Abstract
Background
We aimed at screening for subclinical psoriatic arthritis (PsA) among psoriatic patients without musculoskeletal complaints using ultrasonography of the lower limbs and finding the best predictor for its development.
Results
Subclinical inflammatory articular affection was found by ultrasound in 33 patients, among whom 26 had psoriatic nail affection. According to CASPAR criteria, those 26 patients could be diagnosed as PsA (subclinical). The only statistically significant difference between psoriatic patients with PsA and those without was the mean quadriceps tendon thickness as well as the presence of enthesophytes and bilateral quadriceps thickening. The best and only predictor for subclinical PsA was the presence of enthesophytes.
Conclusion
Ultrasound was more sensitive than clinical examination in detecting subclinical psoriatic arthritis which is highly prevalent in patients with psoriasis even in the absence of manifest arthritic complaints. The best and only predictor for subclinical PsA was the presence of enthesophytes.
Similar content being viewed by others
Explore related subjects
Discover the latest articles, news and stories from top researchers in related subjects.Background
Psoriasis is a chronic inflammatory skin disease that can be associated with psoriatic arthritis (PsA), with a prevalence ranging from 7.6 to 36% according to different populations. Moreover, psoriasis precedes the onset of arthritis which can be destructive, by an average of 7 years. These facts pose great challenges for the urgent need to identify psoriatic patients at increased risk of progression to PsA, which will provide a unique opportunity for early intervention (and possibly even prevention) [1,2,3,4,5].
Enthesitis is a key process in PsA most likely triggered by mechanical stress, and synovitis is secondary to the liberation of proinflammatory cytokines from the enthesis mostly starting in the lower limbs. Based on such a hypothesis, enthesitis should be a prevalent finding in early PsA [6]. Theories related to autoimmunity no longer explain the essence of the disease. Because a prime feature of entheses is stress dissipation, and since “normal” insertions are sites of microdamage, it was proposed that “tissue-specific factors” shared by the joint and skin are critical for disease expression [7]. Enthesitis consists of focal inflammatory lesions which evolve toward fibrous scarring and new bone formation at insertions also known as enthesiophytes. So enthesiophytes are a reaction to a previous bout of entheseal inflammation [8]. Furthermore, a more recent study revealed that enthesiophytes occur early in patients with psoriasis without joint involvement, suggesting that they potentially reflect a common process in psoriasis and PSA [9].
Asymptomatic patients with psoriasis often have subclinical inflammatory articular manifestations, which are often difficult to detect during a clinical examination (CE). Musculoskeletal ultrasound (US) is widely available and inexpensive, can scan repeatedly, and easily demonstrates fluid collections. It has the advantage over CE of being able to directly visualize the enthesis [8] and detect enthesitis [10]. The sonographic soft tissue changes associated with enthesitis include loss of normal fibrillar architecture, hypoechoic changes, and associated thickening [8].
Despite the wide use of classification Criteria for Psoriatic Arthritis (CASPAR) [11], patients who meet the criteria have already clinically confirmed arthritis, which might not improve with treatment. Therefore, early detection of psoriatic patients with subclinical inflammatory articular manifestations by means of US could help with diagnosing subclinical psoriatic arthritis according to CASPAR criteria. This would have an impact on clinical decision-making, such as the use of aggressive treatment at an early stage to prevent damage and functional disability [12].
The aim of the present study was to screen for subclinical psoriatic arthritis among psoriatic patients without musculoskeletal complaints using ultrasonography of the lower limbs for detecting subclinical inflammatory articular manifestations and finding the best predictor for its development.
Methods
This was a cross-sectional study carried out on 36 psoriatic patients (28 males and 8 females) selected from the outpatient clinic of dermatology, main university hospital. The sample size was calculated using EPI-INFO, version 7. The estimated proportion of PsA among patients with psoriasis is 20% [2]. Using a precision of 10% and a 95% confidence interval, the minimum sample size was 35 cases. The inclusion criteria are as follows: age ≥ 18 years; no history of systemic treatment given for psoriasis (i.e., methotrexate, ciclosporin, or retinoids); only phototherapy sessions were given; no history of any inflammatory, microcrystalline, degenerative, or infectious musculoskeletal disease; no history of polytraumatism; and absence of past or present non-traumatic pain in any axial or peripheral musculoskeletal region. An equal number of controls (n = 36) free of any musculoskeletal or dermatologic disease were included to compare sonographic findings in the local general population with the patient group. All included patients signed informed consent for their enrolment in this study. The study was approved by the local ethics committee in the Faculty of Medicine (IRB No: 00007555-FWA NO: 00015712).
All patients and controls were clinically examined to rule out axial or peripheral articular or entheseal affection. The severity of psoriasis was scored according to the Psoriasis Area and Severity Index (PASI) [13]. Also, psoriatic nail affection as well as body mass index (BMI) was assessed.
Ultrasonography of the lower limbs of both patients and controls was performed by an experienced radiologist using a 10-MHz linear array transducer (Siemens-Germany). The examination was done according to Glasgow Ultrasound Enthesitis Scoring System (GUESS score) [14] to the following 5 sites: the superior pole of the patella (quadriceps tendon insertion), the inferior pole of the patella (patellar ligament origin), and the patellar ligament insertion at the tibial tuberosity with the patient in the supine position with the knee flexed at 30°. The Achilles tendon and the plantar aponeurosis were examined with the patient lying prone. At those sites, the following items were evaluated: the thickness of the enthesis (beyond the normal values), the presence or absence of bony erosions, enthesophytes, and bursitis, and each item scored 1. This scoring was carried out bilaterally. The sonographic examination was also carried out on the controls to compare the distribution of any changes found in patients with the local general population.
Psoriatic patients with sonographic signs of inflammation, i.e., thickened enthesis or suprapatellar effusion, were selected. To diagnose those cases as (subclinical) PsA, CASPAR criteria were used. The presence of psoriatic nail dystrophy would allow those patients to score 3 (current skin psoriasis scored 2 and nail affection scored 1) [11].
Data were analyzed using IBM SPSS software package version 25. Quantitative data were presented as the mean and standard deviation, while qualitative data were presented as the number and percentage. We first compared between PsA and non-PsA groups as regards each ultrasound variable using the Mann–Whitney test for not normally distributed data and the independent t test for normal data. Relationships between categorical variables were evaluated by Fisher’s exact test. Spearman’s correlations were conducted to show the relationship between quadricipital tendon thickness and other parameters. Then, on the basis of this univariate analysis, all significant variables with P value ≤ 0.2 were entered in a stepwise multiple logistic regression analysis using the forward LR technique, to build the best model for developing subclinical PsA among psoriatic patients without musculoskeletal complaints. The dependent variable of this study was developing subclinical PsA which has two binary outcomes (non-PsA coded as 1 and PsA coded as 0). The predictor variables considered were the number of thickened tendons, thickness (in mm) of the quadriceps tendon, total GUESS score, enthesophytes (absent = 0, present = 1), and bilateral quadricipital thickening (absent = 0, present 1).
P values ≤ 0.05 were considered statistically significant.
Results
The mean age of the included patients was 46.08 ± 13.24, the mean BMI was 29.74 ± 4.89, and the mean duration of skin psoriasis was 12.74 ± 10.24 years. There was no statistically significant difference between patients and controls as regards their age or BMI. The mean PASI was 13.31 ± 8.09. The US examined 360 entheses in the lower limbs of 36 patients and 100 entheses in 10 controls. Sonographic findings of these entheses are shown in Tables 1 and 2.
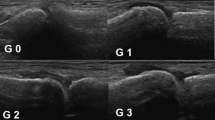
According to the GUESS score, those with quadricipital tendon thickness above 6.1 mm were considered thickened. As for the proximal and distal patellar ligaments, 4 mm or above was considered thickened. Regarding the Achilles tendon and plantar fascia, the thicknesses above 5.29 and 4.4 mm, respectively, were considered thickened. The mean sonographic thickness of the proximal and distal patellar tendons as well as the plantar fascia was significantly higher in patients than in controls (P ≤ 0.05). As for the quadricipital and Achilles tendons, they were still thicker in patients than in controls but have not reached statistical significance. Tendon thickening was found at 98 sites (27.22% of the total examined sites) among 29 patients (23 males and 6 females). Enthesophytes were seen in 50 sites (13.89% of the total examined sites) among 22 patients. All of the 22 patients with enthesophytes had thickened enthesis. Representative US images of pathological findings in psoriatic patients are shown in Fig. 1. The sites most commonly thickened were the distal patellar tendon (41.84% from total thickened tendons) followed by the quadriceps tendon (25.52%), then the proximal patellar tendon (20.41%). Enthesophytes were most commonly seen at the Achilles tendon (48% of total enthesophytes detected) followed by the quadricipital tendon (40%) (Table 2). Suprapatellar bursitis (knee effusion) was found in 21 patients (58.33%), and infrapatellar bursitis was found in 10 patients. On the other hand, none of the controls had knee effusion or infrapatellar bursitis. No erosions were seen at any site in both patients and controls. The total GUESS score in those patients ranged between 0 and 11 with a mean of 5.53 ± 3.19.
A Longitudinal US of the quadriceps tendon in a patient with psoriasis without musculoskeletal complaints of increased thickness (7 mm) and with mild suprapatellar effusion. B Longitudinal US of the plantar fascia of increased thickness (5.2 mm) and enthesophyte in another patient with the same clinical condition. C, D Longitudinal US of distal and proximal patellar ligaments with increased thickness (5.3 and 5 mm, respectively) as well as enthesophytes at ligament insertion. p patella, C calcaneus, TT tibial tuberosity
The number of patients with articular inflammatory findings by US was 33 distributed as 17 (combined knee effusion and thickened entheses), 4 (knee effusion only), and 12 (thickened entheses only). Psoriatic nail affection was found in 27/36 patients (75%) and among 26 patients from those 33 with articular inflammatory findings. According to CASPAR criteria, those 26 patients (20 males and 6 females) scored 3 and hence could be diagnosed as subclinical PsA. Those 26 patients had either enthesitis alone (11 patients) or synovitis alone (3 patients) or both combined (12 patients) together with current skin psoriasis (scored 2) and nail affection (scored 1). The remaining 7 from the 33 with articular inflammatory findings scored only 2 and hence could not be diagnosed as PsA.
The differences between those with subclinical PsA (26 patients) and those without (10) are shown in Tables 3 and 4. The only statistically significant difference between both groups was the mean quadriceps tendon thickness which was significantly higher in those with subclinical PsA as well as the presence of enthesophytes and bilateral quadricipital thickening which were more common in patients with subclinical PsA. Enthesophytes were found in 19/26 patients with subclinical PsA and in 3 of the remaining 10 with a statistically significant difference in distribution. As for bilateral quadricipital thickening, it was found in 10 patients all of whom had subclinical PsA. Also, the mean GUESS score and mean number of thickened tendons per patient were much higher in those with subclinical PsA yet have not reached statistical significance. Sixteen patients out of the 26 with subclinical PsA (61.54%) had at least one site with bilateral tendon thickening, and 14 patients (53.85%) had bilateral enthesophyte distribution. Bilateral tendon thickening was found among 10 patients at the quadricipital tendon and 15 and 7 patients at distal and proximal patellar tendons, respectively. As for bilateral enthesophyte distribution, it was found among 7 and 8 patients at quadricipital and Achilles tendons, respectively.
Quadricipital tendon thickness was then correlated with different factors that could affect it as shown in Table 5. The total GUESS score as well as the total number of thickened tendons or enthesophytes per patient was found to be statistically positively correlated with mean quadricipital tendon thickness and moderate strength. That is, as those variables increased, the mean quadricipital thickness increased too.
Based on findings of univariate analysis, 6 variables proved to be significantly different between the 2 groups (PsA and non-PsA) and had P values < 0.2: the presence of enthesophytes (P = 0.02) and quadriceps tendon thickness (P = 0.006), bilateral quadricipital tendon thickening (P = 0.03), GUESS score (P = 0.07), the total number of thickened tendons (P = 0.09), and the total number of enthesophytes (P = 0.13). Considering multi-collinearity, 5 variables were thus introduced in a stepwise multiple regression to select variables according to their influence on developing subclinical PsA. The results revealed 2 variables to be significant predictors of subclinical PsA: the presence of enthesophytes and thickened tendon per patient as well as increasing quadriceps thickness was associated with an increased likelihood of developing subclinical PsA (OR = 6.3, 4, 5.1, respectively). Results revealed that the presence of enthesophytes was the best and only predictor for developing subclinical PsA (OR = 6.33, 95% CI = 1.27–31.56) which indicates that the presence of enthesophytes had about 6.33-fold higher odds of developing subclinical PsA (Table 6).
Discussion
Psoriatic arthritis is an enthesitis-associated disorder rather than primary synovitic arthropathy. Several criteria have been set for diagnosing these cases, but none of them was statistically derived from a large prospective study as were the CASPAR criteria. CASPAR criteria included the presence of inflammatory articular disease (joint or entheseal) together with ≥ 3 points to be fulfilled to diagnose PsA [11]. Since all our patients were clinically free of any inflammatory articular affection, hence, our aim was to sonographically search for lower limb inflammatory joint or entheseal findings in those asymptomatic and drug-naive psoriatic cases to find those with subclinical PsA who might be clinically recognized thereafter. The lesion of greater interest for this purpose was enthesitis. Entheses of the lower limbs were chosen in our study because they are the most involved in clinical practice and described as “more specific” for spondyloarthritis patients (SpA). The US helped depict inflammatory articular findings in 33 patients, 26 of whom had concomitant psoriatic nail affection. Those 26 patients could be diagnosed by the CASPAR criteria as subclinical PsA as they fulfilled ≥ 3 points. Current psoriasis scored 2 which was present in all our patients and typical psoriatic nail dystrophy scored 1. The remaining 7 of those 33 with articular inflammatory findings scored only 2 because they had no psoriatic nail affection. Those 26 patients (72.22%) with subclinical PsA had enthesitis or joint effusion detected sonographically instead of clinically. The US helped reveal a high frequency of abnormal findings in asymptomatic lower limb entheses in SpA [14]. Another study carried out on psoriatic patients found at least one inflammatory lesion on the hand MRI of 47% of the studied patients [15]. This has recently been proposed that there are three clinically quiet stages after psoriasis onset and before clinically detected PsA; nevertheless, there is still a significant gap in our knowledge on how this disease should be handled at its initial stages [16].
Enthesitis is the first manifestation of psoriatic arthritis but may be asymptomatic and detected only by imaging. Ozçakar et al. found that the percentage of asymptomatic sonographic enthesopathy in psoriatic patients was 56%, but this was based on tendoachilles examination only, which could be much higher if more entheses were examined [17]. In our study, US examined 360 entheses in both lower limbs. The most common abnormality detected was entheseal thickening in 29 patients (80.55%), followed by enthesophytes in 22 patients (61.11%), and finally bursitis (suprapatellar ± infrapatellar) which was detected in 21 patients (58.33%). Defining enthesitis by US is quite difficult because of the numerous structures that can be involved in the inflammatory process. In order to ensure a consensus, a Delphi exercise was conducted and obtained a good to a high agreement (≥ 80%) on the inclusion of increased tendon thickness and enthesophytes. No agreement was obtained, however, for the inclusion of bursitis although its inclusion has been suggested by others [18].
From the clinical point of view, 10% of patients with psoriasis develop PsA over a decade [19], but from the sonographic aspect, more could be affected and with earlier onset. Another study by Tinazzi et al. followed 30 patients with psoriasis for 13 months to detect who will develop PsA and 7 out of those (23.33%) developed frank PsA, but this was still on the clinical level [20]. Another study found this frequency of PsA as high as 40% using more sophisticated techniques [21]. Yet a more recent study found that 47% of patients with psoriasis showed at least one inflammatory lesion on hand MRI and the risk to develop psoriatic arthritis among such population was as high as 60% [16]. Another study by Wang et al. carried out on 852 psoriatic patients using US detected enthesiophytes in 43.5% and joint effusion in 48%, and the quadriceps tendon was the most frequently affected site [12]. The higher percentage in the present study (72.22%) might be because the scanned joints were those of the lower limbs which are more affected than the upper limbs with the inflammatory changes. Also, all of our cases were drug-naive, where none of them has received any DMARDs or retinoids. Hence, there is an urgent need to define the predictive factors for the identification of future PsA in patients with psoriasis. In the study by Tinazzi et al. [20], the baseline thickness of the quadriceps tendon was the best independent predictor. Consequently, we compared the baseline characteristics of those 26 patients with subclinical PsA and the remaining 10. The only significant difference between both groups was in quadriceps tendon thickness by US as well as the presence of enthesophytes and bilateral quadricipital thickening. As for the GUESS score and the total number of thickened tendons per patient, they were much higher in the group of subclinical PsA yet have not reached statistical significance. Quadricipital tendon thickening (≥ 6.1 mm) in our study was found in 15 patients, 13 of whom had subclinical PsA. This thickening was bilateral in only 10 patients all of whom had subclinical PsA. Quadricipital tendon thickness was then correlated with different factors. It was found to be significantly correlated with the total GUESS score, number of thickened tendons per patient (mean = 3.08), and number of enthesophytes per patient (mean = 1.62). This correlation was positive and of moderate strength. That is as those variables increased, the mean quadricipital thickness increased. This common occurrence of subclinical enthesitis in patients with psoriasis is strong evidence for the primacy of this lesion. It is the primary pathology that triggers secondary joint synovitis through the release of proinflammatory mediators [22]. Also, the high prevalence of nail changes is a natural sequelae to the extensive level of enthesis nail interdigitation at the DIP joint [22]. Given that entheses are sites of high joint stressing, it further supports the emerging concept that the link is a mechanical rather than an autoimmune one [23]. In other words, it is an aberrant response to mechanical stresses or autoinflammatory reaction that induces a primary innate immune response, resulting in tissue damage. Similarly, trauma may be responsible for skin lesions in psoriasis, which is a classic example of the Koebner response phenomenon [21].
In one study carried out on psoriatic patients with and without musculoskeletal complaints, enthesitis was found in 33.3% of the asymptomatic group and in 46.7% of the symptomatic group. Serum cartilage oligomeric matrix protein (COMP) level was also measured in both groups but without statistically significant difference between them [24]. This elevated level of COMP in psoriatic patients suggests that there is joint involvement and cartilage breakdown even in asymptomatic cases.
Regarding enthesophytes, they were found in 19/26 patients with subclinical PsA and in 3/10 of the remaining patients (P < 0.05). Enthesophytes may be the end stage of inflammation or may relate to other pathologies such as trauma or degenerative changes [9]. Nevertheless, all patients with enthesophytes in this study had entheseal thickening. Also, it was found to be the best and only predictor for developing subclinical PsA in our study. A definition was proposed that included tendon thickening as signs of acute US enthesitis and included erosions, and enthesophytes as signs of chronic enthesitis [17]. Moreover, in 2005, the Outcome Measures in Rheumatology (OMERACT) US Task Force included the presence of enthesophytes in the definition of enthesopathy. There is extensive evidence to suggest that the stimulus for the formation of bony spurs is mechanical due to microtrauma. Therefore, enthesopathy is more common in the lower than the upper limbs and most typical of the plantar fascia and Achilles tendon [25].
The total GUESS score in our patients ranged between 0 and 11 with a mean of 5.53 ± 3.19, which is comparable to another study (7.9) studying cases without musculoskeletal complaints as well. PsA varies in its clinical presentation from mono-oligoarthritis to symmetric polyarthritis, but in longstanding disease, the symmetric pattern is the most prevalent [26]. In fact, this asymmetry may be a relative asymmetry as the clinical examination is an insensitive tool for identifying articular involvement. The use of US in this study highlighted this symmetry where 16 patients out of the 26 with subclinical PsA (61.54%) had at least one site with bilateral tendon thickening and 14 patients (53.85%) had at least one site with bilateral enthesophyte. Also, bilateral quadricipital tendon thickening was found in 10 patients, all of whom were finally diagnosed as PsA.
The identification of predictors of PsA development in psoriatic patients is a recognized unmet need which needs further exploration.
Conclusion
US was more sensitive than clinical examination in detecting lower limb subclinical inflammatory articular manifestations in psoriatic drug-naive patients without arthritic complaints. Hence, it is more sensitive in detecting subclinical psoriatic arthritis and helps identify patients at risk of developing PsA. Enthesophytes were found to be the best and only predictor for developing subclinical PsA in our study.
Limitations
A major limitation of this study was the small sample size of psoriatic patients, but this could not be overcome because of the inclusion criteria requiring the absence of any musculoskeletal complaint together with the inclusion of drug-naive patients only.
Future research
Comparison between sonographic musculoskeletal findings among psoriatic patients before and after initiation of treatment, to see the ability of treatment of skin disease in controlling the subclinical arthropathy and delaying its evolution into inflammatory articular phase.
Availability of data and materials
The datasets used and/or analyzed during the current study are available from the corresponding author on reasonable request.
Abbreviations
- PsA:
-
Psoriatic arthritis
- CE:
-
Clinical examination
- US:
-
Ultrasound
- CASPAR:
-
Classification Criteria for Psoriatic Arthritis
- PASI:
-
Psoriasis Area and Severity Index
- BMI:
-
Body mass index
- GUESS:
-
Glasgow Ultrasound Enthesitis Scoring System
- COMP:
-
Cartilage oligomeric matrix protein
- OMERACT:
-
Outcome Measures in Rheumatology
References
Scher JU, Ogdie A, Merola JF et al (2019) Preventing psoriatic arthritis: focusing on patients with psoriasis at increased risk of transition. Nature Reviews Rheumatol 15:153–166
Alinaghi F, Calov M, Kristensen LE et al (2019) Prevalence of psoriatic arthritis in patients with psoriasis: a systematic review and meta-analysis of observational and clinical studies. J Am Acad Dermatol 80(1):251-265.e19
Prignano F, Rogai V, Cavallucci E, Bitossi A, Hammen V, Cantini F (2018) Epidemiology of psoriasis and psoriatic arthritis in Italy-a systematic review. Curr Rheumatol Rep 20(7):43. https://doi.org/10.1007/s11926-018-0753-1 (PMID: 29846817)
Sewerin P, Brinks R, Schneider M, Haase I, Vordenbäumen S (2019) Prevalence and incidence of psoriasis and psoriatic arthritis. Ann Rheum Dis 78(2):286–287. https://doi.org/10.1136/annrheumdis-2018-214065 (Epub 2018 Sep 21 PMID: 30242033)
Lindberg I, Lilja M, Geale K, Tian H, Richardson C, Scott A, Osmancevic A (2020) Incidence of psoriatic arthritis in patients with skin psoriasis and associated risk factors: a retrospective population-based cohort study in Swedish routine clinical care. Acta Derm Venereol. 100(18):adv00324. https://doi.org/10.2340/00015555-3682 (PMID: 33135771)
Freeston JE, Coates LC, Helliwell PS, Hensor EM, Wakefield RJ, Emery P et al (2012) Is there subclinical enthesitis in early psoriatic arthritis? A clinical comparison with power Doppler Ultrasound. Arthritis Care Res 64(10):1617–1621
Mc Gonagle D, Tan AL, Benjamin M (2008) The biomechanical link between skin and joint disease in psoriasis and psoriatic arthritis: what every dermatologist needs to know. Ann Rheum Dis 67(1):1–4
D’Agostino MA, Nahal RS, Bouder CH, Brasseur JL, Dougados M, Breban M (2003) Assessment of peripheral enthesitis in the spondylarthropathies by ultrasonography combined with power Doppler. A cross-sectional study. Arth Rheum 48(2):523–533
Simon D, Kleyer A, Faustini F, Englbrecht M, Haschka J, Berlin A, Kraus S, Hueber AJ, Kocijan R, Sticherling M et al (2018) Simultaneous quantification of bone erosions and enthesiophytes in the joints of patients with psoriasis or psoriatic arthritis - effects of age and disease duration. Arthritis Res Ther 20(1):203
Bajri A, Vasili E, Boci F (2015) Subclinical enthesitis: new approach in the clinical examination of psoriatic arthritis and nails. AJMHS 46(1):40–51
Taylor W, Gladman D, Helliwell P, Marchesoni A, Mease P, Mielants H et al (2006) Classification criteria for psoriatic arthritis. Development of new criteria from a large international study. Arthritis Rheum. 54(8):2665–73
Wang Y, Zhang L, Yang M et al (2022) Development of a predictive model for screening patients with psoriasis at increased risk of psoriatic arthritis. Dermatol Ther 12(2):419–433
Langley RG, Ellis CN (2004) Evaluating psoriasis with psoriasis area and severity index, psoriasis global assessment, and lattice system physician’s global assessment. J Am Acad Dermatol 51:563–569
Balint PV, Kane D, Wilson H, McInnes IB, Sturrock RD (2002) Ultrasonography of entheseal insertions in the lower limb in spondyloarthropathy. Ann Rheum Dis 61:905–910
Faustini F, Simon D, Oliveira I, Kleyer A, Haschka J, Englbrecht M et al (2016) Subclinical joint inflammation in patients with psoriasis without concomitant psoriatic arthritis: a cross-sectional and longitudinal analysis. Ann Rheum Dis 75(12):2068–2074
Zabotti A, Tinazzi I, Ayden SZ, McGonagle D (2020) From psoriasis to psoriatic arthritis: insights from imaging on the transition to psoriatic arthritis and implications for arthritis prevention. Curr Rheumatol Rep 22:24
Ozçakar L, Cetin A, Inanici F, Kaymak B, Gurer CK, Kolemen F (2005) Ultrasonographical evaluation of the Achilles tendon in psoriasis patients. Int J Dermatol 44:930–932
Terslev L, Naredo E, Iagnocco A, Balint PV, Wakefield RJ, Aegerter P et al (2014) Defining enthesitis in spondyloarthritis by ultrasound: results of a Delphi process and of a reliability reading exercise. Arthritis Care Res 66(5):741–748
Wilson FC, Icen M, Crowson CS, McEvoy MT, Gabriel SE, Kremers HM (2009) Incidence and clinical predictors of psoriatic arthritis in patients with psoriasis: a populations-based study. Arthritis Rheum 15:233–239
Tinazzi I, McGonagle D, Biasi D, Confente S, Caimmi C, Girolomoni G et al (2011) Preliminary evidence that subclinical enthesopathy may predict psoriatic arthritis in patients with psoriasis. J Rheumatol 38(12):2691–2692
Alenius GM, Stenberg B, Stenlund H, Lundblad M, Dahlqvist SR (2002) Inflammatory joint manifestations are prevalent in psoriasis: prevalence study of joint and axial involvement in psoriatic patients, and evaluation of a psoriatic and arthritis questionnaire. J Rheumatol 29(12):2577–2582
McGonagle D (2009) Enthesitis: an autoinflammatory lesion linking nail and joint involvement in psoriatic disease. JEADV 23(1):9–13
McGonagle D, Tan AL, Benjamin M (2008) The biomechanical link between skin and joint disease in psoriasis and psoriatic arthritis: what every dermatologist needs to know. Ann Rheum Dis 67(1):1–4
Farouk HM, Mostafa AA, Yousef SS, Elbeblawy MMS, Assaf NY, Elokda EE (2010) Value of entheseal ultrasonography and serum cartilage oligomeric matrix protein in the preclinical diagnosis of psoriatic arthritis. Clin Med Insights Arthritis Musculoskelet Disord 3:7–14
Benjamin M, McGonagle D (2009) The enthesis organ concept and its relevance to the spondyloarthropathies. Adv Exp Med Biol 649:57–70. https://doi.org/10.1007/978-1-4419-0298-6_4 (PMID: 19731620)
Delle Sedie A, Riente L, Filippuccie E, Scire CA, IagnoccoA GM et al (2010) Ultrasound imaging for the rheumatologist XXVI. Sonographic assessment of the knee in patients with psoriatic arthritis. Clin Exp Rheumatol 28:147–152
Acknowledgements
Not applicable.
Funding
The authors declare that no financial or material support was provided by any parties, and there are no equity interests, patent rights, or corporate affiliations for this work. Facilities for research are available in our department for all with no restrictions. No commercial party having a direct financial interest in the results of the research supporting this article has or will confer a benefit upon the authors or upon any organization with which the authors are associated.
Author information
Authors and Affiliations
Contributions
S.M.S.: clinical examination, statistical analysis, and writing the manuscript. T.G.: sonographic examination and revision of the manuscript. R.M.G.: dermatological assessment and revision of the manuscript. The authors read and approved the final manuscript.
Corresponding author
Ethics declarations
Ethics approval and consent to participate
This work had been approved by the ethical committee in the Faculty of Medicine, Alexandria University, Egypt (IRB No: 00007555-FWA NO: 00015712), and all patients had signed written informed consent. The present work had been carried out in accordance with the Code of Ethics of the World Medical Association (Declaration of Helsinki) for experiments involving humans.
Consent for publication
Not applicable.
Competing interests
The authors declare that they have no competing interests.
Additional information
Publisher’s Note
Springer Nature remains neutral with regard to jurisdictional claims in published maps and institutional affiliations.
Rights and permissions
Open Access This article is licensed under a Creative Commons Attribution 4.0 International License, which permits use, sharing, adaptation, distribution and reproduction in any medium or format, as long as you give appropriate credit to the original author(s) and the source, provide a link to the Creative Commons licence, and indicate if changes were made. The images or other third party material in this article are included in the article's Creative Commons licence, unless indicated otherwise in a credit line to the material. If material is not included in the article's Creative Commons licence and your intended use is not permitted by statutory regulation or exceeds the permitted use, you will need to obtain permission directly from the copyright holder. To view a copy of this licence, visit http://creativecommons.org/licenses/by/4.0/.
About this article
Cite this article
El-Sherif, S.M., Gaweesh, T. & Genedy, R.M. Diagnosis of subclinical psoriatic arthritis in patients with psoriasis using CASPAR criteria: a sonographic study. Egypt Rheumatol Rehabil 49, 60 (2022). https://doi.org/10.1186/s43166-022-00158-6
Received:
Accepted:
Published:
DOI: https://doi.org/10.1186/s43166-022-00158-6