Abstract
Introduction
Local steroid injection (LSI) in the carpal tunnel is a mainstay of conservative treatment in patients with carpal tunnel syndrome (CTS). Currently, clinicians generally perform a conventional proximal approach (PA) or novel distal approach (DA) for LSI. Recent systematic reviews comparing the two injection methods are lacking. This systematic review and meta-analysis aimed to assess whether LSI using the DA was superior to PA in treating patients with CTS.
Methods
Databases including Pubmed, Embase, and the Cochrane library were searched up to 30 May 2022 to identify relevant randomized controlled trials (RCTs) comparing the DA with the PA steroid injection in patients with CTS. The outcomes mainly included Boston Carpal Tunnel Questionnaire Symptom Severity Scale (BCTQs) and Functional Status Scale (BCTQf), visual analog scores (VAS), electrophysiological outcomes, pain of injection, duration of injection, or adverse events.
Results
Five RCTs involving 339 patients were identified. Pooled analysis showed that the DA group took less time [mean difference (MD) −19.91; 95% CI −34.48 to −5.35; P = 0.007] and acquired better sensory nerve action potential amplitude [standardized mean difference (SMD) −0.37; 95% CI −0.62 to −0.11; P = 0.005]. The two groups were not significantly different in terms of BCTQs and BCTQf, VAS, other electrophysiological outcomes, pain of injection, or adverse events (P > 0.05).
Conclusion
Although providing similar improvement in pain relief or function improvement, the distal approach is superior to the proximal approach in terms of timing, without increasing other side effects. Further high-quality randomized studies are required to confirm these results.
Similar content being viewed by others
Avoid common mistakes on your manuscript.
Recent systematic reviews comparing the novel distal approach and conventional proximal approach for local steroid injection in patients with carpal tunnel syndrome are lacking. |
Based on the current meta-analysis, compared with the proximal approach, the distal approach for the treatment of carpal tunnel syndrome is less time consuming, without increasing other side effects. |
Steroid injection via the distal approach for carpal tunnel is very easy and a feasible alternative for patients with carpal tunnel syndrome. |
Introduction
Carpal tunnel syndrome (CTS) is the most common upper extremity compression neuropathy, and can cause permanent loss of sensation and partial paralysis of the thumb [1]. The prevalence of CTS under different case definitions ranges from 2.5% to 11.0% [2]. There are two conflicting hypotheses regarding the pathogenesis of CTS: one supports an edema-induced increase in carpal tunnel pressure, while another supports fibrosis of the subsynovial connective tissues in the carpal tunnel [3, 4]. Although the optimal management of CTS is still controversial, there is strong evidence for the efficacy of local steroid injection (LSI) [5,6,7]. The therapeutic rationale of LSI is the ability to reduce edema, allowing more space around the carpal tunnel and the median nerve and tendons [8, 9]. For decades, clinicians have conventionally performed a proximal approach (PA: 0–4 proximal to the first crease of the wrist, just to the ulnar side of the palmaris longus tendon) for carpal tunnel injection with satisfactory results [10, 11]. Nevertheless, ischemia, skin depigmentation and atrophy, and injuries to the median nerve and nearby tendons cannot be avoided entirely. Even with correct needle insertion and localization, patients with CTS are prone to injury owing to edema of the median nerve [12]. In this situation, ultrasound-guided injection is gradually applied for accurate localization and acquires favorable outcomes [13,14,15,16,17]. However, landmark-guided injection still seems to be one of the most available management procedures, attributed to good effectiveness, more convenience, and lower cost [18].
Habib et al. [19] pioneered a novel distal approach (DA, 2–3 cm distal to the middle of the wrist crease) for LSI. Özdemir et al. [20] detected decreased pain severity and neural improvement following steroid injection using this novel method in patients with CTS. Badarny et al. [21] found that the rate response of electrophysiological studies was similar to the results of previous studies using the PA. An active debate is currently ongoing in the literature whether the DA results in better treatment outcomes than the conventional PA. This indeterminacy has been magnified by inconsistent results obtained from various clinical trials comparing the effectiveness of distal versus proximal local steroid injection in patients with CTS [10, 19, 22,23,24]. Nair et al. [10] found that the pain perceived during the injections was higher in the DA, while Habib et al. [19] reported a completely opposite result. Two studies [22, 23] showed that the procedure duration in the DA group was significantly shorter than the PA group, while the other two [10, 19] concluded no statistically significant. To provide scientific reference for clinical decision making, we have conducted a systematic review and meta-analysis to consolidate existing evidence from the available literature comparing the two injection methods for LSI in patients with CTS, in terms of symptom severity, functional status, and electrodiagnostic outcomes.
Methods
This systematic review and meta-analysis followed guidelines in the Cochrane Collaboration handbook [25] and Preferred Reporting Items for Systematic Reviews and Meta-Analyses 2020 statement: an updated guideline for reporting systematic reviews [26]. The protocol of our systematic review was registered with PROSPERO (CRD42022350166). This article is based on previously conducted studies and does not contain any new studies with human participants or animals performed by any of the authors.
Search Strategy
A systematic computer-based retrieval for randomized controlled trials (RCTs) comparing the DA with the PA local steroid injection for the management of CTS was performed in the following electronic databases: Pubmed, Embase, and the Cochrane Library up to 30 May 2022. The keywords for the study object (MeSH or free words) were as follows: ‘Carpal Tunnel Syndrome’, ‘Carpal Tunnel Syndromes’, ‘Syndrome, Carpal Tunnel’, ‘Syndromes, Carpal Tunnel’, ‘Amyotrophy, Thenar, Of Carpal Origin’, ‘Median Neuropathy, Carpal Tunnel’, ‘Compression Neuropathy, Carpal Tunnel’, ‘Entrapment Neuropathy, Carpal Tunnel’. For the intervention strategy, the keywords were ‘proximal’, ‘conventional’, ‘distal’, ‘novel’. For the study design strategy, the keywords were ‘Randomized Controlled Trial’, ‘Randomized’, ‘Placebo’. The detailed list of search strategies could be found in the Supplementary Material. Only English language articles were included in our study. The reference lists of retrieved studies and relevant reviews were manually searched to avoid any initially omitted studies.
Inclusion and Exclusion Criteria
Articles eligible for inclusion in this systematic review were as follows: (1) Mild-to-moderate CTS with a clinical diagnosis and neurophysiologic confirmation; (2) RCTs comparing the clinical effectiveness of DA versus PA local steroid injection for CTS, irrespective of the type and dose of corticosteroid or the size of syringe; and (3) Studies reporting at least one outcome of interest: Boston Carpal Tunnel Questionnaire (BCTQ) including the Symptom Severity Scale (BCTQs) and the Functional Status Scale (BCTQf) [27], visual analog scores (VAS), electrophysiological outcomes, pain of injection, duration of injection, or adverse events. Exclusion criteria included: (1) CTS due to trauma or any metabolic disorders (such as thyroid disease, rheumatoid disorders, diabetes mellitus) and (2) non-RCTs, duplicated publications, in vitro studies, and animal studies.
Selection Criteria and Data Extraction
Two authors (Z.Y.T. and D.C.K.) independently evaluated all titles and abstracts of studies identified by the above searches based on the eligibility criteria mentioned above. A full-text review was conducted for any potential studies that met the inclusion criteria, and disagreements were resolved by reaching a consensus among the researchers. Two investigators (Z.Y.T. and D.L.) independently extracted the following characteristics from the included studies: author, publication year, country, interventions (injection methods and drugs), patient information (age, gender, sample size, duration of symptom), duration of follow-up, and outcomes. Data in other forms [i.e., median, interquartile range, and mean ± 95% confidence interval (CI)] were converted to mean ± standard deviation (SD) according to the Cochrane Handbook [25], and figure data was extracted by manual measurement.
Quality Assessment
The methodological quality of included studies was assessed independently by two authors (D.C.K. and Z.J.) according to the Cochrane Collaboration’s Risk of Bias Tool based on the following seven sections: random sequence generation, allocation concealment, blinding of participant and personnel, blinding of outcome assessment, incomplete outcome data, selective reporting, and other bias [28]. Any discrepancies of data extraction and quality assessment were settled by discussing with the third independent author (H.L.M.).
Data Analysis
The data analysis was conducted according to our previous study [29]. All calculations were performed using RevMan 5.3 for Windows (Cochrane Collaboration, Oxford, UK). Continuous data were calculated through the mean difference (MD) or standardized mean difference (SMD) with 95% CI. We calculated risk ratio (RR) with a 95% CI to evaluate adverse events. Heterogeneity across studies was assessed using Cochran's Q and I2 statistics, and P < 0.1 and I2 > 50% was considered statistical heterogeneity [30]. A fixed-effects model was conducted when I2 ≤ 50%; otherwise, a random-effects model was selected. Sensitivity analysis was introduced to detect the stability of the results. The results of this meta-analysis were considered statistically significant if P < 0.05.
Results
Search Results
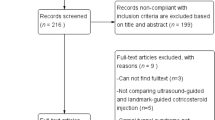
The comprehensive search initially identified a total of 162 potential articles (PubMed 13, Embase 83, the Cochrane Library 65, and additional in the reference lists 1). After removing 22 duplicate and 124 irrelevant studies through screening the titles and abstracts, 16 full-text articles were assessed in more detail for eligibility. Finally, five RCTs were screened out after reading the full text. (Fig. 1).
Characteristics and Risk of Bias of Included Studies
Five related studies involving 339 patients were identified. The characteristics of the included studies are summarized in Table 1. The risk of bias assessment for the five included RCTs is shown in Fig. 2. The random sequence generation was low risk in all studies [10, 19, 22,23,24], and the illustration of allocation concealment was unclear for three trials [19, 23, 24]. The blinding of researcher and outcome assessment was evaluated as “high risk” for one study [22] and “unclear risk” for three trials [19, 23, 24]. There was no attrition or reporting bias for all studies and other biases could not be accurately determined.
Meta-analysis Results
Pooled Analysis of BCTQ
BCTQ was available in three studies [10, 22, 23], including 121 patients in the DA group and 124 patients in the PA group. The summarized estimate of effect size indicated that there was no significant difference in BCTQs (SMD 0.11; 95% CI −0.14 to 0.36; P = 0.39; Fig. 3a) or BCTQf (SMD 0.08; 95% CI −0.17 to 0.33; P = 0.54; Fig. 3b) between the two groups.
Pooled Analysis of VAS
VAS was described in three papers [22,23,24], with 65 patients in the DA group and 68 patients in the PA group. The pooled estimate of effect size showed that the VAS did not differ significantly between the two groups (MD −0.34; 95% CI −0.81 to 0.13; P = 0.15; Fig. 4), and a fixed-effects model was used because no statistical heterogeneity was found among community samples (P = 0.61, I2 = 0%).
Pooled Analysis of Electrophysiological Outcomes
Electrophysiological outcomes of distal motor latency (DML) and sensory nerve conduction velocity (SCV) were recorded in four trials [10, 22,23,24], with 139 patients in the DA group and 144 patients in the PA group. Distal sensory latency (DSL), motor conduction velocity (MCV), compound muscle action potential (CAMP), and sensory nerve action potential amplitude (SNAP) were measured in three studies [10, 22, 23], with 121 patients in the DA group and 144 patients in the PA group. The overall estimates of electrodiagnostic parameters indicated no significant difference in terms of DML (MD −0.05; 95% CI −0.15 to 0.06; P = 0.37; Fig. 5a), DSL (MD −0.02; 95% CI −0.18 to 0.14; P = 0.77; Fig. 5b), MCV (MD 0.22; 95% CI −0.94 to 1.38; P = 0.71; Fig. 5c), SCV (MD −1.40; 95% CI −3.42 to 0.61; P = 0.17; Fig. 5d), and CAMP (MD −0.11; 95% CI −0.81 to 0.59; P = 0.76; Fig. 5e) between the two groups. The overall estimates showed that the DA group had significantly better SNAP than the PA group (SMD −0.37; 95% CI −0.62 to −0.11; P = 0.005; Fig. 5f).
Pooled Analysis of the Pain of Injection
The pain of injection was described in three studies, with a total of 227 patients (113 in the DA group and 114 in the PA group). A random effects model was used. The pain of injection in the DA group was not significantly different from that in the PA group (MD −0.97; 95% CI −3.95 to 2.02; P = 0.53; Fig. 6), with significant heterogeneity between trials (P < 0.00001, I2 = 99%).
Pooled Analysis of the Duration of Injection
Four studies [10, 19, 22, 23] provided relevant data on duration of injection, with 142 patients in the DA group and 145 patients in the PA group. Pooling of data showed significantly less injection duration in the DA group compared with the PA group (MD −19.91; 95% CI −34.48 to −5.35; P = 0.007; Fig. 7). A random effects model was used due to significant statistical heterogeneity (P < 0.00001, I2 = 98%).
Pooled Analysis of the Adverse Events
Related data of adverse events was represent in two studies [10, 19]. The risk of adverse events in the DA group was not significantly different from that in the PA group (RR 0.53; 95% CI 0.20–1.43; P = 0.21; Fig. 8).
Publication Bias and Sensitivity Analysis
Funnel plots of BCTQs and BCTQf were generated for evaluation of publication bias. The distribution of data points indicates mild publication bias (Fig. 9).
The sensitivity analysis was performed by omitting one study in each round to examine the impact on the overall result. SCV in the DA group was not significantly different from that in the PA group when omitting anyone of the studies except EL-Badawy et al. [23]. SNAP was similar between the two groups when omitting studies by EL-Badawy et al. [23]. There was no significant difference in the duration of injection between the two groups when omitting studies by Meshkini et al.[22]. There was no difference in the sensitivity analysis of other outcomes compared with those of the original analysis.
Discussion
To our knowledge, this is the first meta-analysis of RCTs comparing the efficacy and safety of the DA with the PA for LSI in CTS patients. Our study suggests that LSI through the DA for CTS patients would take less operative procedure time and acquire a better SNAP result than through the PA. However, there was no significant difference in the improvement of BCTQ, VAS, or other electrophysiological outcomes between the two methods. Besides, the novel DA did not decrease the risk of adverse events or reduce injection pain.
LSI is the most commonly accepted treatment among the various conservative managements for CTS [5, 31]. Steroid injection may be effective because of the expected anti- inflammatory effect by inhibiting the production of inflammatory cytokines by lymphocytes and macrophages, through an antifibrotic effect via the suppression of collagen expression, and antiedematous effects through reduced vascular permeability [3, 4, 9, 32]. In general, two approaches have been described in the literature: the conventional PA technique [12, 33, 34] and the novel DA technique [10, 19, 22,23,24]. The injection location of the PA approach is usually just ulnar to the tendon of the palmaris longus or flexor carpi radialis muscles, at a distance ranging from 0 to 4 cm proximal to the first crease of the wrist [19]. Theoretically, the PA technique was associated with a higher possibility of injury to the median nerve [34], ulnar nerve [35], transverse carpal ligament [36], or ulnar artery [27] owing to the injection site being adjacent to the above structures. Recently, an extensive prospective study involving 756 patients indicated that injection accuracy (75.7%) was less than reported in previous studies, which noted 82–100% accuracy using the PA technique, and the median nerve was penetrated in 8.7% of attempts [35]. Therefore, the safest injection site of PA remains a paramount concern.
For the above-mentioned, the novel DA technique has been proposed to reduce complications and improve patient satisfaction [19]. Our meta-analysis demonstrates that the DA needs significant shorter procedure duration for the intervention. The injection site through DA is easily located without carefully palpating the distal part of the forearm to distinguish flexor tendons, median nerve, and blood vessels [19]. The physician no longer needs comprehensive care and consideration while performing the injecting and only focuses on the palm [19]. Furthermore, the injection posture is convenient for both the patient and the doctor [23]. These factors probably play significant roles in reducing the procedure duration.
The pooled results show similar improvement of BCTQ, VAS, and electrophysiological parameters in the two groups. The mechanism behind the improvement following steroid injection is believed to be a result of pressure release, improving the spatial relation between the carpal tunnel and the median nerve and tendons [9, 21]. The median nerve compression could occur at the distal part of the tunnel, therefore injection by the DA seems reasonable. Even if the compression area is more proximal, sufficient corticosteroid injection ensures distribution in the lesion area [19]. Thus, these two approaches can achieve similar clinical and electrophysiological improvements.
The pain during injection is an essential factor affecting patient satisfaction. Three studies explored the result, and our meta-analysis indicates that there is no significant difference in injection pain between the two groups. Nair et al. [10] found that the pain perceived during the injections was higher in the DA, which may be attributed to palm skin being more sensitive to pain. Habib et al. [19] considered that the lesser pain expressed by the DA group was probably related to using a syringe needle of smaller diameter and shorter length. In addition, intramuscular injection through the DA could alleviate the effect of needle insertion rather than flexor tendons [19].
Safety is a top concern of clinicians. Adverse effects such as short-lived local pain, tendon rupture, intraneural injection, subcutaneous atrophy, depigmentation, bruising, and facial flushing were estimated at 33% [37]. The probability of serious complications such as median nerve injury and tendon rupture is less than 0.1% [37, 38]. Perhaps due to the sample size, neither of the two studies involved reported severe complications. Some scholars have suggested that local anesthetics should be avoided during the injection to avoid the possibility of masking complications [10, 22]. Our results showed no significant difference in the incidence of minor complications between the two approaches. Therefore, whether the novel DA can reduce the occurrence of serious complications requires further study.
The present study has some limitations. First, heterogeneity may have been caused by different types and doses of corticosteroids, different sizes of syringes, different anesthesia, and different follow-up times. Second, the studies included in our systematic review only discussed the short-term clinical outcomes, and the long-term efficacy remains to be explored. Third, not all studies include all the anticipated outcomes, which may affect generalizability of results. Fourth, the poor quality of most included studies, small number of studies included, and small sample size of each study limits the statistical power of our outcomes; more high-quality RCTs are needed to confirm these results.
Conclusions
Based on the results of this meta-analysis, LSI using the DA for the treatment of CTS is less time consuming without increasing other side effects compared with the PA, and to some extent, yields better electrophysiological outcomes. There was no statistically different in pain relief or function improvement between the two approaches. Steroid injection via the DA to carpal tunnel is a very easy and feasible alternative for patients with CTS.
References
Karjalanen T, Raatikainen S, Jaatinen K, Lusa V. Update on efficacy of conservative treatments for carpal tunnel syndrome. J Clin Med. 2022;11:950.
Yamamoto M, Curley J, Hirata H. Trends in open vs. endoscopic carpal tunnel release: a comprehensive survey in Japan. J Clin Med. 2022;11:4966. https://www.mdpi.com/2077-0383/11/17/4966.
Moon H, Lee BJ, Park D. Change to movement and morphology of the median nerve resulting from steroid injection in patients with mild carpal tunnel syndrome. Sci Rep. 2020;10:1–9. https://doi.org/10.1038/s41598-020-72757-2.
Yamanaka Y, Tajima T, Tsujimura Y, Kosugi K, Mano Y, Zenke Y, et al. Molecular and clinical elucidation of the mechanism of action of steroids in idiopathic carpal tunnel syndrome. J Bone Jt Surg Am. 2021;103:1777–87.
Marshall S, Tardif G, Ashworth N. Local corticosteroid injection for carpal tunnel syndrome. Cochrane Database Syst Rev. 2007;CD001554.
Piazzini DB, Aprile I, Ferrara PE, Bertolini C, Tonali P, Maggi L, et al. A systematic review of conservative treatment of carpal tunnel syndrome. Clin Rehabil. 2007;21:299–314.
Urits I, Smoots D, Anantuni L, Bandi P, Bring K, Berger AA, et al. Injection techniques for common chronic pain conditions of the hand: a comprehensive review. Pain Ther. 2020;9:129–42. https://doi.org/10.1007/s40122-020-00158-4.
Buntragulpoontawee M, Chang K-V, Vitoonpong T, Pornjaksawan S, Kitisak K, Saokaew S et al. The Effectiveness and Safety of Commonly Used Injectates for Ultrasound-Guided Hydrodissection Treatment of Peripheral Nerve Entrapment Syndromes: A Systematic Review. Front Pharmacol 2020;11:621150. https://www.frontiersin.org/articles/https://doi.org/10.3389/fphar.2020.621150/full.
Padua L, Coraci D, Erra C, Pazzaglia C, Paolasso I, Loreti C et al. Carpal tunnel syndrome: clinical features, diagnosis, and management. Lancet Neurol 2016;15:1273–84. https://www.frontiersin.org/articles/https://doi.org/10.3389/fphar.2020.621150/full.
Nair PP, Wadwekar V, Chakkalakkoombil SV, Narayan SK, Marusani R, Murgai A, et al. Comparison of proximal and distal corticosteroid injections for carpal tunnel syndrome. Muscle Nerve. 2020;62:89–94.
Chen PC, Chuang CH, Tu YK, Bai CH, Chen CF, Liaw MY. A Bayesian network meta-analysis: Comparing the clinical effectiveness of local corticosteroid injections using different treatment strategies for carpal tunnel syndrome. BMC Musculoskelet Disord. 2015;16:1–16. https://doi.org/10.1186/s12891-015-0815-8.
Kim DH, Jang JE, Park BK. Anatomical basis of ulnar approach in carpal tunnel injection. Pain Physician. 2013;16:E191–8.
Wang H, Zhu Y, Wei H, Dong C. Ultrasound-guided local corticosteroid injection for carpal tunnel syndrome: A meta-analysis of randomized controlled trials. Clin Rehabil [Internet]. 2021;35:1506–17. http://journals.sagepub.com/doi/https://doi.org/10.1177/02692155211014702.
Zhang S, Wang F, Ke S, Lin C, Liu C, Xin W, et al. The effectiveness of ultrasound-guided steroid injection combined with miniscalpel-needle release in the treatment of carpal tunnel syndrome vs steroid injection alone: a randomized controlled study. Biomed Res Int. 2019;2019:1–9.
Wu YT, Lam KHS, Lai CY, Chen SR, Shen YP, Su YC, et al. Novel motor-sparing ultrasound-guided neural injection in severe carpal tunnel syndrome: a comparison of four injectates. Biomed Res Int. 2022;2022:1–12.
Joon-Sung K, Bomi S, Bo Young H, Seong HL. New technique of ultrasound-guided injection in the carpal tunnel syndrome. Ann Phys Rehabil Med. 2018;61:e169–70. https://doi.org/10.1016/j.rehab.2018.05.386.
Babaei-Ghazani A, Roomizadeh P, Shirin A. Ultrasound-guided vs landmark-guided local corticosteroid injection for carpal tunnel syndrome: a systematic review and meta-analysis. Ann Phys Rehabil Med. 2018;61:e138–9. https://doi.org/10.1016/j.rehab.2018.05.308.
Babaei-Ghazani A, Roomizadeh P, Forogh B, Moeini-Taba SM, Abedini A, Kadkhodaie M, et al. Ultrasound-guided versus landmark-guided local corticosteroid injection for carpal tunnel syndrome: a systematic review and meta-analysis of randomized controlled trials. Arch Phys Med Rehabil. 2018;99:766–75.
Habib GS, Badarny S, Rawashdeh H. A novel approach of local corticosteroid injection for the treatment of carpal tunnel syndrome. Clin Rheumatol. 2006;25:338–40.
Özdemir G, Demir R, Özel L, Ulvi H. The effect of steroid injection by Novel method in carpal tunnel syndrome on pain severity and electrophysiological findings. Dicle Med J/Dicle Tıp Derg. 2014;41:277–81. http://dergipark.gov.tr/doi/https://doi.org/10.5798/diclemedj.0921.2014.02.0416.
Badarny S, Rawashdeh H, Meer J, Abed S, Habib G. Repeated electrophysiologic studies in patients with carpal tunnel syndrome following local corticosteroid injection using a novel approach. Isr Med Assoc J. 2011;13:25–8.
Meshkini M, Fateh HR, Rahimi-Dehgolan S, Azadvari M, Faezi ST. Comparison between distal and proximal approaches for local corticosteroid injection in carpal tunnel syndrome management: a randomized controlled trial. Hand. 2021;155894472110527. http://www.ncbi.nlm.nih.gov/pubmed/34697951.
El-Badawy MAA-F. Electrophysiological and clinical comparison of local steroid injection by means of proximal versus distal approach in patients with mild and moderate carpal tunnel syndrome. Egypt Rheumatol Rehabil. 2015;42:120–7.
Kamanli A, Bezgincan M, Kaya A. Comparison of local steroid injection into carpal tunnel via proximal and distal approach in patients with carpal tunnel syndrome. Bratislava Med J. 2011;112:337–41.
Higgins J, Thomas J, Chandler J, Cumpston M, Cumpston M, Li T et al. Cochrane Handbook for Systematic Reviews of Interventions version 6.0 (updated July 2019) [Internet]. Cochrane; 2019. www.training.cochrane.org/handbook.
Page MJ, McKenzie JE, Bossuyt PM, Boutron I, Hoffmann TC, Mulrow CD et al. The PRISMA 2020 statement: an updated guideline for reporting systematic reviews. BMJ. 2021;372:n71. http://www.ncbi.nlm.nih.gov/pubmed/33782057.
Levine DW, Simmons BP, Koris MJ, Daltroy LH, Hohl GG, Fossel AH, et al. A self-administered questionnaire for the assessment of severity of symptoms and functional status in carpal tunnel syndrome. J Bone Jt Surg. New Zealand; 1993;75:1585–92. http://journals.lww.com/00004623-199311000-00002.
Higgins JPT, Altman DG, Gøtzsche PC, Jüni P, Moher D, Oxman AD, et al. The Cochrane Collaboration’s tool for assessing risk of bias in randomised trials. BMJ. 2011;343:1–9.
Chunke D, Yuting Z, Jun Z, Liang D. Efficacy and safety of pericapsular nerve group (PENG) block in hip surgeries: a systematic review and meta-analysis of randomized controlled trials. Inplasy Protoc. 2022;202270005:1–2. https://inplasy.com/inplasy-2020-7-0022/
Higgins JPT, Thompson SG. Quantifying heterogeneity in a meta-analysis. Stat Med. 2002;21:1539–58.
Ashworth NL, Bland JDP, Chapman KM, Tardif G, Albarqouni L, Nagendran A. Local corticosteroid injection for carpal tunnel syndrome. Cochrane Database Syst Rev 2020;2020.
Yang TH, Gingery A, Thoreson AR, Larson DR, Zhao C, Amadio PC. Triamcinolone Acetonide affects TGF-β signaling regulation of fibrosis in idiopathic carpal tunnel syndrome. BMC Musculoskelet Disord. 2018;19:1–9.
Dubert T, Racasan O. A reliable technique for avoiding the median nerve during carpal tunnel injections. Jt Bone Spine. 2006;73:77–9.
Racasan O, Dubert T. The safest location for steroid injection in the treatment of carpal tunnel syndrome. J Hand Surg Am. 2005;30:412–4.
Green DP, MacKay BJ, Seiler SJ, Fry MT. Accuracy of carpal tunnel injection: a prospective evaluation of 756 patients. Hand. 2020;15:54–8. http://journals.sagepub.com/doi/https://doi.org/10.1177/1558944718787330.
Wong SM, Hui ACF, Tang A, Ho PC, Hung LK, Wong KS, et al. Local vs systemic corticosteroids in the treatment of carpal tunnel syndrome. Neurology. 2001;56:1565–7.
Kaile E, Bland JDP. Safety of corticosteroid injection for carpal tunnel syndrome. J Hand Surg Eur. 2018;43:296–302.
Kim HJ, Park SH. Median nerve injuries caused by carpal tunnel injections. Korean J Pain. 2014;27:112–7.
Acknowledgements
Funding
This work was supported by the Natural Science Foundation of Shaanxi Province (Grant no. 2022JM-546). The Rapid Service Fee was funded by the authors.
Author Contributions
Leiming Hu and Chunke Dong contributed to the study conception and design. Material preparation, data collection and analysis were performed by Yuting Zhu, Jun Zhou, and Liang Dong. The first draft of the manuscript was written by Chunke Dong and Yuting Zhu. All authors commented on previous versions of the manuscript. All authors read and approved the final manuscript.
Authorship
All named authors meet the International Committee of Medical Journal Editors (ICMJE) criteria for authorship of this manuscript, take responsibility for the integrity of the work as a whole, and have given final approval for the version to be published.
Disclosure
Chunke Dong, Yuting Zhu, Jun Zhou, Liang Dong, and Leiming Hu have nothing to disclose.
Compliance with Ethics Guidelines
This article is based on previously conducted studies and does not contain any new studies with human participants or animals performed by any of the authors.
Data Availability
The data sets generated and/or analyzed during the current study are available from the corresponding author on reasonable request.
Author information
Authors and Affiliations
Corresponding author
Supplementary Information
Below is the link to the electronic supplementary material.
Rights and permissions
Open Access This article is licensed under a Creative Commons Attribution-NonCommercial 4.0 International License, which permits any non-commercial use, sharing, adaptation, distribution and reproduction in any medium or format, as long as you give appropriate credit to the original author(s) and the source, provide a link to the Creative Commons licence, and indicate if changes were made. The images or other third party material in this article are included in the article's Creative Commons licence, unless indicated otherwise in a credit line to the material. If material is not included in the article's Creative Commons licence and your intended use is not permitted by statutory regulation or exceeds the permitted use, you will need to obtain permission directly from the copyright holder. To view a copy of this licence, visit http://creativecommons.org/licenses/by-nc/4.0/.
About this article
Cite this article
Dong, C., Zhu, Y., Zhou, J. et al. Comparison of Distal and Proximal Local Steroid Injection for Carpal Tunnel Syndrome: a Systematic Review and Meta-analysis of Randomized Controlled Trials. Pain Ther 11, 1389–1402 (2022). https://doi.org/10.1007/s40122-022-00444-3
Received:
Accepted:
Published:
Issue Date:
DOI: https://doi.org/10.1007/s40122-022-00444-3