Abstract
Trained immunity is a phenomenon in which brief exposure to an infectious agent or a vaccine can induce long-lasting changes in the host's immune system, enhancing protection against subsequent infections. The concept of trained immunity has a significant impact on the field of immunology and has the potential to revolutionize how we approach vaccination and infectious disease control. Investigations into trained immunity are rapidly advancing and have led to the development of new vaccines and immunotherapeutic strategies that harness the power of this phenomenon. While more investigations are needed to fully understand the mechanisms of trained immunity and its potential limitations, the prospects for its future application in clinical practice are promising. Here, we describe trained immunity as a biological process and explore the innate cues, epigenetic changes, and metabolic reprogramming activities that affect how trained immunity is induced.
Similar content being viewed by others
Introduction
Trained immunity is the immunological process through which innate immune cells develop a form of memory, increasing their responsiveness to subsequent triggers. Unlike the acquired immune system, which is traditionally associated with immunological memory, trained immunity represents an adaptive characteristic of the innate immune system. This phenomenon, observed in cells such as natural killer (NK) cells and monocytes, highlights their capacity for increased responsiveness and elasticity (Quintin et al. 2014; Netea and van der Meer 2017; Netea et al. 2020). While innate immune responses typically eliminate invasive pathogens effectively, the functional adaptations observed in trained immunity can sometimes lead to aberrant inflammatory activity, contributing to conditions such as autoimmunity (Ochando et al. 2023). Despite these drawbacks, trained immunity is emerging as a revolutionary immunotherapeutic approach with significant potential for combating secondary infections and other immune-related challenges.
Furthermore, adaptive immune responses, which may be concurrently produced, are driven by dendritic cells (DCs) as well as distinct T and B cells. These lymphocyte-dependent adaptive immune responses produce long-lasting immunological memory despite their slower rate of development (Bonilla and Oettgen 2010). The specific quality of the adaptive immune response is widely accepted to be immunological memory. However, studies suggesting that tissue-resident stem cells and even innate immune cells can display adaptive properties have refuted this paradigm (Bowdish et al. 2007, Christ et al.,2018a, b Naik et al. 2017, Netea et al., 2011b).
In the last ten years, investigations have revealed that trained immunity is highly favorable for host defence, but concerns about possible negative effects in immune-mediated and chronic inflammatory disorders have also been raised. Here, we describe “trained immunity” as a biological process and explore the innate signals, epigenetic changes, and metabolic reprogramming activities that influence how trained immunity is induced. Because hematopoietic stem and progenitor cells are trained, the trained phenotype might last several months or even a year. An interesting framework for creating novel vaccination strategies and pharmaceutical targets for treating inflammatory disorders is trained immunity (Tercan et al. 2021).
Although the notion of trained immunity is intricate, we aim to review its fundamental aspects comprehensively. The main objective of this study is to offer fundamental insights into the potential application of trained immunity in various diseases and conditions. It also provides knowledge on the mechanisms of crosstalk between trained immunity and other innate and adaptive immune responses.
Possibility of trained immunity for treatment
Trained immunity is crucial across many illnesses, such as cancer and inflammation. It is controlled by epigenetic and metabolic reprogramming of hematopoietic stem and progenitor cells, resulting in hyperactive myeloid cells. The treatment of diseases in which dysregulated immune responses play a significant role involves immunotherapy. However, most immunotherapy methods currently being developed use the adaptive immune response. In the last ten years, the innate immune system's myeloid (monocytes, macrophages, and dendritic cells) and lymphoid (natural killer cells and innate lymphoid cells) cell populations have undergone long-term functional programming modifications through metabolic and epigenetic programming (Mulder et al. 2019; Peignier and Parker 2020; Yi et al. 2023; Perzolli et al. 2024).
Conversely, trained immunity can have unfavorable effects in chronic inflammatory circumstances, promote hyperinflammation, and accelerate the development of cardiovascular disease, autoinflammatory syndromes, and neuroinflammation (Bekkering et al. 2021). One study demonstrated that the metabolic sphingolipid‒mitochondrial fission pathway could inhibit tumor metastasis by eliciting trained immunity in prometastatic macrophages (Ding et al. 2023). Another study showed that β-glucan, an inducer of trained immunity, traffics to the pancreas and generates a CCR2-dependent influx of monocytes and macrophages, which stimulates antitumor activity to slow the growth of pancreatic cancer (Geller et al. 2022). Phagocytic myeloid cells naturally interact with nanomaterials, making them perfect tools for controlling trained immunity. Nanomedicines are used in various clinical scenarios to regulate hyperinflammation and maladaptive trained immune responses to improve infection resistance, inducing trained immunity for cancer treatment (van Leent et al. 2022). Owing to the critical roles of stress and an activated immune system in neuropathology, innate immunological training has substantial consequences for our comprehension and treatment of neuropsychiatric diseases (Salam et al. 2018). Exposure to environmental microbes significantly affects lung immune memory and highlights tissue-specific features of trained immunity (Zahalka et al. 2022).
The fact that vaccination with the attenuated strain of Mycobacterium bovis, known as Bacillus Calmette-Guerin (BCG), which is used to elicit protective immunity against tuberculosis (TB), caused a major decline in mortality that could not be elucidated by TB protection alone was one of the principal observations that led to the unearthing of trained immunity. Instead, its defence function appears to cover additional illnesses, such as sepsis and respiratory infections (Li et al. 2024; Kilic et al. 2024). Therefore, strengthening nonspecific immunity to reinfection by bacteria, fungi, or viruses using particular pathogen-derived components is the foundation of training innate immunity (Netea et al. 2016). Researchers were able to modify the innate immune response, lower local bacterial burdens, and induce either LCN2 or CHI3L1 at 24 h after infection when breast injection of lipopolysaccharide (LPS) or lipoteichoic acid (LTA) was combined with local Staphylococcus aureus infection (Breyne et al. 2017). By increasing TNF and IL-6 production in response to commensal bacterial and fungal organisms as well as TLR ligands, S. cerevisiae cells promote trained immunity in monocytes in a strain-dependent manner (Rizzetto et al. 2016). Schleicher et al. reported that TNF inhibited the alternate activation of dendritic cells (DCs) and macrophages by IL-4. TNF reduces histone acetylation, which decreases IL-4-induced arginase 1 (Arg1) production without altering STAT6 phosphorylation or nuclear translocation (Schleicher et al. 2016).
One of the most intriguing and potentially useful concepts in modern immunology regarding commercial and therapeutic applications is the idea of trained immunity. Innate immune cells have been shown to form memories influenced by microbial ligands. Histone modification when pathogen- or danger-associated molecular patterns (PAMPs/DAMPs) are recognized by pattern recognition receptors (PRRs) is a crucial aspect of epigenetic imprinting in this context. Such "trained immunity" impacts targets unrelated to the pathogen that are the intended target and targets that may be visible to the pathogen in the future. Further studies on trained innate immunity and how this understanding might be used in immunotherapeutic strategies are warranted in the context of cancer. A study revealed potential innate immunity-based approaches that could aid in developing host-directed immunotherapies focused on cancer and the potential to include treatments for infectious disorders (Lérias et al. 2020). Trained immunity may be one of the key mechanisms mediating the effects of BCG immunotherapy and may serve as a foundation for future advancements toward a tailored strategy for BCG therapy for nonmuscle-invasive bladder cancer (van Puffelen et al. 2020). The BCG vaccine also responds to other malignancies, such as lymphoma and melanoma (Gupta et al. 2024). Trained immunity could moderate the effects of the GA2LEN/EAACI pocket guide for allergen-specific immunotherapy for allergic rhinitis and asthma (van Puffelen et al. 2020; Walker et al. 2011). They postulated that new and improved methods of immunotherapy for cancer might use TI initiation, either alone or in combination with other immunotherapies (Netea et al. 2017).
Innate immune system activity against infection
In several significant ways, trained immunity differs from classical immunological memory in adaptive immunity (Table 1). It is carried out by a series of cellular populations that vary from one another in terms of their origins and effector capabilities. These cells mostly consist of macrophages, monocytes, natural killer cells, dendritic cells, and myeloid cells (Netea et al. 2020). Even innate lymphoid cells (ILCs) perform differently from traditional immunological memory. The fundamental mechanisms underlying the nonspecific high responsiveness of innate immune cells depend on transcriptional, epigenetic, and metabolic programs after a momentary stimulus (Domínguez-Andrés et al. 2023). Although trained immunity is less long-lasting and more specialized than classical memory is, it nevertheless likely provides an advantage for many viruses because it is driven mostly by epigenetic alterations (Netea et al. 2016). Trained immunity is more general than adaptive immunity and is achieved by the epigenetic reprogramming of nonimmunocompetent cells, mainly natural killer (NK) cells, monocytes, or macrophages. As a consequence, it may cross-protect against diverse pathogens (Vetvicka et al. 2021).
We are constantly exposed to microorganisms that can exist on our skin or mucous membranes or be swallowed. The pathogenicity and competence of host defence mechanisms determine whether these organisms invade and cause disease. The immune system comprises cytokines, lymphoid tissue, cells, and humoral factors. The crucial role of the immune system in host defence is best demonstrated when it malfunctions; underactivity leads to severe infections and tumors of immunodeficiency, and overactivity leads to allergies and autoimmune diseases.
The host's defence against infection relies entirely on innate immunity. Almost all cells can contribute to innate immunity by synthesizing specific innate cytokines, especially type 1 IFNs, and by reacting to these cytokines by inducing new and enhanced intracellular molecular defence mechanisms against infections. However, the key immune cell types responsible for innate immune responses are NK cells, macrophages, and dendritic cells. The quick array of protection provided by innate immunity is established within hours, whereas antigen-specific adaptive immune responses develop within the first few weeks following infection. Adaptive immunity now offers a wider and more refined self- and non-self-antigen recognition repertoire. Antigen-presenting cells, T lymphocytes, and B lymphocytes interact in a highly regulated manner during adaptive immunity, allowing for the expansion of immunologic memory, the activation of pathogen-specific effector pathways, and the control of host immunological homeostasis. The lymphatic system comprises some lymphoid organs where lymphocytes grow and become activated. Genes that code for the distinct antigen receptors of T and B cells are created during development by rearranging and assembling gene segments. The process of receptor reconfiguration creates an incredibly varied repertoire of receptor specificities that can identify elements of any potential pathogen. The development of immunologic memory is another key characteristic of adaptive immunity in addition to specificity. Long-lived memory B and T lymphocytes are created during the initial interaction with an antigen. Memory cells are promptly activated in repeated encounters with the same disease to produce a quicker and more effective protective response (Bonilla and Oettgen 2010). Adaptive immune responses are shaped and initiated by innate immune responses controlled by T and B cells (Iwasaki and Medzhitov 2015; Janeway 1989).
The fundamental relationship between trained immunity and innate immunity
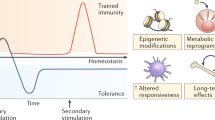
Despite years of intensive study, innate immune memory processes remain largely unexplored. The ability of innate immune system cells to develop immunological memory properties, also known as trained immunity, has recently come to light. The basic mode of action of the immune system is depicted in Fig. 1. Trained immunity is instigated by epigenetic reprogramming, broadly defined as long-term fluctuations in cell physiology and gene expression that do not involve perpetual genetic deviations, such as mutations and recombination, that are requisite for adaptive immunity. Trained immunity depends on a change in the functional state of innate immune cells that lasts weeks to months rather than years after the initial trigger has been abolished. Innate immune cells may become persistently hyperresponsive after brief activation, which is known as trained immunity. Both pathogenic stimuli, such as bacteria, and endogenous chemicals, such as uric acid, oxidized LDL (low-density lipoprotein), and catecholamines, can cause monocytes and macrophages to form memories (Tercan et al. 2021). By facilitating greater innate immune responses and improving defence against variations in microbial stimuli, trained immunity improves the body's reaction to diseases and immunizations. However, trained immunity may also play a role in the pathophysiology of inflammatory, autoimmune, and neurodegenerative illnesses (Domínguez-Andrés et al. 2023). Therapeutically approaching trained immunity might be a promising tool to regulate innate immunity and, subsequently, the adaptive immune responses that are activated and regulated by innate immune cell surface receptors and soluble mediators. Infection and cancer-fighting immune responses can be enhanced by manipulating trained immunity, but autoimmune disorders and allograft rejection can be prevented by reducing immune responses triggered by trained immunity. Several factors affect trained immunity, including ligand‒receptor interactions, metabolic control, and epigenetic regulation.
Boosting the innate immune system via trained immunity
Although heterologous protection against diseases caused by live vaccination can last up to five years, the immunological phenotype of trained immunity has been revealed for at least 3 months and as long as 1 year (Nankabirwa et al. 2015). However, trained immunity is typically less durable and reversible than traditional epitope-specific adaptive immune memory is (Dominguez‐Andres and Netea, 2019). Importantly, recent investigations have indicated that transgenerational effects occur via the induction of trained immunity (Moore et al. 2019). Since trained immunity is less specific than adaptive immunity, it may provide cross-protection against infectious agents. Trained immunity is produced by the epigenetic reprogramming of nonimmunocompetent cells, predominantly monocytes/macrophages and NK cells (Vetvicka et al. 2021). In contrast to a specific transcriptional or functional program, trained immunity refers to long-term innate immune cell adaptation. Indeed, different stimuli (such as LPS, β-glucans, or BCG vaccination) can generate various trained immunity programs.
Initially, studies with mice demonstrated that vertebrates exhibit trained immunity. The mice were shielded from deadly bacterial infection with Staphylococcus aureus by nonspecific compounds such as soluble purified β-glucans (Marakalala et al. 2013). Studies have indicated that through the noncanonical Raf-1 pathway and the glucan receptor Dectin-1, Candida albicans and fungal cell wall β-glucans cause functional reprogramming of monocytes, increasing cytokine production in vivo and in vitro and providing protection against reinfection (Quintin et al. 2012; Rosati et al. 2024). Intriguingly, a study revealed that repeated passages of Candida albicans via the digestive tract of mice, resulting in fungal adaptation toward colonization, increase the potential for trained immunity and improved defence via a lymphocyte-independent approach against nonspecific infections (Tso et al. 2018). Interleukin-22 (IL-22) is produced by dendritic cells after flagellin-induced activation of TLR5 in these cells, which initiates a protective gene expression pathway in intestinal epithelial cells. Additionally, IL-18 is produced by flagellin under the control of NLRC4, and RV-infected cells are immediately eliminated. The ability of flagellin to prevent or treat RV infection was perfectly replicated in mice after IL-22 and IL-18 administration, suggesting that flagellin has potential as a broad-spectrum antiviral agent (Zhang et al. 2014).
According to recent investigations, some vaccines, such as the BCG vaccine, may alter the innate immune system and induce nonspecific memory changes as well as increase the activity of the monocyte-macrophage lineage, which may increase protection against certain infections, such as malaria (Walk et al. 2019). Additionally, it has been suggested that BCG vaccination induces an anticancer kind of trained immunity, primarily in monocyte-macrophage lineage cells, which may effectively treat several cancers, including bladder cancer (Redelman-Sidi et al. 2014). A study revealed a protective effect of BCG vaccination on the risk of lymphoma, with smallpox and BCG immunizations having a protective effect on the future risk of lymphoma and leukemia (Villumsen et al. 2009). Silica-titania hollow nanoparticles (HNPs) have been studied for their effects on cellular internalization, toxicity, and the innate immune response in mouse alveolar macrophages (J774A.1) and human breast cancer (SK-BR-3) cells. The toxicity of HNP-treated macrophages was consistent with their cellular internalization efficiency (Oh et al. 2010). According to previous studies, chemical exposure impacts human immune cells (T cells, B cells, NK cells, dendritic cells, and monocytes) associated with IFN type I/II signaling pathways and the antigen presentation process (Arowolo et al. 2021). Furthermore, graphene nanomaterials can be incorporated into a collagen matrix to promote innate immune training. Nanomaterials can program cells for enhanced inflammatory responses (Yadav and Bachhuka 2023).
Relation and mechanism of action of various components in trained immunity
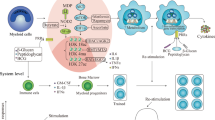
Trained immunity, which is less long-lasting and more specialized than classical memory but probably still provides an advantage during many infections, is driven mostly by epigenetic modifications (Netea et al. 2020) (Fig. 2). In the brain, cannabinoids have anti-inflammatory and neuroprotective effects. This study investigated how endogenous and synthetic cannabinoids affect TNF release in cultured microglia. LPS significantly increased TNFα mRNA expression and release in primary cultures of rat cortical microglia (Facchinetti et al. 2003; Liu et al. 2023). Owing to exposure to the gram-negative bacterial endotoxin LPS, microglia produce large amounts of proinflammatory cytokines, including TNF (Molina-Holgado et al. 1997).
Mechanism of trained immunity and its interaction with the host immune system. This figure illustrates the process of trained immunity and its interaction with the host immune system. Pathogens (viruses, bacteria) and vaccines activate innate immune cells (macrophages, dendritic cells, NK cells) via DAMPs/PAMPs and PRRs, leading to histone modification and epigenetic reprogramming, increasing cytokine production (IL-1β, IL-6, TNF) and creating a hyperresponsive trained immune state. Trained immunity results in enhanced protection and cross-protection against infections through innate immune memory cells. The adaptive immune response (T cells, B cells) provides specific, long-lasting immunity. This interplay between innate and adaptive immunity highlights the potential of trained immunity in disease control and vaccination strategies
β-glucans are good examples of substances that induce trained immunity through changes in the metabolism of innate immune system cells and epigenetic reprogramming. The gut microbial composition, receptor recognition, and downstream signaling are potential processes regulating how β-glucans stimulate the immune system (Petit and Wiegertjes 2016). According to previous findings, the addition of β-glucans to Rabisin® vaccination enhances the immunological response of B lymphocytes, which is crucial for rabies protection. The cytokine secretion profile of PBMCs activated ex vivo indicates that β-glucans frequently stimulate the T-cell response (Paris et al. 2020). Additionally, the potential preventive effects of oral β-glucan on the immunological dysregulation and cytokine storm observed in patients with COVID-19 could be a successful method for enhancing immune responses (Geller and Yan 2020). Hematopoietic cells and multifunctional progenitors are reprogrammed by BCG or glucan through epigenetic reprogramming, metabolomic rewiring, changes in gene expression profiles, and adjustments to the internal and external signaling environments. Myeloid cells, such as monocytes, macrophages, and natural killer (NK) cells, inherit this training, enabling them to react more forcefully to microbial or nonmicrobial stimuli (Kar and Joosten 2020).
Epigenetic reprogramming of monocytes by trained immunity increases cytokine output in response to unrelated infections. A study revealed that a monocyte-dependent approach is safe for preventing Candida albicans reinfection in mice lacking functional B and T lymphocytes. This training is necessary for the noncanonical Raf-1 pathway, the β-glucan receptor Dectin-1, and the effects on the epigenetic mechanisms of H3K4 histone trimethylation (Quintin et al. 2012).
The trained immune system of NK cells has shown tremendous potential for cancer therapy because it enhances effector resistance to restimulation by cancer cells or cytokines. After preactivation, EZH2 is vital for the long-lasting expression of trained immunity in human NK cells (Zhang et al. 2021). BCG vaccination of the healthy body increased proinflammatory cytokine levels following ex vivo stimulation of NK cells with mycobacteria and other irrelevant pathogens up to 3 months after vaccination. NK cells have been demonstrated to have memory-like characteristics during viral diseases, but it is unknown whether these factors play a role in the trained immunity carried out by BCG vaccination (Kleinnijenhuis et al. 2014). The differentiation, functional specialization, and control of NK cell responses are all caused by epigenetic modifications (Cichocki et al. 2013).
One of the key aspects of the formation of innate memory is the epigenetic modification of histones and DNA methylation. Changes in histone methylation are among these epigenetic modifications, and they may either promote or repress gene transcription. Epigenetic modifications during the initial transcriptional response to immune activation and their dynamics as cells return to homeostasis play a key role in the memory of the initial stimulus. While some changes are rapidly reversible, others appear to be long-lasting, with important functional consequences for the stimulus-experienced cell (Sun and Barreiro 2020). Training with β-glucan produces a unique epigenetic signature that reveals a complicated network of enhancers and promoters (Saeed et al. 2014). Ten-eleven translocation (Tet2) increases DNA demethylation and cytokine gene expression activation in T cells, and epigenetic regulation of lineage-specific genes is critical for the differentiation and function of T cells (Ichiyama et al. 2015).
Conclusion
Trained immunity refers to nonspecific immunity after exposure to an infectious agent or vaccination, providing increased protection against subsequent infections. The future of trained immunity may include potential applications in the development of more effective vaccines and immunotherapies and further exploration of the mechanisms underlying this phenomenon to understand its role in overall immune health better. Additionally, investigations into trained immunity may lead to a better understanding of vaccine-induced protection and the design of novel immunization strategies. Various immune-related diseases, including those associated with excessive trained immunity, such as inflammation, autoimmune conditions, allergies, and cardiovascular disease, can be treated with targeted approaches to regulate trained immunity.
Availability of data and materials
All the data generated in this study were extracted from previously published research articles.
References
Arowolo, O., L. Pobezinsky, and A. Suvorov. 2021. Chemical exposures affect innate immune response to SARS-CoV-2. International Journal of Molecular Sciences 22: 12474. https://doi.org/10.3390/ijms222212474.
Arts, R.J., S.J. Moorlag, B. Novakovic, Y. Li, S. Y. Wang, M. Oosting, V. Kumar, R.J. Xavier, C. Wijmenga, and L.A. Joosten. 2018. BCG vaccination protects against experimental viral infection in humans through the induction of cytokines associated with trained immunity. Cell Host, and Microbe 23 (89–100): e5. https://doi.org/10.1016/j.chom.2017.12.010.
Baydemir, I., Dulfer, E., Netea, M. G., and Andrés, J. D. 2024. Trained immunity-inducing vaccines: Harnessing innate memory for vaccine design and delivery. Clinical Immunology, 109930. https://doi.org/10.1016/j.clim.2024.109930.
Bekkering, S., R. J. Arts, B. Novakovic, I. Kourtzelis, C. D. Van Der Heijden, Y. Li, C. D. Popa, R. Ter Horst, J. Van Tuijl, and R. T. Netea-Maier. 2018. Metabolic induction of trained immunity through the mevalonate pathway. Cell 172: 135–146. e9.https://doi.org/10.1016/j.cell.2017.11.025
Bekkering, S., J. Domínguez-Andrés, L. A. Joosten, N. P. Riksen, and M. G. Netea. 2021. Trained immunity: Reprogramming innate immunity in health and disease. Annual Review of Immunology 39: 667–693. https://doi.org/10.1146/annurev-immunol-102119-073855.
Blok, B. A., R. J. Arts, R. Van Crevel, C. S. Benn, and M. G. Netea. 2015. Trained innate immunity as underlying mechanism for the long-term, nonspecific effects of vaccines. Journal of Leucocyte Biology 98: 347–356. https://doi.org/10.1189/jlb.5RI0315-096R.
Bonilla, F. A., and H. C. Oettgen. 2010. Adaptive immunity. Journal of Allergy and Clinical Immunology 125: S33–S40. https://doi.org/10.1016/j.jaci.2009.09.017.
Bowdish, D. M., M. S. Loffredo, S. Mukhopadhyay, A. Mantovani, and S. Gordon. 2007. Macrophage receptors implicated in the “adaptive” form of innate immunity. Microbes and Infection 9: 1680–1687. https://doi.org/10.1016/j.micinf.2007.09.002.
Breyne, K., J. Steenbrugge, K. Demeyere, T. Vanden Berghe, and E. Meyer. 2017. Preconditioning with lipopolysaccharide or lipoteichoic acid protects against Staphylococcus aureus mammary infection in mice. Frontiers in Immunology 8: 833. https://doi.org/10.3389/fimmu.2017.00833.
Cheng, S. C., J. Quintin, R. A. Cramer, K. M. Shepardson, S. Saeed, V. Kumar, E. J. Giamarellos-Bourboulis, J. H. Martens, N. A. Rao, and A. Aghajanirefah. 2014. mTOR-and HIF-1α–mediated aerobic glycolysis as metabolic basis for trained immunity. Science 345: 1250684. https://doi.org/10.1126/science.1250684.
Christ, A., P. Günther, M. A. Lauterbach, P. Duewell, D. Biswas, K. Pelka, C. J. Scholz, M. Oosting, K. Haendler, and K. Baßler. 2018. Western diet triggers NLRP3-dependent innate immune reprogramming. Cell 172 (162–175): e14. https://doi.org/10.1016/j.cell.2017.12.013.
Cichocki, F., J. S. Miller, S. K. Anderson, and Y. T. Bryceson. 2013. Epigenetic regulation of NK cell differentiation and effector functions. Frontiers in Immunology 4: 55. https://doi.org/10.3389/fimmu.2013.00055.
Dagenais, A., C. Villalba-Guerrero, and M. Olivier. 2023. Trained immunity: A “new” weapon in the fight against infectious diseases. Frontiers in Immunology 14: 1147476. https://doi.org/10.3389/fimmu.2023.1147476.
Ding, C., Shrestha, R., Zhu, X., Geller, A. E., Wu, S., Woeste, M. R., Li, W., Wang, H., Yuan, F., and Xu, R. 2023. Inducing trained immunity in pro-metastatic macrophages to control tumor metastasis. Nature Immunology, 1–16. https://doi.org/10.1038/s41590-022-01388-8.
Domínguez-Andrés, J., J.C. Dos Santos, S. Bekkering, W.J. Mulder, J.W. Van Der Meer, N.P. Riksen, L.A. Joosten, and M.G. Netea. 2023. Trained immunity: Adaptation within innate immune mechanisms. Physiological Reviews 103: 313–346. https://doi.org/10.1152/physrev.00031.2021.
Dominguez-Andres, J., and M. G. Netea. 2019. Long-term reprogramming of the innate immune system. Journal of Leukocyte Biology 105: 329–338. https://doi.org/10.1002/JLB.MR0318-104R.
Facchinetti, F., E. Del Giudice, S. Furegato, M. Passarotto, and A. Leon. 2003. Cannabinoids ablate release of TNFα in rat microglial cells stimulated with lypopolysaccharide. Glia 41: 161–168. https://doi.org/10.1002/glia.10177.
Geller, A. E., R. Shrestha, M. R. Woeste, H. Guo, X. Hu, C. Ding, K. Andreeva, J. H. Chariker, M. Zhou, and D. Tieri. 2022. The induction of peripheral trained immunity in the pancreas incites anti-tumor activity to control pancreatic cancer progression. Nature Communications 13: 1–20. https://doi.org/10.1038/s41467-022-28407-4.
Geller, A., and Yan, J. 2020. Could the induction of trained immunity by β-glucan serve as a defense against COVID-19? Frontiers in immunology, 1782. https://doi.org/10.3389/fimmu.2020.01782
Gupta, S., Yadav, S., and Kumar, P. 2024. Efficacy of Bacillus Calmette-Guérin in Cancer Prevention and Its Putative Mechanisms. Journal of Cancer Prevention, 29, 6. https://doi.org/10.15430/JCP.23.036.
Ichiyama, K., T. Chen, X. Wang, X. Yan, B. S. Kim, S. Tanaka, D. Ndiaye-Lobry, Y. Deng, Y. Zou, and P. Zheng. 2015. The methylcytosine dioxygenase Tet2 promotes DNA demethylation and activation of cytokine gene expression in T cells. Immunity 42: 613–626. https://doi.org/10.1016/j.immuni.2015.03.005.
Ifrim, D. C., J. Quintin, L. A. Joosten, C. Jacobs, T. Jansen, L. Jacobs, N. A. Gow, D. L. Williams, J. W. Van Der Meer, and M. G. Netea. 2014. Trained immunity or tolerance: Opposing functional programs induced in human monocytes after engagement of various pattern recognition receptors. Clinical and Vaccine Immunology 21: 534–545. https://doi.org/10.1128/CVI.00688-13.
Iwasaki, A., and R. Medzhitov. 2015. Control of adaptive immunity by the innate immune system. Nature Immunology 16: 343–353. https://doi.org/10.1038/ni.3123.
Janeway, C. A. Approaching the asymptote? Evolution and revolution in immunology. Cold Spring Harbor symposia on quantitative biology, 1989. Cold Spring Harbor Laboratory Press, 1–13. https://doi.org/10.1101/SQB.1989.054.01.003
Kar, U. K., and L. A. Joosten. 2020. Training the trainable cells of the immune system and beyond. Nature Immunology 21: 115–119. https://doi.org/10.1038/s41590-019-0583-y.
Kaufmann, E., J. Sanz, J. L. Dunn, N. Khan, L. E. Mendonça, A. Pacis, F. Tzelepis, E. Pernet, A. Dumaine, and J. C. Grenier. 2018. BCG educates hematopoietic stem cells to generate protective innate immunity against tuberculosis. Cell 172 (176–190): e19. https://doi.org/10.1016/j.cell.2017.12.031.
Kilic, G., P. A. Debisarun, A. Alaswad, M. P. Baltissen, L. A. Lamers, L. C. J. De Bree, C. S. Benn, P. Aaby, H. Dijkstra, and H. Lemmers. 2024. Seasonal variation in BCG-induced trained immunity. Vaccine. https://doi.org/10.2139/ssrn.4751987.
Kleinnijenhuis, J., J. Quintin, F. Preijers, L. A. Joosten, D. C. Ifrim, S. Saeed, C. Jacobs, J. Van Loenhout, D. De Jong, and H. G. Stunnenberg. 2012. Bacille Calmette-Guerin induces NOD2-dependent nonspecific protection from reinfection via epigenetic reprogramming of monocytes. Proceedings of the National Academy of Sciences 109: 17537–17542. https://doi.org/10.1073/pnas.1202870109.
Kleinnijenhuis, J., J. Quintin, F. Preijers, L. A. Joosten, C. Jacobs, R. J. Xavier, J. W. Van Der Meer, R. Van Crevel, and M. G. Netea. 2014. BCG-induced trained immunity in NK cells: Role for non-specific protection to infection. Clinical Immunology 155: 213–219. https://doi.org/10.1016/j.clim.2014.10.005.
Lajqi, T., Köstlin-Gille, N., Bauer, R., Zarogiannis, S. G., Lajqi, E., Ajeti, V., Dietz, S., Kranig, S. A., Rühle, J., and Demaj, A. 2023. Training vs. Tolerance: the yin/yang of the innate immune system. Biomedicines 11: 766. https://doi.org/10.3390/biomedicines11030766
Lérias, J. R., E. De Sousa, G. Paraschoudi, J. Martins, C. Condeço, N. Figueiredo, C. Carvalho, E. Dodoo, A. Maia, and M. Castillo-Martin. 2020. Trained immunity for personalized cancer immunotherapy: Current knowledge and future opportunities. Frontiers in Microbiology 10: 2924. https://doi.org/10.3389/fmicb.2019.02924.
Li, D., and M. Wu. 2021. Pattern recognition receptors in health and diseases. Signal Transduction and Targeted Therapy 6: 291. https://doi.org/10.1038/s41392-021-00687-0.
Li, P., R. Wang, W. Q. Dong, G. Y. Wang, A. D. Zhang, H. C. Chen, and C. Tan. 2024. Systemic neutrophils are triggered by respiratory Bacillus Calmette-Guérin and mediate pulmonary mycobacterial clearance in synergy with the triggering receptor expressed on myeloid cells 1. Microbial Pathogenesis 187: 106535. https://doi.org/10.1016/j.micpath.2024.106535.
Liu, X., L. Zhang, Y. Cao, H. Jia, X. Li, F. Li, S. Zhang, and J. Zhang. 2023. Neuroinflammation of traumatic brain injury: Roles of extracellular vesicles. Frontiers in Immunology 13: 1088827. https://doi.org/10.3389/fimmu.2022.1088827.
Marakalala, M. J., D. L. Williams, J. C. Hoving, R. Engstad, M. G. Netea, and G. D. Brown. 2013. Dectin-1 plays a redundant role in the immunomodulatory activities of β-glucan-rich ligands in vivo. Microbes and Infection 15: 511–515. https://doi.org/10.1016/j.micinf.2013.03.002.
Mitroulis, I., K. Ruppova, B. Wang, L. S. Chen, M. Grzybek, T. Grinenko, A. Eugster, M. Troullinaki, A. Palladini, and I. Kourtzelis. 2018. Modulation of myelopoiesis progenitors is an integral component of trained immunity. Cell 172 (147–161): e12. https://doi.org/10.1016/j.cell.2017.11.034.
Molina-Holgado, F., A. Lledó, and C. Guaza. 1997. Anandamide suppresses nitric oxide and TNF-α responses to Theiler’s virus or endotoxin in astrocytes. NeuroReport 8: 1929–1933. https://doi.org/10.1097/00001756-199705260-00027.
Moore, R. S., R. Kaletsky, and C. T. Murphy. 2019. Piwi/PRG-1 argonaute and TGF-β mediate transgenerational learned pathogenic avoidance. Cell 177 (1827–1841): e12. https://doi.org/10.1016/j.cell.2019.05.024.
Mulder, W. J., J. Ochando, L. A. Joosten, Z. A. Fayad, and M. G. Netea. 2019. Therapeutic targeting of trained immunity. Nature Reviews Drug Discovery 18: 553–566. https://doi.org/10.1038/s41573-019-0025-4.
Naik, S., S. B. Larsen, N. C. Gomez, K. Alaverdyan, A. Sendoel, S. Yuan, L. Polak, A. Kulukian, S. Chai, and E. Fuchs. 2017. Inflammatory memory sensitizes skin epithelial stem cells to tissue damage. Nature 550: 475–480. https://doi.org/10.1038/nature24271.
Nankabirwa, V., Tumwine, J. K., Mugaba, P. M., Tylleskär, T., Sommerfelt, H., and Group, P. E. S. 2015. Child survival and BCG vaccination: a community based prospective cohort study in Uganda. BMC Public Health 15: 1-10. https://doi.org/10.1186/s12889-015-1497-8.
Netea, M. G., and J. W. Van Der Meer. 2017. Trained immunity: An ancient way of remembering. Cell Host & Microbe 21: 297–300. https://doi.org/10.1016/j.chom.2017.02.003.
Netea, M. G., J. Quintin, and J. W. Van Der Meer. 2011. Trained immunity: A memory for innate host defense. Cell Host, and Microbe 9: 355–361. https://doi.org/10.1016/j.chom.2011.04.006.
Netea, M. G., L. A. Joosten, and J. W. Van Der Meer. 2017. Hypothesis: Stimulation of trained immunity as adjunctive immunotherapy in cancer. Journal of Leukocyte Biology 102: 1323–1332. https://doi.org/10.1189/jlb.5RI0217-064RR.
Netea, M. G., J. Domínguez-Andrés, L. B. Barreiro, T. Chavakis, M. Divangahi, E. Fuchs, L. A. Joosten, J. W. Van Der Meer, M. M. Mhlanga, and W. J. Mulder. 2020. Defining trained immunity and its role in health and disease. Nature Reviews Immunology 20: 375–388. https://doi.org/10.1038/s41577-020-0285-6.
Netea, M. G., Joosten, L. A., Latz, E., Mills, K. H., Natoli, G., Stunnenberg, H. G., O’neill, L. A., and Xavier, R. J. 2016. Trained immunity: a program of innate immune memory in health and disease. Science, 352, aaf1098. https://doi.org/10.1126/science.aaf1098
Novakovic, B., E. Habibi, S. Y. Wang, R. J. Arts, R. Davar, W. Megchelenbrink, B. Kim, T. Kuznetsova, M. Kox, and J. Zwaag. 2016. β-Glucan reverses the epigenetic state of LPS-induced immunological tolerance. Cell 167 (1354–1368): e14. https://doi.org/10.1016/j.cell.2016.09.034.
Ochando, J., W. J. Mulder, J. C. Madsen, M. G. Netea, and R. Duivenvoorden. 2023. Trained immunity—basic concepts and contributions to immunopathology. Nature Reviews Nephrology 19: 23–37. https://doi.org/10.1038/s41581-022-00633-5.
Oh, W. K., S. Kim, M. Choi, C. Kim, Y. S. Jeong, B. R. Cho, J. S. Hahn, and J. Jang. 2010. Cellular uptake, cytotoxicity, and innate immune response of silica− titania hollow nanoparticles based on size and surface functionality. ACS Nano 4: 5301–5313. https://doi.org/10.1021/nn100561e.
Paris, S., L. Chapat, N. Martin-Cagnon, P. Y. Durand, L. Piney, C. Cariou, P. Bergamo, J. M. Bonnet, H. Poulet, and L. Freyburger. 2020. β-glucan as trained immunity-based adjuvants for rabies vaccines in dogs. Frontiers in Immunology 11: 564497. https://doi.org/10.3389/fimmu.2020.564497.
Peignier, A., and D. Parker. 2020. Trained immunity and host-pathogen interactions. Cellular Microbiology 22: e13261. https://doi.org/10.1111/cmi.13261.
Perzolli, A., Koedijk, J. B., Zwaan, C. M., and Heidenreich, O. 2024. Targeting the innate immune system in pediatric and adult AML. Leukemia, 1–11. https://doi.org/10.1038/s41375-024-02217-7
Petit, J., and G. F. Wiegertjes. 2016. Long-lived effects of administering β-glucans: Indications for trained immunity in fish. Developmental, and Comparative Immunology 64: 93–102. https://doi.org/10.1016/j.dci.2016.03.003.
Quintin, J., S. Saeed, J. H. Martens, E. J. Giamarellos-Bourboulis, D. C. Ifrim, C. Logie, L. Jacobs, T. Jansen, B. J. Kullberg, and C. Wijmenga. 2012. Candida albicans infection affords protection against reinfection via functional reprogramming of monocytes. Cell Host, and Microbe 12: 223–232. https://doi.org/10.1016/j.chom.2012.06.006.
Quintin, J., S. C. Cheng, J. W. Van Der Meer, and M. G. Netea. 2014. Innate immune memory: Towards a better understanding of host defense mechanisms. Current Opinion in Immunology 29: 1–7. https://doi.org/10.1016/j.coi.2014.02.006.
Redelman-Sidi, G., M.S. Glickman, and B.H. Bochner. 2014. The mechanism of action of BCG therapy for bladder cancer—a current perspective. Nature Reviews Urology 11: 153–162. https://doi.org/10.1038/nrurol.2014.15.
Rizzetto, L., D. C. Ifrim, S. Moretti, N. Tocci, S. C. Cheng, J. Quintin, G. Renga, V. Oikonomou, C. De Filippo, and T. Weil. 2016. Fungal chitin induces trained immunity in human monocytes during cross-talk of the host with Saccharomyces cerevisiae. Journal of Biological Chemistry 291: 7961–7972. https://doi.org/10.1074/jbc.M115.699645.
Rosati, D., Pradhan, A., Van Heck, J. I., Helder, L., Jaeger, M., Gow, N. A., Joosten, L. A., Williams, D. L., Brown, A. J., and Bruno, M. 2024. Candida albicans N-Linked Mannans Potentiate the Induction of Trained Immunity via Dectin-2. The Journal of Infectious Diseases, jiae112. https://doi.org/10.1093/infdis/jiae112
Saeed, S., J. Quintin, H. H. Kerstens, N. A. Rao, A. Aghajanirefah, F. Matarese, S. C. Cheng, J. Ratter, K. Berentsen, and M. A. Van Der Ent. 2014. Epigenetic programming of monocyte-to-macrophage differentiation and trained innate immunity. Science 345: 1251086. https://doi.org/10.1126/science.1251086.
Salam, A. P., A. Borsini, and P. A. Zunszain. 2018. Trained innate immunity: A salient factor in the pathogenesis of neuroimmune psychiatric disorders. Molecular Psychiatry 23: 170–176. https://doi.org/10.1038/mp.2017.186.
Schleicher, U., K. Paduch, A. Debus, S. Obermeyer, T. König, J.C. Kling, E. Ribechini, D. Dudziak, D. Mougiakakos, and P.J. Murray. 2016. TNF-mediated restriction of arginase 1 expression in myeloid cells triggers type 2 NO synthase activity at the site of infection. Cell Reports 15: 1062–1075. https://doi.org/10.1016/j.celrep.2016.04.001.
Sun, S., and L. B. Barreiro. 2020. The epigenetically-encoded memory of the innate immune system. Current Opinion in Immunology 65: 7–13. https://doi.org/10.1016/j.coi.2020.02.002.
Sun, J. C., and L. L. Lanier. 2011. NK cell development, homeostasis and function: Parallels with CD8+ T cells. Nature Reviews Immunology 11:645–657. https://doi.org/10.1038/nri3044.
Tercan, H., N. P. Riksen, L. A. Joosten, M. G. Netea, and S. Bekkering. 2021. Trained immunity: long-term adaptation in innate immune responses. Arteriosclerosis, Thrombosis, and Vascular Biology 41:55–61. https://doi.org/10.1161/ATVBAHA.120.314212.
Tso, G. H. W., J. A. Reales-Calderon, A. S. M. Tan, X. Sem, G. T. T. Le, T. G. Tan, G. C. Lai, K. Srinivasan, M. Yurieva, and W. Liao. 2018. Experimental evolution of a fungal pathogen into a gut symbiont. Science 362: 589–595. https://doi.org/10.1126/science.aat0537.
Van Leent, M. M., B. Priem, D. P. Schrijver, A. De Dreu, S. R. Hofstraat, R. Zwolsman, T. J. Beldman, M. G. Netea, and W. J. Mulder. 2022. Regulating trained immunity with nanomedicine. Nature Reviews Materials 7: 465–481. https://doi.org/10.1038/s41578-021-00413-w.
Van Puffelen, J. H., S. T. Keating, E. Oosterwijk, A. G. Van Der Heijden, M. G. Netea, L. A. Joosten, and S. H. Vermeulen. 2020. Trained immunity as a molecular mechanism for BCG immunotherapy in bladder cancer. Nature Reviews Urology 17: 513–525. https://doi.org/10.1038/s41585-020-0346-4.
Vetvicka, V., P. Sima, and L. Vannucci. 2021. Trained immunity as an adaptive branch of innate immunity. International Journal of Molecular Sciences 22: 10684. https://doi.org/10.3390/ijms221910684.
Villumsen, M., S. Sørup, T. Jess, H. Ravn, T. Relander, J. L. Baker, C. S. Benn, T. I. Sørensen, P. Aaby, and A. Roth. 2009. Risk of lymphoma and leukaemia after bacille Calmette-Guérin and smallpox vaccination: A Danish case-cohort study. Vaccine 27: 6950–6958. https://doi.org/10.1016/j.vaccine.2009.08.103.
Walk, J., L. C. J. De Bree, W. Graumans, R. Stoter, G. J. Van Gemert, M. Van De Vegte-Bolmer, K. Teelen, C. C. Hermsen, R. J. Arts, and M. C. Behet. 2019. Outcomes of controlled human malaria infection after BCG vaccination. Nature Communications 10: 1–8. https://doi.org/10.1038/s41467-019-08659-3.
Walker, S., S. Durham, S. Till, G. Roberts, C. Corrigan, S. Leech, M. Krishna, R. Rajakulasingham, A. Williams, and J. Chantrell. 2011. Immunotherapy for allergic rhinitis. Clinical, and Experimental Allergy 41: 1177–1200. https://doi.org/10.1111/j.1365-2222.2011.03794.x.
Xing, Z., S. Afkhami, J. Bavananthasivam, D. K. Fritz, M. R. D’agostino, M. Vaseghi-Shanjani, Y. Yao, and M. Jeyanathan. 2020. Innate immune memory of tissue-resident macrophages and trained innate immunity: Re-vamping vaccine concept and strategies. Journal of Leucocyte Biology 108: 825–834. https://doi.org/10.1002/JLB.4MR0220-446R.
Yadav, T. C., and A. Bachhuka. 2023. Tuning foreign body response with tailor-engineered nanoscale surface modifications: Fundamentals to clinical applications. Journal of Materials Chemistry B 11: 7834–7854. https://doi.org/10.1039/D3TB01040F.
Yi, M., T. Li, M. Niu, Q. Mei, B. Zhao, Q. Chu, Z. Dai, and K. Wu. 2023. Exploiting innate immunity for cancer immunotherapy. Molecular Cancer 22: 187. https://doi.org/10.1186/s12943-023-01885-w.
Zahalka, S., P. Starkl, M. L. Watzenboeck, A. Farhat, M. Radhouani, F. Deckert, A. Hladik, K. Lakovits, F. Oberndorfer, and C. Lassnig. 2022. Trained immunity of alveolar macrophages requires metabolic rewiring and type 1 interferon signaling. Mucosal Immunology 15: 896–907. https://doi.org/10.1038/s41385-022-00528-5.
Zhang, Q., and X. Cao. 2021. Epigenetic remodeling in innate immunity and inflammation. Annual Review of Immunology 39: 279–311. https://doi.org/10.1146/annurev-immunol-093019-123619.
Zhang, B., B. Chassaing, Z. Shi, R. Uchiyama, Z. Zhang, T.L. Denning, S. E. Crawford, A. J. Pruijssers, J. A. Iskarpatyoti, and M. K. Estes. 2014. Prevention and cure of rotavirus infection via TLR5/NLRC4–mediated production of IL-22 and IL-18. Science 346: 861–865. https://doi.org/10.1126/science.1256999.
Zhang, C., Yin, J., Zheng, J., Xiao, J., Hu, J., Su, Y., Zhou, K., Zhang, Y., Zhang, X., and Zhang, H. 2021. EZH2 identifies the precursors of human natural killer cells with trained immunity. Cancer Biology , and Medicine, 18: 1021. https://doi.org/10.20892/j.issn.2095-3941.2020.0791.
Funding
No external funding was received to conduct this study.
Author information
Authors and Affiliations
Contributions
MS conceptualized and designed the study. MS and SKN wrote the initial draft of the manuscript. MS, MGH, SS, QZ and CZ critically reviewed and revised the manuscript. All the authors approved the final version.
Corresponding authors
Ethics declarations
Ethics approval and consent to participate
It is a review manuscript. Therefore, ethical approval and consent are not needed.
Competing interests
All the authors declare that they have no conflicts of interest regarding the study. Author Chunfu Zheng was not involved in the journal’s review or decisions related to this manuscript.
Additional information
Communicated by Ling Zhao (Associate Editor).
Publisher’s Note
Springer Nature remains neutral with regard to jurisdictional claims in published maps and institutional affiliations.
Rights and permissions
Open Access This article is licensed under a Creative Commons Attribution 4.0 International License, which permits use, sharing, adaptation, distribution and reproduction in any medium or format, as long as you give appropriate credit to the original author(s) and the source, provide a link to the Creative Commons licence, and indicate if changes were made. The images or other third party material in this article are included in the article's Creative Commons licence, unless indicated otherwise in a credit line to the material. If material is not included in the article's Creative Commons licence and your intended use is not permitted by statutory regulation or exceeds the permitted use, you will need to obtain permission directly from the copyright holder. To view a copy of this licence, visit http://creativecommons.org/licenses/by/4.0/. The Creative Commons Public Domain Dedication waiver (http://creativecommons.org/publicdomain/zero/1.0/) applies to the data made available in this article, unless otherwise stated in a credit line to the data.
About this article
Cite this article
Salauddin, M., Nath, S.K., Saha, S. et al. Trained immunity: a revolutionary immunotherapeutic approach. Animal Diseases 4, 31 (2024). https://doi.org/10.1186/s44149-024-00138-7
Received:
Accepted:
Published:
DOI: https://doi.org/10.1186/s44149-024-00138-7