfunction (OR 2.487, 95%CI 1.374–4.501), and physical activity (P = 0.027, OR 4.6).
Conclusion
Waist circumference, poor sleep quality, habitual sleep efficiency, daytime dysfunction, and low physical activity were the real marked predictors for MAFLD severity.
Similar content being viewed by others
Introduction
Non-alcoholic fatty liver disease (NAFLD) is a multifactorial disorder combining genetics, environmental, and metabolic variables. Recent proposals by a group of worldwide experts to redefine the condition metabolic (dysfunction)-associated fatty liver disease (MAFLD) [1]. These are three conditions—overweight or obesity, the presence of type 2 diabetes mellitus (T2DM), or signs of metabolic dysregulation—are the basis for the criteria for MAFLD. The distinction between diagnosing MAFLD and NAFLD is that MAFLD emphasises the role of metabolic dysfunction more so than NAFLD does, and does not need the elimination of heavy alcohol abuse or other forms of chronic liver disease [2].
Growing evidence suggests that MAFLD is a multisystem disorder that impacts a number of extra-hepatic organs and raises the incidence of cardiovascular diseases and chronic kidney disease. This information has been revealed over the past ten years, demonstrating that the global health burden of MAFLD is not limited to potentially progressive hepatic disorder [3].
High calorie consumption and decreased physical exercise are key lifestyle variables that contribute to the occurrence of MAFLD [4]. Furthermore, contrasted to exercise and diet, sleep disorders have received less attention, and there aren't many published findings that link poor sleep to MAFLD [5, 6].
Exercise and nutrition have been demonstrated to be helpful lifestyle therapies for MAFLD prevention and treatment when sufficient weight reduction is accomplished [7]. As a result, this study aims to evaluate the correlation between the lifestyle factors of nutrition, exercise, and sleep quality in individuals with MAFLD and the severity of the condition.
Patients and methods
This cross-sectional analytic study was performed at the outpatient clinic of Endemic Medicine department, Kasr-Al-Ainy Hospital, Cairo University, including 77 patients according to the following inclusion criteria: participants of both sexes aged above 18 years and proved to fulfil the criteria of fatty liver infiltration via abdominal ultrasound. Cases with hepatocellular carcinoma (HCC) and decompensated liver cirrhosis, cases known to have a viral cause hepatitis C virus (HCV), hepatitis B virus (HBV), metabolic disorders (e.g., Wilson’s disease, haemochromatosis), or autoimmune causes for liver disease (e.g., autoimmune hepatitis, primary sclerosing cholangitis, and primary biliary cholangitis) were excluded from the study.
Sampling
Sampling type was non-probability convenient sampling including all patients that fit the criteria of fatty liver as detected by ultrasonography according to inclusion and exclusion criteria. A sample size of 77 was calculated using Open Epi version 3, open-source calculator, with anticipated prevalence of sleep disorders in NAFLD patients of 54% and 95% confidence interval [5].
Data collection and study tools
Structured interview questionnaires
A structured Arabic interview questionnaire recorded by the researcher. It was designed to cover four sections: sociodemographic and medical included questions about the following: personal and sociodemographic history, e.g., age, sex, smoking, residence and occupation; medical history (e.g., diabetes mellitus (DM), hypertension, hypertriglyceridemia, and hypercholesterolemia) and family history of fatty liver disease, DM, hypertension, etc.
A standardised questionnaire is applied to evaluate the features of sleep (sleep duration, total time in bed, sleep efficacy, sleep quality and sleep disturbances), called the Pittsburgh Sleep Quality Index (PSQI). There are 19 self-administered questions in it, and the results yield 7 component scores with subscales from 0 to 3. For the purpose of evaluating the quality of sleep, these component values are added together to get a final score that ranges from 0 to 21. “Poor sleepers” are those with a worldwide score greater than 5, while “excellent sleepers” are those with a score of 5 or less. A short sleep duration was determined to be a self-recorded sleep time of less than 6 h each night [5, 8].
Semi-quantitative food frequency questionnaire (FFQ)
A validated 32-item semi-quantitative FFQ was utilized to assess each person's typical dietary consumption utilizing the typical serving size for Egyptians. Each food item's planned serving frequency throughout the previous year had to be reported by participants on a daily, weekly, or monthly manner. Each food item’s frequency category selection was then translated into a daily consumption. The portion sizes of the foods consumed were converted to grams using standard household measurements. Principal component analysis was used to find the primary eating trends [9,10,11].
International Physical Activity Questionnaire–Short Form (IPAQ)
Detailed history of physical activity (PA) was taken using the International Physical Activity Questionnaire (IPAQ) [12, 13]. Three distinct activity categories, involving vigorous-intensity activities, moderate-intensity activities, and walking, were covered in this questionnaire. Following that, IPAQ assessment computed the metabolic equivalent task (MET) as follows: vigorous MET (minutes/week) = 8.0 × vigorous-intensity activity minutes × vigorous-intensity days, moderate MET (minutes/week) = 4.0 × moderate-intensity activity minutes × moderate days and walking MET (minutes/week) = 3.3 × walking minutes × walking days. Walking, moderate, and vigorous MET (minutes/week) scores add up to the total PA MET (minutes/week). Following the computation of the final MET score, the individuals will be categorised into the following categories:
-
▪Category 1 (low): < 600 MET (minutes/week).
-
▪ Category 2 (moderate): ≥ 600 to < 3000 MET (minutes/week).
-
▪ Category 3 (high): ≥ 3000 MET (minutes/week).
Ultrasonography
Patients whose fatty liver was confirmed by abdominal ultrasound were categorized into three categories rely on the degree of steatosis depending on ultrasound characteristics (the examination was performed in accordance with the manufacturer’s recommendations using 3.5 MHz convex linear transducer to assess hepatic steatosis): gradations of liver echogenicity include mild (grade 1); slight increase in liver echogenicity, moderate (grade 2); visualisation of intrahepatic vessels, mild diaphragm impairment, and severe (grade 3), which includes a significant rise in liver echogenicity, poor penetration of the posterior segment of the right lobe of the liver, and poor or no visualization of the diaphragm and hepatic vessels [14].
Anthropometric measurements
-
1-
Weight: using a digital scale, the individuals’ body weight in light clothing and without shoes was determined and documented to the closest 0.5 kg [15].
-
2-
Height: the patient’s height was determined to the closest 0.1 cm while standing straight up against a wall and without shoes on [15].
-
3-
While standing, the waist circumference was determined midway between the iliac crest and the final rib border.
-
4-
BMI: body weight in kilogrammes was divided by height in square metres (Kg/m2) to get the individuals’ BMI. Standard weight status categories were linked to adult BMI ranges [16].
Data management and statistical methods
The Statistical Package of Social Science software, version 23 (SPSS), was utilized to enter pre-coded data into the computer for statistical analysis. Data were summarized utilizing the mean, standard deviation, range and median with interquartile range (IQR) for quantitative variables. Frequency and percentage were utilized for qualitative variables. Shapiro–Wilk test was used to determine if quantitative variables had a normal distribution. For qualitative data, the chi-square test was utilized to compare the variables. When one or more anticipated cell counts are fewer than five, the Fisher exact test was utilized. The independent t test was utilized to determine quantitative variables between two groups when data were normally distributed. When data were not normally distributed, the Mann–Whitney test was utilized. When grades of hepatic steatosis (mild/moderate and severe) were the dependent variable, multiple logistic regression was utilised to identify important predictors. Statistics were regarded significant at a P value of ≤ 0.05. Utilizing food composition tables for Egyptian foods from the National Nutrition Institute, nutritional intake assessment for the various macronutrients was done [17].
Ethical consideration
The study protocol and the study tools were revised and approved by Public Health and Community medicine Department, Endemic Medicine Department as well as the Research Ethical Committee (REC), Faculty of Medicine, Cairo University. An informed consent was collected from every participant after enough orientation of the study goals. Participation was voluntary, only those who accepted were involved and the participant had the right to discontinue participation at any time.
As per the Helsinki Declaration, strict privacy and confidentiality were upheld via the data collecting, entry, and analysis processes [18].
Results
This cross-sectional study was performed on 77 cases with MAFLD in a period from February 2021 to January 2022. Among the studied population, grading for liver steatosis due to MAFLD using trans-abdominal ultrasound was done revealing that 36%, 34%, and 30% had liver steatosis grades I (mild), II (moderate) and III (severe) respectively. We further classified the cases into 2 groups (group with mild (I) and the other group with moderate-severe steatosis (II–III)), we found that 63.6% of the studied patients were among the moderate-severe steatosis group.
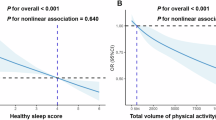
There was no statistically obvious difference between sociodemographic features of the studied population with grades of liver steatosis as revealed in (Table 1). Lifestyle-related factors of MAFLD for the included patients are illustrated in (Table 2). Hypertension and hyperlipidaemia were markedly greater in individuals with moderate-severe steatosis as the prevalence of hypertension and hyperlipidaemia in those cases was 57.1% and 63.3% respectively (p = 0.002 and p = 0.042 respectively). BMI and the waist circumference were markedly greater in cases with moderate-severe steatosis (p = < 0.001 both BMI and waist circumference). Family history of DM was markedly greater in cases with moderate-severe steatosis (p = 0.023). Individuals with moderate-severe fatty liver had markedly greater daily caloric and carbohydrates intake than those with mild fatty liver (p = 0.01 and p = 0.028 respectively). However, no statistically significant differences were detected among participants regarding the daily proteins and fats intake. In the studied population, the spread of poor sleep quality was markedly higher in patients with moderate-severe fatty liver than those with mild fatty liver (p = 0.029) with statistically significant lower grade physical activity among patients with moderate-severe MAFLD (p = 0.006). Concerning the lifestyle predictors of steatosis, it was found that high waist circumference increased the probability to develop severe MAFLD by 11% (OR (95% CI) = 1.19 (1.026–1.22)), poor sleep quality increased the risk of severe NAFLD 3 times (OR (95% CI) = 3.871 (1.075–13.933)) and low physical activity increased the risk 4 times (OR (95% CI) = 4.6 (1.195–17.746)) as illustrated in (Table 3). Table 4 and Fig. 1 showed that very bad sleep latency and fairly bad sleep disturbance and daytime dysfunction were significantly higher in moderate-severe steatosis grade 46.9%, 44.9%, and 40.8 versus 17.9% and 14.3%, 21.4 in mild steatosis. In addition, there was a great positive correlation between the levels of habitual sleep effectiveness, disturbed sleep, and daytime impairment and MAFLD steatosis grades where worse sleep quality scores were associated with moderate-severe fatty liver (r 0.300**, 0.338**, and 0.363** respectively). Table 5 showed that habitual sleep effectiveness and daytime impairment were found to be great predictors for MAFLD severity. Very bad habitual sleep efficiency carries three times risk for moderate-severe steatosis (OR (95% CI) = 3.402 (1.403–8.252)) while very bad daytime dysfunction has two times risk (OR (95% CI) = 2.487 (1.374–4.501)). The significance of waist circumference, BMI, physical activity and sleep duration hours is shown in (Fig. 2).
Discussion
Metabolic dysfunction-associated fatty liver disease (MAFLD) is a growing public health issue globally that needs attention to put plans for its control and prevention in nations where the spread is raising [19]. Thus, this study aimed to assess lifestyle determinants (diet, sleep quality and physical activity) among patients with different grades of MAFLD.
As regards the metabolic syndrome, in the current study, both hypertension and hyperlipidemia were markedly linked with greater grades of liver steatosis (57.1%, 63.3% of cases with moderate-severe liver steatosis, P = 0.002, P = 0.042). In accordance with a cross-sectional study done in Germany where greater grades of hepatic steatosis were significantly linked to greater levels of blood pressure [20]. In accordance with a systematic review, by Oikonomou et al., for all researches that investigated the possible association between hypertension and MAFLD, hypertension was found to be a predictor of new-onset MAFLD diagnosed by ultrasound. This is due to the fact that hypertension causes insulin resistance and renin-angiotensin system-sympathetic nervous system activation [21, 22]. Concerning hyperlipedemia, the same was concluded by the study of Mahaling et al. which observed that elevating levels of MAFLD were significantly linked to raising levels of serum total cholesterol VLDL and LDL and reducing HDL [23]. A cross-sectional Egyptian study done by Rafat et al. has concluded the same results [24]. This could be attributed to the adverse effect of hyperlipidemia on lipoprotein and lipid production in the liver, leading to raised low-density lipoprotein (LDL) and triglycerides (TG) levels and diminished high density lipoprotein (HDL-C) levels that eventually lead to abnormal fatty acid accumulation [25].
Regarding diabetes mellitus, despite that there was no statistically significant difference between the grades of liver steatosis and DM, 30.6% of the recruited patients in this study with moderate-severe steatosis were diabetic which is nearly double the prevalence (17.9%) of DM in patients with mild steatosis however there was significantly higher family history of DM in cases with moderate-severe steatosis (p = 0.023). In alignment with our findings, a cross-sectional prospective study by Loomba et al. concluded that family history of diabetes, particularly in non-diabetics was linked to NASH and fibrosis in MAFLD [26].
The current research reported great association between obesity and waist circumference with moderate-severe grades of liver steatosis (P < 0.001) with a finding that high waist circumference (which reflects central obesity) was a significant predictor for MAFLD severity. The same conclusion was reported by Hasan et al. where BMI and waist circumference were markedly linked to the different grades of steatosis [27]. This also agreed with Shao et al.which concluded that waist circumference was a significant predictor for MAFLD severity in obese and overweight MAFLD cases [28]. A meta-analysis of 8 retrospective and13 prospective studies illustrated that compared to normal weight individuals, obesity independently resulted in a 3.5-fold high risk of developing MAFLD. It also concluded an apparent dose-dependent association between BMI and N = MAFLD risk (per 1-unit increment in BMI: RR = 1.20, 95%CI 1.14 to 1.26, P < 0.001) [29]. This may be due to the fact that there is a close association between obesity and the development of MAFLD and its severity due to insulin resistance and alteration in fatty acid metabolism [30].
On the other hand, many studies concluded that MAFLD can occur in non-obese individuals, and it needs careful evaluation and follow-up [31,32,33]. Lean MAFLD can be attributed to genetic factors.
The relationship between diet and the development of MAFLD is complicated and goes beyond total calorie consumption as diet composition is more important factor [34]. The mechanism by which diet causes MAFLD is through its effect on liver lipogenesis and insulin resistance [4, 35]. Also, excessive ingestion of carbohydrates is a major stimulus for hepatic denovo lipogenesis with more incidence of MAFLD than consumption of dietry fat intake [36]. In this study, individuals with moderate-severe liver steatosis had markedly greater daily caloric and carbohydrates intake than those with mild fatty liver (P = 0.01 and P = 0.028 respectively). Among participants, 72.4% and 64.4% of those with moderate-severe steatosis over consumed fats and proteins respectively versus 27.6% and 35.6% of those with mild steatosis. However, no statistically significant differences were identified between participants regarding the daily proteins and fats intake (P = 0.619 and P = 0.151 respectively). These outcomes match with those of Gonzalez et al. where the relation between steatosis grade and the daily caloric and carbohydrate intake was significant confirming that less calories and less carbohydrate in the diet are linked to less fat in the liver of the cases with MAFLD [37]. On the other hand, Lang and colleagues in Germany had concluded that higher intake of dietary protein was linked to both disease severity and activity in cases with MAFLD [38]. Matching the recommendation of AISF which favors the approach to the Mediterranean diet that includes decreased intake of industrial and refined sugars and this reduces hepatic fat content and decrease cardiovascular risk for MAFLD [39].
Physical inactivity is a unique predisposing factor for MAFLD and its severity regardless of body weight because it is linked to metabolic syndrome and insulin resistance [40]. In the current research, cases with moderate-severe steatosis revealed markedly lower MET than those with mild grade. Physical activity was identified to be a great predictor for MAFLD severity where individuals with low physical activity were four times more liable to develop severe MAFLD. According to the research of Vilar-Gomez et al. MAFLD risk was lower in physically active (≥ 600 metabolic equivalent of task (MET) min/week) versus inactive individuals (< 600 MET min/week) (OR 0.71, p = 0.043) [41]. This association illustrates the protective impact of physical activity.
Owing to the risk factors for development of MAFLD, AASLD recommended a mixture of a hypocaloric diet (daily decrease by 500–1,000 kcal), daily protein intake of 1.2–1.5 g/kg of body weight/day together with moderate-intensity exercise to provide sustained weight loss (7–10% loss target) over time thus reducing hepatic steatosis and regression of fibrosis [42].
The current research revealed that the spread of poor sleep quality was significantly greater in patients with moderate-severe grade of steatosis (71.7%, P = 0.029) with significantly worse scores for sleep latency, sleep disturbance and daytime dysfunction (46.9% very bad, 44.9% and 40.8% fairly bad, respectively) when compared to mild steatosis grade. A significant positive correlation was identified between MAFLD steatosis grades and worse scores of sleep disturbance, habitual sleep efficacy, and daytime dysfunction (r 0.300**, 0.338**, and 0.363** respectively). In Taiwan, Chou et al. study showed comparable results where the spread of poor sleep quality in individuals with moderate-severe liver steatosis was 53.6% [43]. A case control study by Jiahui et al. revealed that liver steatosis degree was positively linked to PSQI score (r = 0.444, P < 0.001) [44]. Using the Epworth sleepiness scale, Bernsmeier et al. have concluded that daytime sleepiness was markedly greater in MAFLD cases especially NASH subgroup in comparison to controls (26.5% vs. 13.6%; p = 0.0228) [45].
The logistic regression analysis in the current research revealed that poor sleep quality (P = 0.038, OR 3.871), habitual sleep efficiency (OR 3.402, 95%CI 1.403–8.252) and daytime dysfunction (OR 2.487, 95%CI 1.374–4.501) were great predictors for severe MAFLD. These results match those of Jiahui et al. where the multivariate logistic regression analysis revealed that a high degree of somnolence (OR = 5.420, P < 0.001), poor sleep quality (OR = 8.493, P < 0.001), and circadian rhythm disturbance (OR = 3.805, P < 0.001) were predisposing factors for MAFLD [44]. According to Takahashi et al., worse daytime dysfunction scores were linked to higher OR for NAFLD in both females (OR 2.08, 95%CI 1.10–3.92) and males (OR 2.82, 95%CI 1.39–5.75). The ratings for habitual sleep efficacy in women and sleep latency in both sexes were similarly linked to NAFLD [6].
Inadequate sleep and short sleep duration were linked to a variety of negative health results, involving type 2 diabetes mellitus, obesity, and the risk and progression of MAFLD [5]. However, there was no statistically significant correlation between grades of steatosis regarding sleep duration. This could be attributed to the fact that despite the sleep duration was adequate, the sleep quality was poor. Chou et al. study has concluded the same results in men but not in women [43]. The stimulation of the hypothalamic–pituitary–adrenal (HPA) axis that cause the release of stress hormones including catecholamines and cortisol, is the primary mechanism through which sleep quality leads to MAFLD and raises the risk of metabolic syndrome [6].
Limitations
The study has limitation concerning the ability to use fibroscan to measure the degree of hepatic steatosis, which may be considered a point for further research.
Conclusion
The study concluded that the actual significant predictors for MAFLD were waist circumference, poor sleep quality, habitual sleep efficiency, daytime dysfunction, and low physical activity. Thus, controlling lifestyle factors is essential in prevention of MAFLD. Further study is recommended on the usage of non invasive methods of diagnosis of liver steatosis (controlled attenuation parameter) and liver function tests in correlation with ultasound staging of hepatic steatosis severity with follow-up of the lifestyle determinants.
Availability of data and materials
The datasets used and/or analyzed during the current study are available from the corresponding author on reasonable request.
References
Eslam M, Sanyal AJ, George J (2020) MAFLD a consensus-driven proposed nomenclature for metabolic associated fatty liver disease. Gastroenterology. 158:1999–2014. (j.gastro..11.312)
Eslam M, Newsome PN, Anstee QM, Targher G, Gomez MR, Zelber-Sagi S et al (2020) A new definition for metabolic associated fatty liver disease: an international expert consensus statement. J Hepatol 73:202–209. (j.jhep.2020.03.039)
Patil R, Sood GK (2017) Non-alcoholic fatty liver disease and cardiovascular risk. World J Gastrointest Pathophysiol 8(2):51
Hallsworth K, Adams LA (2019) Lifestyle modification in NAFLD/NASH: facts and figures. JHEP Rep 1(6):468–479
Marin-Alejandre BA, Abete I, Cantero I, Riezu-Boj JI, Milagro FI, Monreal JI et al (2019) Association between sleep disturbances and liver status in obese subjects with nonalcoholic fatty liver disease: a comparison with healthy controls. Nutrients 11(2):322
Takahashi A, Anzai Y, Kuroda M, Kokubun M, Kondo Y, Ogata T et al (2020) Effects of sleep quality on non-alcoholic fatty liver disease: a cross-sectional survey. BMJ Open 10(10):e039947
European Association for the Study of The Liver, & European Association for the Study of Diabetes (EASD (2016) EASL-EASD-EASO Clinical Practice Guidelines for the management of non-alcoholic fatty liver disease. Obesity Facts. 9(2):65–90
Mollayeva T, Thurairajah P, Burton K, Mollayeva S, Shapiro CM, Colantonio A (2016) The Pittsburgh sleep quality index as a screening tool for sleep dysfunction in clinical and non-clinical samples: A systematic review and meta-analysis. Sleep Med Rev 25:52–73
FAO, WHO & UNU (2001) Human energy requirements: Report of a Joint FAO/WHO/UNU Expert Consultation: Food and Agriculture Organization of the United Nations Rome
NNI, C., Egypt (2008) DNPCNCD: Diet, Nutrition and Prevention of chronic Non-communicable Diseases among Egyptian Adolescents. https://www.unicef.org/egypt/media/296/file
Afif ZE, Shehata RI, El Sayed AF, El Sayed MH, Salem MR (2021) Nutritional status of multiple sclerosis (MS) patients attending Kasr Alainy MS unit: an exploratory cross-sectional study. J Egypt Public Health Assoc 96(1):1–9
Hagströmer M, Oja P, Sjöström M (2006) The International Physical Activity Questionnaire (IPAQ): a study of concurrent and construct validity. Public Health Nutr 9(6):755–762
Ashok P, Kharche JS, Raju R, Godbole G (2017) Metabolic equivalent task assessment for physical activity in medical students. Natl J Physiol Pharm Pharmacol 7(3):236
Ferraioli G, Monteiro LBS (2019) Ultrasound-based techniques for the diagnosis of liver steatosis. World J Gastroenterol 25(40):6053
CDC. (2016a). Anthropometry Procedures Manual. Retrieved from https://www.cdc.gov/nchs/data/nhanes/nhanes_15_16/2016_Anthropometry_Procedures_Manual.pdf
CDC. (2016b, 16–6–2016). Defining adult overweight and obesity. Retrieved from https://www.cdc.gov/obesity/adult/defining.html
NNI, C., Egypt. National Nutrition Institute (NNI) (2006) Food Composition Tables for Egypt. https://www.unicef.org/egypt/media/296/file
-World Medical Association (WMA) (2013). World Medical Association Declaration of Helsinki: ethical principles for medical research involving human subjects. [https://www.wma.net/policies-post/wmadeclaration-of-helsinki-ethical-principles-for-medical-researchinvolving-human-subjects/]. JAMA. 2013;310(20):2191–2194. /jama.2013.281053
Mostafa O, Razik M, Marzaban R, Sayed E, Ahmed M (2019) Epidemiologic profile and predictors of fatty liver: a hospital-based study. Int J Trop Dis Health 36(1):1–11
Lorbeer R, Bayerl C, Auweter S, Rospleszcz S, Lieb W, Meisinger C et al (2017) Association between MRI-derived hepatic fat fraction and blood pressure in participants without history of cardiovascular disease. J Hypertens 35(4):737–744
Oikonomou D, Georgiopoulos G, Katsi V, Kourek C, Tsioufis C, Alexopoulou A et al (2018) Non-alcoholic fatty liver disease and hypertension: coprevalent or correlated? Eur J Gastroenterol Hepatol 30(9):979–985
Cotter TG, Rinella M (2020) Nonalcoholic fatty liver disease 2020: the state of the disease. Gastroenterology 158(7):1851–1864
Mahaling DU, Basavaraj MM, Bika AJ (2013) Comparison of lipid profile in different grades of non-alcoholic fatty liver disease diagnosed on ultrasound. Asian Pac J Trop Biomed 3(11):907–912
Rafat FK, Nabil M, Salah M, Mohamed MA, Ibrahim W (2018) Study of lipid profile in different grades of non-alcoholic fatty liver disease. Egypt J Hosp Med 73(8):7388–7393
Zhang QQ, Lu LG (2015) Nonalcoholic fatty liver disease: dyslipidemia, risk for cardiovascular complications, and treatment strategy. J Clin Transl Hepatol 3(1):78
Loomba R, Abraham M, Unalp A, Wilson L, Lavine J, Doo E et al (2012) Nonalcoholic steatohepatitis clinical research network Association between diabetes, family history of diabetes, and risk of nonalcoholic steatohepatitis and fibrosis. Hepatology 56(3):943–951
Hasan EM, Abd Al Aziz RA, Sabry D, Badary HA, Gaber Y, Yosry A et al (2021) The association of adiponectin gene polymorphisms with susceptibility and progression of NAFLD in a cohort of Egyptian patients. Egyptian Liver Journal 11(1):1–11
Shao C, Ye J, Li F, Feng S, Wang W, Zhong B (2019) Different predictors of steatosis and fibrosis severity among lean, overweight and obese patients with nonalcoholic fatty liver disease. Dig Liver Dis 51(10):1392–1399
Li L, Liu DW, Yan HY, Wang ZY, Zhao SH, Wang B (2016) Obesity is an independent risk factor for non-alcoholic fatty liver disease: evidence from a meta-analysis of 21 cohort studies. Obes Rev 17(6):510–519
Khamseh ME, Malek M, Abbasi R, Taheri H, Lahouti M, Alaei-Shahmiri F (2021) Triglyceride glucose index and related parameters (triglyceride glucose-body mass index and triglyceride glucose-waist circumference) identify nonalcoholic fatty liver and liver fibrosis in individuals with overweight/obesity. Metab Syndr Relat Disord 19(3):167–173
Niriella MA, Kasturiratne A, Pathmeswaran A, De Silva ST, Perera KR, Subasinghe SK et al (2019) Lean non-alcoholic fatty liver disease (lean NAFLD): characteristics, metabolic outcomes and risk factors from a 7-year prospective, community cohort study from Sri Lanka. Hep Intl 13(3):314–322
Zou B, Yeo YH, Nguyen VH, Cheung R, Ingelsson E, Nguyen MH (2020) Prevalence, characteristics and mortality outcomes of obese, nonobese and lean NAFLD in the United States, 1999–2016. J Intern Med 288(1):139–151
Ito T, Ishigami M, Zou B, Tanaka T, Takahashi H, Kurosaki M et al (2021) The epidemiology of NAFLD and lean NAFLD in Japan: a meta-analysis with individual and forecasting analysis, 1995–2040. Hep Intl 15(2):366–379
Stephenson K, Kennedy L, Hargrove L, Demieville J, Thomson J, Alpini G et al (2018) Updates on dietary models of nonalcoholic fatty liver disease: current studies and insights. Gene Expr 18(1):5
Lim JS, Mietus-Snyder M, Valente A, Schwarz JM, Lustig RH (2010) The role of fructose in the pathogenesis of NAFLD and the metabolic syndrome. Nat Rev Gastroenterol Hepatol 7(5):251–264
Basaranoglu M, Basaranoglu G, Bugianesi E (2015) Carbohydrate intake and nonalcoholic fatty liver disease: fructose as a weapon of mass destruction. Hepatobiliary Surg Nutr 4(2):109–116
Gonzalez C, de Ledinghen V, Vergniol J, Foucher J, Le Bail B, Carlier S et al (2013) Hepatic steatosis, carbohydrate intake, and food quotient in patients with nafld. Int J endocrinol 2013(428542):4
Lang S, Martin A, Farowski F, Wisplinghoff H, Vehreschild MJ, Liu J et al (2020) High protein intake is associated with histological disease activity in patients with NAFLD. Hepatol Commun 4(5):681–695
Associazione Italiana per lo Studio del Fegato (AISF), (2022) Società Italiana di Diabetologia (SID) and Società Italiana dell'Obesità (SIO)1Members of the guidelines panel, Non-alcoholic fatty liver disease in adults: a clinical practice guideline of the Italian Association for the Study of the Liver (AISF), the Italian Society of Diabetology (SID) and the Italian Society of Obesity (SIO). Digest Liver Dis 54(2):170–182. https://doi.org/10.1007/s40519-021-01287-1
Windt DJV, Sud V, Hongji Zhang H, Allan Tsung A, Huang H (2018) The s. Gene Expr 18(2):89–101
Vilar‐Gomez E, Nephew LD, Vuppalanchi R, Gawrieh S, Mladenovic A, Pike F, et al (2021) High-quality diet, physical activitym and college education are associated with low risk of NAFLD among the US population. Hepatology 75(6):1491–1506
Chalasani N, Younossi Z, Lavine JE, Charlton M, Cusi K, Rinella M et al (2018) The diagnosis and management of nonalcoholic fatty liver disease: practice guidance from the American Association for the Study of Liver Diseases. Hepatology 67(1):328–357
Chou YT, Cheng HJ, Wu JS, Yang YC, Chou CY, Chang CJ et al (2018) The association of sleep duration and sleep quality with non-alcoholic fatty liver disease in a Taiwanese population. Obes Res Clin Pract 12(6):500–505
Jiahui LYU, Xiaofei LI, Lianyi GUO (2021) Association between metabolic associated fatty liver disease and sleep disorders. J Clin Hepatol 37(12):2854–2860
Bernsmeier C, Weisskopf DM, Pflueger MO, Mosimann J, Campana B, Terracciano L et al (2015) Sleep disruption and daytime sleepiness correlating with disease severity and insulin resistance in non-alcoholic fatty liver disease: a comparison with healthy controls. PLoS One 10(11):e0143293
Acknowledgements
No conflict of interest.
Funding
No particular grant for this research was provided by funding organizations in the public, private, or nonprofit sectors.
Author information
Authors and Affiliations
Contributions
Abeer Attia (AA): analysis, interpretation and conception of data. Nargis Albert Labib (LNA): conception, design, and final revision. Noha Essameldin Elsayed Abdelzaher (ANEE): collection of data and drafting of paper. Sherief Musa (MS): clinical work and revision. Mira Atef (AM): clinical work, drafting of paper, and submission. All authors read and approved the final manuscript.
Corresponding author
Ethics declarations
Ethics approval and consent to participate
This research was approved by Public Health and Community medicine Department, Endemic Medicine Department as well as the Research Ethical Committee (REC), Faculty of Medicine, Cairo University.
Competing interests
The authors declare that they have no conflicts of interest.
Additional information
Publisher’s Note
Springer Nature remains neutral with regard to jurisdictional claims in published maps and institutional affiliations.
Rights and permissions
Open Access This article is licensed under a Creative Commons Attribution 4.0 International License, which permits use, sharing, adaptation, distribution and reproduction in any medium or format, as long as you give appropriate credit to the original author(s) and the source, provide a link to the Creative Commons licence, and indicate if changes were made. The images or other third party material in this article are included in the article's Creative Commons licence, unless indicated otherwise in a credit line to the material. If material is not included in the article's Creative Commons licence and your intended use is not permitted by statutory regulation or exceeds the permitted use, you will need to obtain permission directly from the copyright holder. To view a copy of this licence, visit http://creativecommons.org/licenses/by/4.0/.
About this article
Cite this article
Attia, A., Labib, N.A., Abdelzaher, N.E.E. et al. Lifestyle determinants as predictor of severity of metabolic associated fatty liver disease (MAFLD). Egypt Liver Journal 13, 47 (2023). https://doi.org/10.1186/s43066-023-00282-8
Received:
Accepted:
Published:
DOI: https://doi.org/10.1186/s43066-023-00282-8