Abstract
In an open-air market in southern Italy, we noticed ‘Lady finger’ banana fruit imported from Costa Rica showing a severe rot, whose symptoms consisted of necrotic peel lesions with variable shape and size. Fusarium sacchari and F. proliferatum were consistently isolated from symptomatic fruit. In pathogenicity tests on ‘Lady finger’ banana fruit, F. proliferatum was more virulent than F. sacchari. Quantitative Time-of-Flight Mass Spectrometric analysis of secondary metabolites produced by isolates of these two Fusarium species on three different matrices (banana peel, barley and maize kernels) identified 11 mycotoxins. Seven of them (Fusaproliferin, Fumonisins A1, Fumonisins A2 and Fumonisins B1, Hydrolysed Fumonisin B1, Fusarin C and Moniliformin) were detected in matrices contaminated by F. proliferatum isolates. Fumonisin A1 was the prevalent mycotoxin in both maize kernels and banana peel, while Fumonisin A2 prevailed in barley kernels. Similarly, seven mycotoxins (the cyclic hexadepsipeptides Enniatins B2, B3 and B4, Fumonisins A1 and B2, Hydrolysed Fumonisin B1 and Fusarin C) were detected in matrices contaminated by F. sacchari isolates, but they were only in part the same as those produced by F. proliferatum isolates. Fusarin C prevailed in all three matrices colonized by F. sacchari. Fumonisin A1 was detected exclusively in maize kernels while Enniatins B3 and B4, Fumonisin B2 and Hydrolysed Fumonisin B1 were detected exclusively in barley kernels. Overall, F. proliferatum produced a higher amount of mycotoxins than F. sacchari. Moreover, in banana peel both species produced a lower number and amount of mycotoxins than in the other two matrices.
Similar content being viewed by others
Avoid common mistakes on your manuscript.
Introduction
Banana is one of the most important fruit crops worldwide. It is estimated that there are more than 1000 different banana varieties. According to the Food and Agriculture Organization of the United Nations (FAO), global banana production reached about 120 million tons in 2020 on a cultivated area of more than 5 million hectares. India, which is the largest producer of banana in the world, contributes with 29% of world production, followed by China and Indonesia, which account for 9.60% and 6.40% of world production, respectively (FAOSTAT 2020). Fusarium is known as one of the most economically damaging genera of fungal pathogens of agricultural and ornamental crops. The Fusarium taxonomy, which traditionally was based on phenotypic characters, has been continuously evolving. Over the past decades, molecular systematics studies, which have relied on multilocus DNA sequence data to determine species limits, have revolutionized the taxonomy of Fusarium and provided new insights into species diversity and phyletic relationships within this genus of fungi (O’Donnell et al. 2015). Despite this, the Fusarium taxonomy is still controversial (Crous et al. 2021; Geiser et al. 2021). According to Geiser et al. (2021), the genus includes 23 species complex and more than 300 phylogenetically distinct species that have been identified through molecular phylogenetics. Most of these species are soil-borne and, depending on the ecological context, may have different lifestyles, acting as saprophytes, endophytes, or plant pathogens (Aoki et al. 2014; O’Donnell et al. 2015). One of the most destructive disease of banana crops worldwide is Fusarium wilt, also known as Panama disease, caused by F. oxysporum f. sp. cubense (E.F. Smith) Synder and Hansen (Foc) in the Fusarium oxysporum species complex (FOSC) (Warman and Aitken 2018). It comprises three main races (1, 2 and 4), which are distinguished on the basis of the range of dessert banana cultivars they infect, as well as at least 24 vegetative compatibility groups (VCGs) (Mostert et al. 2017, 2022). Foc race 4 causes disease on most cultivars and has been divided into Foc subtropical race 4 (SR4) and Foc tropical race 4 (TR4), on the basis of its pathogenicity on bananas of the Cavendish group in different environmental conditions. Very probably, this large diversity of Foc depends on its polyphyletic nature (Maryani et al. 2019). One of the most important post-harvest fruit diseases of banana is crown rot, a disease complex caused by several fungi, including among others F. pallidoroseum and F. incarnatum (syn. F. semitectum), sometimes in association with bacteria (Knight et al. 1977; Snowdon 1990; Uribe-Palacio et al. 2022). The infections occur at harvest, but the symptoms appear later, after overseas shipment (Kamel et al. 2016). Several other species or species complex of Fusarium were reported to be associated with crown rot and other post-harvest fruit rots of bananas, including F. camptoceras, F. concentricum, F. concolor, F. fujikuroi, F. equiseti, F. incarnatum, F. oxysporum, F. proliferatum, F. pseudocircinatum, F. sacchari, F. solani and F. verticillioides (Abd Murad et al. 2017; Anthony et al. 2004; Calderón-Santoyo et al. 2022; Ewané et al. 2012; Hirata et al. 2001; Indrakeerthi and Adikaram 2011; John et al. 1996; Kamel et al. 2016; Mirete et al. 2004; Moretti et al. 2004; Xie et al. 2022; Zeng et al. 2013). A post-harvest rot of banana ‘Lady Finger’ caused by F. sacchari was recently reported, for the first time in Italy (Riolo et al. 2020). Symptoms consisted of dark brown to black necrotic lesions of the peel varying in shape and size. Fusarium sacchari was also identified as the causal agent of banana leaf blight (BLB), an emerging disease occurring in Guangdong, China (Cui et al. 2021). This species belongs to the Fusarium fujikuroi species complex (FFSC), which encompasses approximately 50 species (O’Donnell et al. 2015) and is phylogenetically related to FOSC (Maryani et al. 2019). Besides being a pathogen of banana, F. sacchari is a highly destructive pathogen of sugar cane and wheat (Bao et al. 2020; Viswanathan et al. 2017). It was also reported as causal agent of yellow leaf spot of an orchid species (Dekham and Kanchanawatee 2020). Beside F. sacchari, F. proliferatum, another species in the FFSC, was found associated to post-harvest rot of banana ‘Lady Finger’in Italy (Cacciola et al. unpublished data). Fusarium proliferatum was already listed among the fungal species responsible for banana crown rot (Snowdon 1990; Kamel et al. 2016; Uribe-Palacio et al. 2022). It is an extremely polyphagous species, with a host range including both animals and plants. As a plant pathogen, F. proliferatum has been reported on several economically important crops, including beside banana, diverse species of succulent plants, maize, hemp, garlic, onion, peach, red sage and rice (Desjardins et al. 1997; Gwinn et al. 2022; Jerushalmi et al. 2020; Kamali-Sarvestani et al. 2022; Logrieco et al. 1995; Murad et al. 2017; Stankovic et al. 2007; Xie et al. 2018; Yang et al. 2020). Damages caused by infections of Fusarium species include besides yield losses also food contamination by mycotoxins (Bentivenga et al. 2020; Stracquadanio et al. 2021). Mycotoxins are defined as secondary fungal metabolites that exert a toxic action on higher vertebrates and other animals at low concentrations (Bennett 1987). They pose a threat to vertebrates through ingestion, inhalation or skin contact and can enter the food chain via contaminated plant food components or as a consequence of the growth of toxigenic fungi on food (Alshannaq and Yu 2017; Bennett 1987). Agricultural products face the risk of mycotoxin contamination during harvest, transport, warehouse processing and storage, due to improper practices promoting the colonization by opportunistic fungal pathogens (Marin et al. 2013; Williams et al. 2004). Given the heat-stability of many mycotoxins and their endurance of chemical and physical treatments, adopting good hygienic and prophylactic management practices is crucial to reduce contamination risks (Marin et al. 2013; Pitt 2000). Fusarium is known to be a genus of mycotoxigenic fungi (Nesic et al. 2013; Perincherry et al. 2019) and the ability to produce certain types of toxins has been also suggested as a criterion of taxonomic relevance in this genus (Crous et al. 2021; Gwinn et al. 2022; Nirmaladevi et al. 2016; Pasquali et al. 2016; Somma et al. 2014). In particular, members of the FFSC are known as mycotoxin producers (Gwinn et al. 2022). Some of the mycotoxins produced by Fusarium species are also pathogenicity determinants although a direct correlation has not always been found between the amount of toxins produced by the Fusarium isolates tested and their virulence (Alghuthaymi et al. 2020; He et al. 2019; López-Díaz et al. 2018). The ability of Fusarium species, including F. proliferatum, to produce mycotoxins in association with post-harvest rot of banana fruit has been previously reported.(Alghuthaymi et al. 2020; Hirata et al. 2001; Moretti et al. 2004). However, to the best of our knowledge, no F. sacchari isolate from banana was included in these studies. Moreover, F. sacchari was demonstrated to be responsible for post-harvest rot of ‘Lady Finger’ bananas observed in Italy (Riolo et al. 2020), while the etiological role of F. proliferatum, also isolated from these fruit even though in a lower proportion than F. sacchari (38% against 62%, respectively) (Cacciola et al. unpublished), has not been proved.
The aim of this study was to compare the pathogenicity of F. proliferatum and F. sacchari isolates recovered from bananas in Italy and test their mycotoxigenic potential on banana and other food matrices.
Materials and methods
Fusarium isolates
Overall, 17 isolates of Fusarium, obtained from fruit of ‘Lady Finger’ banana (a diploid hybrid of Musa acuminata Colla) with rot symptoms, were included in this study (Table 1). Isolates were sourced in April 2019 from a fruit stock imported into Italy from Costa Rica (Riolo et al. 2020). They were purified by single-conidium subculture on water-agar (WA) in accordance with a standard protocol (Choi et al. 1999) and preserved in the collection of the laboratory of Molecular Plant Pathology at the Department of Agriculture, Food and Environment (Di3A) of the University of Catania, Italy. Reference strains of F. sacchari, F. proliferatum and other Fusarium species from CBS-KNAW and ARS Culture Collection (NRRL) were included for comparison (Table 2).
Morphological characterization of isolates
Isolates were grown in Petri dishes on Potato Dextrose Agar (PDA; Oxoid Ltd., Basingstoke, UK) and Malt Extract Agar (MEA; Sigma-Aldrich, Burlington, MA, USA). Dishes were incubated for 7 days at 25 ± 1 °C in the dark.
Molecular characterization of isolates
Isolates were grown on PDA for 7 days at 25 ± 1 °C, in the dark. Mycelium of each isolate was harvested with a sterile scalpel, and the genomic DNA (gDNA) was extracted using a PowerPlant® Pro DNA isolation Kit (MO BIO Laboratories, Inc., Carlsbad, CA, USA), following the manufacturer’s protocol. The DNA was preserved at −20 °C. A multilocus approach was adopted to characterize and determine the phylogenetic allocation of the isolates obtained from banana fruit. The β-tubulin (Tub 2) and translation elongation factor 1-α (EF-1α) genes of all isolates were amplified from gDNA. The primer pairs used for amplifying these gene regions were βt2α/βt2β (Glass and Donaldson 1995) and EF1/EF2 (O’Donnell et al. 1998), respectively.
PCR amplifications were performed on a GeneAmp PCR System 9700 (Applied Biosystems, Monza-Brianza, Italy). All PCR reactions were carried out by using Taq DNA polymerase recombinant (Invitrogen™) in a total volume of 25 µL containing PCR Buffer (1X), dNTP mix (0.2 mM), MgCl2 (1.5 mM), forward and reverse primers (0.5 µM each), Taq DNA Polymerase (1 U) and 100 ng of genomic DNA. The reaction protocol for Tub2 included an initial preheat at 94 °C for 2 min; followed by 35 cycles of denaturation at 94 °C for 15 s, annealing at 58 °C for 15 s, and extension at 72 °C for 45 s; and final extension at 72 °C for 10 min. The Tef 1 included an initial denaturation at 95 °C for 8 min; followed by 35 cycles of 95, 58, and 72 °C for 15, 20, and 60 s, respectively; and a final extension at 72 °C for 10 min. The amplicons were detected in 1% agarose gel and purified products were sequenced by Macrogen Europe (Amsterdam, The Netherlands). For molecular identification, sequences were aligned using MUSCLE and introduced to MEGA6 for phylogenetic analysis with the Maximum Likelihood method using the Tamura–Nei model (Tamura and Nei 1993). Analyses were performed with 1000 bootstrap replications. In order to maximize the effectiveness of the investigation into the genetic diversity among isolates obtained in the present study, the phylogenetic analysis was conducted using a combined dataset of sequenced markers (EF-1α and Tub 2).
Pathogenicity test
A conidial suspension of each isolate in sterile distilled water (sdw) was prepared by gently scraping 10-day old cultures grown on Potato Dextrose Agar (PDA; Oxoid Ltd., Basingstoke, UK) at 25 °C and used as inoculum. ‘Lady finger’ bananas were surface disinfected with 70% ethanol for 2 min, rinsed with sdw and placed on filter paper for drying. After disinfection bananas were wounded with a sterile needle in an equatorial position (two wounds, 5 cm apart from each other, on the same side of the fruit) and a 20 µl droplet of conidial suspension (104 conidia mL−1 sdw) was pipetted on the surface of the two wounds (Parra et al. 2022; Purwati and Hidayah 2008).
Five isolates for each Fusarium species and 10 fruit per isolate were used in each trial. Ten wounded fruit served as non-inoculated control. Control fruit received a 20 µL droplet of sdw. After inoculation, fruit were incubated in a humid chamber at 23 ± 1 °C, with 80% relative humidity and a photoperiod of 16 h of light and 8 h of darkness. They were regularly monitored up to 10 days post inoculation (dpi) and the external area of the fruit peel with typical brown lesions was measured. The trial was replicated three times.
Preparation of samples to produce mycotoxins
The extraction of mycotoxins from ‘Lady Finger’ banana peel was performed according to Fanelli et al. (2012) with some modifications (Fig. 1). Pieces of banana peel were collected from symptomatic fruit 10 dpi (see section “Pathogenicity test”). Pieces were excised from necrotic lesions around the inoculation site and placed into 15 mL Eppendorf tubes. Each tube contained three fragments from three distinct fruit of the same batch, with three replicates for each batch.
The banana peel pieces were weighted and a 1:5 (w/v) ratio of methanol (MeOH) was added. The samples were incubated at room temperature and continuous stirring (150 rpm) for 30 min. After the incubation period, the supernatant was collected and filtered through a 13 mm/0.22 μm nylon syringe filter (Membrane Solutions) into a 2 mL amber vial for chromatographic analysis. Several dilutions were performed to fit the concentrations among the calibration curves.
To assess the mycotoxigenic potential of Fusarium species isolated from ‘Lady Finger’ bananas, autoclaved maize and barley kernels (10 g each) were inoculated. A 5 mm mycelial plug from each strain was added to 50 mL Falcon tubes containing each matrix, followed by incubation at 30 °C for four weeks in the dark. Control samples of non-inoculated maize and barley kernels underwent the same treatment. This protocol is the same outlined by Serrano et al. (2013) with few modifications. Each cereal sample was homogenized using a grinder (Oster Classic Grinder 220–240 V, 50/60 Hz, 600 W, Oster, Valencia, Spain). Three 5-gram aliquots were transferred to 50 mL plastic Falcon tubes. In each tube, 25 mL of methanol were added, and the samples were homogenized for 3 min using an Ultra Turrax Ultra Ika T18 device (VWR, Staufen, Germany). Then the extracts were centrifuged at 10,000 rpm for five minutes at 5 °C, and the supernatant was transferred to a plastic flask and evaporated to dryness using a Rotavapor R-200 (Büchi Labortechnik AG, Flawil, Switzerland). The remaining material was re-suspended in 5 mL of methanol, transferred to a 15 mL plastic Falcon tube, and evaporated using a Turbovap LV multicampione evaporator (Zymark, Hopkinton, MA, USA) with the assistance of a nitrogen flow. Subsequently, the residue was reconstituted in 1 mL of methanol, filtered through a 13 mm/0.22 μm filter, and transferred to a 1 mL glass chromatography vial for analysis.
Quantitative Time-of-Flight Mass Spectrometry (Q-TOF-MS) analysis
The HPLC system used for the chromatographic determination was an Agilent 1290 (Agilent Technologies, Santa Clara, CA, USA) equipped with a vacuum degasser, autosampler and binary pump. The column was Agilent Zorbax RRHD SB-C18, 2.1 × 50 mm, 1.8 μm column. The mobile phase A was composed of Milli-Q water and acetonitrile was used for mobile phase B (both phases were acidified with 0.1% of formic acid), with gradient elution, as follows: 0 min, 2% B; 22 min 95% B; 25 min, 5% B. The flow rate was 0.4 mL /min, and 5 µL of sample was injected.
Mass spectrometry (MS) analysis was conducted using a Q-TOF-MS (6540 Agilent Ultra High Definition Accurate Mass), equipped with an Agilent Dual Jet Stream electrospray ionization (Dual AJS ESI) interface in positive ionization mode under the following conditions: gas temperature: 325 °C; gas flow: 10 L/min; nebulizer pressure: 40 psig; sheath gas temperature: 295 °C; sheath gas flow: 12 L/min; capillary voltage: 4000 V; nozzle voltage: 500 V; Fragmentor: 120 V; skimmer: 70 V; product ion scan range: 100–1500 Da; MS scan rate: 5 spectra/s; MS/MS scan rate: 3 spectra/s; maximum pre-cursors per cycle: 2; collision energy: 10, 20, 40 eV. The analysis of the metabolites was carried out in triplicate. Untargeted LC/Q-TOF based metabolomics approach was used to identify the differential metabolic profiles of Fusarium species growing on each batch. Integration, data elaboration and identification of metabolites were managed using MassHunter Qualitative Analysis Software B.08.00 and library PCDL Manager B.08.00.
Method validation
The method used in this study was evaluated according to Tamura et al. (2015), with modifications. The linearity, recovery, and detection limit (LOD) of this method were evaluated to validate the analysis. These validations were performed using non-contaminated samples of banana peel, barley kernels, and maize kernels. To measure the linearity of the assay, the samples were spiked with increasing concentrations of the mycotoxins (FUS, FA1, FA2, FB1, HFB1, FC, MON, ENB2, ENB3, and ENB4). The concentrations were 4, 16, 80, 400, and 2000 µg/kg, matching the concentration of the analyte used in the extraction method. To evaluate the recovery of the method, 100 µg/kg of each mycotoxin was added to the analytes, and the extraction procedure was performed on the samples for analysis. The FB1, FB2, MON, and ENB2 used for calibration were obtained from Sigma-Aldrich (Burlington, MA, USA). HFB1 was obtained from Romer Labs (Getzersdorf, Austria). FC, ENB3, and ENB4 were obtained from BenchChem (Austin, TX, USA). FUS was obtained according to Meca et al. (2009). FA1 and FA2 were obtained as described in Tamura et al. (2015).
Statistical analysis of data
Data from pathogenicity tests were normalized by square root transformation and then subjected to analysis of variance (ANOVA) followed by Tukey’s honestly significant difference (HSD) test as a post hoc test (R software). Differences at p ≤ 0.05 were considered significant.
Results
Identification of fungal isolates
On both PDA and MEA, all Fusarium isolates, obtained from infected ‘Lady Finger’ banana fruit (Fig. 2A), produced a fast-growing mycelium, which covered the Petri dishes (9 cm diam.) after 7 d incubation at 25 °C. On PDA, F. sacchari isolates produced aerial mycelium, white at the beginning and turning violet after 7 d at 25 °C, while the mycelium of F. proliferatum isolates was white at the beginning and subsequently turned light purple (Fig. 2B and C).
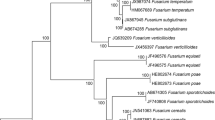
The phylogenetic analysis of the combined data set of sequences from EF-1α and Tub 2 regions of all single-conidium Fusarium isolates sourced in Italy from ‘Lady Finger’ bananas imported from Costa Rica (Table 1), along with sequences of the F. proliferatum and F. sacchari isolates used as references and the reference sequences of other species in the FFSC, produced a phylogenetic tree (Fig. 3) with a similar topology and high concordance with those reported by the authors who revised the systematics of this species complex using multigene sequence analysis (Herron et al. 2015). Eight single-conidium isolates from ‘Lady Finger’ banana were identified as F. sacchari because they clustered (bootstrap values 100%) with the CBS reference isolate of this species (CBS 185.33 and CBS 201.37). Conversely, all remaining single-conidium isolates from ‘Lady Finger’ banana, that showed also a distinct morphotype, clustered (bootstrap values 100%) with the sequences of EF-1α and Tub 2 regions of reference F. proliferatum isolates, including the isolates NRRL 31860, NRRL 66417 and NRRL 22944.
Phylogenetic tree based on the translation elongation factor 1-α (EF-1α) and β-tubulin (Tub 2) regions developed using the Maximum Likelihood Method, based on the Tamura–Nei model. The tree with the greatest log likelihood (-3906.13) is shown. Relationships between the isolates from ‘Lady Finger’ banana and the CBS and NRRL reference isolates of Fusarium fujikuroi species complex. In bold, isolates characterized in this study
All isolates of both F. proliferatum and F. sacchari were pathogenic on ‘Lady Finger’ bananas. However, overall F. proliferatum isolates were more aggressive than F. sacchari isolates. No significant difference in pathogenicity was observed among isolates of the same species (Fig. 4). Symptoms induced by both these two Fusarium species were identical to those observed on banana fruit with natural infections and consisted in necrotic, brown spots of various size and shape (from lenticular to circular), with a regular margin.
All Fusarium isolates were re-isolated from the lesions and identified on the basis of colony morphology and sequencing of EF-1α, and TUB2 regions, thus fulfilling Koch’s postulates.
Detection of mycotoxins produced by F. proliferatum and F. sacchari in different matrices
The mycotxin profiles of the two Fusarium species as determined by Q-TOF-MS were distinct. Q-TOF-MS analysis identified seven diverse mycotoxins in matrices colonized by F. proliferatum isolates, including Fusaproliferin (hereafter referred to as FUS), Fumonisin A1 (FA1), Fumonisin A2 (FA2), Fumonisin B1 (FB1), Hydrolysed Fumonisin B1 (HFB1), Fusarin C (FC) and Moniliformin (MON) (Table 3). Among these mycotoxins, FUS, FA1, FA2, FB1, and FC were consistently detected in all matrices, while FHB1 and MON were detected exclusively in barley kernels. The primary mycotoxin identified in maize kernels and banana peel was FA1, with maximum concentrations of 1757.35 mg/kg and 832.59 mg/kg, respectively. The highest levels of FA2 were detected in barley kernels, with a maximum concentration value of 494.93 mg/kg. In all three matrices, the nine isolates of this species consistently produced FA1, FA2, and FC. Notably, FBA-4 isolate exhibited the highest cumulative mycotoxin production in maize kernels, FBA-2 isolate in barley kernels, and FBA-1 isolate in banana peel, indicating an isolate/matrix interaction. Overall, the highest mycotoxin content was detected in maize kernels, while the lowest mycotoxin content was detected in banana peel.
Overall, seven diverse mycotoxins were detected in matrices colonized by F. sacchari isolates, including the cyclic hexadepsipeptides Enniatins B2, B3 and B4 (ENB2, ENB3 and ENB4, respectively), Fumonisins A1 and B2 (FA1 and FB2, respectively), Hydrolysed Fumonisin B1 (HFB1) and Fusarin C (FC) (Table 4). ENB2 and FC were present in all three matrices, while the remaining five mycotoxins were detected exclusively in maize (FA1) or barley kernels (ENB3, ENB4, FB2, and HFB1). FC was the prevalent mycotoxin in all matrices, reaching the highest concentration in barley kernels with a concentration value of 276.34 mg/kg, followed by maize kernels with a concentration value of 172.01 mg/kg and banana peel with 80.21 mg/kg. In maize kernels, all isolates produced FC, whereas in barley kernel, only FAB-C, FAB-E, and FAB-F isolates and in banana peel only FAB-E isolate produced this toxin. The FBA-C isolate produced the highest amount of FC in maize kernels, while FBA-E isolate produced the highest amount of this toxin in barley kernels and banana peel. Similarly to the samples colonized by F. proliferatum, the mycotoxin production in the banana peel was lower compared to the other two matrices. In fact, only ENB2 and FC were detected in banana peel. The most distinctive qualitative characteristics between the mycotoxin profiles of the two Fusarium species were the presence of Enniatins (mainly ENB1) only in the profile of F. sacchari isolates and the exclusive presence of FA2, FB1 and FUS in the profile of F. proliferatum isolates. In particular, all isolates of the latter species produced FA2 in all three matrices, with mean concentration values in banana peel varying from 48.4 to 416.3 mg/kg (Tables 4 and 5). As for the mycotoxins that were present in the profile of both F. proliferatum and F. sacchari (FA1, FC and HFB1), relevant quantitative differences were observed between the two fungal species. In particular, all isolates of F. proliferatum produced FA2 in all three matrices, with mean concentration values in banana peel varying from 322.16 to 832.59 mg/kg (Table 4). Conversely, only two isolates of F. sacchari produced these mycotoxins, but only in maize kernels and in traces.
Accuracy of the mycotoxin determination method in different matrices
The validation of the method was performed by the analysis of the linearity, recovery, LOD and LOQ and the results are evidenced in the Table 5. The determination of the linearity coefficient evidenced values above 0.99, recovery values were observed to range percentages from 97.3 to 102.8%. Therefore, it can be accepted that the method used in this article evidenced an optimal performance for obtaining the results. The LOD was determined to be 0.71 to 2.11 µg/kg. Detections below these limits were marked as n.d.
Discussion
Results of this study confirm that both F. proliferatum and F. sacchari are able to infect banana fruit. Although the former Fusarium species was isolated in a lower proportion than the latter from symptomatic ‘Finger Lady’ bananas sampled in southern Italy, it was proved to be more virulent in pathogenicity tests, confirming previous findings of other scholars (Abd Murad et al. 2017). No significant intraspecific difference in pathogenicity was observed among diverse isolates of these two species. In line with previous studies investigating post-harvest rots of banana fruit caused by Fusarium species, F. proliferatum and F. sacchari showed significant activity as opportunistic wound pathogens in pathogenicity tests. (Abd Murad et al. 2017; Anthony et al. 2004; Kamel et al. 2016; Riolo et al. 2020). Koch’s postulates were fulfilled for both species, which can be consequently regarded as the causal agents of post-harvest rot of ‘Finger Lady’ banana fruit reported in Italy.
Overall, Q-TOF-MS analysis identified 11 diverse mycotoxins produced by F. proliferatum and F. sacchari in artificially inoculated banana peel, barley and maize kernels. These toxins included Fumonisins A1, A2, B1 and B2, Enniatins B2, B3 and B4, as well as Hydrolysed Fumonisin B1, Fusaproliferin, Fusarin C and Moniliformin.
The fumonisins, a family of carcinogenic mycotoxins, were first isolated from cultures of F. verticillioides (formerly F. moniliforme) on maize kernels (Mostrom 2016; Ostry et al. 2017). Successively, they were shown to be produced by other Fusarium species (Rheeder et al. 2002). Fusarium proliferatum is well known as a producer of fumonisins (Alghuthaymi and Bahkali 2015; Gu et al. 2019; Moretti and Susca 2017; Shi et al. 2017). Shi et al. (2017) classified this species in the group of Fusarium species producing both Fuminosins and Fusaric acid based on the mycotoxigenic profile on three different matrices (PDA, rice and maize), while they classified F. sacchari in the group of Fusarium species producing only Fusaric acid. Indeed, in the present study only three out of eight isolates of F. sacchari produced fumonisins, but exclusively on barley and maize kernels and only in traces. Consistently with the result of this study, Rheeder et al. (2002) listed F. sacchari among the Fusarium species producing Fumonisins. All F. proliferatum isolates produced high amounts of Fuminosins on all three matrices, including the banana peel. Fumonisins, in particular FB1, was shown to exert phytotoxic activity on diverse host plants (Abbas et al. 1991, 1998; Abbas and Boyette 1992; Al Abboud et al. 2012; Doehlert et al. 1994; Kritzinger et al. 2006). In a previous study aimed at characterizing seven Fusarium species, including F. proliferatum, it was found that the virulence of isolates on banana fruit did not correlate with the amount of Fumonisin they produced (Alghuthaymi et al. 2020). Moreover it can not be excluded that diverse mycotoxins produced by this Fusarium species act synergistically as virulence factors.
Xie et al. (2021) demonstrated that FB1 treatment increases the aggressiveness of F. proliferatum on banana fruits by suppressing the fruit’s defense mechanisms through the reduction of key enzyme activities such as phenylalanine ammonia-lyase (PAL), β-1,3-glucanase (GLU), and chitinase (CHI). Additionally, FB1 accelerates cell death in banana fruits, as indicated by increased relative conductivity, malondialdehyde (MDA) content, and transcripts of cell death-related genes. The increased hydrogen peroxide (H2O2) content, likely due to the induction of MaRBOHs, further contributes to reactive oxygen species (ROS)-dependent cell death, thereby reducing the fruit’s resistance to disease (Xie et al. 2021).
Xie et al. (2023) explored the link between FB1 biosynthesis and oxidative stress during F. proliferatum infection. It was observed that while H2O2 treatment inhibits fungal growth, it stimulates FB1 production and increases endogenous ROS levels. This suggests that FB1 helps the fungus tolerate oxidative stress, enhancing its virulence. The construction of a Fusarium proliferatum mutant (ΔFpfum21) with attenuated FB1 biosynthesis confirmed that the production of this mycotoxin is crucial for the fungus’s virulence. This highlights that fumonisins not only contribute to phytotoxicity but are also crucial determinants of the pathogenicity of the Fusarium/banana system, giving F. proliferatum a significant competitive advantage in colonizing and damaging banana fruits.
The literature also evidences the production of several fumonisin analogs, such as FA1 and FA2 detected in this study, and other analogs like the dimethyl and N-3-hydroxypyridinium derivatives which belong to the fumonisin C series and fumonisin P series, respectively, by F. proliferatum. The production of these mycotoxins by this fungal species has been reported in vitro and detected in maize samples (Lazzaro et al. 2013; Tamura et al. 2015; Musser et al. 1995). Notably, this study is the first to report the production of FA1 and FA2 by F. proliferatum in two highly consumed food matrices: barley and banana.
Enniatins belong to the group of cyclodepsipeptides, which comprises also Beauvericins, Beauvenniatins and their analogues (Urbaniak et al. 2020). Originally isolated from cultures of F. orthoceras var. enniatum (later renamed F. oxysporum) from which they were named, these mycotoxins are produced by several Fusarium species, including F. avenaceum, F. sambucinum, F. poae, F. sporotrichioides and F. tricinctum which infect cereals and other commodities (Altomare et al. 2021). Fusarium sacchari was also reported among the Fusarium species that produce both Enniatins and Beauvericin (Mohamed and Al-Ani 2021; Tavakol Noorabadi et al. 2021). The primary toxic action of Enniatins is related to their ionophoric properties. The most important contributors to the dietary exposure of humans and animals to these mycotoxins are cereal grains and cereal grain-based products (EFSA 2014). On the other hand, due to their antimicrobial and cytotoxic properties, Enniatins are candidate as anti-cancer drugs (Urbaniak et al. 2020). Consistently with the data of EFSA (2014), in this study, the greatest amounts of Enniatins were produced by F. sacchari isolates on barley and maize kernels and only two isolates of this Fusarium species produced mycotoxins of this group in banana peel.
Fusaproliferinis are produced by F. proliferatum, after which it was named, and other related Fusarium species (Ćeranić et al. 2021). Several fungi from other distant taxonomic groups were also reported to produce fusaproliferin or the deacetylated derivative, known as siccanol or terpestacin.
Fusarin C, a tetramethylated heptaketide fused to homoserine, is a metabolite with mutagenic properties. Although it is regarded as an emerging mycotoxin it is not legally regulated (Gasser et al. 2023). Originally, Fusarin C was reported as a mycotoxin typically produced by the plant pathogen F. verticillioides in maize (Wentzel et al. 1984). Subsequently, it was reported as a secondary metabolite of a number of other Fusarium species, such as F. avenaceum, F. crookwellense, F. culmorum, F. graminearum, F. poae, F. proliferatum, F. sporotrichioides, F. tricinctum, F. venenatum and F. verticillioides (Desjardins and Proctor 2007; Gaffoor et al. 2005; Gasser et al. 2023; Song et al. 2004; Studt et al. 2012; Thrane 1988). In this study, both F. proliferatum and F. sacchari isolates from banana produced Fusarin C, but their ability to produce this metabolite differed substantially. All isolates of both fungal species produced this mycotoxin in maize kernels and all isolates of F. proliferatum produced it in barley kernels as well as in banana peel. By contrast, only three isolates of F. sacchari out of eight produced Fusarin C in barley kernels and only one isolate out of eight produced it in banana peel. In general, based on the findings of this study the risk of contamination of banana fruit with Fusarin C can be considered low.
Moniliformin is a mycotoxin with low molecular weight primarily produced in cereals by a number of Fusarium species, including F. avenaceum, F. subglutinans and F. proliferatum. Like Fusarin C, it is an unregulated mycotoxin (Gasser et al. 2023). In this study, Moniliformin was detected only in the mycotoxin profile of two F. proliferatum isolates grown on barley kernels.
Overall, of the 11 detected mycotoxins, four (Fumonisins A2 and B1, Fusaproliferin and Moniliformin) were produced exclusively by F. proliferatum, four (Enniatins B2, B3 and B4 and Fumonisin B2) were produced exclusively by F. sacchari and only three (Fusarin C, Fumonisin A1 and Hydrolysed Fumonisin B1) were in common between the two Fusarium species. Substantial quantitative differences were also observed between the mycotoxin profiles of the two species. Moreover, there were noticeable differences in the mycotoxigenic potential among isolates of the same species. Fumonisins A1 and A2 were the prevalent mycotoxins produced by F. proliferatum isolates, with the highest concentrations in maize kernels, varying from 103.68 to 1757.35 mg/kg and from 244.78 to 677.85 mg/kg, respectively. Fusarin C was another major mycotoxin in the metabolite profile of F. proliferatum isolates, with concentrations varying from 89.35 to 258.62, 58.05 to 138.02 and 23.81 to 97.88 mg/kg in maize, barley and banana, respectively. Fusarin C and Enniatin B2 were the prevalent mycotoxins in the metabolite profile of F. sacchari isolates. However, only a single isolate of this Fusarium species produced Fusarin C in banana peel, indicating the ability of producing mycotoxins varies among diverse isolates of the same species and confirming the crucial role of culture medium for the expression of mycotoxigenic potential of Fusarium isolates, as demonstrated in numerous previuos studies (e.g. Shi et al. 2017). The most relevant difference between the mycotoxin profiles of the two Fusarium species was the high amount of Fumonisins A1 and A2 produced by F. proliferatum in the peel of artificially inoculated banana fruit. In general, among the three diverse matrices tested in this study, banana peel was the least conducive for the production of mycotoxins. Other mycotoxins, such as Fusaproliferin, Fumonisin B1, Hydrolysed Fumonisin B1 and Moniliformin were detected only in traces.
Conclusions
In this study, it was demonstrated that F. proliferatum and F. sacchari, singularly or in association, were responsible for the post-harvest rot observed in southern Italy on ‘Lady Finger’ banana fruit imported from Costa Rica. The comparison of the metabolite profile of these two Fusarium species provided enough evidence to hypothesize some mycotoxins they produce, such as Fumonisins, could act as virulence factors in this pathosystem. Understanding the mechanisms by which these mycotoxins affect pathogen-host interactions could pave the way for new approaches to mitigating the impact of Fusarium diseases in banana crops. Moreover, the analysis of the mycotoxigenic potential of F. proliferatum and F. sacchari on different matrices provided information that can contribute to evaluate the risk of contamination by mycotoxins and to improve the legal regulation of mycotoxin concentrations in agricultural products, foods and feeds.
Data availability
The data generated and analyzed during this study are included in the manuscript and all the raw data generated and/or analyzed during the current study are available from the corresponding author on request.
References
Abbas HK, Boyette CD (1992) Phytotoxicity of fumonisin B1 on weed and crop species. Weed Technol 6(3):548–552. https://doi.org/10.1017/S0890037X00035776
Abbas HK, Boyette CD, Hoagland RE, Vesonder RF (1991) Bioherbicidal potential of Fusarium moniliforme and its phytotoxin fumonisin. Weed Sci 39(4):673–677. https://doi.org/10.1017/S004317450008855X
Abbas HK, Duke SO, Merrill AH Jr, Wang E, Shier WT (1998) Phytotoxicity of australifungin, AAL-toxins and fumonisin B1 to Lemna pausicostata. Phytochemistry 47(8):1509–1514. https://doi.org/10.1016/S0031-9422(97)00781-4
Abd Murad NB, Mohamed Nor NMI, Shohaimi S, Mohd Zainudin NAI (2017) Genetic diversity and pathogenicity of Fusarium species associated with fruit rot disease in banana across Peninsular Malaysia. J Appl Microbiol 123(6):1533–1546. https://doi.org/10.1111/jam.13582
Al Abboud MA (2012) Biorepress of fumonisin B1 production and their phytotoxicity on growth and ultrastructures of Maize (Zea mays) seedlings. Egypt. Acad J Biolog Sci 4:9–20. https://doi.org/10.21608/EAJBSG.2012.16655
Alghuthaymi MA, Bahkali AH (2015) Toxigenic profiles and trinucleotide repeat diversity of Fusarium species isolated from banana fruit. Biotechnol Biotechnol Equip 29(2):324–330. https://doi.org/10.1080/13102818.2014.995519
Alghuthaymi M, Alshehri WA, Al-Maary KS, Bahkali NA, AlKahtani MDF, Alarfaj AA, Alnadhari S, Ameen F (2020) Mycotoxigenicity of Fusarium isolated from banana fruit: combining phytopathological assays with toxin concentrations. J King Saud University-Science 32(2):1482–1485. https://doi.org/10.1016/j.jksus.2019.12.001
Alshannaq A, Yu JH (2017) Occurrence, toxicity, and analysis of major mycotoxins in food. Int J Environ Res Public Health 14(6):632. https://doi.org/10.3390/ijerph14060632
Altomare C, Logrieco AF, Gallo A (2021) Mycotoxins and mycotoxigenic fungi: risk and management. A challenge for future global food safety and security https//. https://doi.org/10.1016/B978-0-12-819990-9.00032-9
Anthony S, Abeywickrama K, Dayananda R, Wijeratnam S, Arambewela L (2004) Fungal pathogens associated with banana fruit in Sri Lanka, and their treatment with essential oils. Mycopathologia 157:91–97. https://doi.org/10.1023/B:MYCO.0000012226.95628.99
Aoki T, O’Donnell K, Geiser DM (2014) Systematics of key phytopathogenic Fusarium species: current status and future challenges. J Gen Plant Pathol 80:189–201. https://doi.org/10.1007/s10327-014-0509-3
Bao Y, Xu Y, Wang S, Yao Z, Rao GP, Zhang M (2020) First report of Fusarium Sacchari that causes sugarcane wilt disease in China. Plant Dis 104(8):2289–2289. https://doi.org/10.1094/PDIS-02-20-0229-PDN
Bennett JW (1987) Mycotoxins, mycotoxicoses, mycotoxicology and mycopathologia. Mycopathologia 100:3–5. https://doi.org/10.1007/BF00769561
Bentivenga G, Spina A, Ammar K, Allegra M, Cacciola SO (2020) Screening of durum wheat (Triticum turgidum L. subsp. durum (Desf.) Husn.) Italian cultivars for susceptibility to Fusarium Head Blight incited by Fusarium graminearum. Plants, 10(1), 68. https://doi.org/10.3390/plants10010068
Bolton SL, Brannen PM, Glenn AE (2016) A novel population of Fusarium fujikuroi isolated from Southeastern U.S. winegrapes reveals the need to re-evaluate the species’ Fumonisin production. Toxins 8(9):254. https://doi.org/10.3390/toxins8090254
Calderón-Santoyo M, Iñiguez-Moreno M, Barros-Castillo JC, Miss-Zacarías DM, Díaz JA, Ragazzo-Sánchez JA (2022) Microencapsulation of citral with arabic gum and sodium alginate for the control of Fusarium pseudocircinatum in bananas. Iran Polym J 31(5):665–676. https://doi.org/10.1007/s13726-022-01033-z
Ćeranić A, Svoboda T, Berthiller F, Sulyok M, Samson JM, Güldener U, Schuhmacher R, Adam G (2021) Identification and functional characterization of the gene cluster responsible for fusaproliferin biosynthesis in Fusarium proliferatum. Toxins 13(7):468. https://doi.org/10.3390/toxins13070468
Choi YW, Hyde KD, Ho WH (1999) Single spore isolation of fungi. Fungal Divers 3:29–38
Crous PW, Lombard L, Sandoval-Denis M, Seifert KA, Schroers HJ, Chaverri P, Gené J, Guarro J, Hirooka Y, Bensch K, Kema GHJ, Lamprecht SC, Cai L, Rossman AY, Stadler M, Summerbell RC, Taylor JW, Ploch S, Visagie CM, Yilmaz N, Frisvad JC, Abdel-Azeem AM, Abdollahzadeh J, Abdolrasouli A, Akulov A, Alberts JF, Araújo JPM, Ariyawansa HA, Bakhshi M, Bendiksby M, Ben Hadj Amor A, Bezerra JDP, Boekhout, Câmara MPS, Carbia M, Cardinali G, Castañeda-Ruiz RF, Celis A, Chaturvedi V, Collemare J, Croll D, Damm U, Decock CA, de Vries RP, Ezekiel CN, Fan XL, Fernández NB, Gaya E, González CD, Gramaje D, Groenewald JZ, Grube M, Guevara-Suarez M, Gupta VK, Guarnaccia V, Haddaji A, Hagen F, Haelewaters D, Hansen K, Hashimoto A, Hernández-Restrepo M, Houbraken J, Hubka V, Hyde KD, Iturriaga T, Jeewon R, Johnston PR, Jurjević Z, Karalti İ, Korsten L, Kuramae EE, Kušan I, Labuda R, Lawrence DP, Lee HB, Lechat C, Li HY, Litovka YA, Maharachchikumbura SSN, Marin-Felix Y, Matio Kemkuignou B, Matočec N, McTaggart AR, Mlčoch P, Mugnai L, Nakashima C, Nilsson RH, Noumeur SR, Pavlov IN, Peralta MP, Phillips AJL, Pitt JI, Polizzi G, Quaedvlieg W, Rajeshkumar KC, Restrepo S, Rhaiem A, Robert J, Robert V, Rodrigues AM, Salgado-Salazar C, Samson RA, Santos ACS, Shivas RG, Souza-Motta CM, Sun GY, Swart WJ, Szoke S, Tan YP, Taylor JE, Taylor PWJ, Tiago PV, Váczy KZ, van de Wiele N, van der Merwe NA, Verkley GJM, Vieira WAS, Vizzini A, Weir BS, Wijayawardene NN, Xia JW, Yáñez-Morales MJ, Yurkov A, Zamora JC, Zare R, Zhang CL, Thines M (2021) Fusarium: more than a node or a foot-shaped basal cell. Studies in Mycology, 98, 100116. https://doi.org/10.1016/j.simyco.2021.100116
Cui Y, Wu B, Peng A, Song X, Chen X (2021) The genome of banana leaf blight pathogen Fusarium sacchari str. FS66 harbors widespread gene transfer from Fusarium oxysporum. Front Plant Sci 12:629859. https://doi.org/10.3389/fpls.2021.629859
Dekham K, Kanchanawatee K (2020) The first report of Fusarium sacchari causing yellow leaf spot disease on Rhynchostylis gigantea orchids in Thailand. Am J Agric Biol Sci 15(1):68–74. https://doi.org/10.3844/ajabssp.2020.68.74
Desjardins AE, Proctor R (2007) Molecular biology of Fusarium mycotoxins. Int J Food Microbiol 119(1–2):47–50. https://doi.org/10.1016/j.ijfoodmicro.2007.07.024
Desjardins AE, Plattner RD, Nelson PE (1997) Production of fumonisin B (inf1) and moniliformin by Gibberella fujikuroi from rice from various geographic areas. Appl Environ Microbiol 63(5):1838–1842. https://doi.org/10.1128/aem.63.5.1838-1842.1997
Doehlert DC, Knutson CA, Vesonder RF (1994) Phytotoxic effects of fumonisin B1 on maize seedling growth. Mycopathologia 127:117–121. https://doi.org/10.1007/BF01103067
EFSA Panel on Contaminants in the Food Chain (CONTAM) (2014) Scientific opinion on the risk to human and animal health related to the presence of beauvaricin and enniatins in food and feed. EFSA J 12(8):3892. https://doi.org/10.2903/j.efsa.2014.3802
Ewané CA, Lepoivre P, De Lapeyre de Bellaire L, Lassois L (2012) Involvement of phenolic compounds in the susceptibility of bananas to crown rot. A review. Biotechnol Agron Soc Environ 16(3):393–404
Fanelli F, Schmidt-Heydt M, Haidukowski M, Geisen R, Logrieco A, Mulè G (2012) Influence of light on growth, fumonisin biosynthesis and FUM1 gene expression by Fusarium proliferatum. Int J Food Microbiol 153(1–2):148–153. https://doi.org/10.1016/j.ijfoodmicro.2011.10.031
FAOSTAT (2020) Rome: FAO. http://www.fao.org/faostat/en/#data/QC
Gaffoor I, Brown DW, Plattner R, Proctor RH, Qi W, Trail F (2005) Functional analysis of the polyketide synthase genes in the filamentous fungus Gibberella zeae (anamorph Fusarium graminearum). Eukaryot Cell 4(11):1926–1933. https://doi.org/10.1128/EC.4.11.1926-1933.2005
Gasser K, Sulyok M, Spangl B, Krska R, Steinkellner S, Hage-Ahmed K (2023) Fusarium proliferatum secondary metabolite profile in vitro depends on the origin of the isolates and is clearly reduced in stored garlic. Postharvest Biol Technol 200:112312. https://doi.org/10.1016/j.postharvbio.2023.112312
Geiser DM, Al-Hatmi AMS, Aoki T, Arie T, Balmas V, Barnes I, Bergstrom GC, Bhattacharyya MK, Blomquist CL, Bowden RL, Brankovics B, Brown DW, Burgess LW, Bushley K, Busman M, Cano-Lira JF, Carrillo JD, Chang HX, Chen CY, Chen W, Chilvers M, Chulze S, Coleman JJ, Cuomo CA, de Beer ZW, de Hoog GS, Del Castillo-Múnera J, Del Ponte EM, Diéguez-Uribeondo J, Di Pietro A, Edel-Hermann V, Elmer WH, Epstein L, Eskalen A, Esposto MC, Everts KL, Fernández-Pavía SP, da Silva GF, Foroud NA, Fourie G, Frandsen RJN, Freeman S, Freitag M, Frenkel O, Fuller KK, Gagkaeva T, Gardiner DM, Glenn AE, Gold SE, Gordon TR, Gregory NF, Gryzenhout M, Guarro J, Gugino BK, Gutierrez S, Hammond-Kosack KE, Harris LJ, Homa M, Hong CF, Hornok L, Huang JW, Ilkit M, Jacobs A, Jacobs K, Jiang C, Jiménez-Gasco MM, Kang S, Kasson MT, Kazan K, Kennell JC, Kim HS, Kistler HC, Kuldau GA, Kulik T, Kurzai O, Laraba I, Laurence MH, Lee T, Lee YW, Lee YH, Leslie JF, Liew ECY, Lofton LW, Logrieco AF, López-Berges MS, Luque AG, Lysøe E, Ma LJ, Marra RE, Martin FN, May SR, McCormick SP, McGee C, Meis JF, Migheli Q, Mohamed Nor NMI, Monod M, Moretti A, Mostert D, Mulè G, Munaut F, Munkvold GP, Nicholson P, Nucci M, O’Donnell K, Pasquali M, Pfenning LH, Prigitano A, Proctor RH, Ranque S, Rehner SA, Rep M, Rodríguez-Alvarado G, Rose LJ, Roth MG, Ruiz-Roldán C, Saleh AA, Salleh B, Sang H, Scandiani MM, Scauflaire J, Schmale DG III, Short DPG, Šišić A, Smith JA, Smyth CW, Son H, Spahr E, Stajich JE, Steenkamp E, Steinberg C, Subramaniam R, Suga H, Summerell BA, Susca A, Swett CL, Toomajian C, Torres-Cruz TJ, Tortorano AM, Urban M, Vaillancourt LJ, Vallad GE, van der Lee TAJ, Vanderpool D, van Diepeningen AD, Vaughan MM, Venter E, Vermeulen M, Verweij PE, Viljoen A, Waalwijk C, Wallace EC, Walther G, Wang J, Ward TJ, Wickes BL, Wiederhold NP, Wingfield MJ, Wood AKM, Xu JR, Yang XB, Yli-Mattila T, Yun SH, Zakaria L, Zhang H, Zhang N, Zhang SX, Zhang X (2021) Phylogenomic analysis of a 55.1-kb 19-gene dataset resolves a monophyletic Fusarium that includes the Fusarium solani species complex. Phytopathology, 111(7), 1064–1079. https://doi.org/10.1094/PHYTO-08-20-0330-LE
Glass NL, Donaldson GC (1995) Development of primer sets designed for use with the PCR to amplify conserved genes from filamentous ascomycetes. Appl Environ Microbiol 61(4):1323–1330. https://doi.org/10.1128/aem.61.4.1331-1340.1995
Gu MJ, Han SE, Hwang K, Mayer E, Reisinger N, Schatzmayr D, Park BC, Han SH, Yun CH (2019) Hydrolyzed fumonisin B1 induces less inflammatory responses than fumonisin B1 in the co-culture model of porcine intestinal epithelial and immune cells. Toxicol Lett 305:110–116. https://doi.org/10.1016/j.toxlet.2019.01.013
Gwinn KD, Hansen Z, Kelly H, Ownley BH (2022) Diseases of Cannabis sativa caused by diverse Fusarium species. Front Agron 3:796062. https://doi.org/10.3389/fagro.2021.796062
He X, Dreisigacker S, Singh RP, Singh PK (2019) Genetics for low correlation between Fusarium head blight disease and deoxynivalenol (DON) content in a bread wheat mapping population. Theor Appl Genet 132:2401–2411. https://doi.org/10.1007/s00122-019-03362-9
Herron DA, Wingfield MJ, Wingfield BD, Rodas CA, Marincowitz S, Steenkamp ET (2015) Novel taxa in the Fusarium fujikuroi species complex from Pinus spp. Stud Mycol 80:131–150. https://doi.org/10.1016/j.simyco.2014.12.001
Hirata T, Kimishima E, Aoki T, Nirenberg HI, O’Donnell K (2001) Morphological and molecular characterization of Fusarium verticillioides from rotten banana imported into Japan. Mycoscience 42(2):155–166. https://doi.org/10.1007/BF02464132
Indrakeerthi SRP, Adikaram NKB (2011) Control of crown rot of banana using Carica papaya latex. J Natl Sci Foundation Sri Lanka 39(2):155–162. https://doi.org/10.4038/jnsfsr.v39i2.3176
Jerushalmi S, Maymon M, Dombrovsky A, Freeman S (2020) Fungal pathogens affecting the production and quality of medical cannabis in Israel. Plants 9(7):882. https://doi.org/10.3390/plants9070882
John CD, Dov P, Peter J (1996) Fruit Diseases. In: Ploetz, R. (Ed.)– The mango. Wallingford, UK., CAB International, pp. 257–280
Kamali-Sarvestani S, Mostowfizadeh-Ghalamfarsa R, Salmaninezhad F, Cacciola SO (2022) Fusarium and Neocosmospora species associated with rot of Cactaceae and other succulent plants. J Fungi 8(4):364. https://doi.org/10.3390/jof8040364
Kamel MAM, Cortesi P, Saracchi M (2016) Etiological agents of crown rot of organic bananas in Dominican Republic. Postharvest Biol Technol 120:112–120. https://doi.org/10.1016/j.postharvbio.2016.06.002
Knight C, Cutts DF, Colhoun J (1977) The role of Fusarium semitectum in causing crown rot of bananas. Phytopathologische Z 89(2):170–176
Kritzinger Q, Aveling TAS, Van der Merwe CF (2006) Phytotoxic effects of fumonisin B1 on cowpea seed. Phytoparasitica 34:178–186. https://doi.org/10.1007/BF02981318
Lazzaro I, Falavigna C, Galaverna G, Dall’Asta C, Battilani P (2013) Cornmeal and starch influence the dynamic of fumonisin B, A and C production and masking in Fusarium verticillioides and F. proliferatum. International Journal of Food Microbiology, 166(1), 21–27. https://doi.org/10.1016/j.ijfoodmicro.2013.06.011
Logrieco A, Moretti A, Ritieni A, Bottalico A, Corda P (1995) Occurrence and toxigenicity of Fusarium proliferatum from preharvest maize ear rot, and associated mycotoxins, in Italy. Plant Dis 79(7):727–731
López-Díaz C, Rahjoo V, Sulyok M, Ghionna V, Martín-Vicente A, Capilla J, Di Pietro A, López-Berges MS (2018) Fusaric acid contributes to virulence of Fusarium oxysporum on plant and mammalian hosts. Mol Plant Pathol 19(2):440–453. https://doi.org/10.1111/mpp.12536
Marin S, Ramos AJ, Cano-Sancho G, Sanchis V (2013) Mycotoxins: occurrence, toxicology, and exposure assessment. Food Chem Toxicol 60:218–237. https://doi.org/10.1016/j.fct.2013.07.047
Maryani N, Lombard L, Poerba YS, Subandiyah S, Crous PW, Kema GHJ (2019) Phylogeny and genetic diversity of the banana Fusarium wilt pathogen Fusarium oxysporum f. sp. cubense in the Indonesian centre of origin. Stud. Mycol. 92, 155–194. https://doi.org/10.1016/j.simyco.2018.06.003
Meca G, Sospedra I, Soriano JM, Ritieni A, Valero MA, Mañes J (2009) Isolation, purification and antibacterial effects of fusaproliferin produced by Fusarium subglutinans in submerged culture. Food Chem Toxicology: Int J Published Br Industrial Biol Res Association 47(10):2539–2543. https://doi.org/10.1016/j.fct.2009.07.014
Mirete S, Vázquez C, Mulè G, Jurado M, González-Jaén MT (2004) Differentiation of Fusarium verticillioides from banana fruits by IGS and EF-1α sequence analyses. In: Mulè G, Bailey JA, Cooke BM, Logrieco A (eds) Molecular diversity and PCR-detection of toxigenic fusarium species and ochratoxigenic fungi. Springer, Dordrecht. https://doi.org/10.1007/978-1-4020-2285-2_6
Moretti A, Susca A (2017) Mycotoxigenic fungi. Springer New York. ISSN1064-3745 https://doi.org/10.1007/978-1-4939-6707-0
Moretti A, Mulè G, Susca A, González-Jaén MT, Logrieco A (2004) Toxin profile, fertility and AFLP analysis of Fusarium verticillioides from banana fruits. In: Mulè G, Bailey JA, Cooke BM, Logrieco A (eds) Molecular diversity and PCR-detection of toxigenic fusarium species and ochratoxigenic fungi. Springer, Dordrecht. https://doi.org/10.1007/978-1-4020-2285-2_14
Mostert D, Molina AB, Daniells J, Fourie G, Hermanto C, Chao CP, Fabregar E, Sinohin VG, Masdek N, Thangavelu R, Li C, Yi G, Mostert L, Viljoen A (2017) The distribution and host range of the banana Fusarium wilt fungus, Fusarium oxysporum f. sp. cubense, in Asia. PLoS ONE 12(7):e0181630. https://doi.org/10.1371/journal.pone.0181630
Mostert D, Wicker E, de Jager MM, Al Kaabi SM, O’Neill WT, Perry S, Li C, Ganyun Y, Pegg KG, Mostert L (2022) A polyphasic approach reveals novel genotypes and updates the genetic structure of the banana Fusarium wilt pathogen. Microorganisms 10(2):269. https://doi.org/10.3390/microorganisms10020269
Mostrom M (2016) Mycotoxins: Toxicology. Encyclopedia of Food and Health. Fargo, USA: North Dakota State University, pp 43–48. https://doi.org/10.1016/B978-0-12-384947-2.00480-3
Musser SM, Eppley RM, Mazzola EP, Hadden CE, Shockcor JP, Crouch RC, Martin GE (1995) Identification of an N-acetyl keto derivative of fumonisin B1 in corn cultures of Fusarium proliferatum. J Nat Prod 58(9):1392–1397. https://doi.org/10.1021/np50123a009
Nesic K, Ivanovic S, Nesic V (2013) Fusarial toxins: secondary metabolites of Fusarium fungi. Reviews Environ Contam Toxicol Volume 228:101–120. https://doi.org/10.1007/978-3-319-01619-1_5
Nirmaladevi D, Venkataramana M, Srivastava RK, Uppalapati SR, Gupta VK, Yli-Mattila T, Chandra NS (2016) Molecular phylogeny, pathogenicity and toxigenicity of Fusarium oxysporum f. sp. Lycopersici Sci Rep 6(1):21367. https://doi.org/10.1038/srep21367
O’Donnell K, Cigelnik E, Nirenberg HI (1998) Molecular systematics and phylogeography of the Gibberella fujikuroi species complex. Mycologia 90(3):465–493. https://doi.org/10.1080/00275514.1998.12026933
O’Donnell K, Ward TJ, Robert VA, Crous PW, Geiser DM, Kang S (2015) DNA sequence-based identification of Fusarium: current status and future directions. Phytoparasitica 43:583–595. https://doi.org/10.1007/s12600-015-0484-z
O’Donnell K, Kistler HC, Tacke BK, Casper HH (2000) Gene genealogies reveal global phylogeographic structure and reproductive isolation among lineages of Fusarium graminearum, the fungus causing wheat scab. Proc Natl Acad Sci U S A 97(14):7905–7910. https://doi.org/10.1073/pnas.130193297
Ostry V, Malir F, Toman J, Grosse Y (2017) Mycotoxins as human carcinogens—the IARC monographs classification. Mycotoxin Res 33:65–73. https://doi.org/10.1007/s12550-016-0265-7
Parra MÄ, Gómez J, Aguilar FW, Martínez JA (2022) Fusarium annulatum causes Fusarium rot of cantaloupe melons in Spain. Phytopathologia Mediterranea 61(2):269–277. https://doi.org/10.36253/phyto-13454
Pasquali M, Beyer M, Logrieco A, Audenaert K, Balmas V, Basler R, Boutigny A-L, Chrpová J, Czembor E, Gagkaeva T, González-Jaén MT, Hofgaard IS, Köycü ND, Hoffmann L, Lević J, Marin P, Miedaner T, Migheli Q, Moretti A, Müller MEH, Munaut F, Parikka P, Pallez-Barthel M, Piec J, Scauflaire J, Scherm B, Stanković S, Thrane U, Uhlig S, Vanheule A, Yli-Mattila T, Vogelgsang S (2016) A European database of Fusarium graminearum and F. culmorum trichothecene genotypes. Front Microbiol 7:406. https://doi.org/10.3389/fmicb.2016.00406
Perincherry L, Lalak-Kańczugowska J, Stępień Ł (2019) Fusarium-produced mycotoxins in plant-pathogen interactions. Toxins 11(11):664. https://doi.org/10.3390/toxins11110664
Pitt JI (2000) Toxigenic fungi: which are important? Sabouraudia 38(Supplement1):17–22. https://doi.org/10.1080/mmy.38.s1.17.22
Purwati RD, Hidayah N (2008) Inoculation methods and conidial densities of Fusarium oxysporum f. sp. cubense in Abaca. HAYATI J Biosci 15(1):1–7. https://doi.org/10.4308/hjb.15.1.1
Rheeder JP, Marasas WF, Vismer HF (2002) Production of fumonisin analogs by Fusarium species. Appl Environ Microbiol 68(5):2101–2105. https://doi.org/10.1128/AEM.68.5.2101-2105.2002
Riolo M, Aloi F, Faedda R, Cacciola SO, Pane A (2020) First report of postharvest fruit rot caused by Fusarium sacchari on lady finger banana in Italy. Plant Dis 104(8):2290–2290. https://doi.org/10.1094/PDIS-01-20-0143-PDN
Scauflaire J, Gourgue M, Munaut F (2011) Fusarium temperatum sp. nov. from maize, an emergent species closely related to Fusarium subglutinans. Mycologia 103(3):586–597. https://doi.org/10.3852/10-135
Serrano AB, Font G, Mañes J, Ferrer E (2013) Emerging Fusarium mycotoxins in organic and conventional pasta collected in Spain. Food Chem Toxicol 51(1):259–266. https://doi.org/10.1016/j.fct.2012.09.034
Shi W, Tan Y, Wang S, Gardiner DM, De Saeger S, Liao Y, Wang C, Fan Y, Wang Z, Wu A (2017) Mycotoxigenic potentials of Fusarium species in various culture matrices revealed by mycotoxin profiling. Toxins 9(1):6. https://doi.org/10.3390/toxins9010006
Snowdon AL (1990) A colour atlas of post-harvest diseases and disorders of fruit and vegetables. Volume 1: General introduction and fruit. Wolfe Scientific Ltd. ISBN 9780723409311
Somma S, Petruzzella AL, Logrieco AF, Meca G, Cacciola OS, Moretti A (2014) Phylogenetic analyses of Fusarium graminearum strains from cereals in Italy, and characterisation of their molecular and chemical chemotypes. Crop Pasture Sci 65(1):52–60. https://doi.org/10.1071/CP13314
Song Z, Cox RJ, Lazarus CM, Simpson TJ (2004) Fusarin C biosynthesis in Fusarium moniliforme and Fusarium venenatum. ChemBioChem 5(9):1196–1203. https://doi.org/10.1002/cbic.200400138
Stankovic S, Levic J, Petrovic T, Logrieco A, Moretti A (2007) Pathogenicity and mycotoxin production by Fusarium proliferatum isolated from onion and garlic in Serbia. Eur J Plant Pathol 118:165–172. https://doi.org/10.1007/s10658-007-9126-8
Stracquadanio C, Luz C, La Spada F, Meca G, Cacciola SO (2021) Inhibition of mycotoxigenic fungi in different vegetable matrices by extracts of Trichoderma species. J Fungi 7(6):445. https://doi.org/10.3390/jof7060445
Studt L, Troncoso C, Gong F, Hedden P, Toomajian C, Leslie JF, Humpf H-U, Rojas MC, Tudzynski B (2012) Segregation of secondary metabolite biosynthesis in hybrids of Fusarium fujikuroi and Fusarium proliferatum. Fungal Genet Biol 49(7):567–577. https://doi.org/10.1016/j.fgb.2012.05.005
Tamura K, Nei M (1993) Estimation of the number of nucleotide substitutions in the control region of mitochondrial DNA in humans and chimpanzees. Mol Biol Evol 10(3):512–526. https://doi.org/10.1093/oxfordjournals.molbev.a040023
Tamura M, Mochizuki N, Nagatomi Y, Harayama K, Toriba A, Hayakawa K (2015) Identification and quantification of Fumonisin A1, A2, and A3 in corn by high-resolution liquid chromatography-orbitrap Mass Spectrometry. Toxins 7(2):582–592. https://doi.org/10.3390/toxins7020582
Tavakol Noorabadi M, Masiello M, Taherkhani K, Zare R, Torbati M, Haidukowski M, Somma S, Logrieco AF, Moretti A, Susca A (2021) Phylogeny and mycotoxin profile of Fusarium species isolated from sugarcane in Southern Iran. Microbiol Res 252:126855. https://doi.org/10.1016/j.micres.2021.126855
Thrane U (1988) Screening for Fusarin C production by European isolates of Fusarium species. Mycotoxin Res 4:2–10. https://doi.org/10.1007/BF03192082
Urbaniak M, Waśkiewicz A, Stępień Ł (2020) Fusarium cyclodepsipeptide mycotoxins: Chemistry, biosynthesis, and occurrence. Toxins 12(12):765. https://doi.org/10.3390/toxins12120765
Uribe-Palacio S, Ramírez-Sáncheza M, Umaña-Rojas G, Sáenz-Murillo MV (2022) Studies on the potential for treatment with short wave ultraviolet light (UV-C) to reduce postharvest diseases in banana fruit crown (Musa sp., group AAA, subgroup Cavendish). Fruit 77(1). https://doi.org/10.17660/th2022/005
Viswanathan R, Balaji CG, Selvakumar R, Malathi P, Ramesh Sundar A, Prasanth CN, Chhabra ML, Parameswari B (2017) Epidemiology of Fusarium diseases in sugarcane: a new discovery of same Fusarium sacchari causing two distinct diseases, wilt and pokkah boeng. Sugar Tech 19:638–646. https://doi.org/10.1007/s12355-017-0552-4
Warman NM, Aitken EA (2018) The movement of Fusarium oxysporum f. sp. cubense (sub-tropical race 4) in susceptible cultivars of banana. Front Plant Sci 9:1748. https://doi.org/10.3389/fpls.2018.01748
Wentzel C, Gelderblom A, Thiel P, Marasas WFO, Van der Merwe KJV (1984) Natural occurrence of fusarin C, a mutagen produced by Fusarium moniliforme, in corn. J Agric Food Chem 32(5):1064–1067. https://doi.org/10.1021/jf00125a031
Williams JH, Phillips TD, Jolly PE, Stiles JK, Jolly CM, Aggarwal D (2004) Human aflatoxicosis in developing countries: a review of toxicology, exposure, potential health consequences, and interventions. Am Clin Nutr 80(5):1106–1122. https://doi.org/10.1093/ajcn/80.5.1106
Xie SH, Zhang C, Chen MS, Wei ZP, Jiang YB, Lin X, Zheng YQ (2018) Fusarium proliferatum: a new pathogen causing fruit rot of peach in Ningde, China. Plant Dis 102(9):1858–1858. https://doi.org/10.1094/PDIS-01-18-0103-PDN
Xie L, Wu Y, Wang Y, Jiang Y, Yang B, Duan X, Li T (2021) Fumonisin B1 induced aggressiveness and infection mechanism of Fusarium proliferatum on banana fruit. Environ Pollut 288:117793. https://doi.org/10.1016/j.envpol.2021.117793
Xie L, Wu Y, Duan X, Li T, Jiang Y (2022) Proteomic and physiological analysis provides an elucidation of Fusarium proliferatum infection causing crown rot on banana fruit. Microbiol Res 256:126952. https://doi.org/10.1016/j.micres.2021.126952
Xie L, Yang Q, Wu Y, Xiao J, Qu H, Jiang Y, Li T (2023) Fumonisin B1 biosynthesis is associated with oxidative stress and plays an important role in Fusarium proliferatum infection on banana fruit. J Agric Food Chem 71(13):5372–5381. https://doi.org/10.1021/acs.jafc.3c00179
Yang J, Wang F, Wen Y, Gao S, Lu C, Liu Y, Liu H (2020) First report of Fusarium proliferatum causing root rot disease in Salvia miltiorrhizae in China. Plant Dis. https://doi.org/10.1094/pdis-09-20-1908-pdn
Yilmaz N, Sandoval-Denis M, Lombard L, Visagie CM, Wingfield BD, Crous PW (2021) Redefining species limits in the Fusarium fujikuroi species complex. Persoonia 46:129–162. https://doi.org/10.3767/persoonia.2021.46.05
Zeng L, Zhao Z, Xi Z, Li M, Xi P, Jiang Z (2013) The Fusarium species isolated from banana and their phylogenetic relationships. Mycosystema, 32(4), 617–632. PMID: 24384435
Funding
Open access funding provided by Università degli Studi di Catania within the CRUI-CARE Agreement. This study was supported by the project “Smart and innovative packaging, postharvest rot management, and shipping of organic citrus fruit (BiOrangePack)” under Partnership for Research and Innovation in the Mediterranean Area (PRIMA)– H2020 (E69C20000130001) and the “Italie–Tunisie Cooperation Program 2014–2020” project “PROMETEO «Un village transfrontalier pour protéger les cultures arboricoles méditerranéennes en partageant les connaissances» cod. C-5-2.1-36, CUP 453E25F2100118000.
Open access funding provided by Università degli Studi di Catania within the CRUI-CARE Agreement.
Author information
Authors and Affiliations
Contributions
Conti Taguali S.: Methodology, Data curation, Formal analysis, Writing—original draft; Riolo M.: Investigation, Methodology, Data curation, Formal analysis, Visualization, Supervision, Writing—original draft, Writing—review and editing; Dopazo V.: Investigation, Methodology, Formal analysis; Meca G.: Visualization, Investigation, Methodology; Cacciola SO.: Investigation, Methodology, Visualization, Writing—review and editing, Supervision.
Corresponding author
Ethics declarations
Ethical approval
This article does not contain any studies with human participants or animalsperformed by any of the authors.
Conflict of interest
The authors declare that they have no conflict of interest in the publication.
Additional information
Publisher’s note
Springer Nature remains neutral with regard to jurisdictional claims in published maps and institutional affiliations.
Rights and permissions
Open Access This article is licensed under a Creative Commons Attribution 4.0 International License, which permits use, sharing, adaptation, distribution and reproduction in any medium or format, as long as you give appropriate credit to the original author(s) and the source, provide a link to the Creative Commons licence, and indicate if changes were made. The images or other third party material in this article are included in the article’s Creative Commons licence, unless indicated otherwise in a credit line to the material. If material is not included in the article’s Creative Commons licence and your intended use is not permitted by statutory regulation or exceeds the permitted use, you will need to obtain permission directly from the copyright holder. To view a copy of this licence, visit http://creativecommons.org/licenses/by/4.0/.
About this article
Cite this article
Conti Taguali, S., Riolo, M., Dopazo, V. et al. Characterization of mycotoxins produced by two Fusarium species responsible for postharvest rot of banana fruit. J Plant Pathol (2024). https://doi.org/10.1007/s42161-024-01751-8
Received:
Accepted:
Published:
DOI: https://doi.org/10.1007/s42161-024-01751-8