Abstract
There are only few studies on shallow Antarctic benthic communities associated with habitats affected by intense mineral sedimentation inflow. The analysis of macrofaunal communities associated with two shallow, isolated glacial coves was performed in Admiralty Bay (King George Island) and compared with non-disturbed sites. Multivariate analyses (hierarchical classification, nMDS) clearly separated glacial cove communities (two assemblages) from the sites situated outside both basins (two assemblages). The community influenced by the streamflow of glacial discharge of meltwater situated in the area with sandy–clay–silt sediments had a very low species richness, diversity and abundance. It was dominated by eurytopic, motile deposit feeding polychaetes such as Mesospio moorei, Tharyx cincinnatus and Leitoscoloplos kerguelensis as well as the bivalve Yoldia eightsi. The second glacial community of the area located at a grater distance from the outlet of the stream was characterized by sandy–clay–silt and clay–silt deposits and showed also a low diversity and species richness. The most abundant here were peracarid crustaceans, with the dominant opportunistic feeder Cheirimedon femoratus. Community from the non-disturbed area with silty–clay–sand, and silty–sand sediments had higher species richness and diversity. The assemblage of fauna from the sandy bottom has values of those two indexes similar to those found in the disturbed areas.
Similar content being viewed by others
Avoid common mistakes on your manuscript.
Introduction
Polar regions are affected by a high level of disturbance associated with iceberg scour and glacial sedimentation (Barnes 1999; Gutt 2001; Smale and Barnes 2008); however, not all aspects of these problems were studied equally in the Arctic and in the Antarctic. While ice scour disturbance was intensively studied in both polar regions (Conlan et al. 1998; Gutt and Piepenburg 2003; Brown et al. 2004; Conlan and Kvitek 2005; Gerdes et al. 2008; Smale 2008a), it was recently emphasized by Smale and Barnes (2008) that studies on benthic communities affected by mineral suspension inflow are still lacking from the Southern Ocean.
Arctic benthic communities inhabiting shallow glacial bays were intensively studied, mostly on Spitsbergen (Wlodarska et al. 1996; Wlodarska-Kowalczuk et al. 1998, 1999; Wlodarska-Kowalczuk and Weslawski 2001). In the Antarctic, similar studies are still scarce and often are based on higher taxa abundance and photographic surveys or are focused on only one group of invertebrates (Richardson and Hedgpeth 1977; Sicinski et al. 1996; Gambi et al. 1997; Sahade et al. 1998; Sicinski 2004; Brown et al. 2004; Bowden 2005).
Shallow glacial bays are considered to be very peculiar habitats inhabited mostly by opportunistic, motile species, living under the influence of very serious and long-lasting disturbance (Wlodarska-Kowalczuk et al. 1998, 1999). Among the key factors shaping those habitats are the high rate of mineral sedimentation, low sediment stability, high water turbidity and low primary production. These conditions are accompanied by an increase in freshwater inflow, resulting in temperature and salinity oscillations. This may have a great influence on benthic fauna, especially on sessile suspension feeders, affecting their feeding abilities and/or reproduction (Rhoads 1974; Moore 1977).
The ongoing climate warming observed in the West Antarctic Peninsula region (Walsh 2009), which resulted in strong retreat of glaciers during the last 60 years (Cook et al. 2005), may change the structure of Antarctic benthic communities (Smale and Barnes 2008). According to predictions for both polar regions, the rate of suspension matter inflow driven by climate change will significantly increase in a short period of time (Syvitski and Andrews 1994; Smale and Barnes 2008) and will be especially pronounced in the Antarctic. The main reason for these expectations is the relative unimportance of this factor in the evolution of Southern Ocean shelf benthic communities, which are isolated and dominated by large sessile suspension feeders (Smale and Barnes 2008). It was also observed that some benthic invertebrates are even more sensitive to glacial sedimentation than to the direct impact of ice (Slattery and Bockus 1997). In this context, the studies of shallow glacial basins from the Antarctic, especially from the West Antarctic Peninsula region, are important, because the basic knowledge will be needed to assess possible future changes in those communities.
The aim of this study is to describe and compare benthic, macrofaunal communities associated with two different glacial coves with those from the sites located outside both basins. The first one (Ecology Glacier lagoon) is a very shallow, young glacial cove located in the vicinity of the large tidewater glacier, and the second one (Herve Cove) is deeper and affected by relatively small glacier with tidewater cliff.
Materials and methods
Study area
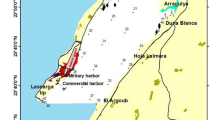
Admiralty Bay is a fjord-like embayment of King George Island. It consists of a central basin and three inlets: Ezcurra Inlet, Martel Inlet and MacKellar Inlet (Fig. 1). The central basin is the deepest part of the bay and it is open to the Bransfield Strait. Glacier cover is distributed mainly along the eastern coast of central basin and in the inlets (Braun and Grossmann 2002). Shores geomorphology of Ezcurra Inlet was described by Marsz (1983). The highest rates of suspended matter in Admiralty Bay were measured in the inner parts of Ezcurra Inlet and in small coves and lagoons located in front of the glaciers (Pecherzewski 1980). In Admiralty Bay, several small glacial coves of different age can be found (Marsz 1983). Herve Cove and Ecology Glacier lagoon are two basins characterized by different stages of development as well as by differences in hydrography and hydrology. Both are to some degree isolated from the open waters of Admiralty Bay (Marsz 1983) (Table 1; Fig. 1).
Herve Cove is a small glacial cove located on the southern coast of Ezcurra Inlet. It is influenced by the shallow tidewater glacier (littoral–shallowest sublittoral)—the edge of the steep outlet glacier (Dera Icefall) (Fig. 1). Mean annual content of suspended matter in the waters of this cove was estimated as 46 mg/dm3. The highest values were noted in the areas located near the outlet of the streamflow of glacial discharge of meltwater. Quantities of mineral suspended matter in this part of Herve Cove reached 270 mg/dm3 (Rakusa-Suszczewski 1995; Sicinski et al. 1996).
Ecology Glacier lagoon is a recently formed glacial cove located on the western shore of central basin in front of the shallow tidewater glacier (littoral–shallowest sublittoral)—the edge of the outlet glacier (Ecology Glacier) (Fig. 1). The amount of suspended matter in Ecology Glacier lagoon reaches the highest values recorded for Admiralty Bay. While the average quantity of suspended matter in open waters of Admiralty Bay was estimated as 12.4 mg/dm3, such values in front of the Ecology Glacier exceeded 150 mg/dm3 (Pecherzewski 1980).
Sampling
Forty-four samples were collected in the summer season of 1993/94 including twenty-seven samples from Herve Cove (HC), six samples from Ezcurra Inlet (EZ) (reference site for Herve Cove), nine samples from Ecology Glacier lagoon (EG) and two samples from the shallow sublittoral outside this basin (EGRS) (reference site for Ecology Glacier lagoon) (Fig. 1).
Samples from Herve Cove were collected using a van Veen grab (0.1 m2). Samples from the shallowest parts of the bottom (all samples from Ecology Glacier lagoon and both reference sites) were collected with the Tvärminne sampler (565 cm2) (Kangas 1972). Abundance values from those samples were converted to 0.1 m2 surface area. All samples were sieved on a 0.5-mm mesh sieve.
Preliminary results from the same set of samples collected in Herve Cove (from HC1 to HC31) were already published by Sicinski et al. (1996). That study was based on the abundance and biomass values mostly of higher taxa (altogether 24 taxa, 11 taxa (mostly polychaetes) of which were identified to the species level) and did not include the sediment analysis. The samples in the present study have the same numeration as used in the paper by Sicinski et al. (1996).
Granulometric analysis
Subsamples for granulometric analysis were taken from 38 out of the 44 collected. The analysis of sediments was carried out using an areometric method. Sediment was sieved on a 1-mm mesh size sieve to separate the skeletal fraction. Fractions with grain diameter below 1 mm were further sorted. On the basis of this analysis, the cumulative curves of granulation were constructed. The content of sand, silt and clay fractions was measured. The classification and nomenclature of sediments proposed by Shepard (1954) (Fig. 2) were used as particularly useful for weakly sorted sediments. The sorting coefficient (So) and median of grain diameter expressed in the units of Md coefficient ф = −log2 d (d—median grain size in millimeters) were also calculated (Krumbein 1934). Those values were used to locate the samples in the coordinate system represented by the sorting coefficient and Md coefficient (Fig. 2).
Data analysis
Bray–Curtis similarity index was used to calculate the similarities between the samples on the basis of density (ind./0.1 m2) of 78 taxa in 44 samples. Hierarchical agglomerative clustering was performed using the group-average method. Non-metric multidimensional scaling (nMDS) was used for sample ordination. Data were square-root-transformed to reduce the influence of dominant species upon the results of the analysis (Clarke and Warwick 1994). Indices of species richness (Margalef d = (S-1)/logN), species diversity (Shannon index H′ = −Σ p i ln p i ) as well as evenness (Pielou J′ = H′/lnS) were measured for each sample (Magurran 2004). The PRIMER package v. 6 was used for this analysis.
Mean values with standard errors and standard deviations were calculated for density values and all richness and diversity indices in each assemblage. Differences between these indices in the distinguished groups were tested using one-way ANOVA. Levene’s test was used to estimate the homogeneity of variance. Tukey’s test for groups with unequal number of replications was used for post hoc multiple comparisons using the STATISTICA 6 package. The group A that contains only 2 samples was excluded from the testing.
Results
Seventy-seven species (26,513 individuals) were recorded in the investigated area (Table 2). Four assemblages were distinguished in the cluster analysis (Fig. 3). Similar pattern was found on the nMDS plot. The relatively low stress value indicates a good two-dimensional representation of multidimensional space (Fig. 4).
Assemblage A
This assemblage represents the shallow bottom areas from the Ecology Glacier lagoon reference site (only 2 samples, both from 6 m depth) (Figs. 1, 3, 4). Only 10 species were found in this assemblage. The most abundant were amphipods: Hippomedon kergueleni (260.0 ± 70.7 ind./0.1 m2, F = 100.0), Cardenio paurodactylus (96.0 ± 0.0 ind./0.1 m2, F = 100) and Monoculodes scabriculosus (29.0 ± 2.8 ind./0.1 m2, F = 100.0). The polychaete Travisia kerguelensis (27.0 ± 9.8 ind./0.1 m2, F = 100.0) was also an important element of this assemblage and the only non-crustacean member of the group (Table 2). The bottom was characterized by sandy deposits (Fig. 2).
Assemblage B
Samples grouped in this cluster were distributed in Ecology Glacier lagoon and in a part of Herve Cove situated in a greater distance from the outlet of streamflow of glacial discharge of meltwater (Figs. 1, 3, 4). This assemblage consists of 40 species. Most of them were amphipod crustaceans with Cheirimedon femoratus (599.9 ± 544.9 ind./0.1 m2, F = 100.0), Djerboa furcipes (176.2 ± 457.1 ind./0.1 m2, F = 47.4) and Orchomenella cf. ultima (16.3 ± 40.7 ind./0.1 m2, F = 57.9) being the most abundant and frequent taxa. The sea anemone Edwardsia sp. was also an important element of these group (19.2 ± 45.1 ind./0.1 m2, F = 47.4) (Table 2). The assemblage is characterized by lowest mean richness, diversity and evenness values (Fig. 5). The sediments were mostly sandy–clay–silt and clay–silt (Fig. 2).
Assemblage C
This cluster grouped the samples from the reference site in Ezcurra Inlet as well as three samples from Herve Cove located close to the submerged moraine (Figs. 1, 3, 4). Fifty-nine species were found in this assemblage (Table 2). It is the group with highest densities, species richness and diversity values (Fig. 5). The most abundant and frequent species were crustaceans Typhlotanais grahami (176.4 ± 160.9 ind./0.1 m2 , F = 77.7), Eudorella splendida (37.7 ± 37.6 ind./0.1 m2, F = 88.8), Heterophoxus videns (48.1 ± 57.6 ind./0.1 m2, F = 100.0) and Philomedes charcoti (66.3 ± 144.5 ind./0.1 m2, F = 77.7), polychaetes: Apistobranchus gudrunae (60.4 ± 99.6 ind./0.1 m2 , F = 66.6), Ceratonereis (C.) antarctica (56.2 ± 62.6 ind./0.1 m2, F = 55.5), Tharyx cincinnatus (75.5 ± 133.4 ind./0.1 m2, F = 88.8) and Rhodine intermedia (60.8 ± 82.0 ind./0.1 m2, F = 100.0) as well as the bivalve Yoldia eightsi (35.4 ± 44.7 ind./0.1 m2, F = 100.0). The sediments in this group of samples can be described as silty–clay–sand and silty–sand (Fig. 2).
Assemblage D
This cluster grouped the samples from a part of Herve Cove situated in a vicinity of the glacial discharge of meltwater outlet (Figs. 1, 3, 4). It is an assemblage with the lowest mean density value. Also, the richness and diversity were relatively low in this group (Fig. 5). Twenty-nine species were found in this assemblage (Table 2). The most abundant and frequent were Mesospio moorei (35.8 ± 52.5 ind./0.1 m2, F = 83.3), Yoldia eightsi (14.1 ± 25.4 ind./0.1 m2, F = 66.6), Tharyx cincinnaus (10.8 ± 14.1 ind./0.1 m2, F = 66.6) and Leitoscoloplos kerguelensis (7.3 ± 14.2 ind./0.1 m2, F = 83.3). The sediments are mostly sandy–clay–silt (Fig. 2).
There were significant differences between the three groups (B, C and D) in respect to Shannon index and Margalef index. Evenness values were significantly different between groups B/C and B/D (Tukey’s test, P < 0.05). No significant differences were found in the pair C/D. Density values were significantly different in pairs B/D and C/D, whereas there were no differences for the pair B/C (Tukey’s test, P < 0.05).
Discussion
Macrobenthic fauna associated with habitats of dynamic sedimentation was not an object of intensive studies in the Antarctic. Only 13% of the Antarctic coast line is covered by glaciers with tidewater cliffs and those sites are scattered all over the continent (Gutt 2001). Besides, in the West Antarctic, the terrigenous sediment deposition originates mostly from grounded and floating ice shelves (Anderson et al. 1982). It was stressed that benthic fauna of the Southern Ocean has not evolved under a strong pressure of the mineral sedimentation inflow and thus may be sensitive to any increase in such processes (Smale and Barnes 2008). On the Antarctic islands, like South Shetlands archipelago, the influence of sedimentation on benthos was already described in the fjords and it is an important mechanism shaping the bottom communities (Sicinski 2004; Momo et al. 2008; Pabis et al. 2011). The sedimentation inflow on a small spatial scale could have a great influence on the composition and diversity of the bottom fauna, especially in areas located close to the glacial termini (Wlodarska-Kowalczuk et al. 1998, 1999; Wlodarska-Kowalczuk and Weslawski 2001; Wlodarska-Kowalczuk and Pearson 2004). The cluster analysis in the present study separated glacial cove communities (assemblages B and D) from the macrobenthic fauna associated with sites located outside both basins (assemblages A and C). The comparison of assemblage A with other groups and literature data may be difficult due to a very small number of samples collected in this bottom area. The outer assemblage of Herve Cove (C) unites the samples from both sides of the moraine. It shows possibility of fauna migration into the cove, as well as the influence of sedimentation, because some of the species building this assemblage are absent from the bottom areas located in the vicinity of the glacial discharge of meltwater (assemblage D). Those species are able to enter into the lagoon but are absent from the glacial assemblage. Both glacial cove communities had low species richness and diversity. The preliminary analysis by Sicinski et al. (1996) based on abundance and biomass values of higher taxa showed similar patterns of benthos distribution in Herve Cove. The total biomass values were the lowest in the vicinity of a glacial discharge of meltwater and had the highest values in samples collected close to submerged moraine (HC15, HC16 and HC18—assemblage C). The benthic fauna from Ecology Glacier lagoon shows similarities with bottom communities in the middle part of Herve Cove even in its deeper part. In Herve Cove, the gradient of changes associated with the distance from the source of disturbance is more pronounced, and this basin seems to be a more complicated system, characterized by more heterogeneous bottom sediments and more diversified faunal groupings than a small and very shallow lagoon in the vicinity of Ecology Glacier.
The community associated with the area located close to the glacial stream (assemblage D) and characterized mostly by sandy–clay–silt deposits was dominated by eurytopic polychaetes: Mesospio moorei, Tharyx cincinnatus and Leitoscoloplos kerguelensis, as well as the bivalve Yoldia eightsi. The similar pattern was observed in the Arctic glacial bays and inner fjord areas with motile deposit feeding polychaetes (Tharyx, Leitoscoloplos, Cosssura) and bivalves like Yoldiidae and Thyasiridae as the important members of the communities (Syvitski et al. 1989; Wlodarska-Kowalczuk et al. 1998, 1999; Wlodarska-Kowalczuk and Pearson 2004). Similar species composition was also observed in sites located close to the glaciers in Arthur Harbor (Richardson and Hedgpeth 1977) and in the polychaete communities inhabiting the shallows of Chile Bay (Gallardo et al. 1988). Polychaetes, like Leitoscoloplos and Tharyx, were among the species colonizing the bottom after iceberg disturbance in the McMurdo Sound (Lenihan and Oliver 1995) and dominated the shallow areas of Terra Nova Bay (Gambi et al. 1997). Mesospio moorei was also a dominant species in shallow parts of the Martel Inlet affected by ice scour disturbance (Bromberg et al. 2000) as well as in shallow areas of Borge Bay (Hardy 1972) and Morbihan Bay (Chardy et al. 1976). Laternula elliptica is sensitive to influence of meltwater streams (Mercuri et al. 2008). On the other hand, this species is able to reduce the metabolic rate under the influence of high sedimentation rate and it is to a certain degree adapted to the changing environmental conditions (Philipp et al. 2011). However, it was pointed out that Y. eightsi, the species adapted to high sedimentation rate, will be more abundant in disturbed sites and may take over the bottom areas of reduced abundance of L. elliptica. In consequence, it may lead to great changes in marine communities facing the ongoing climate warming because L. elliptica is a large, deep burrowing species that has a great influence upon bentho-pelagic carbon flux (Philipp et al. 2011). Results showing the distribution patterns of both species in the studied glacial coves strongly support these scenarios.
The second glacial cove assemblage (assemblage B) was dominated by motile epibenthic species (mostly amphipods), with the most abundant being Cheirimedon femoratus (599.9 ± 544.9, F = 100). The most important polychaete species was Mesospio moorei (1.7 ± 5.7, F = 26.3). C. femoratus is known to bury in bottom deposits during the day time, but it is also most probably a very good vertical swimmer (Bregazzi 1973). It was observed as a species preferring sandy bottom (Bregazzi 1972), while in Herve Cove it was very abundant in the glacially affected area on the sandy–clay–silt and clay–silt sediments. On the other hand, C. femoratus occurred also in high numbers in stony uppermost sublittoral of Admiralty Bay (Jazdzewski et al. 2001). It is the species that was found commonly on animal carcasses, but it is also recognized as an opportunistic feeder (Presler 1986; Smale et al. 2007; Jazdzewska 2009). Mesospio moorei was also recorded as a dominant polychaete species in Herve Cove (Sicinski 2004) and together with Leitoscoloplos kerguelensis, Tharyx cincinnatus and Rhodine intermedia, it belongs to the most abundant and eurytopic species of polychaetes found in Admiralty Bay (Sicinski 2004). The high share of an amphipod Djerboa furcipes in assemblage B could be associated with the fragments of macroalgae that were observed inside small lagoons in Admiralty Bay (Rakusa-Suszczewski 1995). This species feeds on macroalge (Obermüller et al. 2007) and was found in places with decaying macroalgal material (Richardson and Hedgpeth 1977). Generally, the high share of motile species and often secondary consumers (especially amphipods) in glacial coves is similar to general trends described by Smale (2008a) for the sites influenced by ice scour disturbance.
The sea anemone Edwardsia sp. was a dominant species in both glacial cove assemblages. Members of this genus burrow in muddy bottom sediments, and it was often observed in the Antarctic shallows (Williams 1981). Those infaunal anemones were found in polluted sediments around McMurdo Station together with polychaetes like Tharyx or Ophryotrocha claparedei (Lenihan et al. 1995). A closely related Halacampoides was very abundant in the sites affected by strong melt water runoff in Signy Island (Ansell and Peck 2000). This species, together with bivalves, represented also the core of the biomass in Herve Cove (Sicinski et al. 1996).
Almost complete absence of tanaids in both glacial assemblages studied may be due to the low mobility of these crustaceans. Species like Nototanais antarcticus live in the aggregations of tangled corridors, while typhlotanaids are known as tube-dwellers (Hassack and Holdich 1987; Blazewicz-Paszkowycz 2007).
While the highest sedimentation rates were observed in Herve Cove close to the glacial stream (over 200 mg/dm3) and in the proximity of Ecology Glacier (150 mg/dm3) (Pecherzewski 1980; Sicinski et al. 1996; Sicinski 2004) and the bottom deposits clearly reflect the influence of glacial sedimentation in both basins (Fig. 2), these are probably the most important factors structuring the benthos of two coves studied. Glacial stream could also influence the bottom fauna by changes in salinities. Although the surface salinities in a part of Herve Cove located close to the stream outlet were often lower than in open waters of Admiralty Bay (22–26 PSU), on the bottom, even in shallow depth (2–3 m), salinity was again similar (over 34 PSU) to the values found in open waters (Szafranski and Lipski 1982; Kidawa personal communication). Moreover, both basins are isolated from the open waters of Admiralty Bay, and there is no influence of ice scour in these two areas of the bay, unlike as it was observed in Martel Inlet shallows (Echeverria et al. 2005).
The species composition and the patterns of distribution of bottom communities associated with glacial coves and the sites not affected by glacier runoff in Admiralty Bay show similarities with the stages of community recovery after iceberg or anchor ice disturbance (Lenihan and Oliver 1995; Conlan et al. 1998; Bromberg et al. 2000).
The species richness and diversity in assemblage C was higher than in glacial coves. Species composition of both undisturbed bottom areas showed many similarities with other non-disturbed sites in Admiralty Bay. Crustacean species like Cardenio paurodactylus, Hippomedon kergueleni or Monoculodes scabriculosus were among the most abundant inhabitants of shallow water bottom areas of Admiralty Bay central basin (Jazdzewski et al. 1991). The same concerns the polychaete Travisia kerguelensis, which was a very important element of sandy bottom communities in places located far from glaciers (Sicinski and Janowska 1993 and references therein).
In Antarctic larger coves or small basins, but with a direct connection with open waters, many large, sessile species were found, including such pioneer invertebrates, like a bryozoan Fenestrulina rugula and an ascidian Molgula pedunculata as well as some other members of the groups and common sea-urchin Sterechinus neumayeri (Sahade et al. 1998; Brown et al. 2004; Bowden 2005; Smale 2008b). In our study, there were no ascidians and bryozoans present. S. neumayeri was found only in assemblage C but in very small numbers and was absent in the glacial coves. This may be due to the hydrology and hydrography of Herve Cove and Ecology Glacier lagoon, which are both very sheltered and isolated basins. Moreover, large suspension feeders, which may survive the ice scour events in other sites, are also very sensitive to glacial sedimentation. On the other hand, those results may also be associated with differences in sampling methods: van Veen grab in this study and mostly photographic surveys in the others. However, the distribution patterns of megafaunal, suspension feeding communities are similar to results from this study, with the species richness and diversity increasing with the distance from the source of disturbance and almost complete absence of the large suspension feeders in the shallowest and most disturbed areas (Sahade et al. 1998; Smale 2008b). Similar patterns were also described by Barnes and Brockington (2003) at Adelaide Island with increase in diversity and biomass along the depth gradient from 3 to 35 m as a pattern related to ice disturbance. In the same area, the number of encrusting species was 50% lower on site with higher ice scour frequency than on less disturbed site (Brown et al. 2004). The complete lack of similar invertebrates in Herve Cove and Ecology Glacier lagoon showed that the chronic disturbance associated with meltwater streams has even greater impact on those animals than the ice scour, which causes rather patchy distribution but not continuous zonation (Brown et al. 2004; Smale 2008b).
Although both investigated coves are more or less isolated from the open waters of Admiralty Bay, the migration of fauna into these basins is possible. Along with the transport of planktonic larvae and bottom migration of motile species, like some crustaceans or polychaetes, there can be at least two other important ways of fauna transport to these sites. Broken, hooked spits and submerged moraines anyway enable the exchange of cove water with open marine waters. It was also observed in the Ecology Glacier lagoon that marine water entering the cove carries large fragments of macroalge (Rakusa-Suszczewski 1995). Holdfasts of those macroalgae can be an important vector of transport of animals into the cove (Edgar 1987). Polychaetes, like Tharyx cincinnatus, Leitoscoloplos kerguelensis and Rhodine intermedia, were among the most frequent and/or abundant species in the holdfasts of Himantothallus grandifolius in Admiralty Bay (Pabis and Sicinski 2010). Another vector could be associated with the transportation of animal carcasses into the lagoon together with the necrophagous invertebrates. In King George Bay, a large number of amphipods were found on a stranded fur seal carcass (Jazdzewska 2009), including species like Cheirimedon femoratus and Hippomedon kergueleni that were found in both glacial coves. Besides both of those species and Orchomenella cf ultima were very abundant in the shallow sublittoral of Admiralty Bay, especially in the central basin (Jazdzewski et al. 1991, 2001).
Analysis of bottom communities associated with glacial coves shows a clear gradient from the non-disturbed bottom areas to the highly disturbed sites with decrease in species richness and abundance as well as the increasing dominance of mostly motile, highly eurytopic species. This pattern may reflect the possible changes in Antarctic communities associated with the climate change. Similar observations were done in the Arctic (Wlodarska-Kowalczuk and Weslawski 2001). This effect could become more pronounced when climate warming will increase the glacier activity. It may lead to substantial homogenization of shallow bottom habitats and cause larger-scale changes in the richness, diversity and trophic structure of bottom communities in the Arctic as well as in the region of Antarctic Peninsula (Smale and Barnes 2008; Weslawski et al. 2011).
To evaluate properly possible future changes, the baseline knowledge on the bottom fauna associated with sedimentary environments is needed. Particularly small, isolated glacial coves are good sites for studying the influence of sedimentation on benthic fauna. Future studies should be focused on those types of basins in various regions of the Antarctic. Repeated monitoring of sites in several years periods could help to answer further questions concerning the influence of climate change and glacier retreat upon the bottom fauna of the West Antarctic Peninsula region.
References
Anderson JB, Kurtz DD, Weaver F, Weaver M (1982) Sedimentation on the West Antarctic continental margin. In: Craddock C (ed) Antarctic geoscience: symposium on antarctic geology and geophysics. University of Wisconsin Press, Madison, pp 1003–1012
Ansell AD, Peck LS (2000) Burrowing in the Antarctic anemone, Halcampoides sp. from Signy Island. J Exp Mar Biol Ecol 252:45–55
Barnes DKA (1999) The influence of ice on polar nearshore benthos. J Mar Biol Assoc UK 79:401–407
Barnes DKA, Brockington S (2003) Zoobenthic biodiversity, biomass and abundance at Adelaide Island, Antarctica. Mar Ecol Prog Ser 249:145–155
Blazewicz-Paszkowycz M (2007) A revision of the family Typhlotanaidae Sieg, 1984 (Crustacea: Tanaidacea) with the remarks on the Nototanaidae Sieg, 1976. Zootaxa 1598:1–141
Bowden DA (2005) Quantitative characterization of shallow marine benthic assemblages at Ryder Bay, Adelaide Island, Antarctica. Mar Biol 146:1235–1249
Braun M, Grossmann H (2002) Glacial changes in the areas of Admiralty Bay and Potter Cove, King George Island, maritime Antarctica. In: Beyer L, Bolter M (eds) Geoecology of the Antarctic ice-free coastal landscapes. Springer, Berlin, pp 75–90
Bregazzi PK (1972) Habitat selection by Cheirimedon femoratus (Pfeffer) and Tryphosella kergueleni (Miers) (Crustacea: Amphipoda). Br Antarct Surv Bull 31:21–31
Bregazzi PK (1973) Locomotor activity rhythms in Tryphosella kergueleni (Miers) and Cheirimedon femoratus (Pfeffer) (Crustacea, Amphipoda). Br Antarct Surv Bull 33:17–32
Bromberg S, Nonato EF, Corbisier TN, Petti MAV (2000) Polychaete distribution in the near-shore zone of Martel Inlet, Admiralty Bay (King George Island, Antarctica). Bull Mar Sci 6:175–188
Brown KM, Fraser KPP, Barnes DKA, Peck LS (2004) Links between the structure of an Antarctic shallow-water community and ice-scour frequency. Oecologia 141:121–129
Chardy P, Desbruyeres D, Laurec A (1976) Analyse multivariable des taxocenoses annelidiennes du Golfe du Morbihan. Com Nat Franc Rech Antarct 39:97–105
Clarke KR, Warwick RM (1994) Change in marine communities: an approach to statistical analysis and interpretation. Natural Environment Research Council, Plymouth
Conlan KE, Kvitek RG (2005) Recolonization of soft-sediment ice scours on an exposed Arctic coast. Mar Ecol Prog Ser 286:21–42
Conlan KE, Lenihan HS, Kvitek RG, Oliver JS (1998) Ice scour disturbance to benthic communities in the Canadian High Arctic. Mar Ecol Prog Ser 166:1–16
Cook AJ, Fox AJ, Vaughan DG, Ferrigo JG (2005) Retreating glacier fronts on the Antarctic Peninsula over the past half-century. Science 308:541–544
Echeverria CA, Paiva PC, Alves VC (2005) Composition and biomass of shallow benthic megafauna during an annual cycle in Admiralty Bay, King George Island, Antarctica. Antarct Sci 17:312–318
Edgar GJ (1987) Dispersal of faunal and floral propagules associated with drifting Macrocystis pyrifera plants. Mar Biol 95:599–610
Gallardo VA, Medrano SA, Carrasco FD (1988) Taxonomic composition of the sublittoral soft-bottom Polychaeta of Chile Bay (Greenwich Island, South Shetland Islands, Antarctica). Ser Client Inst Antart Chil 37:49–67
Gambi MC, Castelli A, Guizzardi M (1997) Polychaete populations of the shallow soft bottoms off Terra Nova Bay (Ross Sea, Antarctica): distribution, diversity and biomass. Polar Biol 17:199–210
Gerdes D, Isla E, Knust R, Mintenbeck K, Rossi S (2008) Response of Antarctic benthic communities to disturbance: first results from the artificial Benthic disturbance experiment on the eastern Weddell Sea Shelf, Antarctica. Polar Biol 31:1469–1480
Gutt J (2001) On the direct impact of ice on marine benthic communities, review. Polar Biol 24:553–564
Gutt J, Piepenburg D (2003) Scale-dependent impact on diversity of Antarctic benthos caused by grounding of icebergs. Mar Ecol Prog Ser 253:77–83
Hardy P (1972) Biomass estimates for some shallow-water infaunal communities at Signy Island, South Orkney Islands. Br Antarct Surv Bull 31:93–106
Hassack E, Holdich DM (1987) The tubiculous habit amongst the Tanaidacea (Crustacea, Peracarida) with particular reference to deep-sea species. Zool Scri 16:223–233
Jazdzewska A (2009) Antarctic necrophagous lysianassoids from a stranded fur seal carcass. Pol Polar Res 30:29–36
Jazdzewski K, Teodorczyk W, Sicinski J, Kontek B (1991) Amphipod crustaceans as an important component of zoobenthos of the shallow Antarctic sublittoral. Hydrobiologia 223:105–117
Jazdzewski K, De Broyer C, Pudlarz M, Zieliński D (2001) Seasonal fluctuations of vagile benthos in the uppermost sublittoral of a maritime Antarctic fjord. Polar Biol 24:910–917
Kangas P (1972) Quantitative sampling equipment for the littoral benthos. II. IBP Norden 10:9–16
Krumbein WC (1934) Size frequency distributions of sediments. J Sed Petrol 4:65–77
Lenihan HS, Oliver JS (1995) Antropogenic and natural disturbances to marine benthic communities in Antarctica. Ecol App 5:311–326
Lenihan HS, Kiest KA, Conlan KE, Slattery PN, Konar BH, Oliver JS (1995) Patterns of survival and behavior in Antarctic benthic invertebrates exposed to contaminated sediments: field and laboratory bioassay experiments. J Exp Mar Biol Ecol 192:233–255
Magurran AE (2004) Measuring biological diversity. Blackwell Publishing, Carlton
Marsz A (1983) From surveys of the geomorphology of the shores and bottom of the Ezcurra Inlet. Oceanologia 15:209–220
Mercuri G, Tatian M, Momo F, Fuentes V, Sahade R (2008) Massive input of terrigenous sediment into Potter Cove during austral summer and the effects on the bivalve Laternula elliptica: a laboratory experiment. Ber Polar Meeresforsch 571:111–117
Momo FR, Sahade R, Tatian M (2008) Benthic animal communities of Potter Cove (King George Island, Antarctica): observed patterns and explanatory models. Ber Polar Meeresforsch 571:162–167
Moore PG (1977) Inorganic particulate suspensions in the sea and their effects on marine animals. Oceanogr Mar Biol Annu Rev 15:225–363
Obermüller B, Puntarulo S, Abele D (2007) UV-tolerance and instantaneous physiological stress responses of two Antarctic amphipod species Gondogeneia antarctica and Djerboa furcipes during exposure to UV radiation. Mar Env Res 64:267–285
Pabis K, Sicinski J (2010) Polychaete fauna associated with holdfasts of the large brown alga Himantothallus grandifolius in Admiralty Bay, King George Island, Antarctic. Polar Biol 33:1277–1288
Pabis K, Sicinski J, Krymarys M (2011) Distribution patterns in the biomass of macrozoobenthic communities in Admiralty Bay (King George Island, South Shetlands, Antarctic). Polar Biol 34:489–500
Pecherzewski K (1980) Distribution and quantity of suspended matter in Admiralty Bay, King George Island, South Shetland Islands. Pol Polar Res 1:75–82
Philipp EER, Husmann G, Abele D (2011) The impact of sediment deposition and iceberg scour on the Antarctic soft shell clam Laternula elliptica at King George Island, Antarctica. Antarct Sci 23:127–138
Presler P (1986) Necrophagous invertebrates of the Admiralty Bay of King George Island (South Shetland Islands, Antarctica). Pol Polar Res 7:25–61
Rakusa-Suszczewski S (1995) The hydrography of Admiralty Bay and its inlets, coves and lagoons (King George Island, Antarctica). Pol Polar Res 16:61–70
Rhoads DC (1974) Organism-sediment relations on the muddy sea floor. Oceanogr Mar 12:263–300
Richardson MD, Hedgpeth JW (1977) Antarctic soft-bottom, macrobenthic community adaptations to a cold, stable, highly productive, glacially affected environment. In: Llano GA (ed) Adaptations within Antarctic ecosystems. Proceedings of 3-rd SCAR symposium on Antarctic Biology. Smithsonian Institute, Washington, pp 181–196
Sahade R, Tatian M, Kowalke J, Kühne S, Esnal GB (1998) Benthic faunal associations on soft substrates at Potter Cove, King George Island, Antarctica. Polar Biol 19:85–91
Shepard FP (1954) Nomenclature based on salt − silt − clay ratios. J Sed Petrol 24:151–158
Sicinski J (2004) Polychaetes of Antarctic sublittoral in the proglacial zone (King George Island, South Shetland Islands). Pol Polar Res 25:67–96
Sicinski J, Janowska E (1993) Polychaetes of the shallow sublittoral of Admiralty Bay, King George Island, South Shetland Islands. Antarct Sci 5:161–167
Sicinski J, Rozycki O, Kittel W (1996) Zoobenthos and zooplankton of Herve Cove, King George Island, South Shetland Islands, Antarctic. Pol Polar Res 17:221–238
Slattery M, Bockus D (1997) Sedimentation in McMurdo Sound, Antarctica: a disturbance mechanism for benthic invertebrates. Polar Biol 18:172–179
Smale DA (2008a) Ecological traits of benthic assemblages in shallow Antarctic waters: does ice scour disturbance select for small, mobile, secondary consumers with high dispersal potential? Polar Biol 31:1225–1231
Smale DA (2008b) Continuous benthic community change along a depth gradient in Antarctic shallows: evidence of patchiness but not zonation. Polar Biol 31:189–198
Smale DA, Barnes DKA (2008) Likely response of the Antarctic benthos to climate-related changes in physical disturbance during the 21st century, based primarily on evidence from the West Antarctic Peninsula region. Ecography 31:289–305
Smale DA, Barnes DKA, Fraser KPP, Mann PJ, Brown MP (2007) Scavenging in Antarctica: intense variation between sites and seasons in shallow benthic necrophagy. J Exp Mar Biol Ecol 349:405–417
Syvitski JPM, Andrews JT (1994) Climate change: numerical modeling of sedimentation and coastal processes, Eastern Canadian Arctic. Arct Alp Res 26:199–212
Syvitski JPM, Farrow GE, Atkinson RJA, Moore PG, Andrews J (1989) Baffin Island fjord macrobenthos: bottom communities and environmental significance. Arctic 42:232–247
Szafranski J, Lipski M (1982) Characteristic of water temperature and salinity at Admiralty Bay (King George Island) during austral summer 1978–1979. Pol Polar Res 3:7–24
Walsh JE (2009) A comparison of Arctic and Antarctic climate change, present and future. Antarct Sci 21:179–188
Weslawski JM, Kendall MA, Wlodarska-Kowalczuk M, Iken K, Kedra M, Legezynska J, Sejr MK (2011) Climate change effects on Arctic fjord and coastal mecrobenthic diversity—observations and predictions. Mar Biodiv 41:71–85
Williams RB (1981) A sea anemone Edwardsia maridionalis sp. nov., from the Antarctica and preliminary revision of the genus Edwardsia De Quatrefages, 1841 (Coelenterata: Actiniaria). Record Aust Mus 33:325–360
Wlodarska M, Weslawski JM, Gromisz S (1996) A comparison of the macrofaunal community structure and diversity in two arctic glacial bays—a ‘cold’ one off Franz Josef Land and a ‘warm’ one off Spitsbergen. Oceanologia 3:251–283
Wlodarska-Kowalczuk M, Pearson TH (2004) Soft-bottom macrobenthic faunal associations and factors affecting species distribution in an Arctic glacial fjord (Kongsfjord, Spitsbergen). Polar Biol 27:155–167
Wlodarska-Kowalczuk M, Wesławski JM (2001) Impact of climate warming on Arctic benthic biodiversity: a case study of two Arctic glacial bays. Clim Res 18:127–132
Wlodarska-Kowalczuk M, Wesławski JM, Kotwicki L (1998) Spitsbergen glacial bays macrobenthos–a comparative study. Polar Biol 20:66–73
Wlodarska-Kowalczuk M, Szymelfenig M, Kotwicki L (1999) Macro- and meiobenthic fauna of the Yoldiabukta glacial Bay (Isfjorden, Spitsbergen). Pol Polar Res 20:367–386
Acknowledgments
We would like to thank Dr J. Szczechura for the identification of ostracods and Dr W. Teodorczyk for the identification of isopods. Thanks are due as well to E. Janowska MSc for the technical help in sorting the materials. We also want to thank Brigitte Ebbe, Jan Marcin Weslawski and one anonymous reviewer for their valuable comments that helped to improve this article. The study was supported by a grant of Polish Ministry of Science and Higher Education No. 7984/B/P01/2011/40 as well as University of Lodz internal funds. The sampling program was carried out with a support from the Polish Antarctic Station H. Arctowski.
Open Access
This article is distributed under the terms of the Creative Commons Attribution Noncommercial License which permits any noncommercial use, distribution, and reproduction in any medium, provided the original author(s) and source are credited.
Author information
Authors and Affiliations
Corresponding author
Rights and permissions
Open Access This is an open access article distributed under the terms of the Creative Commons Attribution Noncommercial License (https://creativecommons.org/licenses/by-nc/2.0), which permits any noncommercial use, distribution, and reproduction in any medium, provided the original author(s) and source are credited.
About this article
Cite this article
Siciński, J., Pabis, K., Jażdżewski, K. et al. Macrozoobenthos of two Antarctic glacial coves: a comparison with non-disturbed bottom areas. Polar Biol 35, 355–367 (2012). https://doi.org/10.1007/s00300-011-1081-3
Received:
Revised:
Accepted:
Published:
Issue Date:
DOI: https://doi.org/10.1007/s00300-011-1081-3