Abstract
Background
Steatotic liver disease (SLD) is a prevalent metabolic disease. While single component movement behaviors have been related to its development, comprehensive assessments of their joint associations are scarce.
Objective
To investigate the single-component and multi-component associations of moderate and vigorous physical activity (MVPA), light physical activity (LPA), sedentary behavior (SB), and sleep with prevalent SLD in Brazilian adults.
Methods
A cross-sectional analysis using data from the third wave of the ELSA-Brasil cohort (2017–2019). Participants wore an ActiGraph wGT3X-BT in the waist for seven days and completed a sleep diary. SLD was defined by a Fatty Liver Index ≥ 60. To investigate single-component and multi-component associations, we used three exposure modeling approaches based on Poisson models: multivariable-adjusted regression, restricted cubic splines, and compositional data analysis.
Results
Among 8569 participants (55.7% women, mean age 59.2 ± 8.60), 43.9% had SLD. Total activity volume adjusted for covariates was inversely associated with prevalent SLD. Every 1 mg/day increase in total activity volume was associated with a PR of 0.95 in individuals sleeping < 7 h/day (95% CI 0.94–0.97) and 0.95 (95% CI 0.93–0.96) in those sleeping ≥ 7 h/day. Increasing 30 min/day of MVPA was associated with decreasing prevalence of SLD (sleep ≥ 7 h/day [PR 0.83; 95% CI 0.77–0.89]; sleep ≥ 7 h/day [PR 0.78; 95% CI 0.74–0.83]). Sleep, SB, and LPA were not associated with SLD. Associations of total activity volume and MVPA were more pronounced among females. Adjustment with adiposity markers attenuated the associations.
Conclusions
In adults, total activity volume and MVPA were inversely associated with SLD in a dose–response fashion. Substituting lower-intensity behaviors with MVPA was associated with a lower prevalence of SLD independent of sleep duration, sex, and age.
Similar content being viewed by others
Background
Steatotic Liver Disease (SLD) [1, 2] is a prevalent liver condition affecting up to 30% of the population [3, 4]. Its underlying pathophysiology concerns increased hepatic and peripheric insulin resistance, which shares common ground with obesity and type 2 diabetes mellitus (T2DM) [5, 6]. Due to its associated complications, such as liver fibrosis, cirrhosis, and liver cancer, it poses a significant global public health problem [7, 8].
The cornerstone of SLD prevention and management relies on lifestyle measures and weight management [7, 9]. For instance, engaging in moderate and vigorous physical activity (MVPA) lowers the risk of mortality in those with SLD by up to 50% [10]. Conversely, prolonged sedentary behavior (SB) is associated with a higher prevalence of the disease [11,12,13,14]. Sleep patterns are also associated with SLD, albeit with an unclear nature [15,16,17,18].
Studies investigating the associations of device-measured movement behaviors with the occurrence of SLD are scarce [11, 14, 19]. The use of accelerometers allowed for investigations on the effect of movement behaviors on health to consider their multicomponent 24-h pattern rather than single-component behaviors [20,21,22]. Previous studies on this matter focused mostly on single-component movement behaviors, often with limited sample sizes and restricted to Asian and European populations [11, 14, 19]. Furthermore, the influence of sleep duration, age, and sex in these associations has been little explored. In this sense, the large sample size of the ELSA-Brasil study allows for the investigation of possible modifiers of these associations. It also enables the evaluation of the dose–response gradient for movement behaviors and SLD, as well as the theoretical effects of exchanging behaviors, which can help the design of effective interventions.
Thus, our cross-sectional study aims at producing a comprehensive assessment of the association of 24-h movement behaviors with SLD by investigating (1) the dose–response associations of single-component device-measured movement behaviors with SLD; (2) the association of the composition of movement behaviors with SLD prevalence; (3) which theoretical substitutions between movement behaviors could yield maximal benefit in reducing the prevalence of SLD; and (4) the influence of age, sex, and sleep duration on these associations.
Methods
Study design
The Estudo Longitudinal de Saúde do Adulto (Longitudinal Study of Adults’ Health - ELSA-Brasil) is a multicenter cohort investigating noncommunicable disease risk factors in Brazilian adults [23, 24]. After a wide and open invitation to potential participants, we enrolled 15,105 active and retired civil servants of all ranges of occupations from public research, education, and healthcare organizations in six Brazilian states.
At baseline (2008 to 2010), volunteers were aged 35–74. Participants signed an informed consent form, underwent a thorough health assessment, and provided blood and urine samples [25, 26]. For this paper, we analyzed cross-sectional data from participants of wave 3 (2017 to 2019), encompassing 12,636 eligible participants (550 deceased and 1919 lost during follow-up).
Measurements
We obtained overnight 12-h fasting blood samples through venipuncture and conducted a 2-h 75 g oral glucose tolerance test (OGTT) in participants without a prior diagnosis of diabetes [27]. Plasma glucose, glycated hemoglobin (HbA1c), aspartate aminotransferase, alanine transaminase, gamma-glutamyl transferase, triglycerides, and high density lipoprotein were measured in a centralized laboratory [28].
Anthropometric data were collected using a fixed stadiometer (Seca 216, Seca Brasil, Cotia-SP, Brazil) for height and a bioelectrical impedance analyzer (BIA; Inbody230, InBody Co., Ltd., Seoul, Korea) with 8-point tactile electrodes for weight and body composition. Body Mass Index (BMI) was calculated as weight divided by height squared (kg/M1), and body fat percentage was determined as the total fat mass divided by participants’ weight [29]. Waist circumference was the average of two measures between the right iliac crest and the 12th right rib using an inelastic measurement tape.
Blood pressure was measured three times using an oscillometer device (Omron HEM 705CPINT) on the right arm in a quiet room with controlled temperature (20–24 °C) after 5 min of sitting rest [25]. Diabetes classification included previous physician diagnosis; glucose-lowering medication use in the last two weeks (cross-verified against prescriptions) or one laboratory abnormality: fasting plasma glucose ≥ 126 mg/dL (7.0 mmol/L) or 2 h post-load plasma glucose ≥ 200 mg/dL (11.1 mmol/L) or glycated hemoglobin ≥ 6.5% (48 mmol/mol). [30, 31] Certified technicians conducted all measurements following standardized protocols [24].
Movement behaviors
Time in MVPA, LPA, and SB were assessed over 7 days using a waist-worn ActiGraph GT3X + (version 3.2.1, Pensacola, USA). Participants were instructed to wear the devices throughout the 24 h of the day, only removing them when doing activities involving water. We averaged participants’ daily movement behaviors across all valid days of participants with validated wear (≥ 4 days, ≥ 16 h/day) [32,33,34]. We determined the triaxial summary acceleration as the average Euclidean Norm Minus One (ENMO = \(\sqrt{{x}^{2}+{y}^{2}+ {z}^{2}}- 1g\)) for each 5-s epoch. Total activity volume was calculated as the average acceleration during wear. Movement behaviors were defined according to validated thresholds. Specifically, MVPA was characterized by acceleration > 69 mg, LPA 69–15 mg, and SB < 15 mg during wake time [35]. Sleep was calculated using a sleep diary filled by the participant during the same week of the accelerometer use. We then calculated the difference in minutes from the sleep time to the next waking time [36,37,38]. Details an these procedures were previously published [38]. Movement behavior durations were described as arithmetic means, standard deviations (SD), and geometric means.
SLD
We defined SLD as a Fatty Liver Index (FLI) ≥ 60, a composite index including BMI, waist circumference (WC), triglycerides (TG), and gamma-glutamyl transferase (γGT) to predict steatotic liver. The index was calculated as shown below [39].
Validated against abdominal ultrasound, it achieved an area under the curve (AUC) receiver operator characteristic (ROC) of 0.85 (95% CI 0.82 to 0.88). A score of ≥ 60 yielded a positive likelihood ratio (+ LR) of 4.3 [39]. The instrument was assessed against ultrasound in the ELSA-Brasil sample, reaching an area under the curve receiver operator characteristic of 0.82 (95% CI 0.8 to 0.84) and a positive likelihood ratio of 2.93 for moderate steatosis using the threshold of 60 [40].
Covariates
Covariates included study center, age, sex, degree of schooling, household income, employment status (working/retired), smoking status (never, former, current), use of antihypertensive, cholesterol-lowering, and glucose-lowering medication. Total energy intake, estimated from a validated food frequency questionnaire [41], and alcohol consumption in milliliters per week were also considered. Alcohol use was categorized as never, former, or current, with a description of frequency and volume of consumption. Excessive alcohol consumption was defined as ≥ 140 g/week for females and ≥ 210 g/week for males [7, 9].
Race/color was self-declared and grouped using pre-defined categories reflecting sociodemographic similarities particular to the Brazilian population. Individuals identifying as “yellow” (Asian descent), due to small numbers (n = 208), were grouped with white individuals given their socioeconomic similarities [42]. Similarly, “indigenous” (n = 65) were analyzed alongside black and brown individuals, considering their shared historic social vulnerability in Brazil [43].
Statistical analyses
We utilized robust Poisson regression to investigate the associations of single-component movement behaviors with prevalent SLD, estimating prevalence ratios (PR). Cubic splines of single-component movement behaviors were constructed to assess whether the association expressed a linear dose–response relationship or reached a plateau in prevalence reduction. We placed knots at each group’s 10th, 50th, and 90th percentiles, setting the lowest observed exposure level as the reference category for total activity volume and MVPA, and the 5% percentile for LPA and SB. For sleep, the reference was set at 7 h based on international recommendations [44]. We employed compositional data analysis (CoDA) to evaluate the association of the 24-h composition of movement behaviors with the prevalence of SLD [45,46,47,48]. Using compositional models, we estimated the change in SLD prevalence with reallocations of time between behaviors through isotemporal substitution predictions [45, 49]. Supplementary methods are provided in the additional file, along with detailed information on the procedures. Models were adjusted as follows: Model 1 (fully adjusted) study center, age, sex, race/color, degree of schooling, income quintiles, employment, smoking status, alcohol consumption, hypertension, diabetes, and total energy intake. Because BMI might mediate the association between movement behaviors and SLD, our primary analyses are presented without adjustment for BMI. However, adiposity may confound this association, so alternative models are presented including BMI (Model 2a) and percent body fat (%BF) (Model 2b) to Model 1. We chose percent body fat as an alternative measure to mitigate the risk of overfitting the models due to the presence of BMI in defining the outcome (FLI ≥ 60).
We presented results stratified based on achieving more or less than 7 h/day of sleep [22, 50]. In addition, we assessed age and sex as possible modifiers given previous knowledge. Briefly, physical activity and sedentary behavior play a central role in insulin resistance, which increases with aging and leads to metabolic diseases [51]. Furthermore, as individuals age, they tend to develop a less active and more sedentary movement behavior profile [38], which also raises the risk of metabolic diseases. We also evaluated the role of sex in the associations since women usually have less active movement behaviors [38], while men more often develop metabolic diseases. Finally, physiological variability related to age and sex, such as body fat distribution and hormonal differences [52], may also alter the movement behaviors associations with SLD. To assess these potential effect modifications we tested interactions for movement behaviors with sex and age by additionally inserting terms of each ILR with the potentially modifier variables in the models. We considered the interaction worth further investigation if their p-value was lower than 0.2.
Sensitivity analyses included (1) subclassifying SLD in metabolic dysfunction-associated steatotic liver disease (MASLD) using the consensus diagnostic criteria [2] (Supplementary methods, additional file); (2) excluding 534 participants with excessive alcohol consumption that would be classified as metabolic-alcoholic steatotic liver disease (MetALD). Figures for the stratified analysis and sensitivity analyses are provided in the Supplementary file).
We set the significance level at p < 0.05 (two-sided), and estimates are reported with 95% confidence intervals (95% CI). Convergence of the Poisson models was evaluated, and residuals were visually inspected. Collinearity was assessed using the Generalized Variance Inflation Factor (GVIF); a threshold of 2.5 was considered possible collinearity. All data processing and analyses were conducted using the R software version 4.2.1. (R Core Team, 2021, The R Foundation for Statistical Computing, Vienna, Austria). We reported the results according to the STROBE Statement [53]. The checklist is available in the additional file.
Results
After excluding participants with missing data, the analytical sample included 8569 subjects (Fig. 1) with a mean age of 59.2 (8.6) years. As shown in Table 1, there were 3764 (43.9%) prevalent cases of SLD, with higher prevalence among men (p < 0.001), black subjects (p < 0.001), and those with lower education (p < 0.001) and income (p < 0.001).
Individuals with SLD had more metabolic dysfunctions, higher BMI, and a higher prevalence of T2DM (Supplementary Table 1). On average (SD) time engaged in MVPA, LPA, SB, and sleep were respectively 47.4 (25.3) minutes/day, 207 (67.8) minutes /day, 736.8 (99.6) minutes /day, and 448.2 (71.4) minutes /day (Supplementary Table 2). Crude movement distribution differences between participants with and without SLD were minimal (Supplementary Fig. 1).
Single-component behaviors and SLD
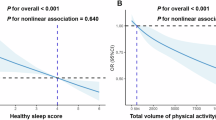
Table 2 shows the associations of total activity volume and movement behaviors with prevalent SLD. A 1mili-g increase in total activity volume was linked to a 3 to 4% lower prevalence of SLD (model 1—sleep < 7 h/day: PR 0.97, 95% CI 0.95 to 0.98; sleep ≥ 7 h/day: PR 0.96, 95% CI 0.95 to 0.98). Decreasing PRs of SLD were observed across the entire range of total activity volume, with a steeper decline from 0 to 10 mg/day (Fig. 2A and B). Incorporating adiposity measures nullified associations in short sleepers. Non-short sleepers had a lower prevalence of SLD with total activity volume exceeding 17 milli-g/day after adjusting for %BF (Model 2b) but not for BMI (Model 2a).
Dose–response associations of physical activity with steatotic liver disease by sleep duration, ELSA-Brasil (2017–2019), n = 8569. Prevalence Ratios (PR) for Steatotic liver disease (SLD) were estimated with Poisson regression models using restricted cubic splines with knots placed at 10th, 50th, and 90th percentiles of the exposure and adjusted for—M1 (blue): study center, sex, age, race/color, income, degree of schooling, smoking, alcohol consumption, diabetes, hypertension, and daily energy intake; M2a (yellow): M1 and further adjustment for body mass index. M2b (red): M1 and further adjustment for % body fat. Continuous lines are the point estimates of PR across the exposure spectra, and the colored hatched area is the 95% confidence interval. The histograms show the sample distribution on the exposure spectra, with the right vertical axis showing the percentage of the study sample. MVPA, moderate-and-vigorous physical activity
Inverse associations between MVPA and SLD were observed regardless of sleep duration (Table 2). Additional 30 min/day of MVPA were associated with PRs of 0.85 (95% CI 0.79 to 0.92) in short sleepers and 0.83 (95% CI 0.78 to 0.87) in non-short sleepers. As depicted in Fig. 2, PRs declined throughout the entire MVPA spectrum. Associations were eliminated among short sleepers when adiposity measures were added. (Fig. 2). In non-short sleepers, adjusting for BMI (Model 3a) nullified associations, while adjusting for %BF (Model 3b) attenuated the association. In this group, the %BF model showed a lower SLD prevalence only with high engagement on MVPA, approximately 70 min/day (Fig. 2D).
We did not observe a significant association of LPA, SB, and sleep duration with the prevalence of SLD in the adjusted models. Table 2 However, in the splines, the group sleeping ≥ 7 h a day showed a slightly lower prevalence of SLD with higher LPA. Additionally, both sleep duration groups expressed a higher prevalence of SLD with very high engagement in sedentary behavior. All these results vanished with adjustment for body fat markers Fig. 2.
Compositional isotemporal substitution and SLD
Figure 3 illustrates the theoretical impacts of bidirectionally reallocating time between movement behaviors. Among short sleepers, substituting LPA or SB with MVPA was linked to a lower prevalence of SLD, regardless of BMI or %BF adjustments. Moreover, reallocating sleep to MVPA was associated with a lower prevalence of SLD with the adjusted model (Model 1) but not after incorporating body adiposity (Models 2a and 2b). For non-short sleepers, reallocating as little as 1 min from other MBs to MVPA was associated with decreased prevalence of SLD irrespective of including %BF, but not with adjustment for BMI. Conversely, reducing MVPA in favor of other movement behaviors was associated with a raised prevalence of SLD in all models. For instance, exchanging 30 min of SB for MVPA resulted in an 11% lower prevalence of SLD (Model 1 – sleep < 7 h/day 0.89 [95% CI 0.86 to 0.92] and sleep ≥ 7 h/day PR 0.89 [95% CI 0.87 to 0.91]), while swapping 30 min of MVPA with SB was associated with a 20 to 37% higher prevalence of SLD (sleep < 7 h/day PR 1.29 [95% CI 1.20 to 1.39]; sleep ≥ 7 h/day PR 1.37 [95% CI 1.28 to 1.46]). No significant associations were observed in the bidirectional substitutions between other movement behaviors.
Exchanging movement behaviors association with prevalent steatotic liver disease by sleep duration ELSA-Brasil (2017–2019), n = 8569. Plots show the predicted prevalence ratios (PR) of Steatotic liver disease (SLD) resulting from reallocating time between movement behaviors using compositional isotemporal substitution robust Poisson models. The vertical continuous black line (x = 0) represents the mean of behaviors of the group as the reference—A = Sleep < 7 h: MVPA: 44.92 min/day, LPA: 209.0 min/day, SB: 803.8 min/day, SLP: 382.2 min/day; B = Sleep ≥ 7 h: MVPA: 38.2 min/day, LPA: 194.8 min/day, SB: 714.0 min/day, SLP: 493.0 min/day. The two behaviors not shown in each plot have their values fixed at the geometric mean for the analysis group. MVPA = moderate and vigorous physical activity; LPA = light physical activity; SB = sedentary behavior. Models were adjusted as follows—Model 1 (M1, blue): study center, sex, age, race/color, income, degree of schooling, smoking, alcohol consumption, diabetes, hypertension, and daily energy intake; Model 2a (M2a, yellow): M1 and further adjustment for body mass index. Model 2b (M2b, red): M1 and further adjustment for % body fat
Subgroup analyses
There was little evidence for a relevant interaction between age and the composition of movement behaviors in relation to the prevalence of SLD (p > 0.2). We observed an interaction between movement behavior composition and sex (p < 0.01), revealing stronger associations in females compared to males (Supplementary Tables 3 and 4). Associations were attenuated in all groups upon adjusting for adiposity measures.
In females sleeping ≥ 7 h/day, when including %BF, a lower prevalence of SLD was observed starting from 16.5 mg/day of total activity volume, the higher 5% of the sample distribution (Supplementary Fig. 2). The same trend was noted for MVPA, with females showing a more substantial association than males (Supplementary Tables 3 and 4). Sex-specific cubic splines are presented in Supplementary Figs. 2 and 3. In compositional models (Supplementary Figs. 4 and 5), females exhibited a more pronounced decrease in the prevalence of SLD with increasing MVPA at the cost of any other MB compared to males. Furthermore, individuals from both sexes in the group with sleep ≥ 7 h/day showed a larger decrease in the prevalence of SLD with increasing MVPA. Lastly, including BMI in the models eliminated associations, while adjustment for %BF did not eliminate associations in males sleeping ≥ 7 h/day. (Supplementary Figs. 4 and 5).
Sensitivity analyses
When applying the SLD subclassification of MASLD, we identified 3762 participants with the condition. Both sensitivity analyses were consistent with the previous results. (Supplementary tables 5 & 6 and Supplementary Figs. 6 and 7). The analysis excluding the 534 participants with high alcohol consumption (187 in the short sleep and 347 in the non-short sleep group) produced results aligning with the primary analysis, demonstrating a larger association magnitude. Notably, in this scenario, including adiposity markers did not eliminate the associations. (Supplementary Table 7 and Supplementary Figs. 8 and 9).
Discussion
Our cross-sectional analyses of a large, multicenter, and ethnically diverse sample of adults explored the dose–response and compositional associations of device-measured movement behaviors with Steatotic Liver Disease. We observed that a 1 mg/day higher daily total activity volume was linked to a 5% lower prevalence of SLD. Previous studies suggest that 1 mg/day measured with wrist accelerometers translates to approximately 500 steps or 5 min of brisk walking a day [54,55,56,57]. Considering the lower acceleration magnitude of hip-worn accelerometers compared to wrist-worn accelerometers [58], it is reasonable to affirm that this value is somewhat higher for measurements in the hip, although no study has directly assessed this question.
Regarding movement behaviors, MVPA—but not LPA, SB, or sleep—exhibited a dose–response association with the prevalence of SLD regardless of sex and sleep duration. However, adjusting for %BF or BMI nullified associations for single-component behaviors. On the other hand, a theoretical increase in time in MVPA at the cost of any other behavior—LPA, SB, or sleep—was associated with a progressively lower prevalence of SLD. Interestingly, reducing MVPA by increasing the other MBs was linked to an asymmetrically higher prevalence of SLD.
Our study aligns with previously described inverse associations of total activity volume and MVPA with the prevalence of SLD, validating results obtained with self-reported and device-based PA measures [13, 14, 59,60,61,62,63,64]. Despite a well-established association, little evidence of a dose–response relationship was available before this study. Nonetheless, a similar study with a Japanese sample found a steep decline in the odds of ultrasound-measured SLD up to 300 weekly minutes (1800 MET-min/week) of device-measured MVPA [14]. Our diverse sample provides new evidence of a dose–response association between MVPA and SLD while considering the non-linear relationship between sleep and SLD and assessing the influence of sex, age, and sleep groups. Our findings also demonstrate a significant decrease in SLD prevalence throughout the whole spectra of exposure to MVPA. We observed a steeper decline in the lower range of exposure up to approximately 420 weekly minutes (60 min/day) of MVPA, supporting the World Health Organization's updated recommendation of 150 to 300 weekly minutes of moderate activity [65]. We still observed a decline in prevalence with exposure higher than 60 min a day, however, its magnitude was smaller than in lower exposure levels. Incorporating markers of adiposity (BMI or %BF) into our model attenuated all associations. However, total activity volume and MVPA associations with SLD and the substitutions remained significant when adjusting for %BF and vanished with BMI adjustment. Given that BMI is a component of the Fatty Liver Index, we argue that %BF adjustments provide better control for adiposity-related confounding. Whether this factor plays the role of a confounder or a mediator in such an association remains an unclear question. However, our analyses suggest that the association between MVPA and SLD is independent of adiposity, with exercise interventions supporting a direct mechanism for this association [66].
Despite participants spending more time in LPA than MVPA, our study did not find an association between LPA and SLD in individuals sleeping less than 7 h, but a slightly lower prevalence of SLD with higher LPA in those sleeping ≥ 7. Three recent cross-sectional studies using device-measured LPA showed inconsistent findings, with two describing an inverse association [11, 19] and a third not observing an association [14]. The evidence suggests that the volume and intensity of activity are essential for preventing this condition, but inconsistencies in cross-sectional studies necessitate further prospective investigations. This hypothesis is corroborated by clinical trials showing a beneficial effect of at least moderate intensity training in liver fat content [67] and an unclear effect of lower intensity modalities [68, 69].
Contrary to our expectations, we observed a higher prevalence of SLD only with very high levels of SB, aligning with other investigations that described a higher prevalence of SLD in the most sedentary participants [13, 63, 70]. One previous similar study did not observe this association [14]. Meanwhile, isotemporal substitution models revealed a direct association of SB with SLD if replaced by MVPA. Factors that explain the inconsistency across studies are a high amount of time in SB (12 h/day) and a small amount of MVPA (3% of the day) in our sample. Considering we observed a higher prevalence of SLD when substituting MVPA with SB, the associations other studies described could have resulted from even lower MVPA levels rather than higher SB levels. Also, the previous study investigating dose–response associations of SB with SLD did not consider sleep duration, which may have distorted associations as sleep duration is likely to have a non-linear association with SLD [15,16,17].
In our compositional analyses, we found that replacing any behavior with MVPA was associated with a decreased prevalence of SLD. Conversely, exchanging MVPA with any other movement behavior was associated with an increased prevalence of SLD, with varying magnitudes. Such results emphasize the importance of considering the total 24-h distribution of movement behaviors in health research, aligning with previous studies. For instance, studies using device-based and self-reported measures showed that replacing SB or LPA with MVPA lowered SLD odds while reallocating time from MVPA to SB or LPA increased SLD odds [14, 64]. Another study in rural Chinese older adults found lower SLD odds by substituting SB with LPA, not SB with MVPA, likely due to high LPA levels in their sample [11]. These findings enhance our understanding of how different movement behaviors influence SLD risk.
The results of the sensitivity analyses were highly consistent with the primary analysis. Exclusion of participants with MASLD and high alcohol consumption, defined as MetALD, reinforced the theoretical framework linking physical activity to metabolic diseases through insulin resistance improvement.
Our study has its limitations. The cross-sectional design limits causal inferences about the association, and its observational component produces the risk of bias due to unmeasured confounders. However, given the absence of longitudinal evidence and the lack of large, well-conducted clinical trials, our study enhances the understanding of the relationship between movement behaviors and the presence of SLD. Secondly, we could not evaluate SB’s postural component, so our operational definition of SB differs from the formal one [71]. However, we used validated thresholds to identify SB, which should address this issue. Also concerning movement behaviors, due to a lack of validated algorithms to identify sleep duration from raw accelerometer data, we have used self-reported measures for this movement behavior. While this approach may be susceptible to measurement bias due to its subjective nature, the detailed sleep diary used was filled during the same time of the accelerometer use, minimizing recall bias Thirdly, we defined SLD using serological markers. Nonetheless, the Fatty Liver Index was validated with satisfactory diagnostic properties and is extensively used in epidemiological research [39, 40]. In regards to the statistical analysis, because our outcome was defined by using the FLI, which includes BMI, adjustment for BMI may generate overfitting of the models. We alternatively adjusted the analyses for %BF, a validated measure of adiposity, to address this issue [29]. Lastly, the multiple testing conducted may raise concerns about type 1 errors.
Our study has notable strengths. Firstly, the ELSA-Brasil study is composed of a highly diverse sample regarding race/ethnicity, cultural, and socioeconomic background. Thus, albeit corroborating previous studies, we provide new insights by enhancing the generalizability to other locations and populations. Secondly, our results are highly reliable due to meticulously standardized data collection and stringent quality control procedures [25, 26]. Additionally, our use of 7-day accelerometry, a more precise approach than questionnaires to assess PA, is uncommon in large epidemiologic studies of low and middle-income countries. Thirdly, our sample size allowed for subgroup analyses, exploring potential effect modifiers such as sleep duration, age, and sex, a novel contribution. Lastly, we used the updated definition of SLD, aligning more closely with the pathophysiological framework and being defined with positive diagnostic criteria rather than using a diagnosis of exclusion.
Conclusion
In conclusion, total activity volume and MVPA were associated with SLD. MVPA exhibited a dose–response relationship, with a decreasing prevalence of SLD up to 420 min per week, aligning with current recommendations. Our compositional analysis highlights that any increase in MVPA correlates with a lower prevalence of SLD regardless of age, sex, sleep duration, and replaced behavior. The findings underscore the message that “every move counts” and emphasize the importance of considering the full 24-h distribution in health studies. Notably, this framework is pivotal when designing lifestyle interventions to address the growing burden of non-communicable diseases at the individual and population levels.
Availability of data and materials
Due to ethical restrictions approved by the ethics committee of each institution (Universidade Federal de Minas Gerais, Universidade de São Paulo, Universidade Federal do Espírito Santo, Universidade Federal do Rio Grande do Sul, Universidade Federal da Bahia e Fundação Oswaldo Cruz) and by the Publications Committee of ELSA-Brasil (publiELSA), the data used in this study can be made available for research proposals by a request to ELSA-Brasil's Data Center (estatisticaelsa@gmail.com) and to ELSA-Brasil's Publications Committee. Additional information can be obtained from the ELSA-Brasil Coordinator of the Research Center of Rio Grande do Sul (maria.schmidt@ufrgs.br).
Abbreviations
- + LR:
-
Positive likelihood ratio
- %BF:
-
Percent body fat
- AUC:
-
Area under the curve
- BMI:
-
Body mass index
- CI:
-
Confidence interval
- ELSA-Brasil:
-
Estudo Longitudinal de Saúde do Adulto
- FLI:
-
Fatty liver index
- FPG:
-
Fasting plasma glucose
- GVIF:
-
Generalized Variance Inflation Factor
- HbA1c:
-
Glycated haemoglobin
- HDL:
-
High density lipoprotein
- LPA:
-
Ligth physical activity
- MASLD:
-
Metabolic dysfunction associated steatotic liver disease
- MB:
-
Movement behaviors
- MetALD:
-
Metabolic-alcoholic steatotic liver disease
- MVPA:
-
Moderate and vigorous physical activity
- NAFLD:
-
Nonalcoholic fatty liver disease
- OGTT:
-
Oral glucose tolerance test
- PR:
-
Prevalence ratios
- ROC:
-
Receiver operator characteristic
- SB:
-
Sedentary behavior
- SLD:
-
Steatotic liver disease
- STROBE:
-
Strengthening the reporting of observational studies in epidemiology
- T2DM:
-
Type 2 diabetes mellitus
- TG:
-
Triglycerides
- WC:
-
Waist circumference
- γGT:
-
Gamma-glutamyl transferase
References
Eslam M, Sanyal AJ, George J, et al. MAFLD: a consensus-driven proposed nomenclature for metabolic associated fatty liver disease. Gastroenterology. 2020;158:1999-2014.e1. https://doi.org/10.1053/j.gastro.2019.11.312.
Rinella ME, Lazarus JV, Ratziu V, et al. A multi-society Delphi consensus statement on new fatty liver disease nomenclature. Hepatology. 2023. https://doi.org/10.1097/HEP.0000000000000520.
Riazi K, Azhari H, Charette JH, et al. The prevalence and incidence of NAFLD worldwide: a systematic review and meta-analysis. Lancet Gastroenterol Hepatol. 2022;7:851–61. https://doi.org/10.1016/S2468-1253(22)00165-0.
Younossi ZM, Koenig AB, Abdelatif D, Fazel Y, Henry L, Wymer M. Global epidemiology of nonalcoholic fatty liver disease-meta-analytic assessment of prevalence, incidence, and outcomes. Hepatology. 2016;64:73–84. https://doi.org/10.1002/hep.28431.
Polyzos SA, Mantzoros CS. Making progress in nonalcoholic fatty liver disease (NAFLD) as we are transitioning from the era of NAFLD to dys-metabolism associated fatty liver disease (DAFLD). Metabolism. 2020;111: 154318. https://doi.org/10.1016/j.metabol.2020.154318.
Valenzuela-Vallejo L, Mantzoros CS. Time to transition from a negative nomenclature describing what NAFLD is not, to a novel, pathophysiology-based, umbrella classification of fatty liver disease (FLD). Metabolism. 2022;134: 155246. https://doi.org/10.1016/j.metabol.2022.155246.
Cusi K, Isaacs S, Barb D, et al. American association of clinical endocrinology clinical practice guideline for the diagnosis and management of nonalcoholic fatty liver disease in primary care and endocrinology clinical settings: co-sponsored by the american association for the study of liver diseases (AASLD). Endocr Pract. 2022;28:528–62. https://doi.org/10.1016/j.eprac.2022.03.010.
Chalasani N, Younossi Z, Lavine JE, et al. The diagnosis and management of nonalcoholic fatty liver disease: practice guidance from the American association for the study of liver diseases. Hepatology. 2018;67:328–57. https://doi.org/10.1002/hep.29367.
European Association for the Study of the Liver (EASL), European Association for the Study of Diabetes (EASD), European Association for the Study of Obesity (EASO). EASL-EASD-EASO clinical practice guidelines for the management of non-alcoholic fatty liver disease. J Hepatol. 2016;64:1388–402. https://doi.org/10.1016/j.jhep.2015.11.004.
Kim D, Murag S, Cholankeril G, et al. Physical activity, measured objectively, is associated with lower mortality in patients with nonalcoholic fatty liver disease. Clin Gastroenterol Hepatol. 2021;19:1240-1247.e5. https://doi.org/10.1016/j.cgh.2020.07.023.
Han Q, Han X, Wang X, et al. Association of accelerometer-measured sedentary behavior patterns with nonalcoholic fatty liver disease among older adults: the MIND-China study. Am J Gastroenterol. 2023;118:569–73. https://doi.org/10.14309/ajg.0000000000002135.
Croci I, Coombes JS, Bucher Sandbakk S, et al. Non-alcoholic fatty liver disease: Prevalence and all-cause mortality according to sedentary behaviour and cardiorespiratory fitness. The HUNT Study. Prog Cardiovasc Dis. 2019;62:127–34. https://doi.org/10.1016/j.pcad.2019.01.005.
Kim D, Vazquez-Montesino LM, Li AA, Cholankeril G, Ahmed A. Inadequate physical activity and sedentary behavior are independent predictors of nonalcoholic fatty liver disease. Hepatology. 2020;72:1556–68. https://doi.org/10.1002/hep.31158.
Tsunoda K, Kitano N, Kai Y, Jindo T, Uchida K, Arao T. Dose–response relationships of accelerometer-measured sedentary behaviour and physical activity with non-alcoholic fatty liver disease. Aliment Pharmacol Ther. 2021;54:1330–9. https://doi.org/10.1111/apt.16631.
Shen N, Wang P, Yan W. Sleep duration and the risk of fatty liver disease: a systematic review and meta-analysis. Sci Rep. 2016;6:31956. https://doi.org/10.1038/srep31956.
Yang J, Zhang K, Xi Z, et al. Short sleep duration and the risk of nonalcoholic fatty liver disease/metabolic associated fatty liver disease: a systematic review and meta-analysis. Sleep Breath. 2022. https://doi.org/10.1007/s11325-022-02767-z.
Imaizumi H, Takahashi A, Tanji N, et al. The association between sleep duration and non-alcoholic fatty liver disease among Japanese men and women. Obes Facts. 2015;8:234–42. https://doi.org/10.1159/000436997.
Um YJ, Chang Y, Jung H-S, et al. Sleep duration, sleep quality, and the development of nonalcoholic fatty liver disease: a cohort study. Clin Transl Gastroenterol. 2021;12: e00417. https://doi.org/10.14309/ctg.0000000000000417.
van Kleef LA, Hofman A, Voortman T, de Knegt RJ. Objectively measured physical activity is inversely associated with nonalcoholic fatty liver disease: the Rotterdam study. Am J Gastroenterol. 2022;117:311–8. https://doi.org/10.14309/ajg.0000000000001584.
Chaput J-P, Carson V, Gray C, Tremblay M. Importance of all movement behaviors in a 24 hour period for overall health. IJERPH. 2014;11:12575–81. https://doi.org/10.3390/ijerph111212575.
Rosenberger ME, Fulton JE, Buman MP, et al. The 24-hour activity cycle: a new paradigm for physical activity. Med Sci Sports Exerc. 2019;51:454–64. https://doi.org/10.1249/MSS.0000000000001811.
24-Hour Movement Guidelines—Canadian 24-Hour Movement Guidelines. 2022. https://csepguidelines.ca/. Accessed 12 Dec 2022.
Aquino EML, Barreto SM, Bensenor IM, et al. Brazilian longitudinal study of adult health (ELSA-Brasil): objectives and design. Am J Epidemiol. 2012;175:315–24. https://doi.org/10.1093/aje/kwr294.
Schmidt MI, Duncan BB, Mill JG, et al. Cohort profile: longitudinal study of adult health (ELSA-Brasil). Int J Epidemiol. 2015;44:68–75. https://doi.org/10.1093/ije/dyu027.
Mill JG, Pinto K, Griep RH, et al. Medical assessments and measurements in ELSA-Brasil. Rev Saude Pub. 2013;47:54–62. https://doi.org/10.1590/S0034-8910.2013047003851.
Schmidt MI, Griep RH, Passos VM, et al. Estratégias e desenvolvimento de garantia e controle de qualidade no ELSA-Brasil. Rev Saúde Pública. 2013;47:105–12. https://doi.org/10.1590/S0034-8910.2013047003889.
World Health Organization. Definition, diagnosis and classification of diabetes mellitus and its complications: report of a WHO consultation Part 1, diagnosis and classification of diabetes mellitus. Geneva: World Health Organization; 1999.
Fedeli LG, Vidigal PG, Leite CM, et al. Logistica de coleta e transporte de material biologico e organizacao do laboratorio central no ELSA-Brasil. Rev Saúde Púb. 2013;47:63–71. https://doi.org/10.1590/S0034-8910.2013047003807.
Malavolti M, Mussi C, Poli M, et al. Cross-calibration of eight-polar bioelectrical impedance analysis versus dual-energy X-ray absorptiometry for the assessment of total and appendicular body composition in healthy subjects aged 21–82 years. Ann Hum Biol. 2003;30:380–91. https://doi.org/10.1080/0301446031000095211.
World Health Organization. International diabetes federation definition and diagnosis of diabetes mellitus and intermediate hyperglycaemia: report of a WHO/IDF consultation. Geneva: World Health Organization; 2006.
ElSayed NA, Aleppo G, Aroda VR, et al. 2. Classification and diagnosis of diabetes: standards of care in diabetes—2023. Diabetes Care. 2023;46:19–40. https://doi.org/10.2337/dc23-S002.
van Hees VT, Fang Z, Langford J, et al. Autocalibration of accelerometer data for free-living physical activity assessment using local gravity and temperature: an evaluation on four continents. J Appl Physiol. 2014;117:738–44. https://doi.org/10.1152/japplphysiol.00421.2014.
van Hees VT, Gorzelniak L, León ECD, et al. Separating movement and gravity components in an acceleration signal and implications for the assessment of human daily physical activity. PLOS ONE. 2013;8: e61691. https://doi.org/10.1371/journal.pone.0061691.
Trost SG, McIver KL, Pate RR. Conducting accelerometer-based activity assessments in field-based research. Med Sci Sports Exerc. 2005;37:S531-543. https://doi.org/10.1249/01.mss.0000185657.86065.98.
Sanders GJ, Boddy LM, Sparks SA, et al. Evaluation of wrist and hip sedentary behaviour and moderate-to-vigorous physical activity raw acceleration cutpoints in older adults. J Sports Sci. 2019;37:1270–9. https://doi.org/10.1080/02640414.2018.1555904.
Itani O, Jike M, Watanabe N, Kaneita Y. Short sleep duration and health outcomes: a systematic review, meta-analysis, and meta-regression. Sleep Med. 2017;32:246–56. https://doi.org/10.1016/j.sleep.2016.08.006.
Jike M, Itani O, Watanabe N, Buysse DJ, Kaneita Y. Long sleep duration and health outcomes: a systematic review, meta-analysis and meta-regression. Sleep Med Rev. 2018;39:25–36. https://doi.org/10.1016/j.smrv.2017.06.011.
De Paula D, Crochemore-Silva I, Griep RH, Duncan BB, Schmidt MI. Accelerometry measured movement behaviors in middle-aged and older adults: cross-sectional analysis of the ELSA-Brasil study. J Phys Act Health. 2023. https://doi.org/10.1123/jpah.2023-0106.
Bedogni G, Bellentani S, Miglioli L, et al. The Fatty Liver Index: a simple and accurate predictor of hepatic steatosis in the general population. BMC Gastroenterol. 2006;6:33. https://doi.org/10.1186/1471-230X-6-33.
Perazzo H, Benseñor I, Mill JG, et al. Prediction of liver steatosis applying a new score in subjects from the Brazilian longitudinal study of adult health. J Clin Gastroenterol. 2020;54:e1-10. https://doi.org/10.1097/MCG.0000000000001007.
Molina MDCB, Benseñor IM, de Cardoso L, et al. Reproducibility and relative validity of the food frequency questionnaire used in the ELSA-Brasil. Cad Saude Pub. 2013;29:379–89.
Chor D, Lima CRDA. Aspectos epidemiológicos das desigualdades raciais em saúde no Brasil. Cad Saúde Púb. 2005;21:1586–94. https://doi.org/10.1590/S0102-311X2005000500033.
Coimbra Junior CE, Santos RV, Escobar AL. Associação Brasileira de Pós-Graduação em Saúde Coletiva. In: Coimbra Junior CE, editor. Epidemiologia e saúde dos povos indígenas no Brasil. Rio de Janeiro: ABRASCO; 2003.
Harrell FE. Regression modeling strategies: with applications to linear models, logistic regression, and survival analysis. New York: Springer; 2001.
Dumuid D, Stanford TE, Martin-Fernández J-A, et al. Compositional data analysis for physical activity, sedentary time and sleep research. Stat Methods Med Res. 2018;27:3726–38. https://doi.org/10.1177/0962280217710835.
Dumuid D, Pedišić Ž, Stanford TE, et al. The compositional isotemporal substitution model: a method for estimating changes in a health outcome for reallocation of time between sleep, physical activity and sedentary behaviour. Stat Methods Med Res. 2019;28:846–57. https://doi.org/10.1177/0962280217737805.
Chastin SFM, Palarea-Albaladejo J, Dontje ML, Skelton DA. Combined effects of time spent in physical activity, sedentary behaviors and sleep on obesity and cardio-metabolic health markers: a novel compositional data analysis approach. PLoS ONE. 2015;10: e0139984. https://doi.org/10.1371/journal.pone.0139984.
Chastin SFM, McGregor DE, Biddle SJ, et al. Striking the right balance: evidence to inform combined physical activity and sedentary behavior recommendations. J Phys Act Health. 2021;18:631–7. https://doi.org/10.1123/jpah.2020-0635.
Aitchison J. The statistical analysis of compositional data. J Roy Stat Soc Ser B (Methodol). 1982;44:139–77.
Consensus Conference Panel, Watson NF, Badr MS, et al. Recommended amount of sleep for a healthy adult: a joint consensus statement of the American academy of sleep medicine and sleep research society. J Clin Sleep Med. 2015;11:591–2. https://doi.org/10.5664/jcsm.4758.
Bracco PA, Gregg EW, Rolka DB, et al. Lifetime risk of developing diabetes and years of life lost among those with diabetes in Brazil. J Glob Health. 2021;11:04041. https://doi.org/10.7189/jgh.11.04041.
Tramunt B, Smati S, Grandgeorge N, et al. Sex differences in metabolic regulation and diabetes susceptibility. Diabetologia. 2020;63:453–61. https://doi.org/10.1007/s00125-019-05040-3.
von Elm E, Altman DG, Egger M, et al. The strengthening the reporting of observational studies in epidemiology (STROBE) statement: guidelines for reporting observational studies. J Clin Epidemiol. 2008;61:344–9. https://doi.org/10.1016/j.jclinepi.2007.11.008.
Lee I-M, Shiroma EJ, Kamada M, Bassett DR, Matthews CE, Buring JE. Association of step volume and intensity with all-cause mortality in older women. JAMA Intern Med. 2019;179:1105–12. https://doi.org/10.1001/jamainternmed.2019.0899.
Chudasama YV, Khunti KK, Zaccardi F, et al. Physical activity, multimorbidity, and life expectancy: a UK Biobank longitudinal study. BMC Med. 2019;17:108. https://doi.org/10.1186/s12916-019-1339-0.
Saint-Maurice PF, Troiano RP, Bassett DR, et al. Association of daily step count and step intensity with mortality among US adults. JAMA. 2020;323:1151–60. https://doi.org/10.1001/jama.2020.1382.
Paluch AE, Bajpai S, Ballin M, et al. Prospective association of daily steps with cardiovascular disease: a harmonized meta-analysis. Circulation. 2023;147:122–31. https://doi.org/10.1161/CIRCULATIONAHA.122.061288.
Hildebrand M, van Hees VT, Hansen BH, Ekelund U. Age group comparability of raw accelerometer output from wrist- and hip-worn monitors. Med Sci Sports Exerc. 2014;46:1816–24. https://doi.org/10.1249/MSS.0000000000000289.
Tsunoda K, Kai Y, Uchida K, Kuchiki T, Nagamatsu T. Physical activity and risk of fatty liver in people with different levels of alcohol consumption: a prospective cohort study. BMJ Open. 2014;4: e005824. https://doi.org/10.1136/bmjopen-2014-005824.
Gerber L, Otgonsuren M, Mishra A, et al. Non-alcoholic fatty liver disease (NAFLD) is associated with low level of physical activity: a population-based study. Aliment Pharmacol Ther. 2012;36:772–81. https://doi.org/10.1111/apt.12038.
Schneider CV, Zandvakili I, Thaiss CA, Schneider KM. Physical activity is associated with reduced risk of liver disease in the prospective UK Biobank cohort. JHEP Rep. 2021;3: 100263. https://doi.org/10.1016/j.jhepr.2021.100263.
Hallsworth K, Thoma C, Moore S, et al. Non-alcoholic fatty liver disease is associated with higher levels of objectively measured sedentary behaviour and lower levels of physical activity than matched healthy controls. Frontline Gastroenterol. 2015;6:44–51. https://doi.org/10.1136/flgastro-2014-100432.
Ryu S, Chang Y, Jung H-S, et al. Relationship of sitting time and physical activity with non-alcoholic fatty liver disease. J Hepatol. 2015;63:1229–37. https://doi.org/10.1016/j.jhep.2015.07.010.
Wang J, Qi Y, Xin Z, et al. Isotemporal substitution of different behaviour patterns with the presence of MAFLD in Chinese adults. Liver Int. 2022;42:2683–95. https://doi.org/10.1111/liv.15439.
Bull FC, Al-Ansari SS, Biddle S, et al. World Health Organization 2020 guidelines on physical activity and sedentary behaviour. Br J Sports Med. 2020;54:1451–62. https://doi.org/10.1136/bjsports-2020-102955.
Babu AF, Csader S, Lok J, et al. Positive effects of exercise intervention without weight loss and dietary changes in NAFLD-related clinical parameters: a systematic review and meta-analysis. Nutrients. 2021;13:3135. https://doi.org/10.3390/nu13093135.
Sabag A, Barr L, Armour M, et al. The effect of high-intensity interval training vs moderate-intensity continuous training on liver fat: a systematic review and meta-analysis. J Clin Endocrinol Metab. 2022;107:862–81. https://doi.org/10.1210/clinem/dgab795.
Keymasi Z, Sadeghi A, Pourrazi H. Effect of pilates training on hepatic fat content and liver enzymes in men with nonalcoholic fatty liver disease. J Appl Health Stud Sport Physiol. 2017;4:49–56. https://doi.org/10.22049/jassp.2019.26576.1241.
Singh AK, Kaur N, Kaushal S, et al. Partitioning of radiological, stress and biochemical changes in pre-diabetic women subjected to Diabetic Yoga Protocol. Diabetes Metab Syndr. 2019;13:2705–13. https://doi.org/10.1016/j.dsx.2019.07.007.
Han Q, Han X, Wang X, et al. Association of accelerometer-measured sedentary behavior patterns with non-alcoholic fatty liver disease among older adults: the MIND-China study. Am J Gastroenterol. 2022. https://doi.org/10.14309/ajg.0000000000002135.
Tremblay MS, Aubert S, et al. Sedentary behavior research network (SBRN)—terminology consensus project process and outcome. Int J Behav Nutr Phys Act. 2017;14:75. https://doi.org/10.1186/s12966-017-0525-8.
Acknowledgements
We would like to thank the ELSA-Brasil volunteer participants and the staff from the ELSA-Brasil study.
Funding
The Brazilian Ministry of Science, Technology, Innovation, and Communications funded the project through grants 405545/2015-0, 405547/2015-3, 405543/2015-8, 405544/2015-4, 405551/2015-0, and 405552/2015-7. de Paula received a scholarship from the Brazilian Coordination for Superior Education of the Ministry of Culture & Education (CAPES)—Finance Code 001. Feter, Griep, Duncan, and Schmidt are supported by the National Council for Scientific and Technological Development (CNPq). The funders had no role in study design, data collection, analysis, decision to publish, or manuscript preparation.
Author information
Authors and Affiliations
Contributions
DP and MIS conceptualized the study and wrote the manuscript. DP and RCPR conducted and revised the statistical analyses. DP prepared all figures. DP, NF, RG, RCPR, BBD, and MIS interpreted the results and contributed substantially to the content of the manuscript. All authors reviewed the manuscript and approved the final version.
Corresponding author
Ethics declarations
Ethics approval and consent to participate
ELSA-Brasil was approved by the National Commission for Ethics in Research (CONEP) and the review boards of all participating institutions (Universidade Federal de Minas Gerais—47125015.4.1001.5149, Universidade de São Paulo—1.801.270, Universidade Federal do Espírito Santo—2.091.107, Hospital de Clínicas de Porto Alegre/Universidade Federal do Rio Grande do Sul—1.893.463, Universidade Federal da Bahia 1.930.817 e Fundação Oswaldo Cruz—1.900.315). All study subjects gave signed consent forms prior to their participation.
Consent for publication
Not applicable.
Competing interests
The authors declare no competing of interest.
Additional information
Publisher's Note
Springer Nature remains neutral with regard to jurisdictional claims in published maps and institutional affiliations.
Supplementary Information
Rights and permissions
Open Access This article is licensed under a Creative Commons Attribution 4.0 International License, which permits use, sharing, adaptation, distribution and reproduction in any medium or format, as long as you give appropriate credit to the original author(s) and the source, provide a link to the Creative Commons licence, and indicate if changes were made. The images or other third party material in this article are included in the article's Creative Commons licence, unless indicated otherwise in a credit line to the material. If material is not included in the article's Creative Commons licence and your intended use is not permitted by statutory regulation or exceeds the permitted use, you will need to obtain permission directly from the copyright holder. To view a copy of this licence, visit http://creativecommons.org/licenses/by/4.0/. The Creative Commons Public Domain Dedication waiver (http://creativecommons.org/publicdomain/zero/1.0/) applies to the data made available in this article, unless otherwise stated in a credit line to the data.
About this article
Cite this article
de Paula, D., Feter, N., dos Reis, R.C.P. et al. Associations of activity, sedentary and sleep behaviors with prevalent steatotic liver disease in middle-aged and older adults: the ELSA-Brasil study. JASSB 3, 16 (2024). https://doi.org/10.1186/s44167-024-00055-7
Received:
Accepted:
Published:
DOI: https://doi.org/10.1186/s44167-024-00055-7