Abstract
Introduction
Acute kidney injury (AKI) is frequently seen in critically ill patients and is associated with high mortality and morbidity. However, the optimal dialysis modality in such patients remains controversial. We examined the hemodynamic tolerability of hemodialysis modalities in critically ill individuals with AKI.
Methodology
Critically ill patients with AKI who underwent Continuous Renal Replacement Therapy (CRRT), Sustained Low-Efficiency Diafiltration (SLEDD-f), or Sustained Low-Efficiency Dialysis (SLED) dialysis were included in the study. In-hospital mortality, number of dialysis sessions, number of sessions terminated pre-maturely, change in blood pressure during dialysis, and hemodynamic instability during dialysis sessions were noted.
Results
A total of 264 patients were included, of which 78 received Continuous Renal Replacement Therapy (CRRT), 62 received Sustained Low-Efficiency Diafiltration (SLEDD-f), and 124 received Sustained Low-Efficiency Dialysis (SLED), with a total of 682 sessions among 264 patients. The commonest cause for AKI was septic shock (32.6%, n=43). All CRRT and SLEDD-f sessions were delivered without anticoagulation, and SLED was delivered without anticoagulation in 88.7% of sessions. There was a significant decrease in mean arterial pressure in CRRT compared to other modalities, with higher mortality. However, patients undergoing CRRT were more sicker. There was no significant difference between SLEDD-f and SLED in terms of outcomes.
Conclusion
SLEDD-f and SLED have good hemodynamic tolerability compared to CRRT. There was no significant difference in hemodynamic disturbances between SLEDD-f and SLED despite a higher proportion of patients on SLEDD-f being more critical.
Similar content being viewed by others
Introduction
Acute kidney injury (AKI) is frequently seen in critically ill patients and causes high mortality and morbidity with prolonged Intensive Care Unit (ICU) stay [1]. Renal Replacement Therapy (RRT) is required in around 4-5% of cases [2]. The optimal dialysis modality of choice in critically ill AKI patients remains controversial. Continuous renal replacement therapy (CRRT) is advocated in patients with hemodynamic instability over conventional intermittent hemodialysis (IHD) [3]. However, randomized control trials have not demonstrated superior survival and hemodynamic stability in patients treated with CRRT compared to IHD, [4, 5] and CRRT is associated with high costs, increased nursing burden, and continuous patient immobility.
Sustained Low-Efficiency Dialysis (SLED) and Sustained Low-Efficiency Diafiltration (SLED-F) have been used as an alternative to CRRT in patients with AKI who have hemodynamic instability. SLED has shown fluid and solute removal comparable to CRRT, [6, 7], but studies on SLED-F (Sustained Low-Efficiency Diafiltration) are lacking. In this study, we examined the hemodynamic tolerability of SLED, SLED-F, and CRRT in critically ill individuals with AKI.
Methodology
Aim
To evaluate the safety and efficacy of various hemodialysis methods in critically ill patients with acute kidney injury.
Design
Prospective Observational study.
Setting
The study was conducted in a tertiary care unit between January 2019 to December 2021.
Methods
After obtaining ethical committee clearance, all critically ill patients with AKI admitted to the critical care unit and needing renal replacement therapy (RRT) were evaluated for inclusion. The patient’s age, gender, and presence of co-morbidities like Diabetes Mellitus (DM), Hypertension (HTN), and cardiac disease (CAD) were noted. The patient’s cause of AKI was obtained from the case records. The treating nephrologist decided on the dialysis modality, such as SLED, SLED-F, or CRRT. Patients undergoing only one session of dialysis or patients initiated on dialysis outside our center were excluded from the analysis. For analysis, 24 hours of CRRT was considered as one complete session. A session was designated as interrupted if the administered time was < 90% of the prescribed time. AKI was defined per the Kidney Disease Improving Global Outcomes (KDIGO) criteria [8]. Baseline characteristics, such as age, sex, presence of co-morbidities, and diagnosis, were noted from the case records. The need and use of vasopressors were also noted from the case records.
CVVHDF (Continuous Veno-Venous Hemodiafiltration) was the CRRT modality used in all patients. CVVHDF was performed using the Gambro Prismaflex system with a high flux dialyzer, with the effluent fluid rate and other parameters prescribed by the attending nephrologist. SLED and SLED-F were performed using the Fresenius 4008S dialysis machine with a Fresenius F6HPS high-flux dialyzer.
SLED was done using a Blood Flow rate of 100-150ml/min and dialysate flow rates of 300ml/min over 6-10 hours per session with fluid removal as prescribed by the nephrologist. SLED-F was performed using blood flow rates of 100-150ml/min, dialysate flow rates of 300ml/hr, and a pre-pump replacement fluid for 6-10 hours per session and fluid removal as prescribed by the nephrologist. The replacement fluid consisted of normal saline or an iso-osmolar mixture of sodium bicarbonate and 0.45% normal saline. Anticoagulation was used as specified by the attending Nephrologist.
The following parameters were monitored every 10 minutes: Systolic Blood Pressure (SBP), Diastolic Blood Pressure (DBP), Mean Arterial Pressure (MAP), and Pulse Pressure (PP). Hemodynamic instability was defined as a> 20% reduction in MAP or an increased requirement of vasopressors. Blood urea and serum creatinine were measured before and after every session, and urea-reduction and creatinine-reduction rates were calculated. The change in blood pressure parameters during dialysis sessions was also noted. The biochemical parameters such as urea and creatinine were measured using standard laboratory techniques.
The following outcomes were noted for each patient: in-hospital mortality, number of dialysis sessions, number of sessions terminated prematurely, change in blood pressure during dialysis, and hemodynamic instability.
Statistical Analysis: The collected data were analyzed with IBM SPSS statistics software 29.0 Version. The continuous variables were expressed as means, standard deviations (SD), or medians as appropriate, and categorical variables as numbers (percentages). The continuous variables were compared using the independent t-test and analysis of variance, while categorical variables were compared using the Fisher exact test and Chi-square test.
Results
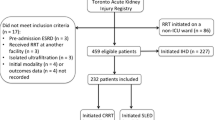
A total of 662 patients with AKI underwent dialysis during the study period, of which 326 patients underwent IHD, and 38 patients received their first dialysis in a non-ICU setting and were excluded. Of the remaining 288 patients, 16 were initiated on RRT at an external facility, while eight patients had incomplete data and were excluded from analysis. Thus, 264 patients who underwent a total of 682 sessions were included for analysis, of which 78 patients received CRRT, 62 received SLEDD-f, and 124 received SLED (Fig. 1). Most of the patients were within the age group of 40-59 years, with a mean age of 51.1 ± 15.15 years, and 62.9% were males. The baseline characteristics of patients in the three groups are shown in Table 1. Among the 264 patients, 42.4% were diabetics, 42.4% were hypertensives, 31.3% had both diabetes and hypertension, and 33.3% had a cardiac disease. The commonest cause for AKI was septic shock (32.6%, n=86), followed by respiratory infection (23.5%, n=62). The remaining cases were caused by cardiac diseases (15.2%, n=40), Neurologic disorders (3%, n=8), Tropical infections (3.8%, n=10), Urosepsis (3%, n=8), and others (18.9%, n=50) like liver disease, poisoning, pregnancy-related AKI, etc. (Fig. 1).
The mean number of sessions per patient was 2.6 ± 0.96, with 64.4% (n=170) of them being on inotropic support. The number of patients on one, two, three, or four inotropes were 80 (30.3%), 64 (24.2%), 20 (7.6%) and 6 (2.3%) respectively. Of the 170 patients on inotropic support at the initiation of dialysis, 27.3% (n=72) needed increased inotropic support during dialysis. Among the 264 patients, 10.6% had hemodynamic instability during dialysis, among which 28.6% did not have an increase in inotropic support, with a mortality rate of 40.9%. An increase in inotropic support was needed in 19.7% without a significant BP drop.
We examined 682 treatment sessions (232 CRRT, 304 SLED, and 146 SLEDD-f sessions) to characterize the RRT delivered to members of the cohort. The mean number of CRRT, SLED, and SLEDD-f sessions were 2.97 ± 1.53, 2.45 ± 0.49, and 2.35 ± 0.48, respectively. On average, 78.9% of the prescribed sessions were delivered. The mean number of sessions terminated prematurely in CRRT, SLED, and SLEDD-f were 19%, 26.3%, and 13.7%, respectively (p<0.01). All CRRT and SLEDD-f sessions were delivered without anticoagulation, and SLED was delivered without anticoagulation in 88.7% of treatment sessions.
In the CRRT group, the mean age was 48.2 ± 13.08 years, with 61.5% males, 41% having DM, 33.3% having HTN, and 41% having CAD. The mean SBP, DBP, and MAP were 94.15 ± 5.48, 62.31 ± 6.01, and 72.92 ± 5.21 mmHg, respectively. A total of 232 sessions of CRRT were done, of which 188 were completed and included for analysis. All the patients undergoing CRRT were on inotropic support, with an increase in inotrope requirement during dialysis needed in 30 patients (38.5%). There was a significant decrease in the SBP, DBP, and MAP after the initiation of CRRT (p<0.001), with an increase in the need for inotropes (p<0.001). The mean drop in MAP was 11.53%, with 28.2% having hemodynamic instability and a mortality rate of 66.7%.
In the SLED group, a total of 124 patients and 304 sessions were done, with a mean age of 52.13 ± 16.58 years, with 61.3% males, 45.2% having DM, 54.8% having HTN, and 32.3% having CAD. The mean number of sessions per patient was 2.45 ± 0.49. The mean SBP, DBP, and MAP were 107.19 ± 17.02, 68.68 ± 9.05, and 81.52 ± 10.77 mmHg, respectively. Among patients undergoing SLED, only 38.7% were on inotropes at initiation, with an increase in inotrope requirement during dialysis needed in 27.4% of patients (n=34). There was a significant marginal increase in the SBP, with a marginal rise in DBP and MAP after initiation of SLED (p<0.001), with an increase in the need for inotropes (p<0.001). The mean drop in MAP was 0.03%, with 3.2% having hemodynamic instability and a mortality rate of 29.8%.
We found statistically significant decreases in Mean Arterial Pressure (MAP) (p<0.0001) and hemodynamic instability (p<0.01) in patients with CRRT as compared to the other modalities. Also, patients on CRRT had significantly higher mortality (p<0.0001) (Fig. 2). There was no significant difference between SLEDD-f and SLED in terms of MAP (p=0.65), Hemodynamic instability (p=0.21), early terminations (p=0.4), or mortality (p=0.83) (Table 2). The percentage of patients on inotropic support before initiation of RRT was 100%, 71%, and 39% in CRRT, SLEDD-f, and SLED groups, respectively. Patients who initiated CRRT had higher SOFA scores at the time of RRT initiation (10.9 ± 3.21) compared to SLED (8.31 ± 2.18) (p<0.001), while there was a non-significant difference when compared to SLEDD-f (10.92 ± 2.15) (p = 0.977). CRRT subjects were significantly more likely to be on multiple inotropes, 2.6 vs. 0.8 vs. 1.5 for CRRT, SLED, and SLEDD-f, respectively (p<0.001). There was a significant difference in SOFA scores (p<0.001) at initiation and in the requirement of inotropes (p<0.05) between SLED and SLEDD-f too. The in-hospital mortality rates in CRRT, SLED, and SLEDD-f were 74.4%, 50%, and 58.1%, respectively (Table 2).
Discussion
In critically ill patients with AKI and hemodynamic instability, the choice of modality for initiating RRT can be a dilemma. Various studies have compared the outcomes and effects of CRRT and SLED, but we also studied the tolerability of SLEDD-f.
We found statistically significant decreases in MAP and hemodynamic instability in patients with CRRT compared to the other modalities. There were no significant differences between SLEDD-f and SLED in terms of a reduction in MAP or hemodynamic instability. The odds ratio for hemodynamic instability with CRRT as compared to SLEDD-f and SLED was 6.07 and 2.85, respectively, while the odds ratio for hemodynamic instability with SLEDD-f as compared to SLED was 0.47. The patients in the CRRT group were sicker compared to other patients and were on significantly more inotropes at initiation and could thus have high mortality. This shows that the higher mortality in the CRRT group could be due to the more severe nature of the illness. Also worth noting is that, despite the higher proportion of patients in the SLEDD-f group being on inotropes before RRT initiation as compared to the SLED group, there was no significant difference between the hemodynamic tolerability between the two groups. This is reflected in the fact that patients on CRRT had significantly higher SOFA scores at baseline.
In the study by Fieghen et al [9], the authors noted that the administration of SLED is feasible and provides comparable hemodynamic control to CRRT in critically patients with AKI. Hemodynamic instability occurred during 22 (56.4%) SLED and 43 (50.0%) CRRT sessions (p = 0.51). In a multivariable analysis that accounted for the clustering of multiple sessions within the same patient, the odds ratio for hemodynamic instability with SLED was 1.20 (95% CI 0.58-2.47), as compared to CRRT. Session interruption occurred in 16 (16.3), 30 (34.9), and 11 (28.2) of IHD, CRRT, and SLED therapies, respectively.
Kitchlu et al. [10] conducted a cohort study comparing SLED (target 8 h/session, blood flow 200 mL/min) to CRRT in four ICUs at an academic medical center. They found similar clinical outcomes for patients treated with SLED and CRRT. The mortality at 30 days was 54 % and 61 % among SLED- and CRRT-treated patients, respectively [adjusted odds ratio (OR) 1.07, as compared with CRRT].
Two randomized controlled trials have compared the tolerability and efficacy of SLED vs CRRT, and their results do not demonstrate the superiority of one over the other. In a study by Abe et al., 60 patients were randomized to receive SLED or CRRT. They found no difference in survival at ICU discharge or 30-days between the two groups [11]. They also concluded that the type of modality did not affect the In-hospital renal recovery. Scwenger et al. conducted the largest trial to compare CRRT and SLED regarding clinical outcomes. They randomized 232 critically ill patients with AKI to either CRRT or SLED and found that there was no difference in survival between the two groups [12]. However, their study's durations of CRRT and SLED were similar, with a mean duration of 15.9±4.2 hrs/session and 14.9±4.4 hrs/session, respectively, which are different from the conventional durations of such modalities.
Sun et al. [13], in their retrospective analysis of 80 patients on SLED and 65 patients on CRRT, observed that the 60-day mortality was similar between both groups, but RRT independence was higher in the CRRT group.
Marshall et al. [14] did SLEDD-f sessions in 24 critically ill patients with AKI and observed that none of the patients developed hypotension or any other complications during the sessions. They found an in-hospital mortality of 46%, which was similar to that expected from the APACHE II critical illness scoring system. They also noted good dialysis adequacy, with a mean Kt/V of 1.02±0.21 per session and good electrolyte control.
Sethi et al. [15] conducted a prospective study to evaluate the feasibility of using SLEDD-f as a step-down modality after CRRT pediatric patients with critical illness and AKI. Patients on less than two inotropes and no response to diuretics were transitioned to SLEDD-f. They evaluated eleven patients who underwent a total of 105 SLEDD-f sessions. The development of hypotension or increased inotrope requirement occurred in 18.18%. They concluded that SLEDD-f is a relatively safe and effective modality to transition down from CRRT.
Deng et al. [16] conducted a retrospective study on patients with wasp-sting-associated AKI. A total of 40 patients were involved, with fourteen patients receiving SLEDD-f, all of whom were older than 60 years. The fourteen patients underwent a total of 50 SLEDD-f sessions. They found that the patients older than 60 years undergoing SLEDD-f had a significantly faster return to normal serum creatinine compared to those undergoing HD. They concluded that SLEDD-f is better than HD in terms of renal recovery of elderly wasp victims.
In our study, the in-hospital mortality was significantly higher in the CRRT group, while it was not significantly different between the SLED and SLEDD-f groups. This could be because patients in the CRRT group had more hemodynamic instability and were sicker at baseline compared to SLED or SLEDD-f. Additionally, there were no significant differences in the duration of hospital stay among the three groups.
Also, the majority of sessions in all three groups could be delivered without the use of anticoagulation. This is compelling since the need for anticoagulation often presents a series of practical challenges in critically ill patients who require RRT. Since clinical outcomes of SLEDD-f seem comparable to CRRT, the ability to deliver RRT without the bleeding and metabolic complications of current anticoagulation options commonly used in CRRT (e.g., heparin or regional citrate anticoagulation) may represent a major benefit of SLEDD-f. Other studies assessing this modality have not reported delivery of SLED predominantly without anticoagulation [11,12,13, 17].
Conclusion
SLEDD-f and SLED have good hemodynamic tolerability compared to CRRT, with fewer incidents of hemodynamic instability, including minor fluctuations. There was no significant difference in the hemodynamic profile or outcomes between SLEDD-f and SLED despite a higher proportion of patients on SLEDD-f being on inotropes. SLEDD-f and SLED could be used as an alternative to CRRT. Full-scale clinical trials to test dialysis adequacy, invasive hemodynamics, and outcomes are required to refine further the grey areas of indications of individual modality and their benefits.
Availability of data and material
The datasets generated and/or analysed during the current study are not publicly available due institutional policy, but are available from the corresponding author on reasonable request.
Abbreviations
- AKI:
-
Acute Kidney Injury
- APACHE:
-
Acute Physiology And Chronic Health Evaluation
- CAD:
-
Coronary Artery Disease
- CRRT:
-
Continuous Renal Replacement Therapy
- CVVHDF:
-
Continuous Veno-Venous Hemodiafiltration
- DBP:
-
Diastolic Blood Pressure
- DM:
-
Diabetes Mellitus
- HTN:
-
Hypertension
- ICU:
-
Intensive Care Unit
- IHD:
-
Intermittent Hemodialysis
- KDIGO:
-
Kidney Disease Improving Global Outcomes
- MAP:
-
Mean Arterial Pressure
- OR:
-
Odds Ratio
- PP:
-
Pulse Pressure
- RRT:
-
Renal Replacement Therapy
- SBP:
-
Systolic Blood Pressure
- SD:
-
Standard Deviation
- SLED:
-
Sustained Low Efficiency Dialysis
- SLEDD-f:
-
Sustained Low-Efficiency Diafiltration
- SOFA:
-
Sequential Organ Failure Assessment
References
Hoste EA, Clermont G, Kersten A, Venkataraman R, Angus DC, De Bacquer D, Kellum JA (2006) RIFLE criteria for acute kidney injury are associated with hospital mortality in critically ill patients: a cohort analysis. Crit Care 10(3):R73
Thakar CV, Christianson A, Freyberg R, Almenoff P, Render ML (2009) Incidence and outcomes of acute kidney injury in intensive care units: a Veterans Administration study. Crit Care Med 37(9):2552–2558
Mehta RL (2005) Continuous renal replacement therapy in the critically ill patient. Kidney Int 67(2):781–795
Mehta RL, McDonald B, Gabbai FB, Pahl M, Pascual MT, Farkas A, Kaplan RM (2001) A randomized clinical trial of continuous versus intermittent dialysis for acute renal failure. Kidney Int 60(3):1154–1163
Augustine JJ, Sandy D, Seifert TH, Paganini EP (2004) A randomized controlled trial comparing intermittent with continuous dialysis in patients with ARF. Am J Kidney Dis 44(6):1000–1007
Kumar VA, Craig M, Depner TA, Yeun JY (2000) Extended daily dialysis: A new approach to renal replacement for acute renal failure in the intensive care unit. Am J Kidney Dis 36(2):294–300
Marshall MR, Golper TA, Shaver MJ, Alam MG, Chatoth DK (2001) Sustained lowefficiency dialysis for critically ill patients requiring renal replacement therapy. Kidney Int 60(2):777–785
Acute Kidney Injury Work Group (2012) Kidney Disease: Improving Global Outcomes (KDIGO) - Clinical Practice Guideline for Acute Kidney Injury. Kidney Int Suppl. 2:1–138
Fieghen et al (2010) The hemodynamic tolerability and feasibility of sustained low-efficiency dialysis in the management of critically ill patients with acute kidney injury. BMC Nephrol 11:32
Kitchlu A, Adhikari N, Burns KEA, Friedrich JO, Garg AX, Klein D, Richardson RM, Wald R (2015) Outcomes of sustained low efficiency dialysis versus continuous renal replacement therapy in critically ill adults with acute kidney injury: a cohort study. BMC Nephrol 16:127
Abe M, Okada K, Suzuki M, Nagura C, Ishihara Y, Fujii Y et al (2010) Comparison of sustained hemodiafiltration with continuous venovenous hemodiafiltration for the treatment of critically ill patients with acute kidney injury. Artif Organs. 34(4):331–8
Schwenger V, Weigand MA, Hoffmann O, Dikow R, Kihm LP, Seckinger J et al (2012) Sustained low efficiency dialysis using a single-pass batch system in acute kidney injury - a randomized interventional trial: the REnal Replacement Therapy Study in Intensive Care Unit PatiEnts. Crit Care. 16(4):R140
Sun Z, Ye H, Shen X, Chao H, Wu X, Yang J (2014) Continuous venovenous hemofiltration versus extended daily hemofiltration in patients with septic acute kidney injury: a retrospective cohort study. Crit Care. 18(2):R70
Marshall MR, Ma T, Galler D, Rankin AP, Williams AB (2004) Sustained low- efficiency daily diafiltration (SLEDD-f) for critically ill patients requiring renal replacement therapy: towards an adequate therapy. Nephrol Dial Transplant 19(4):877–884
Sethi SK, Raina R, Bansal SB, Soundararajan A, Dhaliwal M, Raghunathan V et al (2023) Switching from continuous veno-venous hemodiafiltration to intermittent sustained low-efficiency daily hemodiafiltration (SLED-f) in pediatric acute kidney injury: A prospective cohort study. Hemodial Int. 27(3):308–317
Deng YY, Shen JM, Mao YN, Gou R, Li WW, Ye TT (2019) Sustained low-efficiency diafiltration is superior to hemodialysis in promoting renal function recovery in elderly wasp sting victims with stage III acute kidney injury: a retrospective study. Ren Fail. 41(1):814–820
Fiaccadori E, Regolisti G, Cademartiri C, Cabassi A, Picetti E, Barbagallo M et al (2013) Efficacy and safety of a citrate-based protocol for sustained low- efficiency dialysis in AKI using standard dialysis equipment. Clin J Am Soc Nephrol. 8(10):1670–8
Acknowledgements
None.
Funding
There was no funding obtained for the study.
Author information
Authors and Affiliations
Contributions
VKB collected and summated the data. MS and RE analyzed and interpreted the patient data. RE and JKM supervised the data collection and analysis. VKB and MS contributed in writing the manuscript. RE, and JKM contributed in editing the manuscript. All authors read and approved the final manuscript.
Corresponding author
Ethics declarations
Ethics approval and consent to participate
Ethical clearance and approval was obtained from the institutional ethics committee.
Consent for publication
Not applicable.
Competing interests
The authors declare that they have no competing interests.
Additional information
Publisher's Note
Springer Nature remains neutral with regard to jurisdictional claims in published maps and institutional affiliations.
Rights and permissions
Open Access This article is licensed under a Creative Commons Attribution 4.0 International License, which permits use, sharing, adaptation, distribution and reproduction in any medium or format, as long as you give appropriate credit to the original author(s) and the source, provide a link to the Creative Commons licence, and indicate if changes were made. The images or other third party material in this article are included in the article's Creative Commons licence, unless indicated otherwise in a credit line to the material. If material is not included in the article's Creative Commons licence and your intended use is not permitted by statutory regulation or exceeds the permitted use, you will need to obtain permission directly from the copyright holder. To view a copy of this licence, visit http://creativecommons.org/licenses/by/4.0/.
About this article
Cite this article
Bandi, V.K., Shekar, M., Elumalai, R. et al. Hemodynamic tolerability and efficacy of hemodialysis modalities in critically ill patients. Egypt J Intern Med 36, 88 (2024). https://doi.org/10.1186/s43162-024-00357-x
Received:
Accepted:
Published:
DOI: https://doi.org/10.1186/s43162-024-00357-x