Abstract
Purpose
Isoniazid, a first-line antitubercular drug, is associated with nervous system adverse drug reactions such as seizures, peripheral neuropathy, and psychosis. This systematic review of case reports and case series aimed to characterize the demographic, social, and clinical factors associated with isoniazid-induced psychosis in patients with active tuberculosis (TB) and those who received isoniazid for latent TB infection (LTBI).
Methods
We comprehensively searched the Embase, PubMed, and Scopus databases to identify relevant studies published between the date of inception of the database and June 2024.
Results
A total of 28 studies, including 21 case reports and 7 case series involved 37 patients who developed isoniazid-induced psychosis. A higher frequency of isoniazid-induced psychosis was observed during the first 2 months of treatment, with a relatively early onset observed among patients aged 18 years or less. Delusions and/or hallucinations are the common symptoms of isoniazid-induced psychosis. Psychomotor disturbances, disorganized speech or formal thought disorder, disorganized or abnormal behaviour, and neuropsychiatric symptoms (sleep disturbances, hostility or aggression, confusion, affective symptoms, anxiety symptoms, and cognitive difficulties) were the other symptoms observed in the included studies. More than 80% of cases rechallenged with isoniazid resulted in the recurrence of psychotic symptoms.
Conclusion
Patients with TB and LTBI should be assessed for psychotic and neuropsychiatric symptoms during isoniazid therapy, mainly in the first 2 months. Further research is required to understand the impact of underlying risk factors, such as genetic predisposition and isoniazid pharmacokinetics, as well as the clinical utility and dosage recommendations of pyridoxine for managing isoniazid-induced psychosis.
Similar content being viewed by others
Avoid common mistakes on your manuscript.
Introduction
Isoniazid has been one of the first-line antitubercular drugs used to treat both active tuberculosis (TB) and latent TB infection (LTBI) for several years [1]. Isoniazid is associated with numerous types of adverse drug reactions (ADRs) affecting the central and peripheral nervous system, such as insomnia, headache, muscle twitching, optic neuropathy, peripheral neurotoxicity, psychosis, and restlessness [2]. Isoniazid may cause psychiatric ADRs that include symptoms such as delusions, hallucination, abnormal behaviour, disorganized thoughts, and euphoria [3, 4]. Several case studies have found an association between the development of psychosis and isoniazid use in the treatment and prophylaxis of TB [5, 6]. Several mechanisms have been hypothesized for isoniazid-induced psychosis. One mechanism involves the marginal inhibition of monoamine oxidase (MAO) by isoniazid, which results in elevated levels of monoamines [7, 8]. Another mechanism is the lack of vitamin B6/pyridoxine, a cofactor necessary to produce numerous neurotransmitters, including gamma-aminobutyric acid (GABA) [9]. When isoniazid is metabolized, it produces hydrazine and its metabolites (reactive nitrogen species), which bind with pyridoxal phosphate, an active form of pyridoxine. This binding inhibits the enzymes dependent on pyridoxal phosphate, including transaminases and those involved in amino acid metabolism, leading to a functional pyridoxine deficiency [10]. In addition, isoniazid is linked to increased oxidative stress caused by increased production of reactive oxygen species (ROS), which lowers the density of N-methyl-D-aspartate (NMDA) receptors in the hippocampus. ROS are hypothesized to be produced more often, and subsequently, glutathione levels are depleted [11].
The incidence of isoniazid-induced psychosis has not yet been established. The risk factors for the occurrence of psychosis induced by isoniazid include older age, malnourishment, alcohol consumption, diabetes mellites, uraemia, present, past, and family history of psychiatric illness, hepatocellular dysfunction, and neurological disorder [12,13,14]. Additionally, the isoniazid dose (> 5 mg/kg) and N acetyltransferases (NAT2) slow acetylators status might contribute to the risk of developing psychosis secondary to isoniazid intake [13]. To our knowledge, no epidemiological studies focused on psychosis due to isoniazid for TB treatment and/or LTBI, and most of the available literature includes case reports and case series.
Despite its clinical importance, there is a limited understanding regarding the demographic, social, and clinical determinants of isoniazid-induced psychosis in patients with TB and LTBI. Our systematic review focused on the in-depth clinical profiling of patients who developed isoniazid-induced psychosis. In this systematic review, we assessed the occurrence of isoniazid-induced psychosis based on patient demographics, social factors, and clinical determinants. We investigated whether there were specific periods during which psychosis was more likely to occur in TB and LTBI patients. This review also provides an overview of the pharmacological management of isoniazid-induced psychosis.
Methods
Research question
We conducted our systematic review to address the main research question: What demographic, social, and clinical variables are associated with isoniazid-induced psychosis in patients with active TB or those who received isoniazid for LTBI?
PICO framework
The population-intervention-comparator-outcomes-study design (PICO) framework was used to identify eligible cases [15]. The criteria used were as follows:
-
Population: Active TB/LTBI patients, regardless of age or demographics.
-
Intervention: Individuals received isoniazid for active TB treatment/LTBI.
-
Comparator: Comparator was not applicable.
-
Outcome: The development of psychosis following the initiation of isoniazid therapy as prophylactic or antitubercular treatment.
Eligibility criteria
-
Inclusion criteria: Case studies of isoniazid-induced psychosis was included based on the diagnostic criteria for substance/medication-induced psychosis defined by the Diagnostic and Statistical Manual of Mental Disorders, Fifth Edition (DSM-5) classification [16], published in the format of full text, correspondence, and letters to the editor focused entirely on the case report.
-
Exclusion criteria: Review articles, conference abstract, articles not published in the English language, cases with drug-induced psychosis other than isoniazid, patients with a current or previous history of psychiatric illness, and patients with no symptoms of either hallucination or delusion.
Information source and search strategy
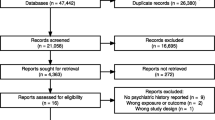
The current study was performed according to the Preferred Reporting Items for Systematic Reviews and Meta-Analyses 2020 (PRISMA) guidelines [17] and the PRISMA checklist in Supplemental Table 1. A systematic search approach was used to identify relevant studies. This included searching electronic databases such as PubMed, Embase, and Scopus and manually reviewing the reference lists of pertinent papers. The search was performed from the date of inception of each database until June 2024. The search keywords used in this systematic review are listed in Supplemental Table 2. Figure 1 illustrates the PRISMA flowchart generated using Microsoft Word.
Two independent reviewers, K.B. and S.N., performed article screening and review. Following an initial search and elimination of duplicates, two independent reviewers (K.B. and S.N.) conducted an extensive eligibility evaluation on the titles and abstracts of the remaining articles. The selected articles were subjected to a systematic full-text screening process, and only those that matched the predetermined inclusion criteria were included in the systematic review. When there were disagreements between the two reviewers regarding the acceptability of an article for inclusion, the other reviewers (L.T. and R.A.) were consulted for arbitration. All excluded reports and the reasons for their exclusion were well-documented, ensuring a rigorous and open selection process.
Selection process and data extraction
Three authors (K.B., L.T., and S.N.) independently extracted the data from each study using a Microsoft Excel spreadsheet. If there was a disagreement among the authors regarding the extracted data, a co-author (R.A. or M.R.) was consulted to arrive at a final decision.
The data extraction involved demographic, social, and clinical variables from the included studies. The variables were as follows: age, sex, body mass index (BMI), type of TB, treatment regimen, comorbidities/previous medical history, alcohol and smoking history, family history of psychosis/psychiatric illness, onset of psychosis since antitubercular treatment initiation, reported psychotic signs and symptoms, rechallenge with isoniazid and its response, pyridoxine dose administered during or after antitubercular treatment, psychotropics given after psychotic episode, withhold/discontinuation of isoniazid, and resolution of psychotic symptoms following therapeutic action. All extracted data were entered into Microsoft Excel for further analysis. We grouped the included studies as follows: cases prescribed only pyridoxine, cases prescribed only psychotropics, cases prescribed pyridoxine and psychotropics, and cases not prescribed or not mentioned regarding pyridoxine and psychotropic administration. GraphPad Prism Version 10.1.2 (GraphPad Software, USA) was used to plot the assessment of the medication, family and social history profile of patients who developed psychosis after the administration of isoniazid (Fig. 2), the relationship between isoniazid dose and age group with the onset of psychosis (Fig. 3), the symptoms experienced by patients who developed isoniazid-induced psychosis (Supplemental Fig. 1), and the risk of bias of studies (Supplemental Fig. 2).
Medication, family and social history profile of patients who developed psychosis after the administration of isoniazid. *Cases that were not isoniazid induced psychosis has been excluded from the case series. Pyridoxine administered before the occurrence of the psychosis. Abbreviations: Fam_hist_Psy_illness, family history of psychiatric illness; NR, not reported; PAS, para-aminosalicylic acid
Quality assessment
The methodological assessment of study quality was performed using a standardized tool adapted from Murad et al. [18]. This scale assesses selection, ascertainment, causation, and reporting domains. Although there were eight questions, question seven was omitted because it was irrelevant to our review. Therefore, the maximum possible score is seven. Each of the seven items in the questionnaire received a binary (yes/no) response. If the criteria were satisfied, a score of one was given; otherwise, a score of zero was given. If the study obtained a score of five to seven, three to four, and under three, we considered the chance of bias to be low, moderate, or high risk of bias, respectively (Supplemental Fig. 2). Four independent reviewers (K.B., L.T., T.B., and S.N.) evaluated the quality of the studies. In case of any discrepancies concerning the overall rating of a paper, R.A. acted as an arbitrator.
Results
Study selection
A comprehensive search of the electronic databases yielded 722 records. After removing 121 duplicates, 601 records were used for title and abstract screening. We sought 59 studies for retrieval, of which five could not be retrieved because of the unavailability of the full text. Finally, 54 studies were deemed relevant and subjected to a full-text review. After thoroughly evaluating full-text articles consisting of case reports, case series, and cases in the form of letters to the editor or short communications, 17 studies met our inclusion criteria. In addition, 11 more studies were identified by manually searching the articles from pertinent literature references. A total of 28 publications were included in our review [5, 6, 12, 14, 19,20,21,22,23,24,25,26,27,28,29,30,31,32,33,34,35,36,37,38,39,40,41,42], which consisted of 37 cases from 13 case reports [5, 6, 12, 14, 19, 20, 24, 27, 30, 31, 34, 36, 39], seven case series (16 cases) [21, 25, 32, 35, 37, 38, 40], seven letters to editors [22, 23, 26, 28, 29, 41, 42], and one short communication [33]. The data extracted from the selected studies are presented in Table 1.
Characteristics of included studies
Our comprehensive search identified 37 cases in 14 countries. Out of the 37 cases, 10 reported the race/ethnicity/immigrant status of the patients [6, 12, 21, 25, 40] (Table 1). The countries from which case studies were reported include India (n = 10) [5, 14, 30,31,32,33, 36, 37, 42], the USA (n = 8; 4 African descent, 1 Filipino, and 3 unknown) [6, 19, 27, 28, 40], the UK (n = 5; 1 Hong Konger, 1 Filipino, 1 Jamaican, 1 African descent, and 1 unknown) [21, 25], Canada (n = 3) [38], Turkey (n = 2) [22, 39], China (n = 1) [26], Nigeria (n = 1) [35], Greece (n = 1) [29], Japan (n = 1) [34], Papua New Guinea (n = 1) [24], the Philippines (n = 1) [41], Portugal (n = 1; 1 African descent) [12], Saudi Arabia (n = 1) [20], and Sri Lanka (n = 1) [23].
The median age of the patients were 31 (IQR 34) years (age range from 3 to 80 years). The frequencies of male and female patients with isoniazid-induced psychosis across the cases were 19 (51.3%) and 18 (48.6%), respectively. Of the 37 cases, 19 were reported to have pulmonary TB (PTB) [14, 20, 21, 25, 26, 29,30,31,32,33, 35,36,37,38, 40, 42], six were extra-pulmonary TB (EPTB) [12, 21, 23,24,25], one had disseminated TB [40], and one had miliary TB [37]. Among the reported cases of EPTB, there were cases of pleural effusion TB (n = 2) [12, 23], TB meningitis (n = 2) [24, 25], spinal TB (n = 1) [21], pericarditis TB (n = 1) [25]. Furthermore, nine additional cases were where isoniazid was administered for LTBI [5, 6, 19, 22, 27, 28, 34, 39, 41]. However, one case did not report the type of TB [38]. Patients diagnosed with TB also had comorbidities, such as anaemia (n = 3) [28, 31, 35] and diabetes mellitus (n = 2) [21, 25]. There was one case with a documented history of smoking [32], one with alcohol consumption [28], and one with both smoking and alcohol consumption [23], respectively (Fig. 2). There were three cases in where the patient had a family history of psychosis [19, 22, 30] (Fig. 2). All 37 patients received isoniazid treatment. The combination of antitubercular drugs administered to patients in conjunction with isoniazid is shown in Fig. 2.
Psychosis was diagnosed at a median of 15 days (IQR 46) after the initiation of isoniazid (n = 37). The dose of isoniazid reported in these studies was between 150 and 600 mg daily. Among the 37 patients included in the study, the most frequently reported dose of isoniazid was a 300 mg daily (n = 17). Assessment of isoniazid daily dose and age groups with the onset time of psychosis (n = 21) revealed that all patients ≤ 18 years developed isoniazid-induced psychosis within 15 days of isoniazid initiation, as shown in Fig. 3.
Approximately 75.8% (n = 25) of patients developed psychosis within the first 60 days of isoniazid therapy. From our findings, we found that the use of pyridoxine during antitubercular treatment has been documented in 11 cases (Fig. 2), whereas 13 cases have reported pyridoxine supplementation following the onset of psychosis. The total daily dose of pyridoxine ranged from 5 to 1000 mg. Delusions (n = 27), hallucinations (n = 26), psychomotor disturbances (n = 25), disorganized speech (or) formal thought disorder (n = 19), disorganized (or) abnormal behaviour (n = 15), sleep disturbances (n = 13), hostility (or) aggression (n = 10), confusion (n = 11), affective symptoms (n = 10), anxiety symptoms (n = 9), and cognitive difficulties (n = 7) were observed in patients with isoniazid-induced psychosis (Supplemental Fig. 1).
From the selected 37 cases, we observed that after the onset of isoniazid-induced psychosis, the isoniazid was dechallenged in 32 cases [5, 6, 12, 14, 19,20,21, 23,24,25, 28,29,30,31,32,33,34,35,36,37,38,39,40,41,42]. Of these 32 cases, in 19 cases, patients who received psychotropics and had complete resolution of psychotic and neuropsychiatric symptoms [12, 14, 19,20,21, 23, 24, 28, 30,31,32,33,34,35,36,37, 39, 41, 42], whereas in 13 patients who did not receive psychotropic intervention [5, 6, 25, 29, 38, 40], nine had complete resolution of the psychotic and neuropsychiatric symptoms [5, 6, 25, 29, 38, 40], two had a partial resolution [40], and two had no improvement in their psychotic/neuropsychiatric symptoms [38]. In one of the cases with partial resolution of symptoms, cognitive disturbance in the form of disorientation persisted, although psychotic symptoms such as auditory and visual hallucinations resolved after 3 months. In this case, the patient had a medical history of peripheral neuropathy and malnutrition. There were no reports of social history (alcohol consumption and smoking habits) or a family history of psychiatric illness. In another case, the patient experienced disturbance even after 8 weeks of discontinuing isoniazid, and there were no reports of any comorbid conditions, social history, or family history of mental illness. Neither patients were rechallenged with isoniazid or prescribed pyridoxine (both prophylactically and therapeutically) and psychotropics [40]. In our review, out of 37 cases, isoniazid was not dechallenged in five cases [21, 22, 26, 27, 37], of which four received psychotropics [21, 22, 26, 37], while one did not receive psychotropic intervention [27] and had complete resolution of the symptoms. Patients were treated with haloperidol (n = 6), lorazepam (n = 5), risperidone (n = 6), olanzapine (n = 4), quetiapine (n = 3), and clonazepam (n = 1), either as monotherapy or in combination therapy for managing psychiatric symptoms (Table 1). Out of 37 cases, eight were rechallenged with isoniazid, of which seven (Table 1) experienced recurrence of psychotic/neuropsychiatric symptoms (Table 1). We found that only five cases were evaluated, which included an assessment of the causality and severity of ADR [30, 32, 33, 37]. The ADR causality assessment tools employed were the World Health Organization (WHO) Uppsala Monitoring Centre (UMC) scale in four cases [32, 33, 37], and the Naranjo scale in four cases [30, 32, 37]. The Hartwig and Siegel severity scale was used in two cases [32, 33]. The grades of the aforementioned scales are listed in Table 2.
Quality of assessment
According to the risk of bias score and classification rules, 25 cases received a score between five and seven (low risk of bias) [5, 12, 14, 19,20,21,22,23,24,25,26,27,28,29,30,31,32, 34,35,36, 39, 41, 42], six cases received a score between three and four (moderate risk of bias) [6, 25, 33, 37, 38], and six cases received a score of less than three (high risk of bias) [38, 40]. A detailed quality assessment of each case is presented in Supplemental Fig. 2.
Discussion
Psychosis is a rare but serious adverse event associated with isoniazid therapy [10]. Our systematic review included 37 patients with psychosis after the isoniazid intake. We found that most cases of isoniazid-induced psychosis were reported in India (n = 10). India is among the top 30 countries with a high TB burden as per the Global Tuberculosis Report 2022 [43]. We identified several variables such as a family history of mental illness, alcohol and smoking history, comorbidities, and the lack of pyridoxine supplementation among patients with isoniazid-induced psychosis. We excluded the studies reporting current or previous history of psychiatric illness. However, the evidence for these associations between ATT-induced psychosis remains insufficient despite some studies being in line with previous research [7, 44,45,46,47]. A recent systematic review and meta-analysis of 53 studies reported a high pooled prevalence (48%) of malnutrition (51 studies used a low BMI to define malnutrition) among PTB patients [48]. Nutritional deficiencies and complications that occur during malnutrition, such as sleep deprivation and metabolic derangements, are known medical causes of psychosis; nutritional rehabilitation and weight restoration may be key interventional strategies for the resolution of psychosis [49]. Lower BMI (< 18.5 kg/m2) was reported for the two cases that have BMI data in our systematic review [20, 32]. There is a high prevalence of TB in patients with human immunodeficiency virus (HIV) co-infection [50]. A meta-analysis reported a pooled prevalence of 23% of the first episodes of psychosis in people living with HIV (PLWHIV) on the African continent [51]. Additionally, the occurrence of psychosis has been reported in the treatment with antiretroviral agents [52, 53]. Co-administration of isoniazid preventive therapy and antiretroviral therapy (ART) was reported to be associated with a higher frequency of psychosis [54]. Further studies assessing the possibility of a synergistic effect of isoniazid and antiretroviral agents in causing psychosis among TB patients co-infected with HIV are required. Hence, factors such as lower BMI/malnutrition, HIV co-infection, and co-administration of antiretroviral agents with isoniazid should be considered as potential confounding variables and/or risk factors in future studies.
The recommended daily dose of isoniazid in adults is 5 mg/kg [55]. Our systematic review identified a daily dose of 300 mg of isoniazid as the most frequently prescribed dose among cases who developed isoniazid-induced psychosis [6, 12, 19, 21, 22, 25, 27,28,29,30,31,32,33, 38, 39, 42]. NAT2 genotype is an important covariate influencing the plasma concentrations of isoniazid. NAT2 slow acetylators achieve higher concentrations of isoniazid than intermediate and fast acetylators [56]. Only one case in our systematic review reported NAT2 phenotype status as a NAT2 fast acetylator [34]. NAT2 single nucleotide polymorphisms (SNPs) and haplotypes were not assessed (or) reported in any of the 37 cases. NAT2 slow acetylators have been implicated as a risk factor for the development of isoniazid-induced ADRs, such as drug-induced liver injury (DILI), particularly during the first month of isoniazid therapy initiation [57, 58]. Future studies should identify and evaluate whether patients with NAT2 genotype/SNPs have an increased risk of developing psychosis due to higher plasma concentrations of isoniazid. Most patients developed isoniazid-induced psychosis within 2 months of isoniazid initiation; although in some cases, it appears even after eight months of therapy. Closer monitoring of isoniazid-induced psychotic symptoms in paediatric patients may be required during the first 2 weeks of treatment. Numerous studies have consistently shown a significant association between the utilization of antibiotics such as cephalosporins and fluoroquinolones and the occurrence of psychosis within a remarkably short period of 2 weeks following the initiation of treatment [44, 59,60,61,62,63,64]. Considering the highly heterogeneous and pleomorphic presentation of isoniazid psychosis, we operationalized the definition based on broad criteria of psychotic disorders as per the International Classification of Diseases (ICD)-10 [65] and DSM-5 [16]. From our review findings, psychotic symptoms (psychomotor disturbance, disorganized speech (or) formal thought disorder, and disorganized (or) abnormal behaviour) and neuropsychiatric symptoms (sleep disturbances, hostility (or) aggression, confusion, affective symptoms, anxiety symptoms, and cognitive difficulties) were the frequently observed symptoms along with delusion and hallucination in the included cases. These findings are consistent with earlier literature [9, 66], which identified comparable symptom patterns in patients with drug-induced psychosis, including drugs such as aminoglycosides, other antitubercular agents, beta-lactam agents, and psychotropic drugs (central nervous system (CNS) stimulants, and CNS depressants) [9, 66]. However, they might serve as significant early-stage indicators and call for further research.
A high frequency of patients had a psychomotor disturbance in our study. Pyridoxine deficiency has been reported to enhance noradrenergic signaling and facilitate behavioural deficits in mice [67]. Pyridoxine (100 mg/day) improved neuroleptic-induced parkinsonism and psychotic symptoms in a patient with schizophrenia [68]. A double-blind placebo-controlled cross-over study in patients with schizophrenia reported pyridoxine as an effective agent for tardive dyskinesia at doses from 300 mg/day [69]. A randomized, double-blind, placebo-controlled study reported that the high-dose pyridoxine-treated patients (1200 mg/day administered as 600 mg BID) had improvement in neuroleptic-induced akathisia symptoms within a few days [70]. The quality of evidence about the effectiveness of pyridoxine for the treatment of neuroleptic-induced tardive dyskinesia among people with schizophrenia and other related psychotic disorders is low [71]. Another study reported that though pyridoxine may be beneficial for ameliorating antipsychotic-induced akathisia, there was no significant difference observed among those who received a low dose (600 mg/day administered as 300 mg BID) or high dose (1200 mg/day administered as 600 mg BID) pyridoxine [72]. Pyridoxine 100 mg was the most prescribed dose; however, several patients have been prescribed pyridoxine doses lesser than 100 mg/day post-isoniazid-induced psychosis.
According to Chan et al., even though pyridoxine supplementation is recommended in patients who are at risk of neuropathy due to underlying medical conditions such as diabetes, uraemia, alcoholism, and HIV infection, it is not an effective treatment for isoniazid-induced psychosis [73]. On the other hand, Yadav et al. suggest that pyridoxine supplementation should be considered after the occurrence of isoniazid-induced psychosis to ensure the reintroduction of isoniazid does not exacerbate psychosis [74]. Only a single study had administered a high dose of pyridoxine of 1000 mg/day (administered as 500 mg intramuscularly BID for 4 days), which, however, did not improve psychosis during the period of administration [25]. The role of pyridoxine supplementation for isoniazid-induced psychosis warrants robust scientific validation for conclusive recommendations. A comparative benefit assessment of different doses of pyridoxine supplementation for the treatment of isoniazid-induced psychosis is also required. Our systematic review shows that approximately 60% (n = 23) of patients have received psychotropic drugs. Notably, in our review, a substantial proportion of the cases did not involve the administration of psychotropic drugs to treat isoniazid-induced psychosis. Among the psychotropics used, lorazepam, which belongs to the class of benzodiazepines [75], is the most frequently administered. The other antipsychotics, which belong to the classes butyrophenones and atypical antipsychotics [76], such as haloperidol, risperidone, quetiapine, and olanzapine, were employed in varying frequencies to treat the psychotic symptoms. In our review, isoniazid-induced psychosis was assessed using numerous ADR assessment tools across the studies, with the WHO UMC scale and the Naranjo scale being the most frequently used. Using different causality assessment tools underscores the importance of standard approaches to establishing the likelihood of isoniazid-induced psychosis [77].
Our systematic review has a few inherent limitations that should be considered. Our review is primarily limited to case reports and case series, which may not provide the strongest degree of evidence in contrast to other study designs. Additionally, by only including articles in English, it is possible that pertinent studies published in different languages were left out. Due to the smaller number of reports and several missing data in these reports, our analysis could affect the precision and generalizability of the findings. Participants with a current or previous history of psychiatric illness studies were excluded from the study. We also excluded grey literature in our review. Some relevant case reports could not be retrieved because the full text was unavailable in the databases. Some case reports have scanty descriptions of the symptoms. However, we included these articles as they mentioned the evaluation of the patient by a mental health professional that led to the diagnosis of isoniazid-induced psychosis. Ascertaining the psychosis diagnosis becomes even more challenging, especially in studies involving the paediatric population. The classificatory systems in psychiatry do get revised over time. The importance of certain symptoms would change with the changing diagnostic guidelines. Including case reports from earlier decades, such as the 1950s, and applying the current classificatory guidelines to relook at the clinical manifestations would have some limitations. We restricted our attention to the relationship between isoniazid and psychosis, which potentially led to an incomplete understanding of the broader mental health implications, such as anxiety and depression. These limitations raise attention to the need for further research to address these gaps and offer a more thorough understanding of the effects of isoniazid on mental health. Future research should focus on well-designed epidemiological studies to determine the incidence/prevalence of isoniazid-induced psychosis and neuropsychiatric symptoms. Our review provides evidence for closer monitoring of psychotic symptoms during the first 2 months of isoniazid treatment as shown in Fig. 3, with a rechallenge of isoniazid resulting in the recurrence of the psychotic and/or neuropsychiatric symptoms in the majority of the patients. However, we could not conclusively conclude on the risk factors that may lead to isoniazid induced psychosis as well as on the clinical utility and dosage recommendations of pyridoxine as a therapeutic agent for the management of isoniazid-induced psychosis symptoms. Further research may focus on retrieval of a greater number of isoniazid-induced psychosis and neuropsychiatric symptoms cases using pharmacovigilance databases such as VigiBase (WHO global database of individual case safety reports), EudraVigilance (European pharmacovigilance database), and the Food and Drug (FDA) adverse event reporting system (FAERS), which may also include unpublished cases for in-depth analysis of the risk factors, severity assessment, and signal detection [78].
Conclusion
Our systematic review identified a higher frequency of isoniazid-induced psychosis during the first 2 months of TB treatment, with a relatively early onset observed among patients of 18 years or less, warranting the need for closer monitoring during this period. Individual patient demographic, social, and clinical characteristics may influence the manifestation of this adverse effect. There is a need for well-controlled studies, to evaluate the influence of genetic variants and isoniazid pharmacokinetics with the development of isoniazid-induced psychosis symptoms and for optimizing pyridoxine therapy.
Data availability
No datasets were generated or analyzed during the current study.
References
Murray JF, Schraufnagel DE, Hopewell PC (2015) Treatment of tuberculosis. A historical perspective. Ann Am Thorac Soc 12(12):1749–1759. https://doi.org/10.1513/AnnalsATS.201509-632PS
Bangert MK, Hasbun R (2019) Neurological and psychiatric adverse effects of antimicrobials. CNS Drugs 33(8):727–753. https://doi.org/10.1007/s40263-019-00649-9
Esposito S, Canevini MP, Principi N (2017) Complications associated with antibiotic administration: neurological adverse events and interference with antiepileptic drugs. Int J Antimicrob Agents 50(1):1–8. https://doi.org/10.1016/j.ijantimicag.2017.01.027
Kass JS, Shandera WX (2010) Nervous system effects of antituberculosis therapy. CNS Drugs 24(8):655–667. https://doi.org/10.2165/11534340-000000000-00000
Sharawat IK, Dawman L (2021) Toddler with intermittent abnormal behavior: is it isoniazid-induced psychosis? Pediatr Emerg Care 37(1):e60–e61. https://doi.org/10.1097/PEC.0000000000001555
Bourgeois JA, Zelenko M, Waraich BS (1996) Psychotic disorder associated with isoniazid. Mil Med 161(11):707. https://doi.org/10.1093/MILMED/161.11.707
Doherty AM, Kelly J, McDonald C et al (2013) A review of the interplay between tuberculosis and mental health. Gen Hosp Psychiatry 35(4):398–406. https://doi.org/10.1016/j.genhosppsych.2013.03.018
Holdiness MR (1987) Neurological manifestations and toxicities of the antituberculosis drugs. A review. Med Toxicol 2(1):33–51. https://doi.org/10.1007/BF03259859
Sternbach H, State R (1997) Antibiotics: neuropsychiatric effects and psychotropic interactions. Harv Rev Psychiatry 5(4):214–226. https://doi.org/10.3109/10673229709000304
Zareifopoulos N, Panayiotakopoulos G (2017) Neuropsychiatric effects of antimicrobial agents. Clin Drug Investig 37(5):423–437. https://doi.org/10.1007/s40261-017-0498-z
Samouco AICB, Alves SP (2023) Isoniazid-induced mania and the history of antidepressant drugs: Case report and literature review. Bipolar Disord 25(1):84–87. https://doi.org/10.1111/bdi.13272
Gomes J, Durães D, Sousa A et al (2019) Isoniazid-induced acute psychosis in a patient with pleural tuberculosis. Case Rep Psychiatry 2019:4272941. https://doi.org/10.1155/2019/4272941
Arya S, Sukhija G, Singh H (2015) Acute psychosis after recent isoniazid initiation. J Clin Diagn Res 9(6):VD01–VD02. https://doi.org/10.7860/JCDR/2015/12450.6130
Prasad R, Garg R, Verma SK (2008) Isoniazid- and ethambutol-induced psychosis. Ann Thorac Med 3(4):149–151. https://doi.org/10.4103/1817-1737.43083
Cumpston MS, McKenzie JE, Thomas J et al (2020) The use of “PICO for synthesis” and methods for synthesis without meta-analysis: protocol for a survey of current practice in systematic reviews of health interventions. F1000Res 9:678
American Psychiatric Association, DSM-5 Task Force (2013) Diagnostic and statistical manual of mental disorders: DSM-5. 5th ed. American Psychiatric Publishing, Inc., Arlington. https://doi.org/10.1176/appi.books.9780890425596
Page MJ, McKenzie JE, Bossuyt PM et al (2021) The PRISMA 2020 statement: an updated guideline for reporting systematic reviews. The BMJ 10(1):89. https://doi.org/10.1186/s13643-021-01626-4
Murad MH, Sultan S, Haffar S et al (2018) Methodological quality and synthesis of case series and case reports. BMJ Evid Based Med 23:60–63. https://doi.org/10.1136/bmjebm-2017-110853
Alao AO, Yolles JC (1998) Isoniazid-induced psychosis. Ann Pharmacother 32:889–891. https://doi.org/10.1345/aph.17377
Alfawaz S, Alattas N, Alhammadi M et al (2020) Acute psychosis secondary to isoniazid in pediatric pulmonary tuberculosis: a case report and literature review. Int J Pediatr Adolesc Med 7(4):196–198. https://doi.org/10.1016/j.ijpam.2020.03.012
Ball R, Rosser R (1989) Psychosis, and anti-tuberculosis therapy. Lancet 2(8654):105. https://doi.org/10.1016/s0140-6736(89)90344-9
Baytunca MB, Erermis S, Bildik T et al (2015) Isoniazid-induced psychosis with obsessive-compulsive symptoms (schizo-obsessive disorder) in a female child. J Child Adolesc Psychopharmacol 25(10):819–820. https://doi.org/10.1089/cap.2014.0065
Demartini B, Camisasca M, Zuin M et al (2013) A case of isoniazid-induced delirium. J Neuropsychiatry Clin Neurosci 25(2):e68–e69. https://doi.org/10.1176/appi.neuropsych.12020022
Duke T, Mai M (1999) Meningitis, or madness: a delicate balance. J Paediatr Child Health 35(3):319–320. https://doi.org/10.1046/j.1440-1754.1999.00344.x
Duncan H, Kerr D (1962) Toxic psychosis due to isoniazid. Br J Dis Chest 56(3):131–138. https://doi.org/10.1016/s0007-0971(62)80045-x
Gnam W, Flint A, Goldbloom D (1993) Isoniazid-induced hallucinosis: response to pyridoxine. Psychosomatics 34(6):537–539. https://doi.org/10.1016/S0033-3182(93)71836-8
Iannaccone R, Sue YJ, Avner JR (2002) Suicidal psychosis secondary to isoniazid. Pediatr Emerg Care 18(1):25–27. https://doi.org/10.1097/00006565-200202000-00008
Ibrahim ZY, Menke JJ (1994) Comment: isoniazid-induced psychosis. Ann Pharmacother 28(11):1311. https://doi.org/10.1177/106002809402801131
Kargiotis O, Marangos M, Polychronopoulos P (2015) An unusual case of isoniazid toxicity. Neurol Sci 36:1037–1039. https://doi.org/10.1007/s10072-015-2105-5
Khanna S, Pant S, Khanna H (2023) Isoniazid-induced psychosis in a patient with pulmonary tuberculosis: a case report. Case Rep Neurol 15(1):76–80. https://doi.org/10.1159/000530779
Madan A, Jain NK, Sharma TN et al (1989) Attempted suicide following treatment with isoniazid. Tubercle 70(2):147–149. https://doi.org/10.1016/0041-3879(89)90041-x
Menon VB, Shaji R, Pereira P (2017) Isoniazid associated psychosis: a case series of 3 patients. Indian J Phar Pract 10(2):142–146. https://doi.org/10.5530/IJOPP.10.2.29
Mukherjee S, Roy S (2023) Acute psychosis secondary to isoniazid in a patient with pulmonary tuberculosis. Bengal Physician J 10(2):73–74
Nagano H, Miura T, Ueda T (2019) Mania induced by isoniazid preventive therapy during steroid treatment for rheumatoid arthritis and organising pneumonia. BMJ Case Rep 12(11):e231919. https://doi.org/10.1136/bcr-2019-231919
Oninla SO, Oyedeji GA, Oninla OA et al (2016) Isoniazid-induced psychosis in 2 children treated for tuberculosis: case reports and literature review. Int J Med Pharm Case Rep 6(4):1–6. https://doi.org/10.9734/IJMPCR/2016/23675
Rajpal S, Kumar R, Dhingra V et al (2000) Isoniazid-induced psychosis – a case report. Indian J Allergy Appl Immunol 14(1):25–27
Singh H, Devi HJG, Chabaria M et al (2018) Isoniazid associated psychosis: case series from a tertiary care centre. Open J Psychiatry Allied Sci 9(2):161–163. https://doi.org/10.5958/2394-2061.2018.00019.8
Jackson SL (1957) Psychosis due to isoniazid. Br Med J 2(5047):743–746. https://doi.org/10.1136/bmj.2.5047.743
Umut G, Dernek B, Kucukparlak I et al (2016) Acute psychotic attack under isoniazid treatment: a case report. Dusunen Adam J Psychiatry Neurol Sci 29(4):379–381. https://doi.org/10.5350/DAJPN2016290411
Wiedorn WS, Ervin F (1954) Schizophrenic like psychotic reactions with administration of isoniazid. AMA Arch Neurol 72(3):321–324. https://doi.org/10.1001/archneurpsyc.1954.02330030055005
Witkowski AE, Manabat CG, Bourgeois JA (2007) Isoniazid-associated psychosis. Gen Hosp Psychiatry 29(1):85–86. https://doi.org/10.1016/j.genhosppsych.2006.10.010
Chaturvedi SK, Upadhyaya M (1988) Secondary mania in a patient receiving isonicotinic acid hydrazide and pyridoxine: case report. Can J Psychiatry 33(7):675–676. https://doi.org/10.1177/070674378803300729
Global Tuberculosis report 2022 (2022) https://www.who.int/publications/i/item/9789240061729. Accessed 3 June 2023
Adams M, Tavakoli H (2006) Gatifloxacin-induced hallucinations in a 19-year-old man. Psychosomatics 47(4):360. https://doi.org/10.1176/appi.psy.47.4.360
Davison K, Roth M (1996) Substance-induced psychosis. Br J Psychiatry 168(5):651. https://doi.org/10.1192/bjp.168.5.651b
Martin SJ, Bowden FJ (2007) Ethambutol toxicity manifesting as acute onset psychosis. Int J STD AIDS 18(4):287–288. https://doi.org/10.1258/095646207780658863
Pachi A, Bratis D, Moussas G et al (2013) Psychiatric morbidity, and other factors affecting treatment adherence in pulmonary tuberculosis patients. Tuberc Res Treat 2013:489865. https://doi.org/10.1155/2013/489865
Li A, Yuan SY, Li QG et al (2023) Prevalence and risk factors of malnutrition in patients with pulmonary tuberculosis: a systematic review and meta-analysis. Front Med (Lausanne) 10:1173619. https://doi.org/10.3389/fmed.2023.1173619
Brodrick BB, Jacobs MA, McAdams CJ (2020) Psychosis in anorexia nervosa: a case report and review of the literature. Psychosomatics 61(2):181–187. https://doi.org/10.1016/j.psym.2019.06.003
Gao J, Zheng P, Fu H (2013) Prevalence of TB/HIV co-infection in countries except China: a systematic review and meta-analysis. PLoS ONE 8(5):e64915. https://doi.org/10.1371/journal.pone.0064915
Chhagan U, Ntlantsana V, Tomita A et al (2021) The dual burden of HIV infection and first-episode psychosis in Africa: a systematic review and meta-analysis. J Nerv Ment Dis 209(8):600–608. https://doi.org/10.1097/NMD.0000000000001366
Wise ME, Mistry K, Reid S (2002) Drug points: neuropsychiatric complications of nevirapine treatment. BMJ 324(7342):879. https://doi.org/10.1136/bmj.324.7342.879
Foster R, Olajide D, Everall IP (2003) Antiretroviral therapy-induced psychosis: case report and brief review of the literature. HIV Med 4(2):139–144. https://doi.org/10.1046/j.1468-1293.2003.00142.x
Masuka JT, Chipangura P, Nyambayo PP et al (2018) A comparison of adverse drug reaction profiles in patients on antiretroviral and antitubercular treatment in Zimbabwe. Clin Drug Investig 38(1):9–17. https://doi.org/10.1007/s40261-017-0579-z
O’Connor C, Patel P, Brady MF (2024) Isoniazid. StatPearls Publishing, Treasure Island (FL)
Thomas L, Raju AP, Chaithra et al (2022) Influence of N-acetyltransferase 2 (NAT2) genotype/single nucleotide polymorphisms on clearance of isoniazid in tuberculosis patients: a systematic review of population pharmacokinetic models. Eur J Clin Pharmacol 78(10):1535–1553. https://doi.org/10.1007/s00228-022-03362-7
Zhang M, Wang S, Wilffert B et al (2018) The association between the NAT2 genetic polymorphisms and risk of DILI during anti-TB treatment: a systematic review and meta-analysis. Br J Clin Pharmacol 84(12):2747–2760. https://doi.org/10.1111/bcp.13722
Thomas L, Raju AP, Chaithra S et al (2024) Influence of N-acetyltransferase 2 polymorphisms and clinical variables on liver function profile of tuberculosis patients. Expert Rev Clin Pharmacol 17(3):263–274. https://doi.org/10.1080/17512433.2024.2311314
Ben-Chetrit E, Rothstein N, Munter G (2013) Ciprofloxacin-induced psychosis. Antimicrob Agents Chemother 57(8):4079. https://doi.org/10.1128/AAC.00672-13
Bernardo M, Gatell JM, Parellada E (1991) Acute exacerbation of chronic schizophrenia in a patient treated with antituberculosis drugs. Am J Psychiatry 148(10):1402. https://doi.org/10.1176/ajp.148.10.1402
Bhalerao S, Talsky A, Hansen K et al (2006) Ciprofloxacin-induced manic episode. Psychosomatics 47(6):539–540. https://doi.org/10.1176/appi.psy.47.6.539
Hakko E, Mete B, Ozaras R et al (2005) Levofloxacin-induced delirium. Clin Neurol Neurosurg 107(2):158–159. https://doi.org/10.1016/j.clineuro.2004.05.006
McDonald CA, Addis S (2003) Cephalosporin-induced psychosis. Aust N Z J Psychiatry 37(5):627–628. https://doi.org/10.1046/j.1440-1614.2003.01243.x
Reeves RR (1992) Ciprofloxacin-induced psychosis. Ann Pharmacother 26(7–8):930–931. https://doi.org/10.1177/106002809202600716
World Health Organization (1992) The ICD-10 classification of mental and behavioural disorders: clinical descriptions and diagnostic guidelines. World Health Organization, Geneva. https://www.who.int/publications/i/item/9241544228. Accessed 02 Jun 2024
Poole R, Brabbins C (1996) Drug induced psychosis. Br J Psychiatry 168(2):135–138. https://doi.org/10.1192/bjp.168.2.135
Toriumi K, Miyashita M, Suzuki K et al (2021) Vitamin B6 deficiency hyperactivates the noradrenergic system, leading to social deficits and cognitive impairment. Transl Psychiatry 11(1):262. https://doi.org/10.1038/s41398-021-01381-z
Sandyk R, Pardeshi R (1990) Pyridoxine improves drug-induced parkinsonism and psychosis in a schizophrenic patient. Int J Neurosci 52(3–4):225–232. https://doi.org/10.3109/00207459009000524
Lerner V, Miodownik C, Kaptsan A et al (2001) Vitamin B(6) in the treatment of tardive dyskinesia: a double-blind, placebo-controlled, crossover study. Am J Psychiatry 158(9):1511–1514. https://doi.org/10.1176/appi.ajp.158.9.1511
Lerner V, Bergman J, Statsenko N et al (2004) Vitamin B6 treatment in acute neuroleptic-induced akathisia: a randomized, double-blind, placebo-controlled study. J Clin Psychiatry 65(11):1550–1554. https://doi.org/10.4088/jcp.v65n1118
Adelufosi AO, Abayomi O (2015) Ojo TM (2015) Pyridoxal 5 phosphate for neuroleptic-induced tardive dyskinesia. Cochrane Database Syst Rev 4:CD010501. https://doi.org/10.1002/14651858.CD010501.pub2
Shams-Alizadeh N, Bakhshayesh H, Rezaei F et al (2018) Effect of vitamin B6 versus propranolol on antipsychotic-induced akathisia: a pilot comparative double-blind study. Iran J Pharm Res 17(Suppl):130–135
Chan TYK (1999) Pyridoxine ineffective in isoniazid-induced psychosis. Ann Pharmacother 33(10):1123–1124. https://doi.org/10.1345/aph.19022
Yadav R, Kumar M, Kumar N et al (2022) Pyridoxine in isoniazid-induced psychosis. Sudan J Paediatr 22(2):190–192. https://doi.org/10.24911/SJP.106-1584524803
Choke A, Perumal M, Howlett M (2007) Lorazepam prescription and monitoring in acute adult psychiatric wards. Psychiatri Bull 31(8):300–303. https://doi.org/10.1192/PB.BP.106.012328
Mackin P, Thomas SH (2011) Atypical antipsychotic drugs. BMJ 342:d1126. https://doi.org/10.1136/bmj.d1126
Atmaja DS, Yulistiani S et al (2022) Detection tools for prediction and identification of adverse drug reactions in older patients: a systematic review and meta-analysis. Sci Rep 12(1):13189. https://doi.org/10.1038/s41598-022-17410-w
Bihan K, Lebrun-Vignes B, Funck-Brentano C et al (2020) Uses of pharmacovigilance databases: an overview. Therapie 75(6):591–598. https://doi.org/10.1016/j.therap.2020.02.022
Acknowledgements
K.B. is thankful to Dr. TMA Pai Ph.D. scholarship from Manipal Academy of Higher Education, Manipal. L.T. and T.B. thank the Indian Council of Medical Research (ICMR) for providing Senior Research Fellowships (No. 45/25/2020/PHA/BMS and No. TB-Fellowship/9/2022-ECD-I, respectively). C. is thankful to the Junior Research Fellowship from ICMR (F.No.5/8/5/45/multicentric study/2019/ECD-1). M.R. is thankful to the ICMR grant (F.No.5/8/5/45/multicentric study/2019/ECD-1).
Funding
Open access funding provided by Manipal Academy of Higher Education, Manipal
Author information
Authors and Affiliations
Contributions
Concept and design: M.R.
Literature search: K.B, R.A, L.T, S.N.
Data extraction: K.B, L.T, S.N.
Quality assessment: K.B, R.A, L.T, T.B.
Drafting of the manuscript: K.B, R.A, M.R.
Reviewing and editing: K.B, R.A, L.T, T.B, S.N, C.S, S.S.M, K.S, K.U, M.R.
Corresponding author
Ethics declarations
Ethics approval
Not applicable.
Consent to participate
Not applicable.
Competing interests
The authors declare no competing interests.
Additional information
Publisher's Note
Springer Nature remains neutral with regard to jurisdictional claims in published maps and institutional affiliations.
Supplementary Information
Below is the link to the electronic supplementary material.
Supplemental Table 1
(DOCX 29 kb)
Supplemental Table 2
(DOCX 14 kb)

Supplemental Fig. 1
Isoniazid induced psychosis symptoms profile. Footnote: * Cases that were not isoniazid induced psychosis has been excluded from the case series. Abbreviation: Form_tht_dis: Formal thought disorder (PNG 153 kb)

Supplemental Fig. 2
Quality assessment of included studies. Footnote: 1Tool for evaluating the methodological quality of case reports and case series developed by Murad et al. 2* Cases that were not isoniazid induced psychosis has been excluded from the case series. 3 Q1: Does the patient(s) represent(s) the whole experience of the investigator (centre) or is the selection method unclear to the extent that other patients with similar presentation may not have been reported? Q2: Was the exposure adequately ascertained? Q3: Was the outcome adequately ascertained? Q4: Were other alternative causes that may explain the observation ruled out? Q5: Was there a challenge/rechallenge phenomenon? Q6: Was there a dose-response effect? Q7: Is the case(s) described with sufficient details to allow other investigators to replicate the research or to allow practitioners make inferences related to their own practice? Abbreviation: Q: Question (PNG 134 kb)
Rights and permissions
Open Access This article is licensed under a Creative Commons Attribution 4.0 International License, which permits use, sharing, adaptation, distribution and reproduction in any medium or format, as long as you give appropriate credit to the original author(s) and the source, provide a link to the Creative Commons licence, and indicate if changes were made. The images or other third party material in this article are included in the article's Creative Commons licence, unless indicated otherwise in a credit line to the material. If material is not included in the article's Creative Commons licence and your intended use is not permitted by statutory regulation or exceeds the permitted use, you will need to obtain permission directly from the copyright holder. To view a copy of this licence, visit http://creativecommons.org/licenses/by/4.0/.
About this article
Cite this article
B, K., Appaji, R., Thomas, L. et al. Characteristics of isoniazid-induced psychosis: a systematic review of case reports and case series. Eur J Clin Pharmacol (2024). https://doi.org/10.1007/s00228-024-03738-x
Received:
Accepted:
Published:
DOI: https://doi.org/10.1007/s00228-024-03738-x