Abstract
Zika virus, a mosquito-borne arbovirus, has repeatedly caused large pandemics with symptoms worsening from mild and self-limiting diseases to Guillain–Barré syndrome in adults and fetal microcephaly in newborns. In recent years, Zika virus diseases have posed a serious threat to human health. The shortage of susceptible small animal models makes it difficult to study pathogenic mechanisms and evaluate potential therapies for Zika virus infection. Therefore, we chose immunocompromised mice (AG129 mice) deficient in IFN-α/β and IFN-γ receptors, which can abolish the innate immune system that prevents Zika virus infection early. AG129 mice were infected with the Zika virus, and this mouse model exhibited replication dynamics, tissue tropism, pathological lesion and immune activation of the Zika virus. Our results suggest that the inoculum dose of Zika virus can affect the viral replication dynamics, cytokine responses and survival rate in AG129 mice. By testing the potential antiviral drug favipiravir, several critical indicators, including replication dynamics and survival rates, were identified in AG129 mice after Zika virus infection. It is suggested that the model is reliable for drug evaluation. In brief, this model provides a potential platform for studies of the infectivity, virulence, and pathogenesis of the Zika virus. Moreover, the development of an accessible mouse model of Zika virus infection will expedite the research and deployment of therapeutics and vaccines.
Similar content being viewed by others
Avoid common mistakes on your manuscript.
Introduction
Zika virus (ZIKV) is a mosquito-borne arbovirus that was first isolated from a sentinel rhesus macaque in Uganda in 1947 during studies of enzootic yellow fever [1]. The genome organization of ZIKV is significantly similar to that of other flaviviruses such as dengue virus, West Nile virus, and yellow fever virus. ZIKV has a single-stranded positive-sense and 11 kilobase RNA genome encoding three structural proteins [capsid (C), precursor membrane (prM), and envelope (E)] and seven nonstructural proteins (NS1, NS2A, NS2B, NS3, NS4A, NS4B, and NS5) [2].
ZIKV was once considered an obscure member of the mosquito-borne flavivirus family for six decades, and human infections with ZIKV were thought to be sporadic, with mild and self-limiting symptoms [3, 4]. However, the first large outbreak emerged on Yap Island, Micronesia, in 2007, with 49 confirmed and 59 probable cases of Zika virus disease [5]. In 2013, there was a larger ZIKV outbreak in French Polynesia [6]. A total of 29,000 people were estimated to have been infected, at least 396 confirmed ZIKV cases were reported [7], and more than 50 cases [8]of Guillain–Barré syndrome (GBS) were linked to ZIKV infection. This is the first study providing evidence for ZIKV infection causing GBS. These data indicate the increased virulence of the ZIKV strain. The earliest confirmed cases of ZIKV infection in the Americas were identified in May 2015 [9]. Then, ZIKV quickly spread, and at least 48 countries and territories in the Americas were affected by the virus by the end of 2016 [10]. Moreover, increasing clinical evidence has shown that ZIKV infection is associated with congenital malformations, including microcephaly, intrauterine growth restriction, and fetal demise [3, 11]. This led the World Health Organization to declare a public health emergency of international concern in 2016 [12]. Although there has been no ongoing Zika virus outbreak around the world since 2017 [13], new research suggests that a single amino acid substitution in the Zika virus genome may increase infectivity, transmission, and pathogenesis, which further highlights the necessity of preparing for another ZIKV outbreak [14,15,16].
The development of animal models is indispensable for future epidemic responses. Research models of ZIKV infection include in vitro and in vivo models. In vitro models are essential for studying the life cycle, infection mechanism, and cytopathic effect of the Zika virus. Some 2D cell lines are available, manageable, and inexpensive. However, most cell lines have been genetically modified for immortalization and may not display an accurate molecular profile of ZIKV infection. Therefore, the results must be interpreted prudently because some modifications can easily impact the pathogenicity of the virus and yield inconsistent experimental results [17]. Models involving three-dimensional (3D) organoids have gradually replaced the 2D monolayer models. Organoids resemble in vivo conditions more than 2D monolayer cell cultures [18]. The number of cases of microcephaly has increased considerably with the increase in the number of cases of ZIKV infection in Brazil. Scientists have examined the effects of ZIKV infection on human neural stem cells growing as neurospheres and brain organoids, and direct evidence has emerged that ZIKV can infect and cause the death of neural stem cells [19]. However, the use of organoids as disease models has several limitations. Current organoid systems have reproducibility concerns as researchers cannot control how cells self-organize into organoids. Existing 3D organoid models are still unable to recapitulate the complex and dynamic inflammatory process that occurs in vivo [18]. In vivo animal models could be used to study the vertical transmission, immune activation, and tissue tropism of ZIKV. Animal models of ZIKV infection involving neuropathology and immunopathology based on nonhuman primates (NHPs) have been studie [20, 21]. Although NHPs have many advantages because of their close resemblance to humans, their use in preclinical studies is limited because of ethical issues and high breeding costs. Another more commonly available species is the domestic pig which shares genetic, physiologic, immunologic, and anatomical features with humans. Another advantage of pigs is that they carry many fetuses. The susceptibility of newborn piglets to ZIKV infection has been reported [22]. However, the large size and long feeding time of pigs cause considerable inconvenience in scientific research. The development of a high-throughput small animal model of ZIKV infection could expedite the testing and screening of drugs and vaccines before more costly evaluation in nonhuman primate and human clinical trials.
Indeed, despite much anticipation of candidate ZIKV vaccines, there have been no pivotal phase III trials demonstrating their safety and efficacy since the incidence of Zika virus cases peaked in early 2016 [23]. Researchers need to continue developing animal models of ZIKV infection. Reportedly, wild-type mice are not readily infected by ZIKV. One reason for this is that viral NS5 proteins can bind and degrade human STAT2 in the antiviral IFN signaling pathway but not mouse STAT2, which enables ZIKV to antagonize type I IFN in humans but not in mice [24,25,26]. Therefore, mice with genetic deficiencies in IFN-I signaling have been developed for ZIKV research. The lack of IFN-α/β and IFN-γ receptors renders AG129 mouse models incapable of responding to type I IFN and type II IFN, and these models have been used successfully to characterize ZIKV infection [27]. Here, we inoculated AG129 mice with the ZIKV strain GZ01 to characterize the infection characteristics, viral dynamics, tissue tropism, pathological features and immune activation of the Zika virus. Moreover, we selected the antiviral drugs favipiravir and testosterone to assess the reliability of the AG129 mouse model, which could contribute to a more comprehensive presentation of this model.
Results
ZIKV infection caused clinical symptoms and viremia in AG129 mice
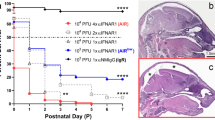
To validate that AG129 mice were susceptible to ZIKV and to determine the viral dose required to achieve robust ZIKV infection, we infected 6 ~ 8-week-old mice by subcutaneous injection of two different doses of ZIKV (GZ01). The experimental design is summarized in (Fig. 1a). In our results similar to those of a previous report [28], AG129 mice were vulnerable to ZIKV infection, began to exhibit ruffled fur, lethargy and a hunched posture at 3–4 days post infection (dpi), and deteriorated rapidly. Mice in 102 TCID50 group became immobile and weak at 7 dpi and died over the next two days, while mice in 104 TCID50 group died at 7dpi (Fig. 1d). After infection, we monitored the temperature and weight daily to measure mouse morbidity and detected the viral load based on blood samples collected from the tail vein. Compared to those in the control group, there were no regular changes in the body temperature fluctuations of these two groups of infected mice (Fig. S1). However, body weight tended to decrease in the infected groups (Fig. 1b). The ZIKV-challenged AG129 mice started to lose weight rapidly on the third day after infection, and mice in the 104 TCID50 group lost weight significantly faster than those in the 102 TCID50 group (Fig. 1b). Moreover, we detected the viral load in mouse blood as early as the second day after infection (Fig. 1c). The viral load in the blood of the 104 TCID50 group peaked at 5 dpi, and that of the 102 TCID50 group peaked at 6 dpi (Fig. 1c). These results showed that AG129 mice were successfully infected with ZIKV and that 104 TCID50 of virus could lead to more severe symptoms and earlier death. Although the viral load in the 104 TCID50 group peaked faster than that in the 102 TCID50 group, the peak viral load seemed not to be related to the viral inoculum.
ZIKV infection caused clinical symptoms and viremia in AG129 mice. a Schematic illustration of AG129 mouse model infected with ZIKV GZ01 strain (related mice elements are designed by Freepik); b Line chart of weight change of AG129 male mice measured from 0 dpi to 7 dpi (n = 6); c Line chart of viral load in blood of AG129 male mice measured from 0 to 7 dpi (n = 6); d Survival percentage for AG129 male mice infected with ZIKV (1*102 TCID50 and 1*104 TCID50) (n = 5). Quantitative data are shown as the mean ± SD (error bars). *P < 0.05, **P < 0.01, ***P < 0.001, ****P < 0.0001 (Sidak's multiple comparisons test)
ZIKV displayed distinct replication dynamics and tissue tropism in AG129 mice
According to previous research, ZIKV exhibits extensive tissue tropism and the ability to replicate in blood, which could affect the function of many organs [17, 29]. Mice of each group were euthanized humanely for tissue sampling at 4 dpi and 7 dpi to evaluate any potential ZIKV RNA in anatomic tissues, including heart, liver, spleen, lung, kidney, duodenum, cerebrum, cerebellum, spinal cord and testicle. We detected the high levels of viral RNA expression in these tissues by RT-qPCR. What is noticeable is that the viral load of most tissues in the 104 TCID50 group is higher than that in the 102 TCID50 group at 4dpi (Fig. 2a), while the viral load of most tissues in the 104 TCID50 group is lower than that in the 102 TCID50 group at 7dpi (Fig. 2b). In addition, the viral load of these tissues at 4 dpi is lower than that at 7 dpi in the 102 TCID50 group, while the viral load of these tissues at 4 dpi is higher than that at 7 dpi in the 104 TCID50 group. The change of viral load in these tissues is consistent with that in blood (Fig. 1c). Meanwhile, we detected viral titer in some tissues of each group at 7 dpi, including heart, lung, kidney, cerebrum, testicle, and blood (Fig. S2). The results indicated that there were infectious viruses in all these tissues. Therefore, we performed immunofluorescence assays of these tissue slices to observe more about the localization and distribution of viral antigens (Fig. 2c).
ZIKV displayed distinct replication dynamics and tissue tropism in AG129 mice. a Viral load in heart, liver, spleen, lung, kidney, duodenum, cerebrum, cerebellum, spinal cord, and testicle of three groups of AG129 mice at 4 dpi (n = 3); b Viral load in heart, liver, spleen, lung, kidney, duodenum, cerebrum, cerebellum, spinal cord, and testicle of three groups of AG129 mice at 7 dpi (n = 3); c Immunofluorescence image of heart, lung, kidney, cerebrum, and testicle of three groups of AG129 mice at 3 dpi and 7 dpi (n = 3). Zika virus capsid protein antibody is used as the first antibody. Green represents ZIKV-infected cells and blue represents DAPI staining. The scale bar represents 50 µm. Quantitative data are shown as the mean ± SD (error bars). *P < 0.05, **P < 0.01, ***P < 0.001, ****P < 0.0001 (Sidak's multiple comparisons test)
Pathological lesions appeared in tissues with infectious virus
To gain insight into the impact of ZIKV infection on heart, lung, kidney, cerebrum, and testicle, these tissue samples were obtained at 4 dpi and 7 dpi for histopathologic analyses (Fig. 3). Compared to the control group, we observed extensive ZIKV-associated pathological lesions in challenged groups. In the myocardium, the inflammatory cells increased, and the local congestion was mild; In addition, pathological lesions of the 102 TCID50 group at 7 dpi were relatively severe. In lung tissue after virus infection, inflammatory cells increased significantly with alveolar septum hemorrhage, pulmonary septum thickening, and bronchial mucosa shedding, and hemorrhage and inflammatory infiltration of the 102 TCID50 group at 7 dpi were more severe. In kidney tissue after virus infection, pathological lesions mainly included vascular congestion, mild local bleeding, and inflammatory cell increase. In the cerebrum, microglia increased. In testicle, tissue was loose, and spermatogonium decreased. Meanwhile, we also found pathologic lesions in liver, spleen, and duodenum (Fig. S3), though no infectious virus was detected in these tissues. In liver, lymphocyte infiltration and mild congestion were observed locally. In spleen, the germinal center disappeared with lymphocyte infiltration and local congestion. In duodenum, lymphocytes increased with local mucosal structure damage.
ZIKV infection resulted in a massive release of cytokines at 4dpi in AG129 mice
To characterize immune activation during ZIKV infection, we compared the change of serum levels of several cytokines, chemokines, and growth factors in AG129 mice of the infection groups. The massive release of KC, IL-12p40, MIP-1β, MCP-1 and RANTES happened on the fourth day after ZIKV infection (Fig. 4a-e, Fig. 4g-k). the massive expression of IFN-γ and G-CSF in the serum of 104 TCID50 group was detected early at 4 dpi (Fig. 4l, Fig. S4g), while that of 102 TCID50 group was detected at 7 dpi (Fig. 4f, Fig. S4b). The expression of KC in the serum of 102 TCID50 group is higher than that of 104 TCID50 group at 4dpi (Fig. 4a,g). On the contrary, the expression of RANTES, IFN-γ, eotaxin and G-CSF in the serum of 102 TCID50 group is lower than that of 104 TCID50 group at 4dpi (Fig. 4e,f,k,l, Fig. S4a,b,f,g). There was no significant difference in the expression of IL-12p40, MIP-1β and MCP-1 in serum of these two infection groups at 4 dpi. ZIKV infection similarly did not affect the expression level of IL-12p70 and TNF-α (Fig. S4d,e,i,j).
ZIKV infection resulted in a massive release of cytokines at 4dpi in AG129 mice. a-f the expression of KC (a), IL-12p40 (b), MIP-1β (c), MCP-1 (d), RANTES (e) and IFN-γ (f) changed in AG129 mice infected with 102 TCID50 of ZIKV (n = 5); g-l the expression of KC (g), IL-12p40 (h), MIP-1β (i), MCP-1 (j), RANTES (k) and IFN-γ (l) changed in AG129 mice infected with 104 TCID50 of ZIKV (n = 5). Quantitative data are shown as the mean ± SD (error bars). *P < 0.05, **P < 0.01, ***P < 0.001, ****P < 0.0001 (one-way ANOVA)
Favipiravir showed antiviral effect in ZIKV-challenged AG129 mice
To prove the practical value of the AG129 mouse model, we evaluated the antiviral activity of favipiravir against ZIKV (1*103TCID50) in female AG129 mice. In the model, we gave mice favipiravir by intragastric administration every day. Favipiravir group displayed a statistically significant reduction of weight compared to the ZIKV group at 7 dpi (Fig. 5a). All untreated mice were immobile and dying at 8 dpi, while all treated mice were alive and active at 9 dpi (Fig. 5b). Viral load in blood of favipiravir group rose more slowly than that of ZIKV group (Fig. 5c). The statistical reduction of viral load in favipiravir group emerged in heart, kidney, cerebrum, cerebellum and spinal cord (Fig. 5d). According to pathological outcome (Fig. 5e), the symptoms of bleeding or inflammatory cell infiltration are milder in the myocardium, liver, spleen, lung, kidney, cerebrum, duodenum and uterus of favipiravir group than ZIKV group. In addition, the pathological changes of myocardial fibrosis, bronchial obstrction, the loss of germinal centers and intestinal mucosal injury were milder in favipiravir group than ZIKV group. We could notice that the difference of pathological lesions between these two groups appeared in heart, liver, spleen, lung, kidney, cerebrum and duodenum based on the pathological score (Fig. S6a). Meanwhile, we also evaluated the antiviral activity of testosterone against ZIKV (1*103TCID50) in male AG129 mice. In the model, we gave mice testosterone by intraperitoneal injection at 2 dpi and 4 dpi. Testosterone group did not show significant antiviral efficacy compared to the ZIKV group according to the results of weight change, survival rate and viral load (Fig. S5a-d). According to the pathological changes (Fig. S5e) and pathological score (Fig. S6b), the symptoms of bleeding and inflammatory cell infiltration are milder in the testicle of testosterone group than ZIKV group. However, we could not observe significant differences in other tissues of these two groups.
Favipiravir showed antiviral effect in ZIKV-challenged AG129 mice. a Line chart of weight change of AG129 female mice of ZIKV group and favipiravir group measured from 0 dpi to 8 dpi (n = 3); b Survival percentage for AG129 female mice of ZIKV group and favipiravir group (n = 3); c Line chart of viral load in blood of AG129 female mice of ZIKV group and favipiravir group measured from 0 to 8 dpi (n = 3); d Viral load in heart, liver, spleen, lung, kidney, duodenum, cerebrum, cerebellum, spinal cord, and uterus of AG129 female mice of ZIKV group and favipiravir group at 8 dpi (n = 3). Quantitative data are shown as the mean ± SD (error bars). *P < 0.05, **P < 0.01, ***P < 0.001, ****P < 0.0001 (Sidak's multiple comparisons test); e Pathological lesions were observed with H&E staining in heart, liver, spleen, lung, kidney, cerebrum, duodenum and uterus of AG129 female mice of ZIKV group and favipiravir group at 8 dpi (n = 3). The scale bar represents 50 µm
Discussion
Mice are the most widely used laboratory animals due to their low cost of breeding, short generation time, and small size. Interestingly, the natural host of the Zika virus comprises mainly mosquitoes, nonhuman primates, and humans [30]. The virus could not infect wild-type mice or immunocompetent mice due to the species-restricted difference in antiviral IFN signaling pathway [26, 31]. The double knockout of IFN-α/β and IFN-γ receptors abolishes innate immunity that prevents ZIKV infection early. Thus, ZIKV could efficiently infect immunocompromised mice that are deficient in IFN-α/β and IFN-γ receptors (AG129 mice) [32]. In this study, we chose AG129 mice as a small animal model to explore the infection characteristics, viral dynamics, pathological features, and cytokines change after ZIKV challenge.
During ZIKV infection, some cells and tissues may become viral reservoirs, contributing to the dissemination of ZIKV to nearby tissues. We have detected viral load in many tissues, including heart, liver, spleen, lung, kidney, duodenum, cerebrum, cerebellum, spinal cord, and testicle, which indicated extensive tissue tropism and was consistent with previous research [33, 34]. It is noteworthy that the viral load in these tissues of the 104 TCID50 group at 4dpi is higher than that of the 102 TCID50 group, but the viral load in these tissues of the 104 TCID50 group at 7dpi is lower than that of the 102 TCID50 group. Interestingly, viral load in these tissues of the 102 TCID50 group increased by approximately 1–4 logs from 4 to 7 dpi, while the viral load in these tissues of the 104 TCID50 group decreased by approximately 0–6 logs during the same time. These results demonstrated viral RNA levels are specific to the injection dose, time point, and tissue type. Meanwhile, we have also detected infectious viruses in some tissues at 7 dpi including heart, lung, kidney, cerebrum, testicle, and blood. As is known to all, the brain and testicle belong to immune-privileged organs. The immune-privileged nature may allow ZIKV to persist in these tissues once the virus enters [35, 36]. In our study, we not only detected viral load by RT-qPCR in the cerebrum and testicle but also infectious virus, which is consistent with previous studies in rhesus macaques and mice [32, 35,36,37,38]. While all of these tissues were found to be susceptible to ZIKV infection, infectious viruses were noticed to be the lowest in the cerebrum. ZIKV replication is cytopathic in neuronal cells, which could cause neurological damage in newborns [39]. These results proved that ZIKV could cross the blood–brain barrier and blood-testis barrier and further confirmed that the virus had the ability of neurotropism and sexual transmission [40,41,42,43]. Previous studies suggest that multiple candidate receptors for ZIKV are not specifically expressed in these tissues, which may explain ZIKV’s broad tissue tropism [44].
In addition, these tissues in which infectious virus were detected have appeared histopathological lesions attributable to infection with ZIKV. ZIKV could uniquely manipulate the host machinery to promote viral replication in a tissue-specific manner, resulting in characteristic pathological disorders [45]. It is noteworthy that related lesions appeared in the cerebrum and testicle. We did not observe more obvious pathological changes associated with microcephaly and male infertility. It is probably because microcephaly happened in fetal mice infected with ZIKV during pregnancy [46]. In a related mouse model, ZIKV infection causes complete destruction of the testicular structure up to two months after infection and leads to male infertility [35]. While our AG129 mice infected with 102 TCID50 of GZ01 died at 8 ~ 9 dpi. Although there was no infectious virus detected in the liver, spleen, and duodenum, pathological lesions happened in these tissues, which might be either due to the focal nature of infection, detection sensitivity or viral clearance during the necropsy of mice or because of a massive release of cytokines activated by ZIKV infection. Therefore, this dysregulated immune response led to multiple organ damage or death, especially liver with ample blood supply. Previous studies have demonstrated that the spleen and intestine as the lymphoid-rich organs were potential targets of ZIKV dissemination [47,48,49]. Our results supported that the levels of cytokines (KC, IL-12p40, MIP-1β, MCP-1, RANTES) in blood increased significantly at 4 dpi, indicating a potential role for these immune-related factors in ZIKV pathogenesis [20, 50]. Moreover, the expression level of several cytokines (KC, IL-12p40, MIP-1β, MCP-1) showed a similar trend with viral load in blood of AG129 mice. These cytokines might join in antiviral response at 3 dpi or 4 dpi. Viral load in blood of 104 TCID50 group decreased faster than that of 102 TCID50 group, which might be attributable to the higher expression of RANTES, IFN-γ, eotaxin and G-CSF in 104 TCID50 group than 102 TCID50 group at 4 dpi. It is noteworthy that the expression of KC of 102 TCID50 group is higher than that of 104 TCID50 group. Combined with more severe leision in heart and lung of 102 TCID50 group at 7 dpi, it might be KC that incurred more severe inflammatory response. Importantly, inflammatory damage appeared in most tissues of AG129 mice at 4 dpi, which indicated a massive release of cytokines due to excessive activation of immune cells destroyed a fragile equilibrium of pro- and anti-inflammation effect. The degree to which IFN-deficient mice model the extent and severity of ZIKV infection in humans is unknown. We recognize that healthy humans may be able to mount an effective antiviral response and prevent viral entry into the central nervous system. However, it remains a possibility that some immunocompromised humans and even some healthy humans may be susceptible in ways modeled by the AG129 mice.
To support the future value of AG129 mouse model, we chose favipiravir as a drug candidate against ZIKV. To ensure that untreated mice die at 8 dpi, we chose the inoculum dose of 103 TCID50. Favipiravir is an RNA polymerase inhibitor. According to experimental results, favipiravir seemed to inhibit the early replication of ZIKV in vivo and could alleviate infection symptoms significantly. Some results support antiviral efficacy of favipiravir against ZIKV in nonhuman primates [51]. Previous research has also proven that testosterone can reduce mortality and attenuate testicular damage in ZIKV-infected A129 mice [52]. However, our models only indicate that the testosterone could relieve ZIKV-induced testicular lesion. We think that the antiviral effect of testosterone may be specific to the ZIKV strains and mouse strains. In addition, the time, frequency, and dose of administration may also affect the experimental results. Therefore, in the future, researchers should focus on the study of pharmacokinetic and pharmacodynamic properties of testosterone in ZIKV-infected AG129 mice and find the optimal dosage regimen.
Therefore, robust animal models are crucial for advancing viral research and screening related drugs. First, our mouse model appeared to reproduce key features of human infection by ZIKV, including viremia, testicular tropism, and neuronal tissue tropism, which is valuable to study fundamental questions about ZIKV pathogenesis. Second, clinical data shows that symptoms associated with ZIKV infection in humans do not appear until 3–11 days after infection [53], which makes it difficult to study early viral infection. However, viral load in the blood of our mouse model could be detected as early as the second day after infection. Third, ZIKV is primarily transmitted to humans by the Aedes aegypti mosquito, and our AG129 mice were infected with the virus by subcutaneous injection, which could mimic mosquito biting to study mosquito-to-vertebrate transmission [54]. Fourth, ZIKV challenge with 102 TCID50 caused AG129 mice to die early at 8dpi, which demonstrated the virulence of GZ01. Rapidly worsening symptoms could shorten the cycle of drug screening in AG129 mice. Fifth, we observed a massive release of cytokines at 4 dpi, which might be a critical factor resulting in extensive pathological lesions of AG129 mice. The discovery can contribute to clinical decision on how to control the balance of pro- and anti-inflammatory responses during ZIKV infection. Finally, we also have noticed that people previously infected with dengue virus may exacerbate the symptoms of ZIKV infection because of antibody-dependent enhancement of the two viruses [55, 56]. Importantly, AG129 mice are susceptible to both dengue and Zika virus infection [54, 57]. In short, the results presented here establish the outstanding potential of the ZIKV mouse model with unique features—specifically effective infectious dose, tissue tropism, pathological lesion, immune response, and early infection, which could significantly contribute to potential vaccination and antiviral strategies. According to our mouse model, we have discovered that the inoculum dose of Zika virus can affect the viral replication dynamics, cytokine responses and survival rate in AG129 mice and that AG129 mice can be used to screen and evaluate antiviral drugs successfully. However, we did not evaluate the vaccine in the AG129 mouse model, which is what we need to improve in the future. In today's world of close interaction, the virus can spread rapidly from one country to another by international routes, potentially bringing the risk of disease and death to every corner of every continent on Earth. Although there is no sign of another ZIKV pandemic outbreak, we believe it is still necessary to study and improve Zika-related animal models constantly. After all, the Zika virus has the mutational potential for increased pathogenicity and enhanced transmissibility [14], and there are no specific drugs and vaccines to inhibit the Zika virus [58].
Materials and methods
Animals, ethics, and biosafety statement
AG129 mice (aged 6 ~ 8 weeks) were purchased from the National Institutes for Food and Drug Control (NIFDC) (manufacturing license: SCXK (JING)2022–0002). All animal procedures were approved by the Institutional Animal Care and Use Committee of the Institute of Medical Biology, Chinese Academy of Medical Science (ethics number: DWSP202108008), and performed in strict accordance with the guidelines for the ABSL-2 facility of the Institute of Medical Biology, Chinese Academy of Medical Science, Yunnan, China.
Cell and virus
Vero cells were self-stored in our laboratory and cultured in Dulbecco’s modified Eagle medium (DMEM) (Gibco) supplemented with 10% fetal bovine serum (FBS) (Gibco) and 1% penicillin/streptomycin at 37 °C with 5% CO2. The ZIKV strain GZ01 (GenBank: KU820898) was obtained from the Institute of Medical Biology, Chinese Academy of Medical Science, and propagated in Vero cells in DMEM (Gibco) supplemented with 2% FBS and 1% penicillin/streptomycin.
AG129 mouse experiment
AG129 mice were maintained and bred in a conventional animal facility. All mice were challenged with ZIKV (1*102 and 1*104 TCID50) via subcutaneous injection in the inner thigh. We selected eleven animals per inoculum dose. Mice were weighed and monitored daily for signs of disease. For all procedures (such as subcutaneous injection, physical examinations, and blood sampling), the animals were anesthetized via inhalation of vaporized isoflurane. Three AG129 mice in each group were sacrificed at 4 dpi and 7 dpi for tissue sample collection. Blood samples were collected from five AG129 mice in each group at 0 dpi, 6 h post infection (hpi), 2 dpi, 4 dpi and 7 dpi.
Viral load
Viral RNA was extracted with a KingFisher Flex Purification System (Thermo Fisher) and a MagMAX™-96 Viral RNA Isolation Kit (Thermo Fisher AM1836). Viral RNA isolated from different tissues was quantified by RT‒qPCR, which was performed using TaqMan Fast Viral 1-Step Master Mix (Thermo Fisher, 4,444,432) on a CFX384 Touch Real-Time PCR Detection System (Bio-Rad, USA). Forward primer (5′-TTGGTCATGATACTGCTGATTGC-3′), reverse primer (5′-CCTTCCACAAAGTCCCTATTGC-3′) and probe (5′-FAM-CGGCATACAGCATCAGGTGCATAGGAG-BHQ1-3′) targeting the ZIKV prM-E gene were used. The reaction system consisted of 2.5 μL qPCR mix, 0.5 μL forward primer, 0.5 μL reverse primer, 0.5 μL probe, 3.5 μL water, 2.5 μL RNA sample, which were added to each well of a 384-well PCR plate. The cycling conditions were as follows: 50 °C for 5 min, 95 °C for 20 s, and 40 cycles of 95 °C for 3 s and 60 °C for 30 s. After the reaction finished, the data were collected, and the number of viral copies was calculated based on the standards.
Viral titration
The virus concentration was quantified as the median tissue culture infective dose (TCID50) in Vero cells. The cells were inoculated with six tenfold serial dilutions of isolated serum from daily blood in DMEM supplemented with 2% fetal bovine serum and 1% penicillin/streptomycin. The cells were incubated for six days. The TCID50 was calculated via the Karber formula.
Immunofluorescence assay
Paraffin sections were sequentially soaked in xylene I, xylene II, xylene III, 100% ethanol, 100% ethanol, 95% ethanol, 85% ethanol, and 75% ethanol, followed by washing three times in PBS. Next, the sections were boiled in sodium citrate buffer in a microwave for 15 min. After the samples were soaked and washed twice in PBS, they were transferred to 0.5% TritonX-100 for permeation and incubated at room temperature for 10 min. Then, the slices were incubated with blocking buffer at room temperature for one hour. After that, Zika virus capsid protein antibody (GeneTex, Irvine, CA, USA, GTX133317) was added to the slices, which were incubated overnight at 4 ℃, followed by soaking and washing three times. Then, goat anti-rabbit IgG H&L conjugated with Alexa Fluor® 488 (Abcam, ab150081) was added as a secondary antibody, and the samples were incubated in the dark for one hour at room temperature, followed by soaking and washing three times. The coverslips were covered after the addition of antifade mounting medium containing DAPI, and the slides were observed under a fluorescence microscope (Leica).
Histopathology
For histopathological evaluation, tissue samples were fixed in 10% formalin and embedded in paraffin. Serial tissues were cut into 5-μm sections and stained with hematoxylin and eosin (HE). These slides were scanned with 3DHISTECH. H&E stained slides were scored by an experienced pathologist using CaseViewer from the manufacturer.
Cytokine measurement
To determine the levels of immune-related factors, we used a Bio-Plex Pro Mouse Cytokine 23-plex Assay #M60009RDPD and performed detection according to the manufacturer’s instructions. Before running the assay, the total number of wells in the experiment was determined, and we calculated the required volumes of coupled beads and detection antibodies. Then, the blood was allowed to clot for at least one hour at room temperature before centrifugation for 15 min at 3500 rpm, and the serum was transferred to a clean polypropylene tube. Next, the coupled beads, samples, standards, blanks, detection antibodies, and streptavidin–phycoerythrin were added to each well according to the manufacturer’s instructions. Finally, the plate was read immediately, and the data were processed.
Drug evaluation
We chose male AG129 mice for testing testosterone and female AG129 mice for testing favipiravir. Three mice in each group were prepared for ZIKV challenge (1*103 TCID50). The testosterone group was treated with 0.05 mg of testosterone in 100 ml of sesame seed oil by intraperitoneal injection on days 2 and 4 after ZIKV infection [52]. The favipiravir group was treated with 300 mg/kg favipiravir by intragastric administration every day [51]. We recorded the body temperature and weight of these mice daily. Moreover, we collected blood samples to detect viremia until the mice died. Finally, we sacrificed these mice at 9 dpi and collected tissue samples to detect the viral load.
Statistical analysis
Statistical analysis was performed with GraphPad Prism (8.0.2). We have described detailed statistics in the figure legends. The following statistical tests were used in this study: Sidak's multiple comparisons test, one-way analysis of variance (ANOVA) and paired t-test. All quantitative data in this study were presented as the mean ± SD. P values < 0.05 were considered significant. Significance values were set as follows: NS, not significant, P > 0.05; *P < 0.05; **P < 0.01; ***P < 0.001; and ****P < 0.0001.
Availability of data and materials
All the data supporting the findings of this current study are available from the corresponding author upon reasonable request.
References
Dick GW, Kitchen SF, Haddow AJ. Zika virus. I. Isolations and serological specificity. Trans R Soc Trop Med Hyg. 1952;46(5):509–20. https://doi.org/10.1016/0035-9203(52)90042-4.
Shankar A, Patil AA, Skariyachan S. Recent perspectives on genome, transmission, clinical manifestation, diagnosis, therapeutic strategies, vaccine developments, and challenges of Zika virus research. Front Microbiol. 2017;8:1761. https://doi.org/10.3389/fmicb.2017.01761.
Liu ZY, Shi WF, Qin CF. The evolution of Zika virus from Asia to the Americas. Nat Rev Microbiol. 2019;17(3):131–9. https://doi.org/10.1038/s41579-018-0134-9.
Cerbino-Neto J, Mesquita EC, Souza TM, Parreira V, Wittlin BB, Durovni B, et al. Clinical manifestations of Zika virus infection, Rio de Janeiro, Brazil, 2015. Emerg Infect Dis. 2016;22(7):1318–20. https://doi.org/10.3201/eid2207.160375.
Duffy MR, Chen TH, Hancock WT, Powers AM, Kool JL, Lanciotti RS, et al. Zika virus outbreak on Yap Island, Federated States of Micronesia. N Engl J Med. 2009;360(24):2536–43. https://doi.org/10.1056/NEJMoa0805715.
Cao-Lormeau VM, Roche C, Teissier A, Robin E, Berry AL, Mallet HP, et al. Zika virus, French polynesia, South pacific, 2013. Emerg Infect Dis. 2014;20(6):1085–6. https://doi.org/10.3201/eid2006.140138.
Ioos S, Mallet HP, LeparcGoffart I, Gauthier V, Cardoso T, Herida M. Current Zika virus epidemiology and recent epidemics. Med Mal Infect. 2014;44(7):302–7. https://doi.org/10.1016/j.medmal.2014.04.008.
Cao-Lormeau VM, Blake A, Mons S, Lastère S, Roche C, Vanhomwegen J, et al. Guillain-Barre Syndrome outbreak associated with Zika virus infection in French Polynesia: a case-control study. Lancet. 2016;387(10027):1531–9. https://doi.org/10.1016/S0140-6736(16)00562-6.
Zanluca C, Melo VC, Mosimann AL, Santos GI, Santos CN, Luz K. First report of autochthonous transmission of Zika virus in Brazil. Mem Inst Oswaldo Cruz. 2015;110(4):569–72. https://doi.org/10.1590/0074-02760150192.
Ikejezie J, Shapiro CN, Kim J, Chiu M, Almiron M, Ugarte C, et al. Zika virus transmission-region of the Americas, May 15,2015-December 15,2016. Morb Mortal Wkly Rep. 2017;66(12):329–34. https://doi.org/10.15585/mmwr.mm6612a4.
Paploski IA, Prates AP, Cardoso CW, Kikuti M, Silva MM, Waller LA, et al. Time lags between exanthematous illness attributed to Zika virus, Guillain-Barre Syndrome, and microcephaly, Salvador. Brazil Emerg Infect Dis. 2016;22(8):1438–44. https://doi.org/10.3201/eid2208.160496.
Kindhauser MK, Allen T, Frank V, Santhana RS, Dye C. Zika: the origin and spread of a mosquito-borne virus. Bull World Health Organ. 2016;94(9):675–86C. https://doi.org/10.2471/blt.16.171082.
Yeasmin M, Molla MMA, Masud HMAA, Saif-Ur-Rahman KM. Safety and immunogenicity of Zika virus vaccine: a systematic review of clinical trials. Rev Med Virol. 2023;33(1):e2385. https://doi.org/10.1002/rmv.2385.
Yuan L, Huang XY, Liu ZY, Zhang F, Zhu XL, Yu JY, et al. A single mutation in the prM protein of zika virus contributes to fetal microcephaly. Science. 2017;358(6365):933–6. https://doi.org/10.1126/science.aam7120.
Song GY, Huang XY, He MJ, Zhou HY, Li RT, Tian Y, et al. A single amino acid substitution in the capsid protein of Zika virus contributes to a neurovirulent phenotype. Nat Commun. 2023;14(1):6832. https://doi.org/10.1038/s41467-023-42676-7.
Liu Y, Liu J, Du S, Shan C, Nie K, Zhang R, et al. Evolutionary enhancement of Zika virus infectivity in Aedes aegypti mosquitoes. Nature. 2017;545(7655):482–6. https://doi.org/10.1038/nature22365.
Shaily S, Upadhya A. Zika virus: molecular responses and tissue tropism in the mammalian host. Rev Med Virol. 2019;29(4):e2050. https://doi.org/10.1002/rmv.2050.
Ho BX, Pek NMQ, Soh BS. Disease modeling using 3D organoids derived from human induced pluripotent stem cells. Int J Mol Sci. 2018;19(4):936. https://doi.org/10.3390/ijms19040936.
Garcez PP, Loiola EC, da Costa RM, Higa LM, Trindade P, Delvecchio R, et al. Zika virus impairs growth in human neurospheres and brain organoids. Science. 2016;352(6287):816–8. https://doi.org/10.1126/science.aaf6116.
Hirsch AJ, Roberts VHJ, Grigsby PL, Haese N, Schabel MC, Wang X, et al. Zika virus infection in pregnant rhesus macaques causes placental dysfunction and immunopathology. Nat Commun. 2018;9(1):263. https://doi.org/10.1038/s41467-017-02499-9.
de Alcantara BN, Imbeloni AA, de Brito Simith Durans D, de Araújo MTF, do Rosário Moutinho da Cruz E, de Carvalho CAM, et al. Histopathological lesions of congenital Zika syndrome in newborn squirrel monkeys. Sci Rep. 2021;11(1):6099. https://doi.org/10.1038/s41598-021-85571-1.
WichgersSchreur PJ, van Keulen L, Anjema D, Kant J, Kortekaas J. Microencephaly in fetal piglets following in utero inoculation of Zika virus. Emerg Microbes Infect. 2018;7(1):42. https://doi.org/10.1038/s41426-018-0044-y.
Wang Y, Ling L, Zhang Z, Marin-Lopez A. Current advances in Zika vaccine development. Vaccines (Basel). 2022;10(11):1816. https://doi.org/10.3390/vaccines10111816.
Li QH, Kim K, Shresta S. Mouse models of Zika virus transplacental transmission. Antiviral Res. 2023;210:105500. https://doi.org/10.1016/j.antiviral.2022.105500.
Marín-Lopez A, Calvo-Pinilla E, Moreno S, Utrilla-Trigo S, Nogales A, Brun A, et al. Modeling arboviral infection in mice lacking the interferon alpha/beta receptor. Viruses. 2019;11(1):35. https://doi.org/10.3390/v11010035.
Grant A, Ponia SS, Tripathi S, Balasubramaniam V, Miorin L, Sourisseau M, et al. Zika virus targets human STAT2 to inhibit type I interferon signaling. Cell Host Microbe. 2016;19(6):882–90. https://doi.org/10.1016/j.chom.2016.05.009.
Rossi SL, Tesh RB, Azar SR, Muruato AE, Hanley KA, Auguste AJ, et al. Characterization of a novel murine model to study Zika virus. Am J Trop Med Hyg. 2016;94(6):1362–9. https://doi.org/10.4269/ajtmh.16-0111.
Aliota MT, Caine EA, Walker EC, Larkin KE, Camacho E, Osorio JE. Characterization of lethal Zika virus infection in AG129 mice. PLoS Negl Trop Dis. 2016;10(4):e0004682. https://doi.org/10.1371/journal.pntd.0004682.
Miner JJ, Diamond MS. Zika virus pathogenesis and tissue tropism. Cell Host Microbe. 2017;21(2):134–42. https://doi.org/10.1016/j.chom.2017.01.004.
Chan JF, Choi GK, Yip CC, Cheng VC, Yuen KY. Zika fever and congenital Zika syndrome: an unexpected emerging arboviral disease. J Infect. 2016;72(5):507–24. https://doi.org/10.1016/j.jinf.2016.02.011.
Hu H, Feng Y, He ML. Targeting type I interferon induction and signaling: how Zika virus escapes from host innate immunity. Int J Biol Sci. 2023;19(10):3015–28. https://doi.org/10.7150/ijbs.83056.
Dowall SD, Graham VA, Rayner E, Atkinson B, Hall G, Watson RJ, et al. A susceptible mouse model for Zika virus infection. PLoS Negl Trop Dis. 2016;10(5):e0004658. https://doi.org/10.1371/journal.pntd.0004658.
Hirsch AJ, Smith JL, Haese NN, Broeckel RM, Parkins CJ, Kreklywich C, et al. Zika virus infection of rhesus macaques leads to viral persistence in multiple tissues. PLoS Pathog. 2017;13(3):e1006219. https://doi.org/10.1371/journal.ppat.1006219.
Baloch Z, Shen Z, Zhang L, Feng Y, Li D, Zhang NN, et al. Recapitulating Zika virus infection in vagina of Tree Shrew (Tupaia belangeri). Front Cell Infect Microbiol. 2021;11:687338. https://doi.org/10.3389/fcimb.2021.687338.
Ma W, Li S, Ma S, Jia L, Zhang F, Zhang Y, et al. Zika virus causes testis damage and leads to male infertility in mice. Cell. 2017;168(3):542. https://doi.org/10.1016/j.cell.2017.01.009.
Aid M, Abbink P, Larocca RA, Boyd M, Nityanandam R, Nanayakkara O, et al. Zika virus persistence in the central nervous system and lymph nodes of rhesus monkeys. Cell. 2017;169(4):610–20.e14. https://doi.org/10.1016/j.cell.2017.04.008.
Berry N, Ferguson D, Ham C, Hall J, Jenkins A, Giles E, et al. High susceptibility, viral dynamics and persistence of South American Zika virus in New World monkey species. Sci Rep. 2019;9(1):14495. https://doi.org/10.1038/s41598-019-50918-2.
Dowall SD, Graham VA, Rayner E, Hunter L, Atkinson B, Pearson G, et al. Lineage-dependent differences in the disease progression of Zika virus infection in type-I interferon receptor knockout (A129) mice. PLoS Negl Trop Dis. 2017;11(7):e0005704. https://doi.org/10.1371/journal.pntd.0005704.
Ravindran S, Lahon A. Tropism and immune response of Chikungunya and Zika viruses: an overview. Cytokine. 2023;170:156327. https://doi.org/10.1016/j.cyto.2023.156327.
Robinson N, Mayorquin Galvan EE, Zavala Trujillo IG, Zavala-Cerna MG. Congenital Zika syndrome: pitfalls in the placental barrier. Rev Med Virol. 2018;28(5):e1985. https://doi.org/10.1002/rmv.1985.
Tsetsarkin KA, Maximova OA, Liu G, Kenney H, Teterina N, Bloom ME, et al. Routes of Zika virus dissemination in the testis and epididymis of immunodeficient mice. Nat Commun. 2018;9(1):5350. https://doi.org/10.1038/s41467-018-07782-x.
Figueiredo CP, Barros-Aragão FGQ, Neris RLS, Frost PS, Soares C, Souza INO, et al. Zika virus replicates in adult human brain tissue and impairs synapses and memory in mice. Nat Commun. 2019;10(1):3890. https://doi.org/10.1038/s41467-019-11866-7.
Khaiboullina SF, Ribeiro FM, Uppal T, Martynova EV, Rizvanov AA, Verma SC. Zika virus transmission through blood tissue barriers. Front Microbiol. 2019;10:1465. https://doi.org/10.3389/fmicb.2019.01465.
Nowakowski TJ, Pollen AA, Di Lullo E, Sandoval-Espinosa C, Bershteyn M, Kriegstein AR. Expression analysis highlights AXL as a candidate Zika virus entry receptor in neural stem cells. Cell Stem Cell. 2016;18(5):591–6. https://doi.org/10.1016/j.stem.2016.03.012.
Giraldo MI, Gonzalez-Orozco M, Rajsbaum R. Pathogenesis of Zika virus infection. Annu Rev Pathol. 2023;18:181–203. https://doi.org/10.1146/annurev-pathmechdis-031521-034739.
Li C, Xu D, Ye Q, Hong S, Jiang Y, Liu X, et al. Zika virus disrupts neural progenitor development and leads to microcephaly in mice. Cell Stem Cell. 2016;19(1):120–6. https://doi.org/10.1016/j.stem.2016.04.017.
Wang T, Li P, Zhang Y, Liu Y, Tan Z, Sun J, et al. In vivo imaging of Zika virus reveals dynamics of viral invasion in immune-sheltered tissues and vertical propagation during pregnancy. Theranostics. 2020;10(14):6430–47. https://doi.org/10.7150/thno.43177.
Tang WW, Young MP, Mamidi A, Regla-Nava JA, Kim K, Shresta S. A mouse model of Zika virus sexual transmission and vaginal viral replication. Cell Rep. 2016;17(12):3091–8. https://doi.org/10.1016/j.celrep.2016.11.070.
Alcendor DJ. Zika virus infection and implications for kidney disease. J Mol Med (Berl). 2018;96(11):1145–51. https://doi.org/10.1007/s00109-018-1692-z.
Pan Y, Cai W, Cheng A, Wang M, Yin Z, Jia R. Flaviviruses: innate immunity, inflammasome activation, inflammatory cell death, and cytokines. Front Immunol. 2022;13:829433. https://doi.org/10.3389/fimmu.2022.829433.
Marlin R, Desjardins D, Contreras V, Lingas G, Solas C, Roques P, et al. Antiviral efficacy of favipiravir against Zika and SARS-CoV-2 viruses in non-human primates. Nat Commun. 2022;13(1):5108. https://doi.org/10.1038/s41467-022-32565-w.
Zheng B, Sun J, Luo H, Yang L, Li Q, Zhang L, et al. Testosterone protects mice against zika virus infection and suppresses the inflammatory response in the brain. iScience. 2022;25(11):105300. https://doi.org/10.1016/j.isci.2022.105300.
Zanluca C, dos Santos CND. Zika virus – an overview. Microbes Infect. 2016;18(5):295–301. https://doi.org/10.1016/j.micinf.2016.03.003.
Baldon LVR, de Mendonça SF, Ferreira FV, Rezende FO, Amadou SCG, Leite THJF, et al. AG129 mice as a comprehensive model for the experimental assessment of mosquito vector competence for arboviruses. Pathogens. 2022;11(8):879. https://doi.org/10.3390/pathogens11080879.
Rey FA, Stiasny K, Vaney MC, Dellarole M, Heinz FX. The bright and the dark side of human antibody responses to flaviviruses: lessons for vaccine design. EMBO Rep. 2018;19(2):206–24. https://doi.org/10.15252/embr.201745302.
Rathore APS, St John AL. Cross-reactive immunity among flaviviruses Front Immunol. 2020;11:334. https://doi.org/10.3389/fimmu.2020.00334.
Byrne AB, García CC, Damonte EB, Talarico LB. Murine models of dengue virus infection for novel drug discovery. Expert Opin Drug Discov. 2022;17(4):397–412. https://doi.org/10.1080/17460441.2022.2033205.
Bifani AM, Chan KWK, Borrenberghs D, Tan MJA, Phoo WW, Watanabe S, et al. Therapeutics for flaviviral infections. Antiviral Res. 2023;210:105517. https://doi.org/10.1016/j.antiviral.2022.105517.
Acknowledgements
We thank all the reviewers and editors who undertook review and editing for this study.
Funding
This study was supported by the National Key R&D Program of China (2021YFC230170402, 2021YFC0864600), CAMS Innovation Fund for Medical Sciences (2022-I2M-CoV19-002), Yunnan Key R&D Project (202103AQ100001), Project supported by the Foundation for Innovative Research Groups of the National Natural Science Foundation of China (82221004), Technical Innovation talents of Yunnan Province, Grant/ Award Number (202205AD160008).
Author information
Authors and Affiliations
Contributions
Shuaiyao Lu and Changfa Fan conceived the ideas and designed the research; Yuhuan Yan, Wenhai Yu, Hao Yang, Yun Yang, Junbin Wang, Yanan Zhou, Cong Tang, Bai Li, Qing Huang, Ran An, Xiaoming Liang and Dongdong Lin performed the experiments and collected and tested samples; YuhuanYan, Wenhai Yu and Hao Yang integrated the data and drafted the manuscript. Shuaiyao Lu and Changfa Fan critically reviewed the manuscript. All authors read and approved the final version of the manuscript.
Corresponding authors
Ethics declarations
Ethics approval and consent to participate
All animal procedures were approved by the Institutional Animal Care and Use Committee of the Institute of Medical Biology, Chinese Academy of Medical Science (ethics number: DWSP202002 001), and performed in strict accordance with the guidelines for the ABSL-2 facility of the Institute of Medical Biology, Chinese Academy of Medical Science, Yunnan, China.
Consent for publication
All the authors have agreed to publish the manuscript.
Competing interests
The authors declare that they have no competing interests.
Additional information
Publisher’s Note
Springer Nature remains neutral with regard to jurisdictional claims in published maps and institutional affiliations.
Supplementary Information
Rights and permissions
Open Access This article is licensed under a Creative Commons Attribution 4.0 International License, which permits use, sharing, adaptation, distribution and reproduction in any medium or format, as long as you give appropriate credit to the original author(s) and the source, provide a link to the Creative Commons licence, and indicate if changes were made. The images or other third party material in this article are included in the article's Creative Commons licence, unless indicated otherwise in a credit line to the material. If material is not included in the article's Creative Commons licence and your intended use is not permitted by statutory regulation or exceeds the permitted use, you will need to obtain permission directly from the copyright holder. To view a copy of this licence, visit http://creativecommons.org/licenses/by/4.0/.
About this article
Cite this article
Yan, Y., Yang, H., Yang, Y. et al. The inoculum dose of Zika virus can affect the viral replication dynamics, cytokine responses and survival rate in immunocompromised AG129 mice. Mol Biomed 5, 30 (2024). https://doi.org/10.1186/s43556-024-00195-x
Received:
Accepted:
Published:
DOI: https://doi.org/10.1186/s43556-024-00195-x