Abstract
Due to limited data on the pathogenicity of Prohemistomum vivax (P. vivax) and its impacts on fish health, this study aimed to determine the morphological, molecular characteristics, pathogenicity, and histopathological alterations in fish infected with P. vivax. Eight hundred (800) Nile tilapia (Oreochromis niloticus) were collected from various farms in Kafr El Sheikh Governorate. The fish were examined for encysted metacercariae (EMC) in different organs. Tissue specimens were collected and underwent histopathological analysis, expression of stress-related genes, and genetic characterization by sequencing of the internal transcribed spacer 2 (ITS2). P. vivax metacercariae were oval to round in shape and were collected from various organs including the muscle, skin, eyes, intestine, liver, kidney, and gills of infected O. niloticus. Sequencing and phylogenetic analysis of the ITS2 region revealed a 507-bp fragment, confirming parasite identity and matching within the same clade as other P. vivax isolates. Infected fish displayed abdominal hydropsy, skin darkening, and emaciation. P. vivax encysted metacercariae were detected during the study period in 620/800 fish, with an overall prevalence of 77.5%. The seasonal prevalence was 95% in summer, 85% in spring, 55% in autumn, and 75% in winter. The intensity of infection was 1–40 cysts per microscopic field. Histopathological examination of muscles revealed parasitic cysts embedded within muscle fibers, causing severe degeneration and necrosis. Upregulation of cytochrome P450 (cpy1a1), heat shock protein 70 (hsp-70), and tumor suppressor p53 (p53) was recorded in both liver and muscle samples of infected tilapia compared to controls. This indicates activation of detoxification, cellular stress, and apoptotic pathways in response to P. vivax infection. There is limited data available on the pathogenicity of P. vivax and its impacts on fish health; thus, this study provides key insights into the morphology, pathogenicity, and histopathological impacts of P. vivax in Nile tilapia.
Similar content being viewed by others
Avoid common mistakes on your manuscript.
Introduction
In recent years, aquaculture has gained increasing significance as a key contributor to global food security, and Egypt has been at the forefront of advancements in fish farming. However, the aquaculture industry faces notable challenges, such as infectious diseases being a primary concern (Soliman and Yacout 2016). Among these challenges, fish parasites, particularly digenetic trematodes, represent a significant health threat to both farmed and wild fish populations (Khan et al. 1993; Taha et al. 2020). These parasites not only compete with fish for resources but also lead to economic losses (Khalil et al. 1997). Furthermore, they can compromise the health of the host fish and reduce their appeal to consumers (Roberts 2001). Understanding these parasites is vital for maintaining ecosystem stability and implementing effective control measures (Avenant 2001). Of particular concern within this context are fish-borne zoonotic trematodes (FBZT), including encysted metacercariae, which pose a significant public health risk (Mutengu and Mhlanga 2018; Younis et al. 2023b). These parasites can potentially be transmitted to humans through the consumption of raw or undercooked fish (Anh et al. 2019; Labony et al. 2020; Salem et al. 2021).
P. vivax utilizes fish as second intermediate hosts, where infective metacercariae encyst in tissues like muscle, skin, and gills before transmission to definitive hosts (birds and mammals) (Scholz et al. 2016). Despite the considerable impact of P. vivax on fish health and its zoonotic potential (Abdelkhalek et al. 2023), fundamental knowledge gaps remain regarding its epidemiology, host-parasite interactions, and molecular pathogenesis. This knowledge gap extends to the impact of stress genes influenced by P. vivax.
The host response to infectious diseases involves complex regulation of gene expression. In fish, parasite infections can prompt major physiological and molecular changes (Da Costa et al. 2021). P. vivax infection frequently causes substantial abnormalities in tilapia, including stunted growth, reproductive loss, and mortality. There is likely a complex interplay between P. vivax and the tilapia immune system, leading to the modulation of various genes related to immunity, stress response, and detoxification (Chuangchaiya et al. 2010; Dayananda et al. 2020). Cytochrome P450 1A1 (CYP1A1), heat shock protein 70 (HSP70), and tumor protein p53 (p53) are three pivotal genes in P. vivax-infected fish; however, it is crucial to emphasize that the impact of P. vivax on the expression of these genes has not been studied before in infected tilapia. CYP1A1, a member of the cytochrome P450 superfamily, plays a crucial role in metabolizing xenobiotics, including environmental carcinogens and toxins (Xin and Shan 2015). Its induction often serves as a biomarker for pollution exposure (Esteves et al. 2021). Investigating CYP1A1 dysregulation by P. vivax can provide insights into how the parasite affects detoxification and overall health. HSP70, a molecular chaperone, is highly conserved across organisms and plays a critical role in the physiological stress response, as well as in defending against protein misfolding and aggregation (Yu et al. 2015). Overexpression of HSP70 is commonly observed in response to various stressors, including infections (Zhi et al. 2018; Gupta et al. 2019). Examining HSP70 expression in P. vivax-infected tilapia can provide valuable insights into the ability of fish to mount a stress response and effectively cope with the pathological consequences of the infection. The well-known tumor suppressor protein, p53, holds a pivotal role in regulating apoptosis, DNA repair, and cell cycling and has been associated with immune and stress signaling pathways in fish (Liu et al. 2022). Assessing p53 expression in P. vivax-infected tilapia can offer valuable insights into the potential role of the p53-mediated defense system against the parasite.
Insufficient data currently exists regarding P. vivax epidemiology. The study aimed to morphological and molecular analysis of P. vivax infection in Nile tilapia, by assessing the modulation of three critical stress genes—CYP1A1, HSP70, and p53—in infected fish, thus elucidating the molecular pathways involved in anti-parasitic defense and stress responses within the infected host. This understanding is pivotal for filling the gap by updating the prevalence, distribution, and impact of P. vivax in tilapia and the development of strategies aimed at mitigating and preventing P. vivax disease.
Material and method
Fish sampling
A total of 800 Nile tilapia (O. niloticus) specimens were randomly collected from farms in El Hamoul area, Kafr El Sheikh Governorate, Egypt (31.19° N 31.9° E) (Fig. 1). These collected fish were carefully transported to the laboratory in oxygen-supplied, water-filled plastic bags. At the laboratory, the fish underwent a comprehensive examination for the identification of digenetic trematode EMC infections in Nile tilapia in Egypt. The sampled fish displayed a size range, with total lengths ranging from 8 to 15 cm and weights varying from 50 to 90 g. Clinical examinations were conducted on the fish samples, during which any abnormal signs were carefully recorded. Both external and internal gross lesions were observed and documented.
Parasitological examination
Each fish was euthanized using a commercial clove oil solution (Ectyocolve®, France) with a concentration of 1239 ppm, mixed at a 1:4 ratio with anhydrous ethanol. Following euthanasia, necropsies were done, and various tissues and organs, including the skin, gills, fins, muscles around the head, muscles around the abdomen, and internal organs, were subjected to examination under a light microscope (Olympus CX41 microscope; Japan). Muscle samples were prepared by compressing them between two slides and subsequently examined under a stereoscopic microscope to detect the presence of EMC (Paperna 1996; Younis et al. 2023b).
ITS2 region sequencing
Genomic DNA was extracted from metacercarial parasites obtained from different infected fish collected from the same locality during various seasons of the year following the protocol of the DNeasy Blood & Tissue extraction Kit (QIAGEN, USA), with Implen NP80 NanoPhotometer (Munich, Germany) assessing quantity and quality. PCR amplification of the internal transcribed spacer 2 (ITS2) utilized the universal primers BD1 (5′-GTCGTAACAAGGTTTCGGTA-3′) and BD2 (5′-TATGCTTAAATTCAGCGGGT-3′) (Bowles and McManus 1993; Bowles et al. 1995). The 25-µl PCR reactions used Maxima® Hot Start PCR Master Mix (Thermo Fisher Scientific, USA). PCR conditions involved an initial phase at 95 °C/5 min, followed by 40 cycles: denaturation (95 °C/45 s), annealing (50 °C/45 s), extension (72 °C/3 min), and a final step at 72 °C/6 min. Four amplicons were visualized by UV light after agarose gel electrophoresis, and purification was performed using GeneJET™ PCR Purification Kit (Thermo Fisher Scientific, USA). Sequencing utilized the Big Dye Terminator v3.1 cycle sequencing kit with the same PCR primers. ABI prism 3730XL (Applied Biosystems, USA) automated sequencer analyzed the sequences. BioEdit software v.7.2 (Hall 1999) facilitated careful sequence assembly, and BLAST programs matched edited sequences against the public genetic sequence database. The final sequences were submitted to GenBank.
Phylogenetic analysis
A phylogenetic tree was constructed to compare ITS2 sequences of the current Prohemistomum species against 27 other accession numbers from various digenetic trematodes, including P. vivax, Diplostomum spathaceum, Tylodelphysimmer, and Cyathocotyle sp. The selected species exhibited over 75% similarity to the current ITS2 sequence of Prohemistomum. Multiple sequence alignment was performed, and the phylogenetic tree was established using maximum likelihood (ML) methodology in MEGA 11 (Tamura et al. 2021). The ML parameters were set based on model selection using Bayesian information criterion (BIC), corrected Akaike information criterion (AIC) scores, and bootstrap confidence values from 1000 replicates. The general time reversible model with gamma-distributed rate variation and invariant sites (GTR + G + I) was chosen as it provided optimal accuracy. Numerous phylogenetic tree building approaches exist, but ML analysis was deemed most suitable for aligning the current parasites at the branch terminals in this study.
Stress-related gene expression analysis
Total RNA extraction and cDNA synthesis
Total RNA extraction was performed using the QIAmp RNA Mini Kit (Qiagen, Hilden, Germany) according to the manufacturer’s recommended protocol. Subsequently, the first-strand cDNA was synthesized with M-MuLV reverse transcriptase (Fermentas, EU).
Real-time PCR (qPCR)
In the real-time PCR (qPCR) analysis, PCR reactions were meticulously prepared using iQ SYBR Green Premix (Bio-Rad 170–880, USA) and carried out in the BIO-RAD iCycler thermal cycler with the MyiQ real-time PCR detection system (Ibrahem and Ibrahim 2014). The primer sequences employed for amplifying the target genes (CYP1A1, HSP70, and p53) were designed based on the sequences available in the gene bank of O. niloticus (Table 1). The qPCR program consisted of an initial pre-incubation step at 95 °C for 10 min, followed by 40 cycles of denaturation at 95 °C for 20 s, annealing at 60 °C for 20 s, and extension at 72 °C for 30 s (Ahmed et al. 2021). Each assay included duplicate reactions for every sample and no-template negative control (Ibrahim et al. 2020). To normalize the expression data, the GAPDH gene was used as an internal control (Ko et al. 2009). The calculation of gene expression data was performed using the 2−ΔΔCT method.
Histopathological examination
Specimens from the muscles, liver, intestine, and kidneys were collected and then fixed in 10% neutral buffered formalin. After fixation, they underwent a series of processes, including washing, dehydration, clearing, and embedding in paraffin. The paraffin-embedded blocks were sectioned to a thickness of 5 µm and stained with hematoxylin and eosin (Bancroft and Gamble 2008) for subsequent histopathological examination. This examination was carried out using a light microscope (Olympus BX50, Japan).
Statistical analysis
All data was displayed as means with standard error of the mean (SEM) and was examined using the SPSS (version 20) program. To compare several groups, one-way analysis of variance (ANOVA) and post hoc Duncan’s test were used. Statistics were judged significant at P ≤ 0.05 (Statistical analysis systems 2013).
Results
Clinical signs
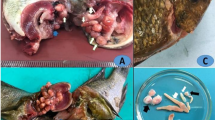
The examined Nile tilapia infected with encysted metacercariae of digenetic trematodes displayed an array of clinical signs including lethargy, loss of appetite, erratic swimming behavior, darkening of the skin, loss of scale, emaciation, excessive mucus production, tail and fin erosions, abdominal hydropsy, respiratory distress, stucked abdomen, and impaired growth (Fig. 2).
Prevalence of infection
Examination of 800 fish specimens revealed widespread P. vivax metacercarial infections, with an overall prevalence of 77.5% (620/800 infected fish). Prevalence displayed seasonal variations, with the highest rates in summer at 95% (190/200 fish), followed by spring at 85% (170/200 fish), autumn at 55% (110/200 fish), and winter at 75% (150/200 fish). The lowest mean infection intensity of 9.4 ± 5.8 cysts per field occurred in winter, while the highest mean intensity of 16.2 ± 15.3 and 36.4 ± 21.5 cysts per field was observed in spring and summer respectively (Table 2, Fig. 3).
Morphological examination
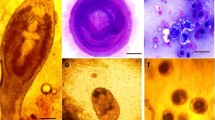
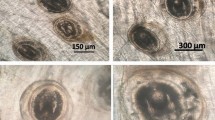
The encysted metacercariae was spherical in shape and had two walls (outer thick and inner hyaline one) that were filled with pigmented brownish granules at two lateral sides. The encysted metacercariae of P. vivax measured 350–395 (385 ± 1.5) µm in length and 315–345 (338 ± 1.0) µm in width (Fig. 4).
Genetic characterization
Molecular analysis of the ITS2 region of encysted metacercariae (EMC) parasites isolated from four Nile tilapia (O. niloticus) specimens representing different seasons revealed a 507-base pair (bp) DNA fragment. Sequences obtained from the four fish were identical and were deposited in GenBank under accession numbers OR794146, OR794147, OR794148, and OR794149. Sequence alignment analysis confirmed the identity of the metacercariae as belonging to the genus Prohemistomum (Trematoda: Digenea: Cyathocotylidae). Genetic analysis of the metacercariae parasites isolated from Nile tilapia confirmed their identity as P. vivax. The ITS2 sequence of P. vivax exhibited a high similarity to other P. vivax isolates and was distinct from other digenetic trematode sequences. BLAST analysis of the ITS2 sequence (OR794146) revealed its unique nature when compared to other digenetic trematode sequences. Distance estimation analysis showed that OR794146 had a high similarity of 99.20% to P. vivax, 92.81% to Cyathocotylidae spp., and 85.46% to Cyathocotyleprussica. It also exhibited a similarity range of 96.51–96.39% to various Diplostomum spp., 95.19–94.74% to Alaria spp. isolates, and 94.33–93.30% to Uvulifer spp. isolates.
The phylogenetic tree (Fig. 5) generated through maximum likelihood analysis revealed the taxonomic placement of P. vivax investigated in this study within the same clade as other P. vivax, supported by a robust bootstrap value. This finding corroborates the molecular identification of P. vivax based on ITS2 sequencing and further confirms the distinctiveness of this parasite species.
Stress-related gene expression analysis
There were clear differences in gene expression between infected and control non-infected normal fishes. The transcript levels of cytochrome P450 (cpy1a1), heat shock protein 70 (hsp-70), and tumor suppressor p53 were significantly upregulated in the liver samples of the infected fish compared to controls (Fig. 6). The greatest difference was noticed in cpy1a1 expression, which was approximately eightfold higher in infected fish. p53 and hsp-70 were upregulated by approximately three- to fourfold. Similarly, the transcript levels of cpy1a1, hsp-70, and p53 were also elevated in the muscle samples of infected fish relative to controls (Fig. 7). The fold changes were smaller than those recorded in the liver, but still statistically significant. Cpy1a1 was upregulated by approximately threefold, while p53 and hsp-70 were upregulated by around twofold in infected muscle tissue.
Histopathological findings
Histopathological examination of muscle tissues revealed the presence of parasitic cysts within muscle fibers (Fig. 8a–d). Parasitic cyst infection led to the destruction and necrosis of the affected muscle fibers (Fig. 8e). Some cases exhibited heavy parasitic infection, characterized by the presence of multiple parasitic cysts in muscle fibers (Fig. 8f, g), resulting in degeneration and necrosis of the muscle fibers. In the liver, embedded EMC were observed within the liver parenchyma between hepatocytes (Fig. 9a). This parasitic infection in the liver coincided with severe degeneration and necrosis of hepatocytes (Fig. 9b, c). Some sections displayed a heavy level of infection in the liver, marked by diffuse degeneration of liver parenchyma (Fig. 9d). The examination of the intestine revealed the occurrence of parasitic cysts in the submucosa, accompanied by minimal tissue reaction (Fig. 9e). Moving on to the kidney, a notable infection was observed with multiple parasitic cysts present in the renal cortex between renal tubules (Fig. 9f). This infection led to the degeneration and necrosis of the tubular lining epithelium (Fig. 9g).
Photomicrograph of fish muscles showing a, b, c, d parasitic cysts of different sizes present embedded in the muscle fibers with no tissue reaction (arrow) (H&EX200); e parasitic cyst (long arrow) with degeneration and destruction of muscle fibers (short arrow) (H&EX200); f heavy infection of muscles with multiple EMC (arrows) (H&EX100); and g higher magnification of the previous photo showing multiple cysts surrounded by connective tissue (arrows) (H&EX200)
Photomicrograph of fish. a Liver showing the presence of EMC embedded in liver parenchyma (arrow) (H&EX100); b EMC embedded in liver tissue (short arrow), with severe degeneration and steatosis of hepatocytes (long arrow) (H&EX200); c liver sowing the presence of large-sized EMC (arrow) (H&EX100); d liver showing heavy infection with EMC (arrows) with diffuse degeneration of liver parenchyma (H&EX200); e intestine showing the presence of parasitic cyst in the submucosa (arrow) (H&EX100); f kidney showing the presence of a large number of parasitic cysts in the renal cortex (arrows) (H&EX100); and g higher magnification of the previous photo showing parasitic cysts in the renal cortex (long arrows) and degeneration and necrosis of tubular lining epithelium (short arrows) (H&EX200)
Discussion
The current study provides novel and multifaceted insights into EMC of P. vivax infections in Nile tilapia. The observed clinical signs aligned with previous reports describing lethargy, loss of appetite, abnormal swimming patterns, skin darkening, excessive mucus secretion, respiratory distress, and stunted growth in association with digenetic trematode infections across various fish species (Abou-Okada et al. 2021; Abdelkhalek et al. 2023; Abd-ELrahman et al. 2023; Younis et al. 2023a). These systemic clinical features indicate the broad pathological impact of chronic metacercarial infections on fish health (Attia et al. 2021; Eissa et al. 2021; Mahdy et al. 2021).
The overall prevalence of 77.5% EMC of P. vivax infections emphasizes these metacercariae are highly prevalent parasites in tilapia. However, it is noteworthy that this finding is higher than the one reported by Abd-ELrahman et al. (2023) and Younis et al. (2023b), who documented an overall prevalence of 58% and 25% respectively for EMC infections in Nile tilapia. This endemicity poses a substantial burden necessitating the development of integrated control strategies to mitigate their impact, particularly considering potential economic losses for aquaculture. Seasonal variations were noted, with prevalence peaking at 95% in summer followed by 85% in spring, then dropping to 55% in autumn and 75% in winter. It is essential to note that while these patterns align with the seasonal fluctuations reported by Abd-ELrahman et al. (2023), our recorded percentages are higher. They reported a prevalence peak of 63% in summer, followed by 51% in spring, dropping to 27% in autumn, and reaching 33% in winter. The heightened summer and spring prevalence correlates with the temperature-dependent life cycle dynamics of digenetic trematodes, which display enhanced transmission, replication, and host interactions at warmer temperatures (Poulin 2006; Studer et al. 2010). This seasonality effect has been consistently documented across fish-infecting heterophyids and should be factored into the design of parasite surveillance and management plans timed to critical transmission periods (Thomas 2002). For instance, strategic anthelmintic treatments could be administered prior to the summer peak as a preventative approach while also monitoring for treatment resistance.
Morphological analysis of cysts from different organs revealed characteristic features of EMC of P. vivax, including a spherical shape with a hyaline double wall surrounded by two lateral lobes or sacs. These attributes align with descriptions of EMC of P. vivax provided in past morphological studies (Abd-ELrahman et al. 2023; El-Seifyet al. 2021). The presence of undistinguishable morphological appearance in our specimens precluded the absence of multiple EMC species, which frequently occur as mixed infections (Mahdy et al. 2021). Molecular analysis of the ITS2 region amplified from metacercariae parasites isolated from tilapia across seasons revealed identical 507-bp sequences. BLAST analysis matched these sequences to the genus Prohemistomum, and alignment confirmed the identity as P. vivax. The ITS2 sequences exhibited 99.2% similarity to other P. vivax isolates but only 85–96% similarity to other digenetic trematodes. Phylogenetic reconstruction placed the P. vivax species investigated herein within the same clade as other P. vivax references with high bootstrap support. This corroborates its molecular identification and distinguishes it from related parasites. The application of molecular techniques enhances species-level identification and confirmation of whether singular or mixed P. vivax metacercariae infections prevail. This approach also contributes to a deeper understanding of the evolutionary relationships among closely related species (Sripalwit et al. 2015; Patarwut et al. 2020; Pitaksakulrat et al. 2022).
Histopathological examination demonstrated that extensive P. vivax metacercarial infections caused severe necrosis and degeneration in multiple organs, particularly muscular tissues, liver, kidney, and intestine. This likely contributes to the observed clinical manifestations of impaired swimming, appetite loss, and stunted growth. The destruction of skeletal muscle fibers and liver hepatocytes disrupts vital metabolic, nutritional, and locomotive functions. The intestinal and renal damage caused by embedded cysts may result in malnutrition and osmoregulatory deficits. Despite the significant tissue damage, the observed connective tissue capsule is considered a tissue reaction in affected fish due to the prolonged irritation caused by parasitic cysts (Aly et al. 2005). This modulatory effect is believed to facilitate metacercarial survival by evading immune elimination.
Gene expression analysis through reverse transcription PCR (RT-PCR) revealed upregulation of three key genes—cytochrome P450 (CYP1A1), heat shock protein 70 (HSP70), and tumor suppressor p53 (p53)—in both the liver and muscle tissues of Nile tilapia infected with P. vivax metacercariae compared to un-infected controls. The cytochrome P450 gene CYP1A1 encodes a monooxygenase enzyme involved in the metabolism and detoxification of endogenous and exogenous compounds (Ge et al. 2022). Its induction serves as a well-established biomarker of contaminant exposure in fish (Addison et al. 1993). Our findings suggest CYP1A1 expression holds significant potential as a biomarker for exposure to specific planar organic pollutants, surpassing other xenobiotic biotransformation genes (Goksoyr 1995; Eggens et al. 1995). The induction of cytochrome P450 gene CYP1A1 points to the activation of detoxification pathways in response to environmental toxins like PAHs and PCBs (Stegeman et al. 1988), or indicating chemical stressors associated with P. vivax metacercariae infection (Zapata-Pérez et al. 2002). Its stimulation in infected fish rather than control fish indicates the parasite elicits a toxic insult, either through direct tissue damage or secretion of bioactive molecules that must be neutralized (Wong et al. 2001). However, persistent CYP1A1 induction could have detrimental effects by diverting energy reserves towards detoxification rather than growth and reproduction (Yeung et al. 2003). However, persistent CYP1A1 activity could deplete cellular antioxidant reserves needed to neutralize its reactive metabolites (Reglero et al. 2018). This may contribute to the oxidative stress often observed during parasitic diseases. CYP1A1 is also entwined with immune signaling networks; thus, its sustained induction may alter inflammatory processes (Monosson and Stegeman 1994).
HSP70 functions as a molecular chaperone facilitating protein folding and stabilization, making it integral to the cellular stress response and preventing degradation under stresses like heat shock and hypoxia that may occur with P. vivax metacercariae infection (Heads et al. 1995). HSP70 serves as a biomarker of stress-induced proteotoxicity, which impairs protein integrity (Sanders 1993). Though few studies have examined the heat shock response in hypoxic fish (Airaksinen et al. 1998; Gamperl et al. 1998), little is known, especially in active organs like the liver (Cumming et al. 1996). The dramatic upregulation of HSP70 implies P. vivax metacercariae infection severely disrupts protein homeostasis (Das et al. 2015). The marked upregulation of HSP70 in infected fish suggests a cytoprotective attempt to mitigate protein misfolding and damage from the severe cellular stress imposed by chronic P. vivax metacercariae infections (Cumming et al. 1996; Kumar et al. 2022). While boosting HSP70 can restore short-term stability, prolonged activation depletes ATP and may preclude other essential chaperone functions (Airaksinen et al. 1998).
For instance, Currie and Tufts (1997) demonstrated that rainbow trout Oncorhynchus mykiss red blood cells could synthesize proteins after 2 h of anoxia but did not boost the synthesis of Hsp70. Although these mechanisms are becoming more understood at the level of model proteins, less is known at the level of the cell, tissue, organ, and entire organism (Feder and Hofmann 1999). Stress proteins reduce the negative effects of stress on individuals, and these mechanisms are mediated through a variety of methods. As a result, research on the stress protein response in organs with high metabolic activity, such as the liver and gills, is very important. In this study, we presented the first proof that in Nile Tilapia exposed to prolonged hypoxia, Hsp70 functions as a biochemical marker associated with hypoxia-caused proteotoxicity.
Finally, increased p53 may stimulate apoptotic pathways to eliminate damaged cells and unchecked p53 activity and constant cell death could lead to the deterioration of tissues and organ function (Liu et al. 2022); the increased expression of P53 in response to stress suggests its possible participation in immune response and stress signaling pathways in tilapia. It could also enable cell cycle arrest for DNA repair. p53 may further interact with immune signaling cascades against the parasite (Ibrahem and Ibrahim 2014; Liu et al. 2022).
Conclusion
This comprehensive study, integrating clinical, histopathological, epidemiological, and molecular data, significantly enhances our understanding of P. vivax infections in tilapia. The findings elucidate clinical manifestations, tissue tropisms, cyst morphology, population prevalence, histopathology, and gene expression profiles. The gene expression results provide valuable insights into the molecular pathways affected during chronic infections, highlighting the importance of such studies in understanding host-parasite relationships. The observed seasonal variations in prevalence offer key epidemiological insights for strategic surveillance and control measures. This multidimensional approach advances fundamental knowledge and demonstrates the benefits of integrating diverse techniques in studying host-parasite dynamics. Future research can build on these insights to further enhance aquaculture sustainability and seafood safety.
Data availability
No datasets were generated or analysed during the current study.
Abbreviations
- EMC:
-
Encysted metacercariae
- P. vivax :
-
Prohemistomum vivax
- ITS2:
-
Internal transcribed spacer 2
- hsp-70:
-
Heat shock protein 70
- cpy1a1:
-
Cytochrome P450 1A1
- p53 :
-
Tumor suppressor p53
- FBZT:
-
Fish-borne zoonotic trematodes
- bp:
-
Base pair
References
Abdelkhalek S, Attia MM, Abdel-Moneam DA, Elgendy MY, Korany RMS, Abdelsalam M (2023) Molecular identification and histopathological alterations associated with Prohemistomum vivax encysted metacercariae infection in farmed African Catfish (Clarias gariepinus). Egypt J Vet Sci 55(4):1055–1065
Abd-ELrahman SM, Gareh A, Mohamed HI, Alrashdi BM, Dyab AK, El-Khadragy MF et al (2023) Prevalence and morphological investigation of parasitic infection in freshwater fish (Nile tilapia) from Upper Egypt. Animals 13(6):1088. https://doi.org/10.3390/ani13061088
Abdelsalam M, Elgendy MY, Elfadadny MR et al (2023) A review of molecular diagnoses of bacterial fish diseases. Aquacult Int 31:417–434. https://doi.org/10.1007/s10499-022-00983-8
Abou-Okada M, AbuBakr HO, Hassan A, Abdel-Radi S, Aljuaydi SH, Abdelsalam M et al (2021) Efficacy of acriflavine for controlling parasitic diseases in farmed Nile tilapia with emphasis on fish health, gene expression analysis, oxidative stress, and histopathological alterations. Aquaculture 541:736791
Addison R, Willis D, Zinck M (1993) Liver microsomal mono-oxygenase induction in winter flounder (Pseudopleuronectes americanus) from a gradient of sediment PAH concentrations at Sydney Harbour. Nova Scotia Marine Environ Res 37(3):283–296. https://doi.org/10.1016/0141-1136(94)90055-8
Ahmed WMS, Abdel-Azeem NM, Ibrahim MA, Helmy NA, Radi AM (2021) Neuromodulatory effect of cinnamon oil on behavioural disturbance, CYP1A1, iNOS transcripts and neurochemical alterations induced by deltamethrin in rat brain. Ecotoxicol Environ Saf 209:111820
Airaksinen S, Råbergh CMI, Sistonen L, Nikinmaa M (1998) Effects of heat shock and hypoxia on the protein synthesis in rainbow trout (Oncorhynchus mykiss) cells. J Exp Biol 201(Pt 17):2543–2551
Aly S, Eissa I, Badran A, Elamie M and Hussain B (2005) Pathological studies on encysted metacercariae infections among some freshwater fish in Egyptian aquaculture. Proceedings of duetscher tropentag. Hohenham Univ., Stuttgart, Germany
Attia MM, Elgendy MY, Abdelsalam M, Hassan A, Prince A, Salaeh NM et al (2021) Morpho-molecular identification of Heterophyes heterophyes encysted metacercariae and its immunological and histopathological effects on farmed Mugil cephalus in Egypt. Aquacult Int 29(3):1393–1407
Avenant-Oldewage A (2001). Protocol for the assessment of fish health based on the health index. Report and a manual for training of field workers to rand water, Vereeniging. Report No. 2001/03/ 31.BIOM.GEN. (H 1)
Bancroft JD, Gamble M (2008) Theory and practice of histological techniques, 6th Edn. Elsevier, Churchill Livingstone
Chuangchaiya S, Jangpatarapongsa K, Chootong P, Sirichaisinthop J, Sattabongkot J, Pattanapanyasat K et al (2010) Immune response to Plasmodium vivax has a potential to reduce malaria severity. Clin Exp Immunol 160(2):233–9. https://doi.org/10.1111/j.1365-2249.2009.04075.x
Cumming DV, Heads RJ, Watson A, Latchman DS, Yellon DM (1996) Differential protection of primary rat cardiocytes by transfection f specific heat stress proteins. J Mol Cell Cardiol 28(12):2343–2349. https://doi.org/10.1006/jmcc.1996.0227
Currie S, Tufts B (1997) Synthesis of stress protein 70 (Hsp70) in rainbow trout (Oncorhynchus mykiss) red blood cells. J Exp Biol 200(Pt 3):607–614. https://doi.org/10.1242/jeb.200.3.607
da Costa JC, de Souza SS, Castro JDS, Amanajás RD, Val AL (2021) Climate change affects the parasitism rate and impairs the regulation of genes related to oxidative stress and iono regulation of Colossomam acropomum. Sci Rep 11(1):22350
Das S, Mohapatra A, Sahoo PK (2015) Expression analysis of heat shock protein genes during Aeromonas hydrophila infection in rohu, Labeorohita, with special reference to molecular characterization of Grp78. Cell Stress Chaperones 20(1):73–84. https://doi.org/10.1007/s12192-014-0527-2
Dayananda KK, Achur RN, Gowda DC (2018) Epidemiology, drug resistance, and pathophysiology of Plasmodium vivax malaria. J Vector Borne Dis 55(1):1–8
Eggens M, Bergman A, Vethaak D, Van der Weiden M, Boon JPE, Steves F et al (2021) The central role of cytochrome P450 in xenobiotic metabolism-a brief review on a fascinating enzyme family. J Xenobiot 11(3):94–114
Eissa AE, Attia MM, Elgendy MY, Ismail GA, Sabry NM, Prince A et al (2021) Streptococcus, Centrocestus formosanus and Myxobolus tilapiae concurrent infections in farmed Nile tilapia (Oreochromis niloticus). Microb Pathog 158:105084
El-Seify MA, Sultan K, Elhawary NM, Satour NS, Marey NM (2021) Prevalence of heterophyid infection in tilapia fish “Orechromas niloticus” with emphasize of cats role as neglected reservoir for zoonotic Heterophyes heterophyes in Egypt. J Parasit Dis 45:35–42
Feder ME, Hofmann GE (1999) Heat-shock proteins, molecular chaparones, and the stress response: evolutionary and ecological physiology. Annu Rev Physiol 61:243–282
Ge J, Huang Y, Lv M, Zhang C, Talukder M, Li JY et al (2022) Cadmium induced Fak-mediated anoikis activation in kidney via nuclear receptors (AHR/CAR/PXR)-mediated xenobiotic detoxification pathway. J Inorg Biochem 227:111682
Goksoyr A (1995) Use of cytochrome P4501A (CYP1A) in fish as a biomarker of aquatic pollution. Arch Toxicol Suppl 1995(17):80–95. https://doi.org/10.1007/978-3-642-79451-3_7
Gupta DK, Dembele L, Voorberg-van der Wel A, Roma G, Yip A, Chuenchob V et al (2019) The Plasmodium liver-specific protein 2 (LISP2) is an early marker of liver stage development. Elife 16(8):e43362. https://doi.org/10.7554/eLife.43362
Heads RJ, Yellon DM, Latchman DS (1995) Differential cytoprotection against heat stressor hypoxia following expression of specific stress protein genes in myogenic cells. J Mol Cell Cardiol 27:1669–1678
Ibrahem MD, Ibrahim MA (2014) The potential effects of Spirulina platensis (Arthrospira platensis) on tissue protection of Nile tilapia (Oreochromis niloticus) through estimation of P53 level. J Adv Res 5(1):133–136
Ibrahim MA, Radwan MI, Kim HK, Han J, Warda M (2020) Evaluation of global expression of selected genes as potential candidates for internal normalizing control during transcriptome analysis in dromedary camel (Camelus dromedarius). Small Rumin Res 184:106050
Khalil LF, Polling L (1997) Checklist of the helminth parasites of African freshwater fishes. University of the North, Pietersburg, Limpopo, South Africa, p 185
Khan RA, Ryan K, Barker DE, Lee EM (1993) Effect of a single Lernae ocerabranchialis (Crustacea: Copepoda) on growth of Atlantic cod. Journal of Parasitology 79(6):954–958. https://doi.org/10.2307/3283738
Ko JH, Ibrahim MA, Park WS, Ko EA, Kim N, Warda M et al (2009) Cloning of large-conductance Ca(2+)-activated K(+) channel alpha-subunits in mouse cardiomyocytes. Biochem Biophys Res Commun 389(1):74–79
Kumar V, Roy S, Behera BK, Das BK (2022) Heat shock proteins (Hsps) in Cellular homeostasis: a promising tool for health management in crustacean aquaculture. Life (Basel) 12(11):1777. https://doi.org/10.3390/life12111777
Labony SS, Alim MA, Hasan MM, Hossain MS, Islam A, Alam MZ et al (2020) Fish-borne trematode infections in wild fishes in Bangladesh. Pathog Glob Health 114(2):91–98. https://doi.org/10.1080/20477724.2020.1727217
Liu S, Luo L, Zuo F, Geng Y, Ou Y, Chen D et al (2022) Immunosuppression and apoptosis activation mediated by p53-Bcl2/Bax signaling pathway -the potential mechanism of goldfish (Carassius auratus Linnaeus) gill disease caused by Myxobolus ampulli capsulatus. Front Immunol 30(13):998975
Mahdy OA, Abdel-Maogood SZ, Abdelsalam M, Shaalan M, Abdelrahman HA, Salem MA (2021) Epidemiological study of fish borne zoonotic trematodes infecting Nile tilapia with first molecular characterization of two heterophyid flukes. Aquac Res 52(9):4475–4488
Monosson E, Stegeman JJ (1994) Induced cytochrome P45011A in winter flounder, Pleuronectes americanus, from offshore and coastal sites. Can J Fish Aquat Sci 51:933–941
Paperna I (1996) Parasite, infections and disease of fishes in Africa—an update. CIFA Tech Pap 31:1–220
Patarwut L, Chontananarth T, Chai JY, Purivirojkul W (2020) Infections of digenetic trematode metacercariae in Wrestling halfbeak, Dermogenys pusilla from Bangkok Metropolitan Region in Thailand. Korean J Parasitol 58(1):27
Pitaksakulrat O, Sithithaworn P, Kopolrat KY, Kiatsopit N, Saijuntha W, Andrews RH et al (2022) Molecular identification of trematode parasites infecting the freshwater snail Bithynia siamensis goniomphalos in Thailand. J Helminthol 20(96):e49. https://doi.org/10.1017/S0022149X22000402
Poulin R (2006) Global warming and temperature-mediated increases in cercarial emergence in trematode parasites. Parasitology 132:143–151
Roberts RJ (2001) Fish pathology, 3rd edn. W.B. Saunders, Philadelphia
Salem MA, Abdel-Maogood SZ, Abdelsalam M, Mahdy OA (2021) Comparative morpho-molecular identification of Clinostomum phalacrocoracis and Clinostomum complanatum metacercaria coinfecting Nile tilapia in Egypt. Egyptian J Aquatic Biol Fisheries 25(1):461–476
Sanders BM (1993) Stress proteins in aquatic organisms: an environmental perspective. Crit Rev Toxicol 23(1):49–75. https://doi.org/10.3109/10408449309104074
Scholz T, Besprozvannykh VV, Boutorina TE, Choudhury A, Cribb TH, Ermolenko AV et al (2016) Trematode diversity in freshwater fishes of the Globe I: “Old World.” Syst Parasitol 93(3):257–69. https://doi.org/10.1007/s11230-016-9630-3
Sripalwit P, Wongsawad C, Chontananarth T, Anuntalabhochai S, Wongsawad P, Chai JY (2015) Developmental and phylogenetic characteristics of Stellantchasmus falcatus (Trematoda: Heterophyidae) from Thailand. Korean J Parasitol 53:201–207
Stegeman JJ, Hahn ME (1994) Biochemistry and molecular biology of monooxygenases: current perspectives on forms, functions, and regulation of cytochrome P450 in aquatic species. In: Malins DC, Ostrander GK (eds) Aquatic toxicology, molecular, biochemical and cellular perspectives. Lewis Publishers, Boca Raton, pp 87–206
Stegeman JJ, Woodin BR, Goksoyr A (1988) Apparent cytochrome P-450 induction as an indication of exposure to environmental chemicals the flounder Platichthys flesus. Mar Ecol Prog Ser 46(55–60):0171–8630
Studer A, Theiltges DW, Poulin R (2010) Parasites and global warming: net effects of temperature on an intertidal host–parasite system. Mar Ecol Prog Ser 415:11–22
Statistical Analysis Systems, SAS (2013) The SAS system for windows, release 9.4. Statistical Analysis Systems Institute, Cary, NC, pp 556
Thomas JD (2002) The ecology of fish parasites with particular reference to helminth parasites and their salmonid fish hosts in Welsh rivers: a review of some of the central questions. Adv Parasitol 52:1–154. https://doi.org/10.1016/s0065-308x(02)52011-x
Wong CK, Yeung HY, Woo PS, Wong MH (2001) Specific expression of cytochrome P4501A1 gene in gill, intestine and liver of tilapia exposed to coastal sediments. Aquatic Toxicology (amsterdam, Netherlands) 54(1–2):69–80. https://doi.org/10.1016/s0166-445x(00)00173-9
Xin H, Shan F (2015) Role of metabolic enzymes P450 (CYP) on activating procarcinogen and their polymorphisms on the risk of cancers, current drug metabolism; 16(10). https://doi.org/10.2174/138920021610151210164501
Yamaguti S (1958) Systema Helminthum, vol I. Intersience Publishers, New York & London, The digenetic trematodes of vertebrates-Part II
Ye H, Lin Q, Luo H (2018) Applications of transcriptomics and proteomics in understanding fish immunity. Fish Shellfish Immunol 77:319–327. https://doi.org/10.1016/j.fsi.2018.03.046
Yeung HY, Wong CC, Wong MH, Wong CK (2003) Differential expression of CYP1A1 mRNA in gill, intestine and liver of tilapia fed with PCB Aroclor-1254 and Aroclor-1260 spiked food. Chemosphere 52(9):1659–1665. https://doi.org/10.1016/S0045-6535(03)00543-5
Younis NA, Elgendy MY, El-Samannoudy SI, Abdelsalam M, Attia MM (2023) Cyathocotylidae spp and motile aeromonads co-infections in farmed Nile tilapia (Oreochromis niloticus) causing mass mortality. Microb Pathog 174:105897
Younis NA, Thabit H, El-Samannoudy SI, Attia MM (2023) The immune responses of Oreochromis niloticus against Prohemistomum vivax encysted metacercariae infection with the evaluation of different biomarkers stressors. Sci Rep 13(1):1–7. https://doi.org/10.1038/s41598-023-38809-z
Yu A, Li P, Tang T, Wang J, Chen Y, Liu L (2015) Roles of Hsp70s in stress responses of microorganisms, plants, and animals. Biomed Res Int 2015:510319. https://doi.org/10.1155/2015/510319
Zapata-Pérez O, Gold-Bouchot G, Ortega A, López T, Albores A (2002) Effect of pyrene on hepatic cytochrome P450 1A (CYP1A) expression in Nile Tilapia (Oreochromis niloticus). Arch Environ Contam Toxicol 42(4):477–485. https://doi.org/10.1007/s00244-001-0018-1
Zhi T, Xu X, Chen J, Zheng Y, Zhang S, Peng J et al (2018) Expression of immune-related genes of Nile tilapia Oreochromis niloticus after Gyrodactylus cichlidarum and Cichlidogyrus sclerosus infections demonstrating immunosupression in coinfection. Fish Shellfish Immunol 80:397–404
Funding
Open access funding provided by The Science, Technology & Innovation Funding Authority (STDF) in cooperation with The Egyptian Knowledge Bank (EKB).
Author information
Authors and Affiliations
Contributions
Conceptualization, M.M.A., S.A; M.A: Methodology, D. A: Original draft writing, R.K: Histopathological examination; M.I: Gene Expression; Writing−review and editing: M.M.A.,S.A; M.A. All authors read and approved the final manuscript.
Corresponding author
Ethics declarations
Competing interests
The authors declare no competing interests.
Ethical approval
The study protocol was reviewed and approved by the Research Ethics Committee of the Faculty of Veterinary Medicine, Cairo University, Egypt (VET-CU-IACUC-09092023797).
Conflict of interest
The authors declare no competing interests.
Additional information
Handling Editor: Amany Abbass
Publisher's Note
Springer Nature remains neutral with regard to jurisdictional claims in published maps and institutional affiliations.
Rights and permissions
Open Access This article is licensed under a Creative Commons Attribution 4.0 International License, which permits use, sharing, adaptation, distribution and reproduction in any medium or format, as long as you give appropriate credit to the original author(s) and the source, provide a link to the Creative Commons licence, and indicate if changes were made. The images or other third party material in this article are included in the article's Creative Commons licence, unless indicated otherwise in a credit line to the material. If material is not included in the article's Creative Commons licence and your intended use is not permitted by statutory regulation or exceeds the permitted use, you will need to obtain permission directly from the copyright holder. To view a copy of this licence, visit http://creativecommons.org/licenses/by/4.0/.
About this article
Cite this article
Abdelkhalek, S., Attia, M.M., Ibrahim, M.A. et al. Alterations in histopathology and stress-associated gene expression induced by infection with Prohemistomum vivax encysted metacercariae in Nile tilapia. Aquacult Int 32, 5107–5124 (2024). https://doi.org/10.1007/s10499-024-01418-2
Received:
Accepted:
Published:
Issue Date:
DOI: https://doi.org/10.1007/s10499-024-01418-2