Abstract
Purpose
This study examined the metazoan ectoparasites of the Critically Endangered giant shovelnose ray, Glaucostegus typus, in the eastern Indian Ocean.
Methods
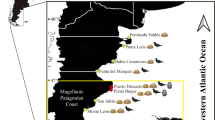
We screened 186 G. typus for ectoparasites in four coastal regions of Western Australia between 2020 and 2022: the Pilbara Region, Exmouth Gulf, Ningaloo Coast and Shark Bay.
Results
Five parasite taxa were encountered on 186 G. typus: Caligus furcisetifer (Copepoda: Caligidae), Dermopristis cairae (Monopisthocotyla: Microbothriidae), Branchellion plicobranchus and Stibarobdella macrothela (Hirudinida: Piscicolidae), and praniza larvae of unidentified gnathiid isopod/s (Isopoda: Gnathiidae). Two of these species, B. plicobranchus and S. macrothela, are reported for the first time on G. typus. Only C. furcisetifer and S. macrothela were relatively common, encountered on 31% and 40% of G. typus, respectively. Gnathiids were observed infrequently, encountered on 13% of G. typus, and D. cairae and B. plicobranchus were scarce, encountered on 1% and 2% of G. typus, respectively. Intensity of infection for C. furcisetifer and gnathiids increased with host length. Likelihood of infection varied seasonally for C. furcisetifer, being considerably lower in summer, and regionally for gnathiids, being greatest at Shark Bay. Intensity and likelihood of infection for S. macrothela increased with host length and varied regionally, being greatest at Shark Bay.
Conclusion
These findings improve our understanding of the downstream impacts for dependent parasites that might arise should populations of G. typus continue to decline.
Similar content being viewed by others
Avoid common mistakes on your manuscript.
Introduction
Parasites are important components of ecosystems, in terms of species richness, biomass and maintaining ecosystem function [1, 2]. However, parasites also likely comprise the majority of fauna that are threatened with extinction [3,4,5,6], being vulnerable to direct threats, such as anthropogenic impacts [6], and also indirectly, via coextinction with their hosts [5, 7, 8]. Parasites with density-dependent transmission and specificity for threatened hosts face the greatest risk of extinction, because transmission may decrease below the threshold required to maintain the parasite population as the host population declines [5, 9]. Predicting outcomes for dependent parasites following a decline in the host population necessitates an understanding of those taxa [10, 11], yet most parasites remain poorly understood and perhaps the majority are still unknown to science [12]. If they are to be considered in host conservation plans, it is imperative that parasites of threatened host species are identified [5].
The giant shovelnose ray, Glaucostegus typus (Anonymous [Bennett], 1830), is a large species of giant guitarfish (Glaucostegidae) that has suffered severe population declines and fragmentation throughout much of its range as a consequence of overfishing [13,14,15,16]. Collectively, the giant guitarfishes are one of the most imperilled vertebrate families, with all seven extant species classified as Critically Endangered on the International Union for Conservation of Nature’s (IUCN) Red List of Threatened Species [14, 17,18,19,20,21,22]. It is estimated that populations of G. typus have been reduced by more than 80% over three generations across the northern limit of its distribution in the Indo-West Pacific [14, 15]. Although it is unprotected throughout its entire range, G. typus is afforded some refuge in Australian waters, including along the mid to northern coastline of Western Australia, where it is common as far south as Shark Bay [14, 16, 23, 24].
The metazoan parasite fauna of G. typus is relatively well characterised and better investigated than any other glaucostegid, comprising 28 reported taxa (Table 1). Of these, 11 species are known only from G. typus, although three have been considered species inquirenda [25] and a further three are known only from Borneo, where identifications of G. typus are seemingly ambiguous [26]. These investigations are limited to studies of a single parasite species or group and there are no reports of parasites of G. typus from the expansive coastline of Western Australia in the eastern Indian Ocean. The aims of this study were to characterise the metazoan ectoparasites infecting G. typus in the eastern Indian Ocean, and to examine intrinsic (size and sex of the host) and extrinsic (season and region) factors influencing infections.
Materials and Methods
Host Sampling and Parasite Collection
Targeted sampling for G. typus was conducted at multiple sites in four coastal regions in Western Australia (Fig. 1): the Pilbara region between October 2020 and August 2022; Exmouth Gulf between August 2020 and July 2022; Ningaloo Coast in February 2022; and Shark Bay between March and November 2022 (Table 2), which is considered the southern limit of the distribution of G. typus in the eastern Indian Ocean [16, 27,28,29]. Individual G. typus were caught using set nets, throw nets, encircled by monofilament gillnets in shallow waters, or by hand. Upon capture, rays were held on their backs in the extreme shallows with their gills submerged, inducing a state of tonic immobility. Stretched total length (TL; to the nearest mm) and sex were recorded. Subsequent estimates of age class were based on the clasper morphology of males (i.e., calcification) and growth data reported by White et al. [30]. Examinations for ectoparasites were conducted first on the ventral surface, after which the host was righted for examination of the dorsal surface, before being released. The gills, nasal lamellae and cloaca were not examined, because these areas could not be inspected non-invasively. Parasite attachment sites were recorded according to general body location (Fig. 2). Parasites were removed using forceps and most were immediately preserved in 100% ethanol, with some copepods and monopisthocotylan specimens fixed in 10% formalin for subsequent morphological study. Measurements of water temperature were recorded in each region using a YSI Professional Plus Multiparameter Meter (YSI Inc., Yellow Springs, United States of America) (Supplementary Table S1).
Morphological Study
Parasite identifications were based solely on morphology. For mounting of select copepods and monopisthocotylans, specimens initially preserved in formalin were later transferred to absolute ethanol using a graded ethanol series: 40, 60, 75 and twice at 100% for approximately 1 h per stage. Copepod specimens were cleared in lactophenol and mounted (unstained) in Canada balsam. Monopisthocotylans were treated as described in Ingelbrecht et al. [31, 32]. Leeches and isopods were examined as uncleared and unstained wet mounts in absolute ethanol. Slide-mounted specimens were examined and photographed using an Olympus BX50 compound microscope, with Nomarski interference contrast, fitted with an Olympus DP71 digital microscope camera and U-CMAD3 adaptor (Olympus Inc., Tokyo, Japan). Wet mounts were examined and photographed using an Olympus SZX7 stereo microscope with an Olympus DF PLAPO auxiliary lens. Select material has been deposited with the Crustacea and Worms collection of the Western Australian Museum (WAM).
Data Analyses
Prevalence (proportion of infected hosts), intensity of infection (mean number of parasites per infected host) and parasite abundance (equivalent to prevalence × intensity) were calculated for each ectoparasite species using the online tool QPweb v.1.0.15 [33]. Bias-corrected and accelerated bootstrap 95% confidence intervals were calculated for mean infection intensity. For ectoparasite species with adequate sample sizes (i.e., present on ≥ 10 host individuals), aggregation was investigated in QPweb from variance/mean ratios (s2/m) and negative binomial exponent values (k), with aggregation indices calculated across all screened G. typus.
Predictors of parasite abundance were investigated for ectoparasite species with adequate sample sizes, using a series of models in R v.4.2.3 [34]. Because parasite abundances are aggregated by nature, two distributions were compared to account for overdispersion: zero-inflated negative binomial and zero-inflated Poisson. These models assume that excess zeros are generated by a separate process from count data and are therefore modelled separately, with the first modelled distribution relating to the odds of infection for a host (i.e. whether it is infected or not) and the second relating to the intensity of infection. Fixed predictors of abundance incorporated into initial models included host TL and sex, sampling season and region. Models using all combinations of predictor variables and their interactions were created. Samples from the Ningaloo Coast were not included due to inadequate sample size (i.e., < 10 screened G. typus). The best-fit model from this set was chosen through examination of Akaike’s Information Criterion (AIC), using the lowest AIC score, or, if the lowest AIC scores were within two units, the model with the fewest degrees of freedom (DF) was selected.
Results
A total of 186 G. typus (84 females, 102 males) were examined for ectoparasites (Table 2). Ectoparasites were detected on 98 G. typus, of which 9% were estimated to be young of the year (YOY) (< 520 mm TL), 80% were juveniles older than one year (523–1880 mm TL) and 11% were mature (> 1880 mm TL).
Parasite Taxa
Five ectoparasite taxa were detected on G. typus (Table 3): Caligus furcisetifer Redkar, Rangnekar & Murti, 1949 (Copepoda: Caligidae), Dermopristis cairae Whittington & Kearn, 2011 (Monopisthocotyla: Microbothriidae), Branchellion plicobranchus Sanjeeva Raj, 1953 and Stibarobdella macrothela (Schmarda, 1861) (Hirudinida: Piscicolidae), and praniza larvae of one or more unidentified gnathiid isopod species (Isopoda: Gnathiidae). Seventeen parasite specimens from G. typus were deposited as vouchers: four C. furcisetifer (WAM C84062–C84065), four D. cairae (WAM V12775–V12778), one B. plicobranchus (WAM V12770), four S. macrothela (WAM V12771–V12774) and four gnathiid praniza larvae (WAM C84058–C84061).
Caligus furcisetifer (Fig. 3) were identified on the basis of body size of specimens (4.2–4.7 mm body length; n = 3); rounded cephalothorax, approximately 1.1 times longer than wide, with maximum width approximately half of the distance from the anterior end, comprising approximately 63% of total body length (Fig. 3a); minute lunules, shifted laterally on the frontal plate (Fig. 3b); presence of a small, triangular sclerite on the ventral surface that projects distally over maxillule dentiform process base; genital complex with rounded corners, approximately 1.3 times wider than long (Fig. 3c); sternal furca with widely spaced, slightly divergent tines (Fig. 3d); presence of an accessory process on middle and inner terminal spines and reduced apical seta on the terminal exopodal segment of leg 1 (Fig. 3e); and subequal middle and outer spines on the terminal exopodal segment of leg 4 (Fig. 3h) [35,36,37]. Caligus furcisetifer was encountered in all sampled regions (Table 3) and on all exterior surfaces of the host body (i.e., excluding the nares, spiracles and buccal cavity), but were predominantly encounterd on the head, which accounted for 60% of the combined total infections by this species (Table 4).
Caligus furcisetifer from the giant shovelnose ray, Glaucostegus typus, in Western Australia. a Habitus, dorsal view. b Right lunule and side of frontal plate, ventral view. c Right maxillule, ventral view. d Sternal furca, ventral view. e Right leg 1 exopod, ventral view. f Right leg 2 exopod, ventral view. g Abdomen with caudal rami and egg sacs, ventral view. h Right leg 4 exopod, ventral view. Scale bars: a = 1 mm; b–e = 50 μm; g, h = 100 μm
Dermopristis cairae (Fig. 4) was identified on the basis of body size and shape of specimens (4.6–5.3 mm body length, 4.9–5.5 mm body width; n = 3); absence of transverse ridges on the ventral surface (Fig. 4b); absence of a seminal receptacle; simple, hookless haptor (Fig. 4c); and fine details of gut diverticula adjacent to the vas deferens and the oötype (Fig. 4d) [38]. All specimens were found on the dorsal surface of G. typus (Table 4), occurring almost exclusively adjacent to the series of thorns running along the mid-line (Fig. 4a), and were encountered only at Shark Bay (Table 3).
Dermopristis cairae from the giant shovelnose ray, Glaucostegus typus, in Western Australia. a In situ adjacent to the series of thorns along the dorsal mid-line (Photograph by Leon Deschamps). b Habitus, ventral view. c Simple haptor, ventral view. d Fine details of gut diverticula and oötype, posterior to tubular male reproductive tract, ventral view. Scale bars: b = 1 mm; c, d = 200 μm
Only a single, immature specimen of B. plicobranchus was encountered, which was identified based on the 33 pairs of leaf-like branchiae (Fig. 5); absence of eyespots on the oral sucker; and absence of an obvious bilobed hump on the ventral surface of segment VII [39]. This specimen was recovered from the head of a G. typus on the Ningaloo Coast (Table 3).
Stibarobdella macrothela (Fig. 6) was identified based on the large, wart-like tubercles present both dorsally and ventrally on the annuli of each trachelosome and urosome segment of specimens (Fig. 6c); two large, trumpet shaped ocular patches on the oral sucker (Fig. 6d); and large caudal sucker [40, 41]. Additionally, two new variations in pigmentation were observed on specimens in situ: predominantly black with some white streaks occurring laterally along the length of the urosome (Fig. 6a), and uniformly dark red; the latter of which was observed infrequently and only for immature specimens (Fig. 6b). Most S. macrothela were found on the head, particularly proximal to the nares (63%) and buccal cavity (18%), which were occasionally heavily infected (Table 4). Stibarobdella macrothela was encountered in all regions except for the Ningaloo Coast (Table 3).
Stibarobdella macrothela from giant shovelnose ray, Glaucostegus typus, in Western Australia. a In situ in the naris of a G. typus in Shark Bay, with predominantly black pigmentation (Photograph by Leon Deschamps). b In situ in the buccal cavity and, or adjacent to, the nares of G. typus in the Pilbara region, including with dark red pigmentation (bottom right) (Photograph by David Morgan). c Habitus, lateral view. d Oral sucker, lateral view, with ocular patch. Abbreviations: caudal, caudal sucker; oral, oral sucker. Scale bars: c = 1 mm; d = 200 μm (colour figure online)
Gnathiid taxonomy is typically based on the morphology of adult males [42]; therefore, no attempt has been made to determine the specific identity of recovered pranizae. Few species descriptions of gnathiids include pigmentation as a characteristic [43], and colouration often disappears through fixation [44]. Nevertheless, several distinct body pigmentations were noted in situ on collected pranizae during this study: predominantly yellow, with occasional uniform, brown streaks on the cephalosome, pereon and pleon (Fig. 7a); as well as predominantly white pigment, with sparse, light spots on the pleon (Fig. 7b). Gnathiids were encountered in all regions except for the Pilbara (Table 3), and exclusively on the heads of rays, with most infections (68%) occurring in the nares (Table 4).
Aggregation, Presence and Intensity
Caligus furcisetifer, S. macrothela and gnathiids were each highly aggregated (s2/m = 5.51, 13.52, 8.02 and k = 0.19, 0.20, 0.08, respectively). The AIC values indicated a zero-inflated negative binomial model was the best fit for all three taxa (see Supplementary Table S2). For C. furcisetifer, the best fit model included host TL and season as predictors of infection presence (AIC = 377.29, DF = 8), with odds of infection increasing with host TL and considerably lower in summer (Fig. 8) (Supplementary Table S3). Host TL was maintained in the best-fit model as the sole predictor of C. furcisetifer infection intensity, which increased with TL, especially on host individuals > 1300 mm in length (Fig. 8) (Supplementary Table S4).
Sampled (data points) and model-predicted (curve) abundance of Caligus furcisetifer, infecting the giant shovelnose ray, Glaucostegus typus (n = 185), in Western Australia. Abundance is displayed relative to host total length and sampling season. Standard error margins for model-predicted intensity are denoted by grey shading either side of curve. Sample from the Ningaloo Coast not included
For S. macrothela, host TL and sample region, and their interaction, were in the best-fit model as predictors of infection presence and intensity (AIC = 511.32, DF = 13) (Supplementary Table S3, S4). Odds and intensity of infection increased with host TL, especially on host individuals > 1500 mm TL, and were considerably higher in Shark Bay compared to other regions (Fig. 9).
Sampled (data points) and model-predicted (curve) abundance of Stibarobdella macrothela infecting the giant shovelnose ray, Glaucostegus typus (n = 185), in Western Australia. Abundance is displayed relative to host total length and sampling region. Standard error margins for model-predicted intensity are denoted by grey shading either side of curve. Sample from the Ningaloo Coast not included
For gnathiids, the best-fit model had sample region as the sole predictor of infection presence (AIC = 165.83, DF = 6) (Supplementary Table S3), with odds of infection greatest in Shark Bay. Host TL was the sole predictor of gnathiid infection intensity (Supplementary Table S4), which increased with host TL, especially on host individuals > 1500 mm TL (Fig. 10).
Sampled (data points) and model-predicted (curve) abundance of gnathiid praniza larvae infecting the giant shovelnose ray, Glaucostegus typus (n = 185), in Western Australia. Abundance is displayed relative to host total length and sampling region. Standard error margins for model-predicted intensity are denoted by grey shading either side of curve. Sample from the Ningaloo Coast not included
Discussion
This is the first study to investigate parasites exploiting the Critically Endangered giant shovelnose ray, Glaucostegus typus, in Western Australia. These are the first records of B. plicobranchus and S. macrothela infecting G. typus, although Burreson [40] speculated that the former likely infects this host. This research extends the geographical range of B. plicobranchus and C. furcisetifer south in the eastern Indian Ocean to the Ningaloo Coast and Shark Bay, respectively. Dermopristis cairae was originally described based on specimens collected during freshwater bathing treatment of G. typus at Cairns Marine Aquarium Supply in Queensland, Australia, with ambiguity pertaining to its microhabitat [38], which this study clarifies (occurring mostly along the thorn ridge on the dorsal midline). The microhabitat of D. cairae is distinct from its congeners; Dermopristis pterophila Ingelbrecht, Morgan & Martin, 2022, exhibits affinity for the pectoral and pelvic fin bases of the green sawfish, Pristis zijsron Bleeker, 1851 [32], and Dermopristis paradoxa Kearn, Whittington & Evans-Gowing, 2010, occurs proximal to the mouth of the largetooth sawfish, Pristis pristis (Linnaeus, 1758) [45].
In Western Australia alone, C. furcisetifer is known to infect P. pristis, P. zijsron and the eyebrow wedgefish, Rhynchobatus palpebratus Compagno & Last, 2008, whereas B. plicobranchus has been encountered on P. zijsron, and S. macrothela has been encountered on P. zijsron, as well as the sandbar shark, Carcharinus plumbeus (Nardo, 1827), the blacktip reef shark, Carcharhinus melanopterus (Quoy & Gaimard, 1824), and an unidentified wobbegong (Orectolobidae) [31, 36, 40].
Seasonal and Regional Variations in Infections
In our analyses, the best-fit models demonstrated that G. typus were less likely to be infected by C. furcisetifer during summer. Seasonal fluctuations of C. furcisetifer prevalence are possibly related to variations in copepod recruitment or host behaviour that affect the probability of parasite transmission [46, 47]. The activity space of G. typus is known to increase significantly during winter, likely in response to a decrease in resource availability, which may increase the probability of contact with the infectious copepodid stage of C. furcisetifer [48]. Conversely, habitat use is confined to smaller areas throughout the warmer months of the year, particularly in summer, which may limit contact with copepodids [48]. An alternative, but not mutually exclusive, explanation for this seasonal pattern in prevalence is a lack of immigration of infected G. typus into the study area in summer when activity is relatively low [48]. It seems unlikely that seasonal variation in prevalence of C. furcisetifer is directly related to water temperature, because temperatures recorded in autumn and summer were similar (mean = 26.5 ± 0.1 °C and 27.3 ± 0.2 °C, respectively) and this species is known to occur on P. pristis in the Fitzroy River estuary, Western Australia, and the Leichhardt River estuary, Queensland, where water temperatures occasionally exceed 30 °C [36, 49], although seasonal differences in parasite abundance can vary among host species [50]. Therefore, additional work is needed to understand the driver of the seasonal fluctuation in C. furcisetifer prevalence.
Regional variations among parasite populations are common, with differences in parasite diversity and abundance typically increasing with geographical distance [51,52,53]. For S. macrothela and gnathiids, likelihoods of infection were greatest in Shark Bay, with S. macrothela intensity of infection also greatest in this region. In Australian waters, previous encounters with S. macrothela have occurred mostly north of the Tropic of Capricorn [40], suggesting it is perhaps a predominantly tropical species. It is therefore intriguing that the likelihood and intensity of infections by S. macrothela were greatest in Shark Bay, which signifies a transition zone between tropical and temperate conditions, although this species has previously been detected as far south as Point Peron, almost 700 km south of Shark Bay [40]. These results suggest that S. macrothela could in fact be most suited for subtropical conditions and that previous records simply reflect where this species has been recorded, rather than where it is most abundant. The differences in infections of S. macrothela between the Pilbara, Exmouth Gulf and Shark Bay may also be a consequence of biotic or abiotic conditions that vary between regions, such as salinity or turbidity. However, additional work is required to determine this. Considering the presence of gnathiids also correlated positively with latitude, it is highly plausible that the gnathiid pranizae encountered during this study are most suited for subtropical or temperate conditions.
Importance of Host Size
Our analyses demonstrated that larger G. typus are more likely to be infected by C. furcisetifer and S. macrothela, and carry a greater intensity of infection of these species, as well as gnathiids, across the study area. Correlations between host size and parasite intensity are common in wild populations; larger, older hosts provide greater surface area for parasites to colonise, have had more time to accumulate parasites and often have home ranges that expand with growth, which may increase their exposure to parasites [54,55,56, 62]. Such trends have been reported for numerous species, including for C. furcisetifer on P. zijsron in Western Australia, where copepod abundance was found to increase exponentially with host TL, as well as for gnathiid pranizae on several labrid species in Queensland, Australia [31, 58, 59]. Like P. zijsron, the activity space of G. typus is known to increase with age, with mature individuals also utilising a greater variety of habitats than juveniles, likely increasing their exposure to parasites [60, 61]. As such, these data suggest that as host individuals grow, they may increasingly contribute to the spread and proliferation of parasites including C. furcisetifer, S. macrothela and gnathiid pranizae within and among populations.
Conclusion
Of the ectoparasites encountered during this study, D. cariae is the only species considered to be specific to G. typus. Collectively, species of Dermopristis are known only from rhinopristiform fishes in Australian waters, and D. cairae has been previously reported only from G. typus within captive settings [38, 45]. Modifications to the classification criteria for the IUCN Red List of Threatened Species have been proposed to classify parasites via referencing the threatened status of their host/s [5, 62]. However, this could result in an underestimation of the true extinction risk faced by parasites, due to the over-dispersed nature of infections, particularly species that are host-specific [5, 63]. Considering that D. cairae, which is presumably restricted to G. typus, was encountered at a very low prevalence (2%) and in only a single locality, these findings suggest that the threat of extinction faced by D. cairae is likely greater than that of its host, which is considered to be a species of Least Concern within Australian waters and Critically Endangered globally [14, 24].
Data Availability
The data supporting the findings of this study are available from the corresponding author upon reasonable request.
References
Dobson A, Lafferty KD, Kuris AM, Hechinger RF, Jetz W (2008) Homage to Linnaeus: how many parasites? How many hosts? Proc Natl Adac Sci 105:11482–11489. https://doi.org/10.1073/pnas.0803232105
Kuris AM, Hechinger RF, Shaw JC, Whitney KL, Aguirre-Macedo L, Boch CA, Dobson AP, Dunham EJ, Fredensborg BL, Huspeni TC (2008) Ecosystem energetic implications of parasite and free-living biomass in three estuaries. Nature 454:515–518. https://doi.org/10.1038/nature06970
Carlson CJ, Dallas TA, Alexander LW, Phelan AL, Phillips AJ (2020) What would it take to describe the global diversity of parasites? Proc R Soc B 287:20201841. https://doi.org/10.1098/rspb.2020.1842
Dougherty ER, Carlson CJ, Bueno VM, Burgio KR, Cizauskas CA, Clements CF, Seidel DP, Harris NC (2016) Paradigms for parasite conservation. Conserv Biol 30:724–733. https://doi.org/10.1111/cobi.12634
Lymbery AJ, Smit NJ (2023) Conservation of parasites: a primer. Int J Parasitol 21:255–263. https://doi.org/10.1016/j.ijppaw.2023.07.001
Carlson CJ, Burgio KR, Dougherty ER, Phillips AJ, Bueno VM, Clements CF, Castaldo G, Dallas TA, Cizauskas CA, Cumming GS, Dona J, Harris NC, Jovani R, Mironov S, Muellerklein OC, Proctor HC, Getz WM (2017) Parasite biodiversity faces extinction and redistribution in a changing climate. Sci Adv 3:e1602422. https://doi.org/10.1126/sciady.1602422
Dunn RR, Harris NC, Colwell RK, Koh LP, Sodhi NS (2009) The sixth mass coextinction: are most endangered species parasites and mutualists? Proc R Soc B Biol Sci 276:3037–3045. https://doi.org/10.1098/rspb.2009.0413
Lafferty KD (2012) Biodiversity loss decreases parasite diversity: theory and patterns. Phil Trans R Soc Lond B Biol Sci 367:2814–2827. https://doi.org/10.1098/rstb.2012.0110
Thompson RA, Lymbery AJ, Godfrey SS (2018) Parasites at risk–insights from an endangered marsupial. Trends Parasitol 34:12–22. https://doi.org/10.1016/j.pt.2017.09.001
Clark NJ, Clegg SM, Sam K, Goulding W, Koane B, Wells K (2018) Climate, host phylogeny and the connectivity of host communities govern regional parasite assembly. Divers Distrib 24:13–23. https://doi.org/10.1111/ddi.12661
Moss WE, McDevitt-Galles T, Calhoun DM, Johnson PT (2020) Tracking the assembly of nested parasite communities: Using β-diversity to understand variation in parasite richness and composition over time and scale. J Anim Ecol 89:1532–1542. https://doi.org/10.1111/1365-2656.13204
Carlson CJ, Hopkins S, Bell KC, Doña J, Godfrey SS, Kwak ML, Lafferty KD, Moir ML, Speer KA, Strona G, Torchin M, Wood CL (2020) A global parasite conservation plan. Biol Conserv 250:108596. https://doi.org/10.1016/j.biocon.2020.108596
D’Alberto BM, Carlson JK, Pardo SA, Simpfendorfer CA (2019) Population productivity of shovelnose rays: Inferring the potential for recovery. PLoS One 14:e0225183. https://doi.org/10.1371/journal.pone.0225183
Kyne PM, Rigby CL, Dharmadi, Gutteridge AN, Jabado RW (2019) Glaucostegus typus. The IUCN red list of threatened species. https://www.iucnredlist.org/species/104061138/68623995. Accessed 26 Aug 2023
Kyne PM, Jabado RW, Rigby CL, Gore MA, Pollock CM, Herman KB, Cheok J, Ebert DA, Simpfendorfer CA, Dulvy NK (2020) The thin edge of the wedge: extremely high extinction risk in wedgefishes and giant guitarfishes. Aquat Conserv 30:1337–1361. https://doi.org/10.1002/aqc.3331
Last P, Naylor G, Séret B, White W, de Carvalho M, Stehmann M (2016) Rays of the world. CSIRO Publishing, Clayton
Kyne PM, Jabado RW (2019) Glaucostegus cemiculus. The IUCN red list of threatened species. https://www.iucnredlist.org/species/104050689/104057239. Accessed 02 Sep 2023
Kyne PM, Jabado RW (2019) Glaucostegus halavi. The IUCN red list of threatened species. https://www.iucnredlist.org/species/161408/124479984. Accessed 10 Sep 2023
Kyne PM, Jabado RW (2021) Glaucostegus obtusus (amended version of 2019 assessment). The IUCN red list of threatened species. https://www.iucnredlist.org/species/60170/207283191. Accessed 10 Sep 2023
Kyne PM, Jabado RW (2021) Glaucostegus thouin (amended version of 2019 assessment). The IUCN red list of threatened species. https://www.iucnredlist.org/species/60175/207731709. Accessed 10 Sep 2023
Kyne PM, Haque AB, Charles R, Jabado RW (2022) Glaucostegus granulatus. The IUCN red list of threatened species. https://www.iucnredlist.org/species/60166/215829219. Accessed 09 Sep 2023
Kyne PM, Haque AB, Charles R, Jabado RW (2022) Glaucostegus younholeei. The IUCN red list of threatened species. https://www.iucnredlist.org/species/214418407/214418413. Accessed 13 Jul 2023
Bateman RL, Morgan DL, Wueringer BE, McDavitt M, Lear KO (2024) Collaborative methods identify a remote global diversity hotspot of threatened, large bodied rhino rays. Aquat Conserv 34:e4047. https://doi.org/10.1002/aqc.4047
Kyne PM, Heupel MR, White WT, Simpfendorfer CA (2021) The action plan for australian sharks and rays 2021. National Environmental Research Program Marine Biodiversity Hub. https://www.nespmarine.edu.au/document/action-plan-australian-sharks-and-rays-2021. Accessed 02 Feb 2024
Herzog KS, Caira JN, Kar PK, Jensen K (2023) Novelty and phylogenetic affinities of a new family of tapeworms (Cestoda: Rhinebothriidea) from endangered sawfish and guitarfish. Int J Parasitol 53:347–362. https://doi.org/10.1016/j.ijpara.2023.02.007
Naylor GJP, Caira JN, Jensen K, Rosana KAM, White WT, Last PR (2012) A DNA sequence–based approach to the identification of shark and ray species and its implications for global elasmobranch diversity and parasitology. Bull Am Mus Nat Hist 2012:1–262. https://doi.org/10.1206/754.1
Claudino-Sales V (2019) Coastal world heritage sites. Springer, Dordrecht
Heithaus MR (2004) Fish communities of subtropical seagrass meadows and associated habitats in Shark Bay, Western Australia. Bull Mar Sci 75:79–99
Spalding MD, Fox HE, Allen GR, Davidson N, Ferdaña ZA, Finlayson M, Halpern BS, Jorge MA, Lombana A, Lourie SA (2007) Marine ecoregions of the world: a bioregionalization of coastal and shelf areas. Bioscience 57:573–583. https://doi.org/10.1641/B570707
White J, Simpfendorfer CA, Tobin AJ, Heupel MR (2014) Age and growth parameters of shark-like batoids. J Fish Biol 84:1340–1353. https://doi.org/10.1111/jfb.12359
Ingelbrecht J, Lear KO, Martin SB, Lymbery AJ, Norman BM, Boxshall GA, Morgan DL (2024) Ectoparasites of the Critically Endangered green sawfish Pristis zijsron and sympatric elasmobranchs in Western Australia. Parasitol Int 101:102900. https://doi.org/10.1016/j.parint.2024.102900
Ingelbrecht J, Morgan DL, Lear KO, Fazeldean T, Lymbery AJ, Norman BM, Martin SB (2022) A new microbothriid monogenean Dermopristis pterophilus n. sp. from the skin of the Critically Endangered green sawfish Pristis zijsron Bleeker, 1851 (Batoidea: Pristidae) in Western Australia. Int J Parasitol Parasites Wildl 17:185–193. https://doi.org/10.1016/j.ijppaw.2022.01.006
Reiczigel J, Marozzi M, Fábián I, Rózsa L (2019) Biostatistics for parasitologists–a primer to quantitative parasitology. Trends Parasitol 35:277–281. https://doi.org/10.1016/j.pt.2019.01.003
R Core Team (2023) R: a language and environment for statistical computing. http://www.R-project.org/
Boxshall G (2018) The sea lice (Copepoda: Caligidae) of Moreton Bay (Queensland, Australia), with descriptions of thirteen new species. Zootaxa 4398:1–172. https://doi.org/10.11646/ZOOTAXA.4398.1.1
Morgan DL, Tang D, Peverell SC (2010) Critically endangered Pristis microdon (Elasmobranchii), as a host for the Indian parasitic copepod, Caligus furcisetifer Redkar, Rangnekar et Murti, 1949 (Siphonostomatoida): New records from northern Australia. Acta Parasitol 55:419–423. https://doi.org/10.2478/s11686-010-0050-2
Redkar MV, Rangnekar PG, Murti NN (1949) Four new species of parasitic copepods from the marine fishes of Bombay. J Univ Bombay 18:36–50
Whittington ID, Kearn GC (2011) A new species of Dermopristis Kearn, Whittington & Evans-Gowing, 2010 (Monogenea: Microbothriidae), with observations on associations between the gut diverticula and reproductive system and on the presence of denticles in the nasal fossae of the host Glaucostegus typus (Bennett) (Elasmobranchii: Rhinobatidae). Syst Parasitol 80:41–51. https://doi.org/10.1007/s11230-011-9308-9
Sanjeeva Raj PJ (1974) A review of the fish-leeches of the Indian Ocean. J Mar Biol Assoc India 16:381–397
Burreson EM (2020) Marine and estuarine leeches (Hirudinida: Ozobranchidae and Piscicolidae) of Australia and New Zealand with a key to the species. Invertebr Syst 34:235–259. https://doi.org/10.1071/IS19048
Llewellyn LC (1966) Pontobdellinae (Piscicolidae: Hirudinea) in the British Museum (Natural History) with a review of the subfamily. Bull Br Mus Nat Hist Zool 14:391–439
Wilson GDF, Sims CA, Grutter AS (2011) Toward a taxonomy of the Gnathiidae (Isopoda) using juveniles: the external anatomy of Gnathia aureamaculosa zuphea stages using scanning electron microscopy. J Crustac Biol 31:509–522. https://doi.org/10.1651/10-3432.1
Hale HM (1924) Notes on Australian Crustacea. Hassell Press, Melbourne
Smit NJ, Davies AJ (2004) The curious life-style of the parasitic stages of gnathiid isopods. In: Baker JR, Muller R, Rollinson D (eds) Advances in parasitology. Academic Press, Cambridge, pp 289–391
Kearn GC, Whittington ID, Evans-Gowing R (2010) A new genus and new species of microbothriid monogenean (Platyhelminthes) with a functionally enigmatic reproductive system, parasitic on the skin and mouth lining of the largetooth sawfish, Pristis microdon, in Australia. Acta Parasitol 55:115–122. https://doi.org/10.2478/s11686-010-0019-1
Chubb JC (1977) Seasonal occurrence of helminths in freshwater fishes Part I. Monogenea. Adv Parasitol 15:133–199. https://doi.org/10.1016/S0065-308X(08)60528-X
Rohde K (1982) Ecology of marine parasites. University of Queensland Press, Brisbane
Henderson CJ, Stevens T, Gilby BL, Lee SY (2018) Spatial conservation of large mobile elasmobranchs requires an understanding of spatio-temporal seascape utilization. ICES J Mar Sci 75:553–561. https://doi.org/10.1093/icesjms/fsx192
Lear KO, Morgan DL, Whitty JM, Whitney NM, Byrnes EE, Beatty SJ, Gleiss AC (2020) Divergent field metabolic rates highlight the challenges of increasing temperatures and energy limitation in aquatic ectotherms. Oecologia 193:311–323. https://doi.org/10.1007/s00442-020-04669-x
Poulin R (2020) Meta-analysis of seasonal dynamics of parasite infections in aquatic ecosystems. Int J Parasitol 50:501–510. https://doi.org/10.1016/j.ijpara.2020.03.006
Behnke JM, Bajer A, Harris PD, Newington L, Pidgeon E, Rowlands G, Sheriff C, Kuliś-Malkowska K, Siński E, Gilbert FS, Barnard CJ (2008) Temporal and between-site variation in helminth communities of bank voles (Myodes glareolus) from NE Poland. 2. The infracommunity level. Parasitology 135:999–1018. https://doi.org/10.1017/S0031182008004484
Nekola JC, White PS (1999) The distance decay of similarity in biogeography and ecology. J Biogeogr 26:867–878. https://doi.org/10.1046/j.1365-2699.1999.00305.x
Poulin R (2003) The decay of similarity with geographical distance in parasite communities of vertebrate hosts. J Biogeogr 30:1609–1615. https://doi.org/10.1046/j.1365-2699.2003.00949.x
Adou YE, Blahoua KG, KamelanN’douba TMV (2017) Prevalence and intensity of gill monogenean parasites of Tilapia guineensis (Bleeker, 1862) in man-made Lake Ayamé 2, Côte d’Ivoire according to season, host size and sex. Int J Biol Chem Sci 11:1559–1576. https://doi.org/10.4314/ijbcs.v11i4.13
Ibrahim MM (2012) Variation in parasite infracommunies of Tilapia zillii in relation to some biotic and abiotic factors. Int J Zool Res 8:59
Poulin R (2000) Variation in the intraspecific relationship between fish length and intensity of parasitic infection: biological and statistical causes. J Fish Biol 56:123–137. https://doi.org/10.1111/j.1095-8649.2000.tb02090.x
Tekin-Özan S, Kir I, Barlas M (2008) Helminth parasites of common carp (Cyprinus carpio L., 1758) in Beyşehir Lake and population dynamics related to month and host size. Turk J Fish Aquat Sci 8:201–205
Grutter AS (1994) Spatial and temporal variations of the ectoparasites of seven reef fish species from Lizard Island and Heron Island, Australia. Mar Ecol Prog Ser 115:21–30
Grutter AS (1996) Parasite removal rates by the cleaner wrasse Labroides dimidiatus. Mar Ecol Prog Ser 130:61–70
White J, Simpfendorfer CA, Tobin AJ, Heupel MR (2014) Spatial ecology of shark-like batoids in a large coastal embayment. Environ Biol Fish 97:773–786. https://doi.org/10.1007/s10641-013-0178-7
Morgan DL, Ebner BC, Allen MG, Gleiss AC, Beatty SJ, Whitty JM (2017) Habitat use and site fidelity of neonate and juvenile green sawfish Pristis zijsron in a nursery area in Western Australia. Endanger Species Res 34:235–249. https://doi.org/10.3354/esr00847
Moir ML, Brennan KE (2020) Incorporating coextinction in threat assessments and policy will rapidly improve the accuracy of threatened species lists. Biol Conserv 249:108715. https://doi.org/10.1016/j.biocon.2020.108715
Churcher T, Ferguson N, Basanez MG (2005) Density dependence and overdispersion in the transmission of helminth parasites. Parasitology 131:121–132. https://doi.org/10.1017/S0031182005007341
Kik MJL, Janse M, Benz GW (2011) The sea louse Lepeophtheirus acutus (Caligidae, Siphonostomatoida, Copepoda) as a pathogen of aquarium-held elasmobranchs. J Fish Dis 34:793–799. https://doi.org/10.1111/j.1365-2761.2011.01295.x
Kabata Z (1993) Two new species of Copepoda (Crustacea) parasitic on marine fishes. Syst Parasitol 26:233–239. https://doi.org/10.1007/BF00009731
Gleeson RJ, Bennett MB, Adlard RD (2010) First taxonomic description of multivalvulidan myxosporean parasites from elasmobranchs: Kudoa hemiscylli n. sp. and Kudoa carcharhini n. sp. (Myxosporea: Multivalvulidae). Parasitology 137:1885–1898. https://doi.org/10.1017/S0031182010000855
Sprent JFA (1990) Some ascaridoid nematodes of fishes: Paranisakis and Mawsonascaris ng. Syst Parasitol 15:41–63. https://doi.org/10.1007/BF00009917
Jones MK, Beveridge I (2001) Echinobothrium chisholmae n. sp. (Cestoda, Diphyllidea) from the giant shovel-nose ray Rhinobatos typus from Australia, with observations on the ultrastructure of its scolex musculature and peduncular spines. Syst Parasitol 50:41–52. https://doi.org/10.1023/A:1011809006300
Olson PD, Caira JN, Jensen K, Overstreet RM, Palm HW, Beveridge I (2010) Evolution of the trypanorhynch tapeworms: parasite phylogeny supports independent lineages of sharks and rays. Int J Parasitol 40:223–242. https://doi.org/10.1016/j.ijpara.2009.07.012
Ivanov VA, Caira JN (2012) Description of three new species of Echinobothrium (Cestoda: Diphyllidea) from Indo-Pacific elasmobranchs of the genus Glaucostegus (Rajiformes: Rhinobatidae). J Parasitol 98:365–377. https://doi.org/10.1645/GE-2731.1
Caira JN, Jensen K, Healy CJ (1999) On the phylogenetic relationships among tetraphyllidean, lecanicephalidean and diphyllidean tapeworm genera. Syst Parasitol 42:77–151. https://doi.org/10.1023/A:1006192603349
Olson PD, Caira JN (1999) Evolution of the major lineages of tapeworms (Platyhelminthes: Cestoidea) inferred from 18S ribosomal DNA and elongation factor-1α. J Parasitol 85:1134–1159. https://doi.org/10.2307/3285679
Cielocha JJ, Jensen K, Caira JN (2014) Floriparicapitus, a new genus of lecanicephalidean tapeworm (Cestoda) from sawfishes (Pristidae) and guitarfishes (Rhinobatidae) in the Indo-West Pacific. J Parasitol 100:485–499. https://doi.org/10.1645/13-468.1
Butler SA (1987) Taxonomy of some tetraphyllidean cestodes from elasmobranch fishes. Aust J Zool 35:343–371. https://doi.org/10.1071/ZO9870343
Healy CJ, Caira JN, Jensen K, Webster BL, Littlewood DTJ (2009) Proposal for a new tapeworm order, Rhinebothriidea. Int J Parasitol 39:497–511. https://doi.org/10.1016/j.ijpara.2008.09.002
Jadhav BV, Shinde GB (1982) A review of the genus Balanobothrium Hornell, 1912 with four new species. Helminthologia 19:185–194
Jadhav BV, Shinde GB (1979) Balanobothrium veravalensis n. sp. (Cestoda: Lecanicephalidae) from a marine fish. Indian J Parasitol 3:83–85
Schaeffner BC, Beveridge I (2013) Dollfusiella Campbell & Beveridge, 1994 (Trypanorhyncha: Eutetrarhynchidae) from elasmobranchs off Borneo, including descriptions of five new species. Syst Parasitol 86:1–31. https://doi.org/10.1007/s11230-013-9435-6
Beveridge I, Schaeffner BC (2018) Trypanorhynch cestodes (Platyhelminthes) parasitic in elasmobranchs and crustaceans in Moreton Bay, Queensland. Mem Qld Mus Nat 61:109–142. https://doi.org/10.17082/j.2204-1478.61.2018.2017-13
Beveridge I, Jones MK (2000) Prochristianella spinulifera n. sp. (Cestoda: Trypanorhyncha) from Australian dasyatid and rhinobatid rays. Syst Parasitol 47:1–8. https://doi.org/10.1023/A:1006486713630
Miquel J, Świderski Z (2006) Ultrastructure of the spermatozoon of Dollfusiella spinulifera (Beveridge and Jones, 2000) Beveridge, Neifar and Euzet, 2004 (Trypanorhyncha, Eutetrarhynchidae). Parasitol Res 99:37–44. https://doi.org/10.1007/s00436-005-0094-7
Świderski Z, Miquel J, Młocicki D, Neifar L, Grytner-Zięcina B, Mackiewicz JS (2006) Ultrastructural and cytochemical studies on vitellogenesis in trypanorhynch cestode Dollfusiella spinulifera Beveridge, Neifar et Euzet, 2004 (Eutetrarhynchidae). Acta Parasitol 51:182–193. https://doi.org/10.2478/s11686-006-0029-1
Schaeffner BC, Beveridge I (2014) The trypanorhynch cestode fauna of Borneo. Zootaxa 3900:21–49. https://doi.org/10.11646/zootaxa.3900.1.2
Kearn GC, Whittington ID, Evans-Gowing R (2011) Spermatophores in Dermopristis cairae Whittington et Kearn, 2011 (Monogenea, Microbothriidae). Acta Parasitol 56:371–376. https://doi.org/10.2478/s11686-011-0078-y
Perkins EM, Donnellan SC, Bertozzi T, Chisholm LA, Whittington ID (2009) Looks can deceive: molecular phylogeny of a family of flatworm ectoparasites (Monogenea: Capsalidae) does not reflect current morphological classification. Mol Phylogent Evol 52:705–714. https://doi.org/10.1016/j.ympev.2009.05.008
Whittington ID, Barton DP, Lester RJG (1989) A redescription of Calicotyle australis Johnston, 1934 (Monogenea: Monocotylidae) from a new host, Rhinobatos batillum (Batiformes: Rhinobatidae), from Moreton Bay, Queensland. Syst Parasitol 14:145–156. https://doi.org/10.1007/BF00016909
Cribb BW, Armstrong WD, Whittington ID (2004) Simultaneous fixation using glutaraldehyde and osmium tetroxide or potassium ferricyanide-reduced osmium for the preservation of monogenean flatworms: an assessment for Merizocotyle icopae. Microsc Res Tech 63:102–110. https://doi.org/10.1002/jemt.20015
Kritsky DC, Bullard SA, Bakenhaster MD, Scharer RM, Poulakis GR (2017) Resurrection of Mycteronastes (Monogenoidea: Monocotylidae), with description of Mycteronastes caalusi n. sp. from olfactory sacs of the smalltooth sawfish, Pristis pectinata (Pristiformes: Pristidae), in the Gulf of Mexico off Florida. J Parasitol 103:477–485. https://doi.org/10.1645/17-40
Mollaret I, Jamieson BGM, Adlard RD, Hugall A, Lecointre G, Chombard C, Justine J (1997) Phylogenetic analysis of the Monogenea and their relationships with Digenea and Eucestoda inferred from 28S rDNA sequences. Mol Biochem Parasitol 90:433–438. https://doi.org/10.1016/S0166-6851(97)00176-X
Whittington ID, Armstrong WD, Cribb BW (2004) Mechanism of adhesion and detachment at the anterior end of Neoheterocotyle rhinobatidis and Troglocephalus rhinobatidis (Monogenea: Monopisthocotylea: Monocotylidae). Parasitol Res 94:91–95. https://doi.org/10.1007/s00436-004-1171-z
Beverley-Burton M, Williams A (1989) Merizocotyle icopae, sp. nov., and Thaumatocotyle australensis, sp. nov., (Monogenea: Monocotylidae) from the nasal cavities of rajiform elasmobranchs of the Great Barrier Reef. Aust J Zool 37:25–35. https://doi.org/10.1071/ZO9890025
Chisholm LA (1998) Ciliated cells and chaetotaxy of the larvae of seven species of monocotylid monogeneans (Platyhelminthes) from Heron Island, Great Barrier Reef, Australia. Parasitol Res 84:828–834. https://doi.org/10.1007/s004360050495
Chisholm LA, Whittington ID (1996) Descriptions of the larvae of six species of monocotylid monogeneans from Himantura fai (Dasyatididae) and Rhinobatos typus (Rhinobatidae) from Heron Island, Great Barrier Reef, Australia. Syst Parasitol 35:145–156. https://doi.org/10.1007/BF00009823
Chisholm LA, Whittington ID (1999) A revision of the Merizocotylinae Johnston and Tiegs, 1922 (Monogenea: Monocotylidae) with descriptions of new species of Empruthotrema Johnston and Tiegs, 1922 and Merizocotyle Cerfontaine, 1894. J Nat Hist 33:1–28. https://doi.org/10.1080/002229399300452
Chisholm LA, Whittington ID (2000) Egg hatching in 3 species of monocotylid monogenean parasites from the shovelnose ray Rhinobatos typus at Heron Island, Australia. Parasitology 121:303–313. https://doi.org/10.1017/S003118209900637X
Chisholm LA, Whittington ID (2002) Efficacy of praziquantel bath treatments for monogenean infections of the Rhinobatos typus. J Aquat Anim Health 14:230–234. https://doi.org/10.1577/1548-8667(2002)014%3c0230:EOPBTF%3e2.0.CO;2
Chisholm LA, Whittington ID (2003) Invasion of the shovelnose ray (Rhinobatos typus) by Neoheterocotyle rhinobatidis and Merizocotyle icopae (Monogenea: Monocotylidae). Parasitol 127:561–570. https://doi.org/10.1017/S0031182003004062
Chisholm LA, Morgan JA, Adlard RD, Whittington ID (2001) Phylogenetic analysis of the Monocotylidae (Monogenea) inferred from 28S rDNA sequences. Int J Parasitol 31:1253–1263. https://doi.org/10.1016/S0020-7519(01)00223-5
Cribb BW, Chisholm LA, Gould RJ, Whittington ID (2003) Morphology, ultrastructure, and implied function of ciliated sensory structures on the developmental stages of Merizocotyle icopae (Monogenea: Monocotylidae). Microsc Res Tech 62:267–276. https://doi.org/10.1002/jemt.10387
Hamwood TE, Cribb BW, Halliday JA, Kearn GC, Whittington ID (2002) Preliminary characterisation and extraction of anterior adhesive secretion in monogenean (platyhelminth) parasites. Folia Parasitol 49:39–49. https://doi.org/10.14411/fp.2002.010
Chisholm LA, Whittington ID (2012) Three new species of Merizocotyle Cerfontaine, 1894 (Monogenea: Monocotylidae) from the nasal tissues of dasyatid rays collected off Malaysian and Indonesian Borneo. Syst Parasitol 82:167–176. https://doi.org/10.1007/s11230-012-9358-7
Kearn GC (1978) Early development and microhabitat of the monogenean Horricauda rhinobatidis, with observations on the related Troglocephalus rhinobatidis, from Rhinobatos batillum from Queensland, Australia. Int J Parasitol 8:305–311. https://doi.org/10.1016/0020-7519(78)90095-4
Chisholm LA, Whittington ID (1997) A revision of Neoheterocotyle (Monogenea: Monocotylidae) with descriptions of the larvae of N. rhinobatis and N. rhynchobatis from Heron Island, Great Barrier Reef, Australia. Int J Parasitol 27:1041–1060. https://doi.org/10.1016/S0020-7519(97)00072-6
Cribb BW, Gould RJ, Whittington ID (2001) A comparison of anterior adhesive areas and secretions in Troglocephalus rhinobatidis and Neoheterocotyle rhinobatidis (Monogenea: Monocotylidae) from the gills of the shovelnose ray, Rhinobatos typus (Rhinobatidae). Aust J Zool 49:577–587. https://doi.org/10.1071/ZO01028
Watson NA (1997) Spermiogenesis and sperm ultrastructure in Troglocephalus rhinobatidis, Neoheterocotyle rhinobatidis and Merizocotyle australensis (Platyhelminthes, Monogenea, Monopisthocotylea, Monocotylidae). Int J Parasitol 27:389–401. https://doi.org/10.1016/S0020-7519(96)00203-2
Kritsky DC, Chisholm LA (2020) Monocotylids (Monogenoidea) infecting elasmobranchs in Moreton Bay, Queensland, Australia, with descriptions of Calicotyle cutmorei n. sp. (Calicotylinae) and Dendromonocotyle raiae n. sp. (Monocotylinae). Syst Parasitol 97:569–589. https://doi.org/10.1007/s11230-020-09946-0
Young PC (1967) A taxonomic revision of the subfamilies Monocotylinae Gamble, 1896 and Dendromonocotylinae Hargis, 1955 (Monogenoidea: Monocotylidae). J Zool 153:381–422. https://doi.org/10.1111/j.1469-7998.1967.tb04070.x
Cribb TH, Chick RC, O’Connor W, O’Connor S, Johnson D, Sewell KB, Cutmore SC (2017) Evidence that blood flukes (Trematoda: Aporocotylidae) of chondrichthyans infect bivalves as intermediate hosts: indications of an ancient diversification of the Schistosomatoidea. Int J Parasitol 47:885–891. https://doi.org/10.1016/j.ijpara.2017.05.008
Cutmore SC, Cribb TH, Yong RQY (2018) Aporocotylids from batoid and elopomorph fishes from Moreton Bay, Queensland, Australia, including a new genus and species of blood fluke infecting the giant shovelnose ray, Glaucostegus typus (Rhinopristiformes: Glaucostegidae). Parasitol Int 67:768–775. https://doi.org/10.1016/j.parint.2018.08.003
Caira JN, Jensen K, Barbeau E (2023) Global Cestode Database. World Wide Web electronic publication. www.tapewormdb.uconn.edu. Accessed Sep, 2023
Acknowledgements
We thank Leon Deschamps, Matthew Cross, Timothy Winship, Paul de Lestang, Steve Moore, Andrew Slater, Carlos Estrabeau, Danielle Spreitzer, Davey Jones, Jasmin O’Brien, Jade Pursell, Robert Blackburn, Heather Kay, Amy Beck, Alanah Kilner, Anouska Freedman, Manuel Pel, Samantha Reynolds and Joselyn Davis for assisting with sample collection. We also thank Eugene Burreson, Colin Simpfendorfer, Patricia Charvet, Samuel Lymbery, the Malgana Sea Rangers, including Gaven Poland and Richard Cross, and the Chevron Indigenous Sea Rangers, including Chloe Davidson and Stevie Sweeting, for assistance with the research, and recognise and thank the Malgana, Thalanyji, and Baiyungu peoples of the land on which this work took place. Sample collection was funded and/or supported by Chevron Australia, Murdoch University and the Shark Ark Project.
Funding
Open Access funding enabled and organized by CAUL and its Member Institutions. This work was supported by The Shark Ark Project, Chevron Australia, Protect Ningaloo, The Cape Conservation Group, and Murdoch University. JI was supported by the Research Training Program stipend administered by Murdoch University and the Holsworth Wildlife Research Endowment administered by the Ecological Society of Australia. SBM is supported by the Australian Biological Resources Study (ABRS) National Taxonomy Research Grant G046WN7.
Author information
Authors and Affiliations
Contributions
JI, KOL, AJL, BMN and DLM conceptualized the study; JI, KOL, RLB, BMN, TF and DLM were responsible for sample collection; JI and SBM were responsible for species identifications; JI, KOL and AJL conducted the formal analysis; all authors contributed to the writing and editing of the manuscript.
Corresponding author
Ethics declarations
Conflict of Interest
The authors declare that there is no conflict of interest associated with this research.
Ethical Approval
Handling and sampling of G. typus was conducted under Murdoch University Animal Ethics Approval RW3191/19, Western Australian Government Department of Primary Industries and Regional Development fisheries exemption no. 3378 and 3553, Department of Fisheries Regulation 178 (SPA 11–11), and Department of Environment and Conservation Permit SF007889.
Additional information
Publisher's Note
Springer Nature remains neutral with regard to jurisdictional claims in published maps and institutional affiliations.
Supplementary Information
Below is the link to the electronic supplementary material.
Rights and permissions
Open Access This article is licensed under a Creative Commons Attribution 4.0 International License, which permits use, sharing, adaptation, distribution and reproduction in any medium or format, as long as you give appropriate credit to the original author(s) and the source, provide a link to the Creative Commons licence, and indicate if changes were made. The images or other third party material in this article are included in the article's Creative Commons licence, unless indicated otherwise in a credit line to the material. If material is not included in the article's Creative Commons licence and your intended use is not permitted by statutory regulation or exceeds the permitted use, you will need to obtain permission directly from the copyright holder. To view a copy of this licence, visit http://creativecommons.org/licenses/by/4.0/.
About this article
Cite this article
Ingelbrecht, J., Lear, K.O., Lymbery, A.J. et al. Ectoparasites of the Critically Endangered Giant Shovelnose Ray Glaucostegus typus in the Eastern Indian Ocean, with a Summary of the Known Metazoan Parasites. Acta Parasit. (2024). https://doi.org/10.1007/s11686-024-00918-8
Received:
Accepted:
Published:
DOI: https://doi.org/10.1007/s11686-024-00918-8