Abstract
CD146 was originally identified as a melanoma cell adhesion molecule (MCAM) and highly expressed in many tumors and endothelial cells. However, the evidence that CD146 acts as an adhesion molecule to mediate a homophilic adhesion through the direct interactions between CD146 and itself is still lacking. Recent evidence revealed that CD146 is not merely an adhesion molecule, but also a cellular surface receptor of miscellaneous ligands, including some growth factors and extracellular matrixes. Through the bidirectional interactions with its ligands, CD146 is actively involved in numerous physiological and pathological processes of cells. Overexpression of CD146 can be observed in most of malignancies and is implicated in nearly every step of the development and progression of cancers, especially vascular and lymphatic metastasis. Thus, immunotherapy against CD146 would provide a promising strategy to inhibit metastasis, which accounts for the majority of cancer-associated deaths. Therefore, to deepen the understanding of CD146, we review the reports describing the newly identified ligands of CD146 and discuss the implications of these findings in establishing novel strategies for cancer therapy.
Similar content being viewed by others
Introduction
In 1987, Johnson et al. first found that a tumor antigen, MUC18, was expressed most strongly on metastatic lesions and advanced primary melanoma with rare detection in benign lesions. Due to the high sequence homology between MUC18 with cell adhesion molecules (CAMs), this melanoma antigen was given an official name, melanoma CAM (MCAM).1 With an increasing number of discoveries about MCAM by various research groups, more alias names were given to this protein, including P1H12, MUC18, A32 antigen, S-Endo-1, Mel-CAM, MET-CAM, HEMCAM, or CD146.1,2,3
CAM is a kind of proteins located on the cell surface and mediates contacting and binding of cell to cell or cell to extracellular matrix (ECM).4 These dynamic interactions provide signals input into the cellular decision-making process such as cell growth, survival, migration, and differentiation,5 essential for embryonic development and for maintaining the integrity of tissue architecture in adults.6,7 Dependent on adhesion, some CAMs can initiate the formation of complexes composed of extracellular ligands, kinases, and cytoskeletal proteins.8 Abnormal expression of CAMs can cause various diseases, such as cancer and inflammatory disorders.9,10
There are three forms of CD146 proteins in human, mouse, and chicken. The two membrane-anchored forms of CD146 are encoded by cd146 gene and soluble form of CD146 (sCD146) is generated by the proteolytic cleavage of the membrane forms.11,12,13 Soluble CD146 can be detected in cell culture supernatants, serum, and interstitial fluids from either healthy or unhealthy subjects.14,15,16 Because sCD146 does not have either transmembrane or cytoplasmic regions, it is not competent in cellular adhesion.17,18 Therefore, we will not describe sCD146, its ligands and its functions in this review, although it is a potential target in tumor microenvironment of CD146-positive invasive tumors.19
Recent evidence has revealed that membrane-bound CD146 may act as a cell-surface receptor to bind with various ligands involved in cellular signaling transduction independent of the adhesion properties. In order to deepen the understanding of the functions of CD146 in physiological and pathological processes, we summarize the various newly identified ligands of CD146 and the ligand-elicited roles in signal transduction and discuss the implications of CD146 in remodeling interactions between the cancerous cells with the elements of their surrounding microenvironments.
The CD146 protein
Membrane CD146 protein has two isoforms: long form (CD146-l) has a long cytoplasmic tail; short form (CD146-s) has a short cytoplasmic tail.17,18 These two CD146 isoforms are produced from different exon splicing strategies and the premature molecules have a signal peptide located on the anterior region of the amino terminal.20 In human, mature CD146 protein is composed of an extracellular sections with five distinct Ig-like domains that exist in a V–V–C2–C2–C2 structural motif, a hydrophobic transmembrane region and a short cytoplasmic tail.21 The cytoplasmic domain in both isoforms contains two potential recognition sites for protein kinases C (PKC), an ERM (protein complex of ezrin, radixin, and moesin) binding site, a motif with microvilli extension, and a double leucine motif for baso-lateral targeting.21 The two isoforms co-exist as monomers and dimers and the dimerization is mediated through a disulfide bond between cysteine residues in the C2 domain most proximal to the membrane.20,22 However, the information about CD146 crystal structure, including secondary and tertiary, is still lacking.
CD146 is a highly glycosylated type I transmembrane protein and belongs to the immunoglobulin superfamily. Based on bioinformation analysis, eight putative N-glycosylation sites are present in the extracellular fragment across species.23 In clear cell renal cell carcinoma and prostate cancer, CD146 glycosylation levels were upregulated.24,25 In 2018, it was reported that CD146 glycosylation is favorably carried out by b-1,3-galactosyl-Oglycosyl-glycoprotein b-1,6-N-acetylglucosaminyltransferase-3, which was overexpressed in highly metastatic melanomas. Such glycosylations can extend CD146 protein stability, upregulate CD146 protein levels, and lead to elevation of CD146-mediated cellular motility in melanoma cells.26 These observations suggest that the degree of CD146 glycosylation may be directly related to malignant progression of tumors, especially CD146-positive neoplasms.
The expression profile of CD146 protein
Based on literature, metazoan CD146 has been detected in majority of cell types, including vessel constituting cells (endothelium, pericyte, and smooth muscle cell), epithelia, fibroblasts, mesenchymal stem cells, and lymphocytes, except erythrocytes.21 Under physiological conditions, CD146 expression is restricted to limited adult normal tissues and its adhesive strength is relatively weak, in contrast to most other CAMs, which show wide expression patterns in normal adult tissues and strong adhesion strength.21,23 However, CD146 expression is broadly and highly detected in embryonic tissues compared to its abundance in normal adult tissues.21 In quickly proliferating cells, increased expression of CD146 may allow cells to actively interact with each other and with the elements of the cellular microenvironment, promoting cell proliferation, and migration.
Under pathological conditions, such as inflammation and tumorigenesis, CD146 was upregulated in the related cells and has been identified as a reliable marker for numerous types of cancers. Accumulating evidence shows that CD146 overexpression has been linked to either the initial development of the primary lesion or progression to metastases of most of cancer types, primarily including melanoma,1,27,28,29 breast,6,30,31 ovarian,32,33,34,35 lung,36,37 prostate,38,39,40 glioma,41 kidney,42 hepatic,43,44 and gastric cancers.21,45 In 2017, Nollet et al. reported that TsCD146 mAb (for tumor specific anti-CD146 monoclonal antibody) can specifically recognize CD146 expressed in cancer cells but not CD146 in physiological vessels, suggesting that structural features of cancer CD146 differ from those of physiological CD146.28
Recognition of CD146 ligands in history
The recognition of CD146 ligands and analysis of their functions was undertaken over a prolonged period in history. Because CD146 is highly expressed in vessel cells and cancer cells, it is likely that CD146 within these cells contributes to cancer metastasis through the mediation of a homophilic adhesion between cancerous cells and vascular endothelia, a key part of the metastatic process. However, evidence of the direct interactions between CD146 and itself is still lacking.46,47,48 Accordingly, it is possible that CD146-mediated adhesion between cancerous cells with vascular endothelia as well as with their surrounding elements occurs through the bidirectional heterophilic interactions between CD146 with its ligands, but not the homophilic interaction with itself.
In 1991, the first CD146’s ligand was found using chicken smooth muscle cells. Taniura et al. discovered that neurite outgrowth factor (NOF) was a ligand of chicken CD146 (Gicerin) and that binding of NOF to CD146 is essential for the development of the chicken retina.49,50 However, at that time, due to technological limitations, the molecular characteristics of NOF were not determined. In 2012, Laminin 411 was revealed as the ligand of CD146, facilitating the entry of blood lymphocytes into the central nervous system (CNS). In this report, the authors claimed that Laminin 411 is a major tissue ligand for CD146 on lymphocytes.51 In 2014, Ishikawa et al. finally determined the identity of NOF, Laminin 421, which has the same α4 subunit as Laminin 411.52
In 2012, our laboratory identified that CD146 can bind with vascular endothelial growth factor receptor 2 (VEGFR2) as a co-receptor required for the activation by vascular endothelial growth factor-A (VEGF-A).53 Because VEGF-A is a well-known growth factor with strong pro-angiogenesis effects, this finding provided the mechanism underlying the roles of CD146 in tumor angiogenesis, especially in sprouting stage. Subsequently, our laboratory identified an array of pro-angiogenetic growth factors, including Wingless/integrase (Wnt)5a,54 Netrin-1,55 fibroblast growth factor (FGF)4,56 VEGF-C,57 and Wnt1,58 as the ligands of CD146. In 2017, we further identified that CD146 on endothelia can directly bind with platelet-derived growth factor receptor-β (PDGFR-β) on pericyte, required for PDGF-B-induced PDGFR-β activation.59 Because PDGF-B/PDGFR-β plays crucial roles in recruiting adjacent pericytes to the endothelia, this finding indicates that CD146 is required for vessel integrity.
Until now, there had been a total of 13 molecules or complexes identified as the CD146 ligands (Table 1). According to the characteristics of these ligands, they can be categorized into three groups: components of the ECM, pro-angiogenic factor receptors, and growth factors. All these ligands have been sown to directly interact with CD146 in physiological and pathological processes are involved in the promotion of CD146-mediated angiogenesis and tumor metastasis. Here, we will review the various CD146′ heterophilic ligands and discuss the implications of these findings in tumoral context.
CD146 is the receptor of proteins in relation to the ECM
One of the critical features of malignant proliferation is cancer metastatic plasticity affected by its microenvironment. This plasticity is a major reason for the failure of inhibition of cancer metastasis. The metastatic process involves epithelial mesenchymal transition (EMT), attachment of metastatic cells to the endothelium of the vascular or lymphatic vessels, and invasion into distant metastatic tissues.60 It is well known that the aberrant high expression of CD146 is involved in nearly every step of development and progression in almost all types of malignant cancers.21 The findings that several ECM-related proteins, including Laminin 411 and 421, Galectin-1 and -3, S100A8/A9, and matriptase, are specific ligands of CD146, may elucidate the mechanism underlying the function of CD146 in remodeling tumor microenvironments during tumor development, especially metastasis via vascular and lymphatic vessels (Fig. 1).
Schematic representation of CD146 as the receptor of proteins related in the ECM. CD146-ligand binding-induced events are indicated by the red arrows. The immunoglobulin domains of CD146 are illustrated as balls and the pink and blue color represent the V and C2 immunoglobulin domains, respectively. The green rectangle and the yellow trapezoid represent the PKC phosphorylation site and the dileucine motif, respectively
Laminins 411 and 421
Laminins are a family of large heterotrimeric αβγ proteins with over 15 different isoforms. Five laminin α chains (α1–α5), four laminin β chains (β1–β4), and three laminin γ chains (γ1–γ3) constitute αβγ heterotrimers. They are denominated according to their chain composition; for example, laminin α4β1γ1 is designated as Laminin 411.61 Laminins are predominantly found in basement membranes that compartmentalize different tissues and surround blood vessels, nerves, and adipocytes.62,63 They play a crucial role in physiological and pathological remodeling of the ECM during angiogenesis, wound healing, embryogenesis, and tumor metastasis. Remodeling of the ECM during metastasis allows tumor cells to invade their surrounding ECM, spread via the vascular or lymphatic circulation, and extravasate into distant organs.
Laminin isoforms, particularly the laminin α chain, are expressed in a cell and tissue specific manner and are distinctly bound by almost ten different integrins and other cell-surface receptors.62,63 The α4-laminins are mesenchymal laminins expressed by the cells of mesenchymal origin, such as vascular and lymphatic endothelial cells, pericytes, and leukocytes, and are required for normal development of the cardiovascular and neurological system in mice.64,65,66 Under pathological conditions, α4-laminins are expressed and secreted by various tumor cells, such as melanoma and glioma,67,68,69,70,71,72 or tumor stroma, lymphatic and vascular vessels, nervous system.69,73,74
Laminin 411
Laminin 411 is expressed along the vascular endothelium.68,73,75 This laminin isoform is recognized by various integrins, including α6β1, α3β1, α6β4, and αVβ3, which promote the migration of several cell types along vascular or nervous system tracks.76,77,78,79,80
In 2012, Laminin 411 on the vascular endothelia was discovered as a specific ligand for CD146 on a subset of human CD4+ T helper (Th) cells.51 This subset of human T cells expresses CD146 and can enter tissues to promote pathogenic autoimmune responses. To determine the CAMs involved in the migratory capacity of Th17 cells into tissues, researchers used purified Laminin 411 to identify its receptor. In this study, the authors demonstrated that purified CD146-Fc binds to Laminin 411 with high affinity (27 nM) and that this binding disappeared when the endogenous Laminin 411 was specifically deleted. Correspondingly, blocking this binding by CD146 antibody in vivo also reduced Th17 lymphocyte infiltration into the CNS. Therefore, the authors concluded that Laminin 411 is a major tissue ligand for CD146+ lymphocyte.
However, the role of Th17 cells in the pathogenesis of malignant tumors is still remains controversial. Some studies revealed that increased percentage of Th17 lymphocytes among cells infiltrating ovarian cancer cells stimulate tumor progression;81 whereas other studies showed that Th17 lymphocytes have anticancer activity and can reduce tumor growth and metastasis.82 Therefore, the roles of CD146+ Th17 cells in cancer development may be worthy of further investigations.
Laminin 421
CD146 is a reliable biomarker of endothelia and is concentrated at the intercellular junctions of endothelial cells of vessel system.21 Most cancer cells, including melanoma, migrate along the abluminal sides of vascular and/or lymphatic vessels, as they disseminate throughout the body.83 Laminin 421 is major laminins of along the tumor-dissemination tracks (blood and lymphatic vessels, nerves, and tumor stroma).84,85,86
To determine the mechanism of CD146 roles in metastasis, researchers used melanoma cells to test what laminin isoforms, other than Laminin 411, can bind with the melanoma marker of CD146. Therefore, they used all laminin α chains to examine the binding affinity with human CD146 in a solid-phase ligand binding assay.87 Finally, they found that only Laminin 421, of several laminin isoforms, readily bound to CD146, suggesting that Laminin 421 is a primary ligand for CD146 in melanoma. Accordingly, a function-blocking mAb to CD146 inhibited tumor cell migration on Laminin 421, but not on laminins 411 or 521. In addition, this investigation determined that the identity of NOF, previously identified as a ligand for chicken CD146 (gicerin), is actually Laminin 421.
In this study, the authors also determined that Laminin 411 and especially Laminin 421 are capable of stimulating migration of a broad panel of cancer cell lines through a filter. This investigation is consistent with the observation that the α4-laminins, including Laminins 411 and 421, expressed and secreted by various carcinoma cells, have already emerged as “onco-laminins.”67,68,69,70,71,72 Melanoma CD146 binds with Laminin 421 but not 411, whereas lymphocyte CD146 only binds with Laminin 411; suggesting that the epitopes of CD146 on somatic cancer cells are different from those of CD146 on blood lymphocytes. Therefore, the infiltration of CD146+ invasive cancers into tumor-dissemination tracks is likely dependent on the interaction between CD146 and Laminin 421, and blocking their binding may affect the efficacy of cell–cell interactions and interfere metastasis.
Galectin-1 and -3
CD146 is a highly glycosylated junctional CAM involved in the control of vascular vessel integrity. Sequence analysis predicts the presence of eight putative N-glycosylation sites at residue positions 56, 418, 449, 467, 508, 518, 527, and 544.21 It has been estimated that 35% of the CD146 molecular mass is attributed to glycans.88 The galactose residues in glycans can bind with galectins, and such binding can be inhibited by lactose.
Galectins are a family of soluble carbohydrate-binding lectins that modulate cell-to-cell and cell-to-ECM adhesions.89 Up to now, 15 galectins have been identified in mammals and 11 are found in humans. Among them, Galectin-1, -3, and -9 are three best-investigated galectins and Galectin-1 and -3 promote tumor development, progression, and immune escape.90 Galectin-1 and -3 can hamper antitumor responses and are considered multifunctional targets for cancer therapy.91,92 The underlying mechanisms include interfering with drug efficacy/delivery or reducing the antitumor effect of immune cells. For instance, Galectin-1 confers drug resistance via inducing the expression of multidrug resistance protein 1, which in turn helps tumor cells to pump out cytotoxic drugs, facilitating cancer cells to combat anticancer drugs.93 Regarding the immunosuppressive effects of Galectin-1 and -3 on T cells, in a mouse melanoma model, targeted inhibition of Galectin-1 enhanced T cell-mediated tumor clearance;94 Galectin-3 can neutralize glycosylated IFN-γ in tumor matrices, ablating the immune response to tumors.95 To increase overall responsiveness of tumors to chemo- or immune-therapy, inhibitors of Galectin-196,97 and -398,99 have been used in combination with anti-CTLA-4 or anti-PD-1 to treat cancer patients in clinical trials.
Because both CD146 and galectins are involved in the modulation of angiogenesis, researchers hypothesized that some galectins may be the ligands of CD146 and the interactions between them are required for functional CD146 in angiogenesis, as well as in cancer metastasis. To date, two galectins, 1 and 3, have been identified as the ligands of CD146.
Galectin-1
Galectin-1 prefers to bind with the branched N-glycans of cell-surface glycoproteins and mediates a glycosylation-dependent angiogenesis.91,100,101,102,103 It has been reported that increased secretion of Galectin-1 in the ECM facilitates cancer cell proliferation and resistance to cancer therapy in prostate cancer104 and Kaposi’s sarcoma.105 Mechanistic investigation has revealed that Galectin-1 can bind to N-glycans on VEGFR2 to activate VEGF-like signaling in anti-VEGF-A refractory tumors, promoting tumor progression. Accordingly, disruption of the Galectin-1-N-glycan axis inhibits tumor growth by promoting vascular remodeling.101 This research highlights the importance of Galectin-1 in tumor angiogenesis and cancer metastasis. However, these studies cannot exclude the fact that other cell-surface proteins with branched N-glycans are also involved in this glycosylation-dependent pro-angiogenesis pathway.
Early in 2011, it was reported that the co-expression of Galectin-1 and CD146 is required for tumor vascularization in a human mesenchymal stem cell strain with significant angiogenic potential.106 In 2013, Jouve et al. reported that Galectin-1 binds to CD146 on endothelial cells, facilitating cell survival.107 In this report, they explained that CD146 glycosylation is mainly composed of branched N-glycans. They showed that the interaction of CD146 with Galectin-1 is carbohydrate-mediated using both an enzyme-linked immunosorbent assay and surface plasmon resonance assays. In addition, they demonstrated that the interaction between Galectin-1 and CD146 protects endothelial cells against apoptosis induced by Galectin-1. Thus, it is tempting to speculate that CD146 could be a decoy receptor for Galectin-1, preventing the Galectin-1 from binding to pro-apoptotic receptors.107 However, whether this interaction affects tumor cell survival remains unknown. In 2015, Yazawa et al. thus further analyzed the functions of this interaction on melanoma and found that when Galectin-1 binds to CD146, it helps maintain intrinsic malignant features.108 The authors examined the expression, identity, and function of Galectin-1 ligands in melanoma progression and demonstrated that CD146 is the major Galectin-1 ligand on melanoma cells.
These findings provide a perspective on the interactions between CD146 and its ligands, such as Galectin-1, as contributors to cancer malignancy. Indeed, various membrane glycoconjugates have been identified as binding partners of Galectin-1 such as β1 integrins, CD2, CD3, CD4, CD43, CD45, and GM1 ganglioside. In addition, Galectin-1 can bind to a number of ECM components in a dose-dependent and β-galactoside-dependent manner. For instance, laminin and fibronectin, which are highly N-glycosylated, interact with Galectin-1.109 Because it has been reported that CD146 can interact with Laminin 411, Laminin 421, and β1 integrin, it is reasonable to speculate that CD146 may also interact with all of those Galectin-1 interactors within cancerous cells.
Since the tumor vasculature is an easily accessible target for cancer therapy, understanding how galectins influence cancer angiogenesis is important for the translational development of therapies intended to prevent tumor progression. Based on the fact that VEGF-targeted therapies often fail when tumors receive continued treatment,110 targeting glycosylation-dependent Galectin-1-receptor interactions, such as Galectin-1-CD146-VEGFR2 may increase the efficacy of anti-VEGF treatment.
Galectin-3
Like Galectin-1, Galectin-3 can also bind to various galactose-terminated glycans of cell-surface receptors and proteins of ECM and is involved in many physiological and pathological processes from cell adhesion and migration to cell activation.111,112 In cancer cells, it modulates cell–cell and cell–microenvironment communications, contributing to cancer development, progression, and metastasis.113,114,115,116,117,118,119,120 Patients with metastatic diseases tend to have higher concentrations of circulating Galectin-3 than those with only localized tumors.121 Increased circulating Galectin-3 promotes blood-borne metastasis due to the interaction of Galectin-3 with receptors on vascular endothelial cells, further causing endothelial secretion of several metastasis-promoting cytokines.
To identify the Galectin-3-binding molecules on the endothelial cell surface, using the Galectin-3 affinity purification method, Colomb et al. found that CD146 was the major cell-surface receptor to strongly bind and co-localize with Galectin-3, compared with other glycosylated receptors, CD31, CD144, and CD106. They also showed that Galectin-3 bound to N-linked glycans on CD146 and induced CD146 dimerization and subsequent activation of protein kinase B (AKT) signaling. Correspondingly, suppression of CD146 expression abolishes Galectin-3-induced secretion of metastasis-promoting cytokine from the endothelial cells. Thus, they concluded that CD146 is the functional Galectin-3-binding receptor on the endothelial cell surface responsible for Galectin-3-induced secretion of cytokines, and therefore influences cancer progression and metastasis.122
Subsequently, the binding moieties of CD146 by Galectin-3 have been further identified. The authors demonstrated that Galectin-3 interacts with the highly glycosylated Domain 5 in the CD146 extracellular fragment regardless of the presence or absence of lactose. These findings provide a better understanding of how Galectin-3 interacts with cell-surface receptors to mediate endothelial cell migration and the secretion of cytokines.123,124
The endothelial galectins are confined to four family members, i.e., Galectin-1, -3, -8, and -9, which contribute to tumor angiogenesis.92 Tumor-induced angiogenesis is a pathologic condition in which tumor cells secrete growth factors, such as VEGFs, to promote the growth of new blood vessels.125,126 These growth factors activate quiescent endothelial cells in host tissue to facilitate them to invade into the tumor stroma for growth of new capillaries.127 Endothelial galectins binding with glycoconjugates on tumors are involved in different processes during tumor-induced angiogenesis. Because Galectin-1 and -3 binding of glycoconjugates on tumor cells mediates many key processes in angiogenesis and elevated levels of Galectin-1 and -3 in the endothelium are correlated with tumor vascularization,105,128,129,130,131 the promotion of tumor vascular remodeling by tumor CD146 may be due to the interactions between CD146 with Galectin-1 and -3.
S100A8/A9
S100 proteins
In humans, there are at least 21 members of the S100 protein family,132 which have 2 EF-hand calcium-binding motifs and are 100% soluble in saturated ammonium sulfate.133 S100 family members typically form homodimers, as well as heterodimers, trimers and tetramers, etc.132,134 S100 proteins are typically cytoplasmic proteins, but several family members are secreted by cells as extracellular factors.134,135,136,137 Thus, they contribute to a broad array of intracellular and extracellular functions.134,135 Upregulation of S100 proteins promotes pro-inflammatory responses that contribute to the development and progression of cancer and autoimmune and chronic inflammatory diseases.138,139,140,141
The secreted S100 proteins bind with several cell-surface receptors, including advanced glycation end products (RAGE),142,143,144,145,146 toll-like receptor 4 (TLR4),147 CD36,148 FGFR1,149 ALCAM,150 CD68,151 and ErbB4.152 However, how the cell-surface receptors mediate extracellular S100 signaling is lacking and how S100 protein secretion is dynamically regulated in biological processes also still remains unknown.
S100A8/A9 heterodimer
The secreted S100A8/A9 proteins are the best characterized soluble S100 proteins. Most inflammatory processes seem to require the release of the S100A8/A9 heterodimer into the ECM.153,154,155 Significant upregulation of S100A8/A9 has been observed in many tumors, including lung, gastric, esophageal, colon, pancreatic, bladder, ovarian, thyroid, breast, and skin cancers.156,157 The upregulation of S100A8/A9 is caused either by the infiltrating immune cells of tumor microenvironment158 or by the tumor itself,156,157 contributing to the establishment of a pre-metastatic niche in the tumor microenvironment.159
Mechanistic investigations demonstrated that upregulated S100A8/A9 induces the expression of serum amyloid 3, which in turn recruits myeloid-derived suppressor cell (MDSC), producing a pro-inflammatory environment during metastasis of aggressive disease.160,161,162,163,164,165,166,167 In addition, enhanced expression of S100A8/A9 is also associated with poor prognosis.168
S100A8/A9 proteins mediate these effects by binding to plasma membrane elements, including heparan sulfate proteoglycan (HSPG),169 N-glycans,170 TLR4,171 and RAGE.172,173 In a melanoma lung metastasis model, Hiratsuka et al. clearly demonstrated that lung S100A8/A9, as a strong chemokine, interacts with TLR4 on melanoma to attract distant cancer cells to the lungs.174 Recently, it has also been shown that CD146, on melanoma and breast cancer, can respond to lung S100A8/A9 to induce lung-specific metastasis of melanoma175,176 and breast cancer.177
S100A8/A9 as the ligand of CD146
The expression levels of S100A8 and S100A9 were higher in the lungs than in other organs and the higher expression levels were induced by the primary tumor itself.162 In lung-associated MDSC and endothelial cells, tumor-derived transforming growth factor-beta (TGF-β) and VEGF-A can upregulate the expression and secretion of S100A8/A9.162 Thus, it has been recognized that S100A8/A9 plays a critical role in lung tropic metastasis and the subsequent growth of cancer cells in the lungs.26,178 During metastasis, lung S100A8/A9 might act as a guiding protein for cancer cells that possess high expression levels of CD146.162
In 2016, Ruma et al. revealed that S100A8/A9 uses CD146 as a receptor during lung-specific metastasis of melanoma cells.175 In this study, they demonstrated that S100A8/A9 binding to CD146 activates nuclear factor-kappa B (NF-κB) and induces reactive oxygen species formation, significantly increasing cell adhesion, growth, and invasion. Notably, this study proposed that CD146 governs cancer invasion toward the lungs by sensing the cancer microenvironment as a soil sensor receptor of lung S100A8/A9.175 Therefore, the authors conclude that S100A8/A9 plays a crucial role in lung tropic cancer metastasis by helping to establish an immunosuppressive metastatic niche, to which it then attracts remote cancer cells by interacting with CD146 on the cancer cell surfaces.
In 2019, Chen et al. further determined the importance of the S100A8/A9-CD146 axis in melanoma dissemination in a skin lesion, a critical early step for metastasis of melanoma. This mechanistic study revealed that S100A8/A9-CD146 binding activates a cascade of functions; it leads to significant activation of the transcription factor, ETS translocation variant 4 (ETV4), and the subsequent induction of matrix metalloproteinase-25. The activation of MAP3K8/ETV4 by S100A8/A9-CD146 binding finally results in lung tropic metastasis of melanoma.176
Breast cancer cells prefer the lung, liver, bone, and brain as their metastatic sites. This organ-tropic metastasis is known as the “seed and soil” theory.179 This conclusion was reached because CD146 was remarkably overexpressed in metastatic breast cancer cells.180,181,182 In 2019, in breast cancer cells, the S100A8/A9-CD146 axis-elicited downstream signals that produce the driving force for distant metastasis were identified. This study revealed how S100A8/A9 binding to CD146 accelerates breast cancer growth and metastasis. They found that S100A8/A9 acts as an extracellular cytokine to activate the CD146/ETV4 axis, which upregulates a very high level of ZEB1, a strong EMT inducer. ZEB1 in turn induced a mobile phenotype, i.e., EMT in cells. In contrast, the downregulation of CD146/ETV4 axis repressed S100A8/A9-induced EMT, resulting in greatly weakened tumor growth and lung metastasis. Thus, this report suggested that S100A8/A9 contributes to these signaling processes through CD146.177
Since metastasis accounts for the majority of cancer-associated deaths, studies on metastasis mechanisms are needed to establish innovative strategies for cancer treatments. These findings that CD146, as a novel receptor for S100A8/A9, mediates the transition of malignant cancers to metastatic sites, suggest that strategies modulating the interaction between CD146 and S100A8/A9 may be useful for interference with cancer metastasis, especially in the progression of pre-metastatic tumors to the lungs.
Matriptase
Matriptase is an epithelial-specific membrane-anchored serine protease that proteolytically degrades targets, such as ECM components and the pro-forms of growth factors.183,184,185,186 Because most of solid tumors are originated from epithelia, matriptase is thus critically involved in cancer invasive growth through degradation events related to breaching the basement membrane, reorganization of the ECM, and activation of oncogenic signaling pathways.187
During neurogenesis, matriptase, expressed on neural stem/progenitor (NS/P), plays a critical role in cell-contact signaling between NS/P and brain endothelial cells.188 In 2017, the direct binding between brain endothelial CD146 and NS/P matriptase, was identified to be involved in the direct endothelia-NS/P contact.189 Such binding can activate the downstream signaling cascades from CD146, including p38 and canonical Wnt/β-catenin pathways in endothelia, leading to secretion of cytokines and chemokines. These factors in turn act on NS/P cells to induce differentiation and migration for the adequate neurogenesis. Consistently, none of these signaling events occurred when either matriptase or CD146 is deleted. Thus, this study suggests that CD146, expressed on stromal cells in tumor microenvironment, plays essential roles in tumor invasion by interaction with matriptase expressed on invading cancer cells originated from epithelia.
Recent years, matriptase has received considerable attention in the field of cancer research, because it is upregulated in many cancers and is required for the degradation of the ECM and the maturation of a variety of oncogenic pro-growth factors.184 Mice with reduced levels of matriptase display a significant delay in oncogene-induced mammary tumor formation and growth.190,191 Therefore, interference of the interaction between CD146 on stromal cells and matriptase on cancers is a reasonable strategy to turn off the signaling pathway and invasive responses in cancers, especially epithelia-originated neoplasms.
CD146 is the co-receptor of pro-angiogenic factor receptors
Angiogenesis refers to the physiological process by which new blood vessels are formed from preexisting blood vessels. This highly ordered process relies on extensive signaling networks both among and within endothelial cells, their associated mural cells (vascular smooth muscle cells and pericytes) and other cell types (e.g., immune cells). VEGF-A is the principal mediator of angiogenesis and contributes to the formation of a pioneering tip cells of angiogenic sprouts.192 With further vessel maturation, endothelial cells-secreted platelet-derived growth factor (PDGF)-B is the major player for recruitment of adjacent pericytes and vascular smooth muscle cells to the endothelial surface.193,194 We found that CD146 can directly interact with VEGFR253 and PDGFR-β195 to promote tumor angiogenesis and cerebrovascular development, respectively (Fig. 2).
CD146 as the co-receptor for VEGFR2 in tumor angiogenesis
There are five members of the human VEGF family: VEGF-A, -B, -C, -D and placental growth factor (PlGF).196,197 VEGF-A is the best characterized family member and the most potent stimulator of angiogenic processes.192 VEGF-A has at least nine different splicing forms with different binding affinities within its receptors and ECM components.198 VEGF-A is secreted by many cell types, such as endothelial cells,199,200 fibroblasts,201 smooth muscle cells,202 platelets,203 neutrophils,204 macrophages, and ~60% of all tumors.205 VEGF-A secretion is induced by ischemia and inflammatory stimuli.206 Cellular responses to VEGF-A are mainly driven by their binding to its cognate receptors—the VEGFRs.
VEGFRs belong to the class IV receptor tyrosine kinase family and are expressed by endothelial cells, macrophages, hematopoietic cells and smooth muscle cells.207,208,209,210 In humans, there are three VEGFR subtypes, which are encoded by separate genes: VEGFR1 (FLT1), VEGFR2 (KDR), and VEGFR3 (FLT4). Among them, VEGFR1 and VEGFR2 show high structural similarity.198,211 In endothelial cells, VEGFR2, but not VEGFR1, mediates the full range of VEGF responses.212,213
VEGFR2 activation leads to distinct activation patterns, including proliferation via mitogen activated protein kinase (MAPK),214 cell migration via phosphoinositide-specific phospholipase C (PLC)-γ215 and focal adhesion kinase (FAK),216,217 and cell survival through phosphatidylinositol-4,5-bisphosphate 3-kinase (PI3K)/AKT.218 In addition, VEGFR2 interaction with its co-receptors is essential for its functions. Till now, the well-established VEGFR2 co-receptors include Neuropilin-1, CD44, vascular endothelial cadherin, and β integrins.219 Although the exact molecular mechanisms of how VEGFR2 induces diverging downstream signals have not been completely understood, ligand diversity and availability as well as interactions with co-receptors might explain most of these effects.
Similar to the functions of VEGFR2 in endothelia, CD146 also contributes to endothelial cell proliferation, migration, and angiogenesis.220,221,222 Different from VEGFR2 with kinase activity, CD146 does not show any kinase activity. But similarly to VEGFR2, CD146 also can activate numerous signaling pathways, known as the downstream cascades of VEGFR2, such as focal adhesion formation mediated by FAK,14 cell migration mediated by PLC-γ,14 cell survival through PI3K/AKT,223 proliferation via MAPK and NF-κB.224
In 2012, we found that CD146 interacts with VEGFR2 on endothelia and acts as a co-receptor.53 This interaction occurs in the extracellular protein domain of CD146 independently of VEGF-A. When CD146 was inhibited using the blocking antibody AA98 or CD146 siRNA, VEGF-A induced phosphorylation of VEGFR2 was suppressed in human umbilical vein endothelial cells (HUVECs), demonstrating that the interplay of CD146 with VEGFR2 is mandatory for functional VEGFR2 signaling.
In addition to the extracellular binding of CD146 to VEGFR2, intracellular CD146 signaling is also required for VEGF-A-induced signal transduction via VEGFR2. When associated with VEGFR2, the cytoplasmic tail of CD146 recruited ERM proteins and the actin cytoskeleton, to assemble a “signalosome,” which is required for signal transduction from VEGFR2 to AKT and P38 MAPK. Inhibition of CD146 by blocking antibody AA98 hinders the interaction between VEGFR2 and CD146, resulting in abrogation of the downstream cascade of p38 MAPK and AKT signaling. Therefore, our study revealed that the interaction of CD146 with VEGFR2 improves pro-angiogenesis signaling as part of such a signalosome in which CD146 binding to VEGFR2 enables the reorganization of the cytoskeletal network during angiogenesis.
Using a preclinical pancreas carcinoma xenograft model, we found that the efficacy of combined treatment with the anti-VEGF antibody Bevacizumab and the anti-CD146 antibody AA98 was significantly higher than treatment with either one of the two agents. The clinical benefit from VEGF-targeted therapies is always compromised during continued treatment with anti-VEGF antibodies, such as Bevacizumab in pancreatic carcinoma.110,225,226 Thus, our findings indicate the possibility that the clinical efficacy of anti-VEGF therapy can be augmented by targeting CD146 treatment in the future.
Several mechanisms by which tumor angiogenesis may proceed in the presence of anti-VEGF have been identified. For example, pro-inflammatory cytokines and Galectin-1 produced from tumors activate VEGF-like pro-angiogenic pathways.101,227 Based on our findings, we speculate that the interaction between CD146 and VEGFR2 might also underlie the mechanisms of anti-VEGF treatment failure. Thus, in the future, investigating the interaction network of Galectin-1, CD146, and VEGFR2 will be necessary for a better understanding of the mechanisms underlying the angiogenesis and metastasis of carcinoma.
CD146 as the co-receptor of PDGFR-β in vessel integrity
PDGF family members, including PDGF-A, -B, -C, and -D, play a crucial role in embryologic development based on the fact that all PDGF knockout mice are embryonic lethal. Similar to VEGFs, all PDGFs are dimers of disulfide-linked polypeptide chains.228 In the mouse embryo, PDGF-B is strongly expressed in the developing vascular endothelium,229 tip cells of angiogenic sprouts, and in the endothelia of growing arteries, where pericytes are actively recruited.230,231 During angiogenesis, PDGF-BB released from endothelial cells promotes the recruitment of adjacent pericytes to the endothelial surface.193,194
Both PDGFs and their receptors (PDGFR-α and -β) are implicated in tumor angiogenesis and lymphangiogenesis.232,233,234,235 PDGF-AA and PDGF-CC mainly bind to PDGFR-α, whereas PDGF-BB binds to PDGFR-β.236 PDGFR-β is expressed in the mesenchyme, particularly in vascular smooth muscle cells and pericytes.230,237 PDGF-BB/PDGFR-β signaling elicits several well-characterized signaling cascades, such as Ras-MAPK, PI3K, and PLC-γ. The PDGFR-β-Ras-MAPK cascade leads to the stimulation of cell proliferation, differentiation, and migration.238,239 The PDGFR-β-PI3K branch promotes cell growth and cytoskeletal reorganizations.240 PDGFR-β-PLC-γ activates intracellular Ca2+ mobilization, stimulating Ca2+-dependent secondary signaling, such as tube formation and cell motility.241,242 In addition, in adult mice, PDGF-BB/PDGFR-β signaling exerts a neuroprotective function by the recruitment of pericytes.243
In 2017, we revealed that CD146 controls pericyte recruitment and vessel stabilization through interactions with PDGFR-β to protect the CNS.59 First, we extensively examined the expression patterns of CD146 in brain endothelial cells and pericytes throughout development and adulthood. In mouse brains, CD146 is first expressed in the cerebrovascular endothelial cells of premature blood vessels without pericyte coverage; with increased coverage of pericytes, CD146 could not be detected in cerebrovascular endothelial cells, it could only be found in pericytes. Furthermore, we studied the role of CD146 in endothelial-pericyte communication through its selective deletion in both cell types. We found that a knockdown in either cell type leads to the breakdown of the blood-brain barrier (BBB), which initiates the invasion of the endothelial cells toward the CNS and the recruitment of pericytes to the nascent vessels during embryogenesis. Furthermore, we demonstrated that CD146 functions as a co-receptor of PDGFR-β to mediate pericyte recruitment to cerebrovascular endothelial cells, indicating that pericyte CD146 was important for pericyte recruitment and vessel stabilization. The attached pericytes in turn downregulate endothelial CD146 by secreting TGF-β1 to promote further BBB maturation.
Because both CD146 and PDGFR-β are involved in regulation of growth and survival of different cell types, including cancer cells, further investigation of functions of CD146 and PDGFR-β interaction in cancers may help deeply understand the dysregulation of spatio-temporally controlled PDGF-BB induced signaling during tumor development and progression, especially tumor angiogenesis and lymphangiogenesis.
CD146 is the receptor for growth factors
Interestingly, all the ligands identified by our laboratory are well-known growth factors and mitogens, which include Wnt5a, Wnt1, Netrin-1, FGF4, and VEGF-C. These ligands and their cognate receptors are not only involved in almost all cellular processes required for embryogenesis, development, and adult life, but also effective targets for numerous anticancer treatments (Fig. 2).
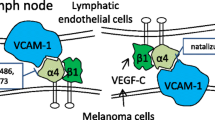
CD146 as the receptor of VEGF-C in lymphangiogenesis
VEGF-C belongs to the VEGF family.197,244 Different from VEGF-A in angiogenesis, VEGF-C is the principal driver of lymphangiogenesis and controls the whole process of lymphangiogenesis.245,246,247 Genetic studies in mice have revealed that loss of VEGF-C impairs the development of the lymphatic vasculature.247,248 On the other hand, overexpression of VEGF-C in specific tissues induces in situ lymphangiogenesis, such as in the skin,249,250 pancreas,251 and lung.252
Full-length VEGF-C undergoes proteolytic processing and becomes a fully processed form, which increases its affinity for VEGFR2 and VEGFR3.253,254 VEGF-C regulation of proliferation and migration of lymphatic endothelial cells is mainly through binding with VEGFR3.255,256,257 VEGF-C/VEGFR3 activates the ERK-MAPK and PI3K/AKT pathways and enhances diverse physiological effects in lymphatic endothelial cells, such as growth, proliferation, mobility, and invasiveness. Therefore, the signaling axis of VEGF-C/VEGFR3 is under extremely sophisticated control.258,259,260 However, the receptors that mediate VEGF-C signal transduction for lymphatic sprouting, the initiating step of lymphangiogenesis, remain elusive.
In 2017, we found that VEGF-C is also the ligand of CD146 and the binding site exists between D4 and D5 domains of the extracellular domain of CD146.57 VEGF-C activates CD146 to mediate sprouting during lymphangiogenesis, although the exact amino acid residues in the intracellular domains of CD146 responsible for transmitting VEGF-C signals still need to be defined. Using a zebrafish model, we discovered why CD146 is required for the lymphatic sprouting during development. Knockdown of CD146 inhibited phosphorylation of p38 and ERK, while knockdown of VEGFR3 inhibited phosphorylation of AKT and ERK. Furthermore, we confirmed that inhibition of p38 mainly reduced sprouting of lymphatic endothelial cells during lymphangiogenesis.
Maladjustment of VEGF-C-elicited signaling results in tumor metastasis, especially via lymphatic vessels.261,262,263,264,265 Lymphatic metastasis is a challenge for clinical treatment of tumors and is the cause of death for some cancers,266,267 such as breast cancer,268 lung cancer,269 and melanoma.270 Soluble VEGFR3,271,272 VEGF-C inhibitor,273 VEGFR3 antibodies,274 and VEGF-C siRNA275 have been used in the treatment of lymphatic metastasis. Our findings suggest that targeting CD146 may also be an effective therapeutic strategy to treat lymphatic metastasis.
CD146 as the receptor of Wnt5a in cell migration
The Wnt family includes several secreted glycoproteins that are involved in the regulation of a wide variety of normal and pathological processes, including embryogenesis, differentiation, and tumorigenesis.276 In human, there are 19 wnt genes that encode functionally distinct Wnt proteins, which can bind to their receptors, FZD/LRP heterodimers. Wnt signaling pathways include canonical and non-canonical cascades. The canonical pathway causes stabilization and nuclear translocation of β-catenin, which regulates transcription of Wnt target genes. The non-canonical pathway is β-catenin-independent and can be further divided into Wnt/planar cell polarity (PCP), Wnt/Ror2, and Wnt/Ca2+ signaling cascades.277 The PCP pathway is activated by c-Jun N-terminal kinases (JNKs).278
Wnt5a transmits signals through either canonical or non-canonical Wnt pathway.279,280 In 2013, we found that CD146 is the receptor of Wnt5a and is required for the Wnt5a-controlled cell migration and convergent extension during zebrafish embryogenesis.54 The biochemical experiments revealed that CD146 binds to Wnt5a with the high affinity, leading to activation of JNK-PCP pathway and downregulation of β-catenin expression.54 Further analysis demonstrated that CD146 can interact with Dvl2, and this interaction is enhanced under Wnt5a treatment. Accordingly, knockout of CD146 results in dysregulation of the Wnt/PCP pathway. Thus, our findings provide the first direct evidence that CD146 turns on the non-canonical Wnt signaling branch as a functional Wnt5a receptor in cell migration during development.
Wnt5a is upregulated in various types of human cancers.281,282 Meanwhile, Wnt5a activation of JNK is linked with cytoskeletal remodeling and cell motility in various cell systems.283,284,285 For example, in melanoma, Wnt5a is thought to directly affect cell motility and metastasis.286 In this view, CD146 may represent the prime target to develop more effective and less toxic therapies toward Wnt5a/CD146/JNK activation for meeting the challenges from tumor metastasis.
CD146 as the receptor of Wnt1 in fibroblast activation
Wnt1, originally known as oncogene int-1, was initially discovered by analysis of host cell sequences adjacent to viral integration sites in tumors of mice infected with mouse mammary tumor virus.287,288 Subsequent evidence suggests that the oncogenic functions of Wnt1 is mediated via upregulation of proliferative genes by canonical β-catenin pathway.
Similar with CD146, Wnt1 protein expression levels are high at developmental stage and low in adults, and ectopic expression of Wnt1 causes tumor development.289 In 2018, we found that CD146 can directly bind with Wnt1 in fibroblast, activating fibroblast via canonical Wnt/β-catenin pathway. Such interaction is essential for Wnt1-induced fibroblast proliferation and ECM production.58
Because cancer-associated fibroblasts (CAFs), as the main cellular constituent of the cancer-associated stroma, can drive cancer cell invasion but can also impair the migration and activation of T cells. Herein, researchers should further examine whether CD146 is a marker of CAFs, and if so, inhibiting the interaction of CD146 with Wnt1 on CAFs may benefit cancer treatment.
CD146 as the receptor of Netrin-1 in angiogenesis
Netrin-1 belongs to a family of Laminin related secreted proteins that control axonal and cellular migration during the development of the nervous system.290,291,292,293 Netrin-1 is a 640 amino acid protein and its carboxy-terminal sequence is enriched in basic amino acids.294,295,296 This sequence can bind HSPGs with high affinity, contributing to retaining them in the ECM.297 Netrin-1 is not only expressed in the nervous system but also in other non-neuronal organs.290,291,298 It regulates diverse processes, such as neuronal navigation, cellular adhesion, motility, proliferation, and differentiation during development.299,300,301,302 Dysregulation of Netrin-1 is involved in diverse pathological processes, such as cancer, cardiovascular disease, and kidney disease, making it an attractive potential therapeutic target.301,303,304
Netrin-1 acts through two main receptors, DCC (deleted in colorectal cancer) and UNC5B (uncoordinated-5 homolog), to alter the architecture of the cytoskeleton networks.305,306 Binding to its receptors activates chemotropic responses and adhesive mechanisms, regulating inflammation, angiogenesis, and apoptosis.299,301,302
Our laboratory clarified that netrin-1 binds CD146 with a higher affinity than the classical UNC5B receptor.55 Netrin-1 preferentially binds CD146 at low concentrations (50–200 ng/mL) and binds UNC5B at high concentrations (1000–2000 ng/mL). In addition, our study demonstrated the dual action of Netrin-1 on angiogenesis: the pro-angiogenic roles through CD146 and the antiangiogenic functions through UNC5B. CD146 silencing or deletion inhibits Netrin-1-induced HUVEC proliferation, migration, and tube formation, as well as VEGFR2, p38, and extracellular regulated MAP kinase (ERK)1/2 activation. In contrast, Netrin-1 binding with UNC5B at high concentrations triggers signals that counteract the CD146-mediated pro-angiogenic pathway. CD146 can also interact with VEGFR2 as a co-receptor;53 because of this, CD146-Netrin-1 binding may further improve VEGF-A signaling-mediated angiogenesis.
Thus, this finding highlights the importance of CD146 in Netrin-1-induced angiogenesis, although other factors are also likely contributors in the absence of CD146.303 Similarly to CD146, Netrin-1 also takes part in tumor growth, these properties of Netrin-1 in cancer offer reasonable therapeutic approaches. Therefore, preclinical and clinical studies should be planned to investigate the therapeutic potential of the Netrin-1/CD146 pathway in tumor angiogenesis.
CD146 as the receptor of FGF4 in apical-basal polarization
FGFs were initially recognized as fibroblast-specific growth factors, and it is now recognized that FGFs regulate multiple biological functions in diverse cell types beyond fibroblasts. FGFs constitute a large family with 23 members,307 which share sequences and structural similarities.308 These factors include paracrine or endocrine forms and participate in important pathophysiological processes such as cell proliferation, survival, migration, angiogenesis, wound healing, differentiation, and endocrine secretion regulation during development and adult life.309,310,311
Among FGFs, only FGF4 and eight have been revealed to possess the chemotactic activity and FGF4 acts as a chemo-attractant during morphogenesis.312 FGF4 belongs to the paracrine FGFs that signal FGFRs by forming a tripartite complex with FGFRs (1–4) and HSPGs.313 Although extensive investigations focus on the FGF signaling, there has been no indication that FGF ligands can bind with other receptors beyond FGFRs. Meanwhile, no direct evidence has indicated that any one of the FGFRs mediates the FGF4-elicited chemo-attracting activities.
The chief intracellular substrates of FGF signaling are FGF receptor substrate 2 (FRS2) and PLC-γ. Activated FRS2 activates the transcription factors of activator protein 1 (AP-1) and forkhead box protein O (FOXO) through RAS–ERK or the PI3K-pyruvate dehydrogenase kinase pathway, respectively, whereas PLCγ leads to the activation of Ca2+-dependent nuclear factor of activated T cells (NFAT).313 There are several points where other pathways can cross-talk with FGF signaling, for instance, activated JNK is required for AP-1 activation and FOXO nuclear translocation.314
In 2017, we found that CD146 is an independent receptor of FGF4.56 The binding affinity between CD146 and FGF4 is higher than that between FGF4 with its receptors (FGFR1-4). In addition, the presence of HSPG is not required for the binding of FGF4 to CD146. Using zebrafish as the model system, we found that CD146, but not FGFR, is the responsive receptor for FGF4. It provided local spatial cues to organize apical-basal polarity in participating cells during morphogenesis. An in vitro lumen formation assay further confirmed the migration of CD146, but not FGFR1, toward FGF4 as the key activity during FGF4-induced establishment of the apical-basal polarity. By investigating the effects of CD146 on FGF signaling output, we found that the cooperative actions between CD146-dependent activation of JNK and NFAT together with FGF signaling-dependent activation of ERK ensure that CD146+ cells concomitantly upregulate the transcriptional activity of AP-1 and NFAT during organ morphogenesis.
Thus, our findings suggest the essential roles of CD146 in FGF4-elicited morphogenetic signaling. In light of the coincident spatiotemporal distribution of CD146/FGF4 on developmental embryos, CD146 may be a more preferable receptor in FGF4 biological responses than FGFRs. The cooperation of CD146/FGF4 in cell polarity establishment suggests that CD146 could be the genuine responsive receptor in all of FGF4-executed chemo-attracting actions. Because FGF4 possesses the potent pro-angiogenetic activity and fails to bind heparan sulfate in the heart and blood vessels,315 it is possible that CD146-FGF4 is also a critical partner in angiogenesis. For this reason, in the therapeutic regimes of pathological angiogenesis, simultaneous targeting of both CD146 and FGF4 could have better efficacy than settings with that singular target either.
Conclusions
Overall, CD146 expression is frequently increased in fast-proliferating cells, such as cells in their developmental stages and cancer cells.21 From the functional perspective of CD146 as a CAM, upregulation of CD146 expression enhances the interactions between CD146 and its ligands in the ECM, including Laminin 411, Laminin 421, Galectin-1, Galectin-3, S100A8/A9, and matriptase, shifting the balance between cell–cell and cell-matrix adhesion while increased secretion of pro-metastatic cytokines causes cells to invade their surrounding ECM, spread via the vascular or lymphatic circulation, and extravasate into distant organs. As a co-receptor of VEGFR2 and PDGFR-β, or an independent receptor of growth factors of Wnt5a, Wnt1, Netrin-1, FGF4, and VEGF-C, highly expressed CD146 can enhance pro-angiogenesis signaling and promotes cell growth, proliferation, differentiation, and survival. Therefore, these novel scenarios highlight the importance of CD146 in proliferating cells and facilitate a better understanding of the mechanisms and implications of the interactions between CD146 with its ligands in invasive growth, proliferation, and motility of cancer cells.
It has been reported that there are three blocking antibodies to membrane CD146; ABX-MA1 raised by Bar-Eli’s laboratory, AA98 raised by our laboratory, and TsCD146 raised by Blot-Chabaud’s laboratory. They exhibit powerful inhibitory activity on membrane CD146’s function. ABX-MA1 can effectively decrease cell–cell adhesion and cell invasion in vitro, as well as decrease primary tumor growth and lung metastases in vivo.316,317,318 AA98 displays prevailing inhibitory activity on cancer progression in various models of tumor bearing mice.181,319,320,321,322 TsCD146 can specifically recognize and internalize cancer CD146 without interfering with physiological CD146 on vessels, suggesting great potential in tumor diagnostic and/or therapeutic applications.28 Because of this, CD146-targeted immunotherapy has promising therapeutic value in tumor treatment because manifold dose regimens of the antibodies could be administered to the patients without increasing the immune reaction.
Immunotherapy, including immune checkpoint blockades, has revolutionized the field of cancer therapy in the last decades. However, due to the inability of T cells to access the tumor microenvironment, there are still a substantial number of patients do not benefit from current forms of immunotherapy. Given the various roles of CD146 in the remodeling tumor microenvironment, immunotherapy against CD146 could provide a possibility for overcoming this impediment. Metastasis accounts for the majority of cancer-associated deaths, therefore establishing innovative strategies for modulating CD146 and ligand interactions are needed for cancer treatment in the future.
References
Lehmann, J. M. et al. Discrimination between benign and malignant cells of melanocytic lineage by two novel antigens, a glycoprotein with a molecular weight of 113,000 and a protein with a molecular weight of 76,000. Cancer Res. 47, 841–845 (1987).
Lehmann, J. M., Riethmuller, G. & Johnson, J. P. Muc18, a marker of tumor progression in human-melanoma, shows sequence similarity to the neural cell-adhesion molecules of the immunoglobulin superfamily. Proc. Natl. Acad. Sci. USA 86, 9891–9895 (1989).
Shi, I. M. The role of CD146 (Mel-CAM) in biology and pathology. J. Pathol. 189, 4–11 (1999).
Trzpis, M., McLaughlin, P. M. J., De Leij, L. M. F. H. & Harmsen, M. C. Epithelial cell adhesion molecule. Am. J. Pathol. 171, 386–395 (2007).
Aplin, A. E., Howe, A. K. & Juliano, R. L. Cell adhesion molecules, signal transduction and cell growth. Curr. Opin. Cell Biol. 11, 737–744 (2000).
Zabouo, G. et al. CD146 expression is associated with a poor prognosis in human breast tumors and with enhanced motility in breast cancer cell lines. Breast Cancer Res. 11, R1 (2009).
McGary, E. C., Lev, D. C. & Bar-Eli, M. Cellular adhesion pathways and metastatic potential of human melanoma. Cancer Biol. Ther. 1, 459–465 (2002).
Juliano, R. L. Signal transduction by cell adhesion receptors and the cytoskeleton: functions of integrins, cadherins, selectins, and immunoglobulin-superfamily members. Annu Rev. Pharmacol. Toxicol. 42, 283–323 (2002).
Korthuis, R. J., Anderson, D. C. & Granger, D. N. Role of neutrophil-endothelial cell adhesion in inflammatory disorders. J. Crit. Care 9, 47–71 (1994).
Albelda, S. M. Role of integrins and other cell adhesion molecules in tumor progression and metastasis. Lab Investig. 68, 4–17 (1993).
Bardin, N. et al. Soluble CD146, a novel endothelial marker, is increased in physiopathological settings linked to endothelial junctional alteration. Thromb. Haemost. 90, 915–920 (2003).
Bardin, N., Frances, V., Combes, V., Sampol, J. & Dignat-George, F. CD146: biosynthesis and production of a soluble form in human cultured endothelial cells. FEBS Lett. 421, 12–14 (1998).
Bardin, N. et al. CD146 and its soluble form regulate monocyte transendothelial migration. Arterioscler Thromb. Vasc. Biol. 29, 746–753 (2009).
Anfosso, F. et al. Activation of human endothelial cells via S-endo-1 antigen (CD146) stimulates the tyrosine phosphorylation of focal adhesion kinase p125(FAK). J. Biol. Chem. 273, 26852–26856 (1998).
Wang, D. et al. Soluble CD146, a cerebrospinal fluid marker for neuroinflammation, promotes blood-brain barrier dysfunction. Theranostics 10, 231–246 (2020).
Kaspi, E. et al. Identification of CD146 as a novel molecular actor involved in systemic sclerosis. J. Allergy Clin. Immunol. 140, 1448–1451 e1446 (2017).
Johnson, J. P., Rothbacher, U. & Sers, C. The progression associated antigen MUC18: a unique member of the immunoglobulin supergene family. Melanoma Res. 3, 337–340 (1993).
Dye, D. E. et al. hShroom1 links a membrane bound protein to the actin cytoskeleton. Cell Mol. Life Sci. 66, 681–696 (2009).
Stalin, J. et al. Therapeutic targeting of soluble CD146/MCAM with the M2J-1 monoclonal antibody prevents metastasis development and procoagulant activity in CD146-positive invasive tumors. Int. J. Cancer, https://doi.org/10.1002/ijc.32909 (2020).
Bu, P. et al. Visualization of CD146 dimerization and its regulation in living cells. Biochim. Biophys. Acta 1773, 513–520 (2007).
Wang, Z. & Yan, X. CD146, a multi-functional molecule beyond adhesion. Cancer Lett. 330, 150–162 (2013).
Stowell, S. R. et al. Ligand reduces galectin-1 sensitivity to oxidative inactivation by enhancing dimer formation. J. Biol. Chem. 284, 4989–4999 (2009).
Vainio, O. et al. HEMCAM, an adhesion molecule expressed by c-kit+ hemopoietic progenitors. J. Cell Biol. 135, 1655–1668 (1996).
Gbormittah, F. O., Lee, L. Y., Taylor, K., Hancock, W. S. & Iliopoulos, O. Comparative studies of the proteome, glycoproteome, and N-glycome of clear cell renal cell carcinoma plasma before and after curative nephrectomy. J. Proteome Res. 13, 4889–4900 (2014).
Hubbard, S. C., Boyce, M., McVaugh, C. T., Peehl, D. M. & Bertozzi, C. R. Cell surface glycoproteomic analysis of prostate cancer-derived PC-3 cells. Bioorg. Med. Chem. Lett. 21, 4945–4950 (2011).
Sumardika, I. W. et al. beta-1,3-Galactosyl-O-Glycosyl-Glycoprotein beta-1,6-N-Acetylglucosaminyltransferase 3 increases Mcam Stability, Which Enhances S100A8/A9-mediated cancer motility. Oncol. Res. 26, 431–444 (2018).
Jouve, N. et al. CD146 mediates VEGF-induced melanoma cell extravasation through FAK activation. Int J. Cancer 137, 50–60 (2015).
Nollet, M. et al. A novel anti-CD146 antibody specifically targets cancer cells by internalizing the molecule. Oncotarget 8, 112283–112296 (2017).
Luca, M. et al. Direct correlation between MUC18 expression and metastatic potential of human melanoma cells. Melanoma Res. 3, 35–41 (1993).
Zeng, Q. Q. et al. CD146, an epithelial-mesenchymal transition inducer, is associated with triple-negative breast cancer. Proc. Natl. Acad. Sci. USA 109, 1127–1132 (2012).
Li, W. D. et al. Increased expression of CD146 and microvessel density (MVD) in invasive micropapillary carcinoma of the breast: Comparative study with invasive ductal carcinoma-not otherwise specified. Pathol. Res. Pract. 207, 739–746 (2011).
Wu, G. J. & Dickerson, E. B. Frequent and increased expression of human METCAM/MUC18 in cancer tissues and metastatic lesions is associated with the clinical progression of human ovarian carcinoma. Taiwan J. Obstet. Gynecol. 53, 509–517 (2014).
Ma, X. et al. Targeting CD146 in combination with vorinostat for the treatment of ovarian cancer cells. Oncol. Lett. 13, 1681–1687 (2017).
Onisim, A., Vlad, C., Simon, I., Dina, C. & Achimas Cadariu, P. The role of CD146 in serous ovarian carcinoma. J. Buon 24, 1009–1019 (2019).
Zhou, P. et al. Clinical significance of melanoma cell adhesion molecule CD146 and VEGFA expression in epithelial ovarian cancer. Oncol. Lett. 17, 2418–2424 (2019).
Kristiansen, G. et al. Expression of the cell adhesion molecule CD146/MCAM in non-small cell lung cancer. Anal. Cell Pathol. 25, 77–81 (2003).
Zhang, X. et al. MCAM expression is associated with poor prognosis in non-small cell lung cancer. Clin. Transl. Oncol. 16, 178–183 (2014).
Fritzsche, F. R. et al. CD146 protein in prostate cancer: revisited with two different antibodies. Pathology 40, 457–464 (2008).
Liu, J. W. et al. Hypermethylation of MCAM gene is associated with advanced tumor stage in prostate cancer. Prostate 68, 418–426 (2008).
Wu, G. J., Wu, M. W., Wang, C. & Liu, Y. Enforced expression of METCAM/MUC18 increases tumorigenesis of human prostate cancer LNCaP cells in nude mice. J. Urol. 185, 1504–1512 (2011).
Yang, Y. et al. Targeting CD146 with a 64Cu-labeled antibody enables in vivo immunoPET imaging of high-grade gliomas. Proc. Natl. Acad. Sci. USA 112, E6525–E6534 (2015).
Feng, G. et al. CD146 gene expression in clear cell renal cell carcinoma: a potential marker for prediction of early recurrence after nephrectomy. Int. Urol. Nephrol. 44, 1663–1669 (2012).
Jiang, G. et al. CD146 promotes metastasis and predicts poor prognosis of hepatocellular carcinoma. J. Exp. Clin. Cancer Res. 35, 38 (2016).
Hernandez, R. et al. CD146-targeted immunoPET and NIRF imaging of hepatocellular carcinoma with a dual-labeled monoclonal antibody. Theranostics 6, 1918–1933 (2016).
Liu, W. F. et al. CD146 expression correlates with epithelial-mesenchymal transition markers and a poor prognosis in gastric cancer. Int J. Mol. Sci. 13, 6399–6406 (2012).
Johnson, J. P., Bar-Eli, M., Jansen, B. & Markhof, E. Melanoma progression-associated glycoprotein MUC18/MCAM mediates homotypic cell adhesion through interaction with a heterophilic ligand. Int J. Cancer 73, 769–774 (1997).
Guezguez, B. et al. Dual role of melanoma cell adhesion molecule (MCAM)/CD146 in lymphocyte endothelium interaction: MCAM/CD146 promotes rolling via microvilli induction in lymphocyte and is an endothelial adhesion receptor. J. Immunol. 179, 6673–6685 (2007).
Staquicini, F. I. et al. A subset of host B lymphocytes controls melanoma metastasis through a melanoma cell adhesion molecule/MUC18-dependent interaction: evidence from mice and humans. Cancer Res. 68, 8419–8428 (2008).
Taniura, H., Kuo, C. H., Hayashi, Y. & Miki, N. Purification and characterization of an 82-kD membrane protein as a neurite outgrowth factor binding protein: possible involvement of NOF binding protein in axonal outgrowth in developing retina. J. Cell Biol. 112, 313–322 (1991).
Taira, E., Takaha, N., Taniura, H., Kim, C. H. & Miki, N. Molecular cloning and functional expression of gicerin, a novel cell adhesion molecule that binds to neurite outgrowth factor. Neuron 12, 861–872 (1994).
Flanagan, K. et al. Laminin-411 is a vascular ligand for MCAM and facilitates TH17 cell entry into the CNS. PLoS ONE 7, e40443 (2012).
Ishikawa, T. et al. Laminins 411 and 421 differentially promote tumor cell migration via alpha6beta1 integrin and MCAM (CD146). Matrix Biol. 38, 69–83 (2014).
Jiang, T. et al. CD146 is a coreceptor for VEGFR-2 in tumor angiogenesis. Blood 120, 2330–2339 (2012).
Ye, Z. et al. Wnt5a uses CD146 as a receptor to regulate cell motility and convergent extension. Nat. Commun. 4, 2803 (2013).
Tu, T. et al. CD146 acts as a novel receptor for netrin-1 in promoting angiogenesis and vascular development. Cell Res. 25, 275–287 (2015).
Gao, Q. et al. The signalling receptor MCAM coordinates apical-basal polarity and planar cell polarity during morphogenesis. Nat. Commun. 8, 15279 (2017).
Yan, H. et al. CD146 is required for VEGF-C-induced lymphatic sprouting during lymphangiogenesis. Sci. Rep. 7, 7442 (2017).
Zhang, L. et al. CD146: a potential therapeutic target for systemic sclerosis. Protein Cell 9, 1050–1054 (2018).
Chen, J. et al. CD146 coordinates brain endothelial cell-pericyte communication for blood-brain barrier development. Proc. Natl. Acad. Sci. USA 114, E7622–E7631 (2017).
Al-Mehdi, A. B. et al. Intravascular origin of metastasis from the proliferation of endothelium-attached tumor cells: a new model for metastasis. Nat. Med. 6, 100–102 (2000).
Aumailley, M. et al. A simplified laminin nomenclature. Matrix Biol. 24, 326–332 (2005).
Miner, J. H. & Yurchenco, P. D. Laminin functions in tissue morphogenesis. Annu. Rev. Cell Dev. Biol. 20, 255–284 (2004).
Durbeej, M. Laminins Cell Tissue Res. 339, 259–268 (2010).
Wang, J. et al. Cardiomyopathy associated with microcirculation dysfunction in laminin alpha4 chain-deficient mice. J. Biol. Chem. 281, 213–220 (2006).
Thyboll, J. et al. Deletion of the laminin alpha4 chain leads to impaired microvessel maturation. Mol. Cell Biol. 22, 1194–1202 (2002).
Wallquist, W. et al. Impeded interaction between Schwann cells and axons in the absence of laminin alpha4. J. Neurosci. 25, 3692–3700 (2005).
Fujiwara, H., Gu, J. & Sekiguchi, K. Rac regulates integrin-mediated endothelial cell adhesion and migration on laminin-8. Exp. Cell Res. 292, 67–77 (2004).
Fujiwara, H., Kikkawa, Y., Sanzen, N. & Sekiguchi, K. Purification and characterization of human laminin-8. Laminin-8 stimulates cell adhesion and migration through alpha3beta1 and alpha6beta1 integrins. J. Biol. Chem. 276, 17550–17558 (2001).
Vainionpaa, N., Lehto, V. P., Tryggvason, K. & Virtanen, I. Alpha4 chain laminins are widely expressed in renal cell carcinomas and have a de-adhesive function. Lab Investig. 87, 780–791 (2007).
Kawataki, T. et al. Laminin isoforms and their integrin receptors in glioma cell migration and invasiveness: Evidence for a role of alpha5-laminin(s) and alpha3beta1 integrin. Exp. Cell Res. 313, 3819–3831 (2007).
Takkunen, M. et al. Epithelial-mesenchymal transition downregulates laminin alpha5 chain and upregulates laminin alpha4 chain in oral squamous carcinoma cells. Histochem. Cell Biol. 130, 509–525 (2008).
Oikawa, Y. et al. Melanoma cells produce multiple laminin isoforms and strongly migrate on alpha5 laminin(s) via several integrin receptors. Exp. Cell Res. 317, 1119–1133 (2011).
Petajaniemi, N. et al. Localization of laminin alpha4-chain in developing and adult human tissues. J. Histochem. Cytochem. 50, 1113–1130 (2002).
Franz, M. et al. Stromal laminin chain distribution in normal, hyperplastic and malignant oral mucosa: relation to myofibroblast occurrence and vessel formation. J. Oral. Pathol. Med. 39, 290–298 (2010).
Pedraza, C. et al. Monocytic cells synthesize, adhere to, and migrate on laminin-8 (alpha 4 beta 1 gamma 1). J. Immunol. 165, 5831–5838 (2000).
Lian, J., Dai, X., Li, X. & He, F. Identification of an active site on the laminin alpha4 chain globular domain that binds to alphavbeta3 integrin and promotes angiogenesis. Biochem. Biophys. Res. Commun. 347, 248–253 (2006).
Li, J. et al. Overexpression of laminin-8 in human dermal microvascular endothelial cells promotes angiogenesis-related functions. J. Investig. Dermatol. 126, 432–440 (2006).
Geberhiwot, T. et al. Blood platelets contain and secrete laminin-8 (alpha4beta1gamma1) and adhere to laminin-8 via alpha6beta1 integrin. Exp. Cell Res. 253, 723–732 (1999).
Kortesmaa, J., Doi, M., Patarroyo, M. & Tryggvason, K. Chondroitin sulphate modification in the alpha4 chain of human recombinant laminin-8 (alpha4beta1gamma1). Matrix Biol. 21, 483–486 (2002).
Kortesmaa, J., Yurchenco, P. & Tryggvason, K. Recombinant laminin-8 (alpha(4)beta(1)gamma(1)). Production, purification,and interactions with integrins. J. Biol. Chem. 275, 14853–14859 (2000).
Kato, T. et al. Expression of IL-17 mRNA in ovarian cancer. Biochem. Biophys. Res. Commun. 282, 735–738 (2001).
Kryczek, I., Wei, S., Szeliga, W., Vatan, L. & Zou, W. Endogenous IL-17 contributes to reduced tumor growth and metastasis. Blood 114, 357–359 (2009).
Lugassy, C. et al. Pericytic-like angiotropism of glioma and melanoma cells. Am. J. Dermatopathol. 24, 473–478 (2002).
Hansen, K. & Abrass, C. K. Laminin-8/9 is synthesized by rat glomerular mesangial cells and is required for PDGF-induced mesangial cell migration. Kidney Int. 64, 110–118 (2003).
Berfield, A. K., Hansen, K. M. & Abrass, C. K. Rat glomerular mesangial cells require laminin-9 to migrate in response to insulin-like growth factor binding protein-5. Am. J. Physiol. Cell Physiol. 291, C589–C599 (2006).
Saito, N. et al. Laminin-421 produced by lymphatic endothelial cells induces chemotaxis for human melanoma cells. Pigment Cell Melanoma Res. 22, 601–610 (2009).
Ishikawa, T. et al. Monoclonal antibodies to human laminin alpha4 chain globular domain inhibit tumor cell adhesion and migration on laminins 411 and 421, and binding of alpha6beta1 integrin and MCAM to alpha4-laminins. Matrix Biol. 36, 5–14 (2014).
Shih, I. M. The role of CD146 (Mel-CAM) in biology and pathology. J. Pathol. 189, 4–11 (1999).
Thijssen, V. L., Rabinovich, G. A. & Griffioen, A. W. Vascular galectins: regulators of tumor progression and targets for cancer therapy. Cytokine Growth Factor Rev. 24, 547–558 (2013).
Chou, F. C., Chen, H. Y., Kuo, C. C. & Sytwu, H. K. Role of galectins in tumors and in clinical immunotherapy. Int. J. Mol. Sci. 19, https://doi.org/10.3390/ijms19020430 (2018).
Barondes, S. H. et al. Galectins: a family of animal beta-galactoside-binding lectins. Cell 76, 597–598 (1994).
Barondes, S. H., Cooper, D. N., Gitt, M. A. & Leffler, H. Galectins. Structure and function of a large family of animal lectins. J. Biol. Chem. 269, 20807–20810 (1994).
Luo, W. et al. Identification of galectin-1 as a novel mediator for chemoresistance in chronic myeloid leukemia cells. Oncotarget 7, 26709–26723 (2016).
Rubinstein, N. et al. Targeted inhibition of galectin-1 gene expression in tumor cells results in heightened T cell-mediated rejection; a potential mechanism of tumor-immune privilege. Cancer Cell 5, 241–251 (2004).
Gordon-Alonso, M., Hirsch, T., Wildmann, C. & van der Bruggen, P. Galectin-3 captures interferon-gamma in the tumor matrix reducing chemokine gradient production and T-cell tumor infiltration. Nat. Commun. 8, 793 (2017).
Paz, H. et al. Treatment of B-cell precursor acute lymphoblastic leukemia with the Galectin-1 inhibitor PTX008. J. Exp. Clin. Cancer Res. 37, 67 (2018).
Leung, Z. et al. Galectin-1 promotes hepatocellular carcinoma and the combined therapeutic effect of OTX008 galectin-1 inhibitor and sorafenib in tumor cells. J. Exp. Clin. Cancer Res. 38, 423 (2019).
Harrison, S. A. et al. Randomised clinical study: GR-MD-02, a galectin-3 inhibitor, vs. placebo in patients having non-alcoholic steatohepatitis with advanced fibrosis. Aliment Pharmacol. Ther. 44, 1183–1198 (2016).
Capalbo, C., Scafetta, G., Filetti, M., Marchetti, P. & Bartolazzi, A. Predictive Biomarkers for Checkpoint Inhibitor-Based Immunotherapy: The Galectin-3 Signature in NSCLCs. Int. J. Mol. Sci. 20, https://doi.org/10.3390/ijms20071607 (2019).
Stowell, S. R. et al. Galectin-1, -2, and -3 exhibit differential recognition of sialylated glycans and blood group antigens. J. Biol. Chem. 283, 10109–10123 (2008).
Croci, D. O. et al. Glycosylation-dependent lectin-receptor interactions preserve angiogenesis in anti-VEGF refractory tumors. Cell 156, 744–758 (2014).
Hsieh, S. H. et al. Galectin-1, a novel ligand of neuropilin-1, activates VEGFR-2 signaling and modulates the migration of vascular endothelial cells. Oncogene 27, 3746–3753 (2008).
Fischer, I. et al. Inhibiton of RET and JAK2 signals and upregulation of VEGFR3 phosphorylation in vitro by galectin-1 in trophoblast tumor cells BeWo. Placenta 30, 1078–1082 (2009).
Laderach, D. J. et al. A unique galectin signature in human prostate cancer progression suggests galectin-1 as a key target for treatment of advanced disease. Cancer Res. 73, 86–96 (2013).
Croci, D. O. et al. Disrupting galectin-1 interactions with N-glycans suppresses hypoxia-driven angiogenesis and tumorigenesis in Kaposi’s sarcoma. J. Exp. Med. 209, 1985–2000 (2012).
Burns, J. S. et al. Decellularized matrix from tumorigenic human mesenchymal stem cells promotes neovascularization with galectin-1 dependent endothelial interaction. PLoS ONE 6, e21888 (2011).
Jouve, N. et al. The involvement of CD146 and its novel ligand Galectin-1 in apoptotic regulation of endothelial cells. J. Biol. Chem. 288, 2571–2579 (2013).
Yazawa, E. M. et al. Melanoma cell Galectin-1 ligands functionally correlate with malignant potential. J. Investig. Dermatol 135, 1849–1862 (2015).
Camby, I., Le Mercier, M., Lefranc, F. & Kiss, R. Galectin-1: a small protein with major functions. Glycobiology 16, 137R–157R (2006).
Welti, J., Loges, S., Dimmeler, S. & Carmeliet, P. Recent molecular discoveries in angiogenesis and antiangiogenic therapies in cancer. J. Clin. Investig. 123, 3190–3200 (2013).
Kaltner, H. & Gabius, H. J. A toolbox of lectins for translating the sugar code: the galectin network in phylogenesis and tumors. Histol. Histopathol. 27, 397–416 (2012).
Smetana, K. Jr., Andre, S., Kaltner, H., Kopitz, J. & Gabius, H. J. Context-dependent multifunctionality of galectin-1: a challenge for defining the lectin as therapeutic target. Expert Opin. Ther. Targets 17, 379–392 (2013).
Nakahara, S., Oka, N. & Raz, A. On the role of galectin-3 in cancer apoptosis. Apoptosis 10, 267–275 (2005).
Zubieta, M. R. et al. Galectin-3 expression correlates with apoptosis of tumor-associated lymphocytes in human melanoma biopsies. Am. J. Pathol. 168, 1666–1675 (2006).
Liu, F. T. & Rabinovich, G. A. Galectins as modulators of tumour progression. Nat. Rev. Cancer 5, 29–41 (2005).
Newlaczyl, A. U. & Yu, L. G. Galectin-3-a jack-of-all-trades in cancer. Cancer Lett. 313, 123–128 (2011).
Zhao, Q. et al. Circulating galectin-3 promotes metastasis by modifying MUC1 localization on cancer cell surface. Cancer Res. 69, 6799–6806 (2009).
Yu, L. G. et al. Galectin-3 interaction with Thomsen-Friedenreich disaccharide on cancer-associated MUC1 causes increased cancer cell endothelial adhesion. J. Biol. Chem. 282, 773–781 (2007).
Sindrewicz, P., Lian, L. Y. & Yu, L. G. Interaction of the Oncofetal Thomsen-Friedenreich antigen with galectins in cancer progression and metastasis. Front. Oncol. 6, 79 (2016).
Taylor-Papadimitriou, J., Burchell, J., Miles, D. W. & Dalziel, M. MUC1 and cancer. Biochim. Biophys. Acta 1455, 301–313 (1999).
Barrow, H. et al. Serum galectin-2, -4, and -8 are greatly increased in colon and breast cancer patients and promote cancer cell adhesion to blood vascular endothelium. Clin. Cancer Res. 17, 7035–7046 (2011).
Colomb, F. et al. Galectin-3 interacts with the cell-surface glycoprotein CD146 (MCAM, MUC18) and induces secretion of metastasis-promoting cytokines from vascular endothelial cells. J. Biol. Chem. 292, 8381–8389 (2017).
Zhang, Z., Zheng, Y., Wang, H., Zhou, Y. & Tai, G. CD146 interacts with galectin-3 to mediate endothelial cell migration. FEBS Lett. 592, 1817–1828 (2018).
Zhang, Z. et al. NMR-based insight into galectin-3 binding to endothelial cell adhesion molecule CD146: evidence for noncanonical interactions with the lectin’s CRD beta-sandwich F-face. Glycobiology 29, 608–618 (2019).
Griffioen, A. W. & Molema, G. Angiogenesis: potentials for pharmacologic intervention in the treatment of cancer, cardiovascular diseases, and chronic inflammation. Pharmacol. Rev. 52, 237–268 (2000).
Folkman, J., Merler, E., Abernathy, C. & Williams, G. Isolation of a tumor factor responsible for angiogenesis. J. Exp. Med. 133, 275–288 (1971).
Hanahan, D. & Folkman, J. Patterns and emerging mechanisms of the angiogenic switch during tumorigenesis. Cell 86, 353–364 (1996).
Thijssen, V. L. et al. Galectin-1 is essential in tumor angiogenesis and is a target for antiangiogenesis therapy. Proc. Natl. Acad. Sci. USA 103, 15975–15980 (2006).
Nangia-Makker, P. et al. Galectin-3 induces endothelial cell morphogenesis and angiogenesis. Am. J. Pathol. 156, 899–909 (2000).
Funasaka, T., Raz, A. & Nangia-Makker, P. Galectin-3 in angiogenesis and metastasis. Glycobiology 24, 886–891 (2014).
Jia, W., Kidoya, H., Yamakawa, D., Naito, H. & Takakura, N. Galectin-3 accelerates M2 macrophage infiltration and angiogenesis in tumors. Am. J. Pathol. 182, 1821–1831 (2013).
Zimmer, D. B., Eubanks, J. O., Ramakrishnan, D. & Criscitiello, M. F. Evolution of the S100 family of calcium sensor proteins. Cell Calcium 53, 170–179 (2013).
Moore, B. W. A soluble protein characteristic of the nervous system. Biochem. Biophys. Res. Commun. 19, 739–744 (1965).
Donato, R. et al. Functions of S100 proteins. Curr. Mol. Med. 13, 24–57 (2013).
Ryckman, C., Vandal, K., Rouleau, P., Talbot, M. & Tessier, P. A. Proinflammatory activities of S100: proteins S100A8, S100A9, and S100A8/A9 induce neutrophil chemotaxis and adhesion. J. Immunol. 170, 3233–3242 (2003).
Marenholz, I., Heizmann, C. W. & Fritz, G. S100 proteins in mouse and man: from evolution to function and pathology (including an update of the nomenclature). Biochem. Biophys. Res. Commun. 322, 1111–1122 (2004).
Yan, W. X. et al. Mast cell and monocyte recruitment by S100A12 and its hinge domain. J. Biol. Chem. 283, 13035–13043 (2008).
Balkwill, F. & Mantovani, A. Inflammation and cancer: back to Virchow? Lancet 357, 539–545 (2001).
Ruegg, C. Leukocytes, inflammation, and angiogenesis in cancer: fatal attractions. J. Leukoc. Biol. 80, 682–684 (2006).
Ott, H. W. et al. Calgranulins in cystic fluid and serum from patients with ovarian carcinomas. Cancer Res. 63, 7507–7514 (2003).
Gebhardt, C. et al. Calgranulins S100A8 and S100A9 are negatively regulated by glucocorticoids in a c-Fos-dependent manner and overexpressed throughout skin carcinogenesis. Oncogene 21, 4266–4276 (2002).
Koch, M. et al. Structural basis for ligand recognition and activation of RAGE. Structure 18, 1342–1352 (2010).
Park, H., Adsit, F. G. & Boyington, J. C. The 1.5 A crystal structure of human receptor for advanced glycation endproducts (RAGE) ectodomains reveals unique features determining ligand binding. J. Biol. Chem. 285, 40762–40770 (2010).
Yatime, L. et al. The Structure of the RAGE:S100A6 Complex Reveals a Unique Mode of Homodimerization for S100 Proteins. Structure 24, 2043–2052 (2016).
Penumutchu, S. R., Chou, R. H. & Yu, C. Structural insights into calcium-bound S100P and the V domain of the RAGE complex. PLoS ONE 9, e103947 (2014).
Xie, J. et al. Hexameric calgranulin C (S100A12) binds to the receptor for advanced glycated end products (RAGE) using symmetric hydrophobic target-binding patches. J. Biol. Chem. 282, 4218–4231 (2007).
Ehrchen, J. M., Sunderkotter, C., Foell, D., Vogl, T. & Roth, J. The endogenous Toll-like receptor 4 agonist S100A8/S100A9 (calprotectin) as innate amplifier of infection, autoimmunity, and cancer. J. Leukoc. Biol. 86, 557–566 (2009).
Tondera, C., Laube, M. & Pietzsch, J. Insights into binding of S100 proteins to scavenger receptors: class B scavenger receptor CD36 binds S100A12 with high affinity. Amino Acids 49, 183–191 (2017).
Riuzzi, F., Sorci, G. & Donato, R. S100B protein regulates myoblast proliferation and differentiation by activating FGFR1 in a bFGF-dependent manner. J. Cell Sci. 124, 2389–2400 (2011).
von Bauer, R. et al. CD166/ALCAM mediates proinflammatory effects of S100B in delayed type hypersensitivity. J. Immunol. 191, 369–377 (2013).
Okada, K. et al. CD68 on rat macrophages binds tightly to S100A8 and S100A9 and helps to regulate the cells’ immune functions. J. Leukoc. Biol. 100, 1093–1104 (2016).
Pankratova, S. et al. The S100A4 Protein Signals through the ErbB4 Receptor to Promote Neuronal Survival. Theranostics 8, 3977–3990 (2018).
Foell, D., Frosch, M., Sorg, C. & Roth, J. Phagocyte-specific calcium-binding S100 proteins as clinical laboratory markers of inflammation. Clin. Chim. Acta 344, 37–51 (2004).
Thorey, I. S. et al. The Ca2+-binding proteins S100A8 and S100A9 are encoded by novel injury-regulated genes. J. Biol. Chem. 276, 35818–35825 (2001).
Katz, A. B. & Taichman, L. B. A partial catalog of proteins secreted by epidermal keratinocytes in culture. J. Investig. Dermatol. 112, 818–821 (1999).
Gebhardt, C., Nemeth, J., Angel, P. & Hess, J. S100A8 and S100A9 in inflammation and cancer. Biochem Pharmacol. 72, 1622–1631 (2006).
Salama, I., Malone, P. S., Mihaimeed, F. & Jones, J. L. A review of the S100 proteins in cancer. Eur. J. Surg. Oncol. 34, 357–364 (2008).
Kerkhoff, C. et al. Novel insights into the role of S100A8/A9 in skin biology. Exp. Dermatol 21, 822–826 (2012).
Greten, F. R. et al. IKKbeta links inflammation and tumorigenesis in a mouse model of colitis-associated cancer. Cell 118, 285–296 (2004).
Acharyya, S. et al. A CXCL1 paracrine network links cancer chemoresistance and metastasis. Cell 150, 165–178 (2012).
Cheng, P. et al. Inhibition of dendritic cell differentiation and accumulation of myeloid-derived suppressor cells in cancer is regulated by S100A9 protein. J. Exp. Med. 205, 2235–2249 (2008).
Hiratsuka, S., Watanabe, A., Aburatani, H. & Maru, Y. Tumour-mediated upregulation of chemoattractants and recruitment of myeloid cells predetermines lung metastasis. Nat. Cell Biol. 8, 1369–1375 (2006).
Ichikawa, M., Williams, R., Wang, L., Vogl, T. & Srikrishna, G. S100A8/A9 activate key genes and pathways in colon tumor progression. Mol. Cancer Res. 9, 133–148 (2011).
Hauschild, A. et al. S100B protein detection in serum is a significant prognostic factor in metastatic melanoma. Oncology 56, 338–344 (1999).
Laouedj, M. et al. S100A9 induces differentiation of acute myeloid leukemia cells through TLR4. Blood 129, 1980–1990 (2017).
Miller, P. et al. Elevated S100A8 protein expression in breast cancer cells and breast tumor stroma is prognostic of poor disease outcome. Breast Cancer Res. Treat. 166, 85–94 (2017).
Tidehag, V. et al. High density of S100A9 positive inflammatory cells in prostate cancer stroma is associated with poor outcome. Eur. J. Cancer 50, 1829–1835 (2014).
Arai, K. et al. S100A8 and S100A9 overexpression is associated with poor pathological parameters in invasive ductal carcinoma of the breast. Curr. Cancer Drug Targets 8, 243–252 (2008).
Robinson, M. J., Tessier, P., Poulsom, R. & Hogg, N. The S100 family heterodimer, MRP-8/14, binds with high affinity to heparin and heparan sulfate glycosaminoglycans on endothelial cells. J. Biol. Chem. 277, 3658–3665 (2002).
Srikrishna, G. et al. Two proteins modulating transendothelial migration of leukocytes recognize novel carboxylated glycans on endothelial cells. J. Immunol. 166, 4678–4688 (2001).
Vogl, T. et al. Mrp8 and Mrp14 are endogenous activators of Toll-like receptor 4, promoting lethal, endotoxin-induced shock. Nat. Med. 13, 1042–1049 (2007).
Turovskaya, O. et al. RAGE, carboxylated glycans and S100A8/A9 play essential roles in colitis-associated carcinogenesis. Carcinogenesis 29, 2035–2043 (2008).
Ghavami, S. et al. S100A8/A9 at low concentration promotes tumor cell growth via RAGE ligation and MAP kinase-dependent pathway. J. Leukoc. Biol. 83, 1484–1492 (2008).
Hiratsuka, S. et al. The S100A8-serum amyloid A3-TLR4 paracrine cascade establishes a pre-metastatic phase. Nat. Cell Biol. 10, 1349–1355 (2008).
Ruma, I. M. et al. MCAM, as a novel receptor for S100A8/A9, mediates progression of malignant melanoma through prominent activation of NF-kappaB and ROS formation upon ligand binding. Clin. Exp. Metastasis 33, 609–627 (2016).
Chen, Y. et al. Melanoma cell adhesion molecule is the driving force behind the dissemination of melanoma upon S100A8/A9 binding in the original skin lesion. Cancer Lett. 452, 178–190 (2019).
Chen, Y. et al. Critical role of the MCAM-ETV4 axis triggered by extracellular S100A8/A9 in breast cancer aggressiveness. Neoplasia 21, 627–640 (2019).
Kinoshita, R. et al. exSSSRs (extracellular S100 soil sensor receptors)-Fc fusion proteins work as prominent decoys to S100A8/A9-induced lung tropic cancer metastasis. Int. J. Cancer 144, 3138–3145 (2019).
Paget, S. The distribution of secondary growths in cancer of the breast. Lancet 133, 571–573 (1889).
Zeng, Q. et al. Quantitative proteomics reveals ER-alpha involvement in CD146-induced epithelial-mesenchymal transition in breast cancer cells. J. Proteom. 103, 153–169 (2014).
Zeng, Q. et al. CD146, an epithelial-mesenchymal transition inducer, is associated with triple-negative breast cancer. Proc. Natl. Acad. Sci. USA 109, 1127–1132 (2012).
Mishra, R. et al. Semaphorin 3A upregulates FOXO 3a-dependent MelCAM expression leading to attenuation of breast tumor growth and angiogenesis. Oncogene 34, 1584–1595 (2015).
Ustach, C. V. et al. A novel signaling axis of matriptase/PDGF-D/ss-PDGFR in human prostate cancer. Cancer Res. 70, 9631–9640 (2010).
List, K. Matriptase: a culprit in cancer? Future Oncol. 5, 97–104 (2009).
Hurst, N. J. Jr, Najy, A. J., Ustach, C. V., Movilla, L. & Kim, H. R. Platelet-derived growth factor-C (PDGF-C) activation by serine proteases: implications for breast cancer progression. Biochem. J. 441, 909–918 (2012).
Szabo, R. & Bugge, T. H. Membrane-anchored serine proteases in vertebrate cell and developmental biology. Annu. Rev. Cell Dev. Biol. 27, 213–235 (2011).
Forbs, D. et al. In vitro inhibition of matriptase prevents invasive growth of cell lines of prostate and colon carcinoma. Int J. Oncol. 27, 1061–1070 (2005).
Tung, H. H. & Lee, S. L. Neural transmembrane protease and endothelial Gs protein activation in cell contact-dependent signaling between neural stem/progenitor cells and brain endothelial cells. J. Biol. Chem. 287, 22497–22508 (2012).
Tung, H. H. & Lee, S. L. Physical binding of endothelial MCAM and neural transmembrane protease matriptase-novel cell adhesion in neural stem cell vascular niche. Sci. Rep. 7, 4946 (2017).
Zoratti, G. L. et al. Targeting matriptase in breast cancer abrogates tumour progression via impairment of stromal-epithelial growth factor signalling. Nat. Commun. 6, 6776 (2015).
Lin, C.-Y., Chen, Y.-W., Xu, Z. & Johnson, M. D. in Handbook of Proteolytic Enzymes 3rd edn (eds Neil D. Rawlings & Guy Salvesen) Ch. 14, 2969–2975 (Academic Press, 2013).
Ferrara, N. VEGF and intraocular neovascularization: from discovery to therapy. Transl. Vis. Sci. Technol. 5, 10 (2016).
Sweeney, M. D., Ayyadurai, S. & Zlokovic, B. V. Pericytes of the neurovascular unit: key functions and signaling pathways. Nat. Neurosci. 19, 771–783 (2016).
Armulik, A., Genove, G. & Betsholtz, C. Pericytes: developmental, physiological, and pathological perspectives, problems, and promises. Dev. Cell 21, 193–215 (2011).
Chen, J. et al. CD146 is essential for PDGFRbeta-induced pericyte recruitment. Protein Cell 9, 743–747 (2018).
Ferrara, N. VEGF-A: a critical regulator of blood vessel growth. Eur. Cytokine Netw. 20, 158–163 (2009).
Hoeben, A. et al. Vascular endothelial growth factor and angiogenesis. Pharmacol. Rev. 56, 549–580 (2004).
Koch, S., Tugues, S., Li, X., Gualandi, L. & Claesson-Welsh, L. Signal transduction by vascular endothelial growth factor receptors. Biochem. J. 437, 169–183 (2011).
Uchida, K. et al. Glomerular endothelial cells in culture express and secrete vascular endothelial growth factor. Am. J. Physiol. 266, F81–F88 (1994).
Namiki, A. et al. Hypoxia induces vascular endothelial growth factor in cultured human endothelial cells. J. Biol. Chem. 270, 31189–31195 (1995).
Nissen, N. N. et al. Vascular endothelial growth factor mediates angiogenic activity during the proliferative phase of wound healing. Am. J. Pathol. 152, 1445–1452 (1998).
Brogi, E., Wu, T., Namiki, A. & Isner, J. M. Indirect angiogenic cytokines upregulate VEGF and bFGF gene expression in vascular smooth muscle cells, whereas hypoxia upregulates VEGF expression only. Circulation 90, 649–652 (1994).
Banks, R. E. et al. Release of the angiogenic cytokine vascular endothelial growth factor (VEGF) from platelets: significance for VEGF measurements and cancer biology. Br. J. Cancer 77, 956–964 (1998).
Gaudry, M. et al. Intracellular pool of vascular endothelial growth factor in human neutrophils. Blood 90, 4153–4161 (1997).
Berse, B., Brown, L. F., Van de Water, L., Dvorak, H. F. & Senger, D. R. Vascular permeability factor (vascular endothelial growth factor) gene is expressed differentially in normal tissues, macrophages, and tumors. Mol. Biol. Cell 3, 211–220 (1992).
Franco, M., Roswall, P., Cortez, E., Hanahan, D. & Pietras, K. Pericytes promote endothelial cell survival through induction of autocrine VEGF-A signaling and Bcl-w expression. Blood 118, 2906–2917 (2011).
Alexander, S. P. et al. The concise guide to PHARMACOLOGY 2015/16: catalytic receptors. Br. J. Pharm. 172, 5979–6023 (2015).
Witmer, A. N., Dai, J., Weich, H. A., Vrensen, G. F. & Schlingemann, R. O. Expression of vascular endothelial growth factor receptors 1, 2, and 3 in quiescent endothelia. J. Histochem. Cytochem. 50, 767–777 (2002).
Ishida, A. et al. Expression of vascular endothelial growth factor receptors in smooth muscle cells. J. Cell Physiol. 188, 359–368 (2001).
Kabrun, N. et al. Flk-1 expression defines a population of early embryonic hematopoietic precursors. Development 124, 2039–2048 (1997).
Smith, G. A., Fearnley, G. W., Tomlinson, D. C., Harrison, M. A. & Ponnambalam, S. The cellular response to vascular endothelial growth factors requires co-ordinated signal transduction, trafficking and proteolysis. Biosci. Rep 35, https://doi.org/10.1042/BSR20150171 (2015).
Meyer, R. D., Mohammadi, M. & Rahimi, N. A single amino acid substitution in the activation loop defines the decoy characteristic of VEGFR-1/FLT-1. J. Biol. Chem. 281, 867–875 (2006).
Waltenberger, J., Claesson-Welsh, L., Siegbahn, A., Shibuya, M. & Heldin, C. H. Different signal transduction properties of KDR and Flt1, two receptors for vascular endothelial growth factor. J. Biol. Chem. 269, 26988–26995 (1994).
Takahashi, T., Ueno, H. & Shibuya, M. VEGF activates protein kinase C-dependent, but Ras-independent Raf-MEK-MAP kinase pathway for DNA synthesis in primary endothelial cells. Oncogene 18, 2221–2230 (1999).
Takahashi, T., Yamaguchi, S., Chida, K. & Shibuya, M. A single autophosphorylation site on KDR/Flk-1 is essential for VEGF-A-dependent activation of PLC-gamma and DNA synthesis in vascular endothelial cells. EMBO J. 20, 2768–2778 (2001).
Abu-Ghazaleh, R., Kabir, J., Jia, H., Lobo, M. & Zachary, I. Src mediates stimulation by vascular endothelial growth factor of the phosphorylation of focal adhesion kinase at tyrosine 861, and migration and anti-apoptosis in endothelial cells. Biochem. J. 360, 255–264 (2001).
Chen, T. T. et al. Anchorage of VEGF to the extracellular matrix conveys differential signaling responses to endothelial cells. J. Cell Biol. 188, 595–609 (2010).
Gerber, H. P. et al. Vascular endothelial growth factor regulates endothelial cell survival through the phosphatidylinositol 3’-kinase/Akt signal transduction pathway. Requirement for Flk-1/KDR activation. J. Biol. Chem. 273, 30336–30343 (1998).
Orian-Rousseau, V. & Ponta, H. Adhesion proteins meet receptors: a common theme? Adv. Cancer Res. 101, 63–92 (2008).
Kang, Y. et al. Knockdown of CD146 reduces the migration and proliferation of human endothelial cells. Cell Res. 16, 313–318 (2006).
Bardin, N. et al. Identification of the S-Endo 1 endothelial-associated antigen. Biochem. Biophys. Res. Commun. 218, 210–216 (1996).
Bardin, N. et al. Identification of CD146 as a component of the endothelial junction involved in the control of cell-cell cohesion. Blood 98, 3677–3684 (2001).
Li, G. et al. Reciprocal regulation of MelCAM and AKT in human melanoma. Oncogene 22, 6891–6899 (2003).
Bu, P. et al. Anti-CD146 monoclonal antibody AA98 inhibits angiogenesis via suppression of nuclear factor-kappaB activation. Mol. Cancer Ther. 5, 2872–2878 (2006).
Kindler, H. L. et al. Gemcitabine plus bevacizumab compared with gemcitabine plus placebo in patients with advanced pancreatic cancer: phase III trial of the Cancer and Leukemia Group B (CALGB 80303). J. Clin. Oncol. 28, 3617–3622 (2010).
Abdollahi, A. & Folkman, J. Evading tumor evasion: current concepts and perspectives of anti-angiogenic cancer therapy. Drug Resist. Updates 13, 16–28 (2010).
Phan, V. T. et al. Oncogenic RAS pathway activation promotes resistance to anti-VEGF therapy through G-CSF-induced neutrophil recruitment. Proc. Natl. Acad. Sci. USA 110, 6079–6084 (2013).
Heldin, C. H. & Westermark, B. Mechanism of action and in vivo role of platelet-derived growth factor. Physiol. Rev. 79, 1283–1316 (1999).
Lindahl, P., Johansson, B. R., Leveen, P. & Betsholtz, C. Pericyte loss and microaneurysm formation in PDGF-B-deficient mice. Science 277, 242–245 (1997).
Hellstrom, M., Kalen, M., Lindahl, P., Abramsson, A. & Betsholtz, C. Role of PDGF-B and PDGFR-beta in recruitment of vascular smooth muscle cells and pericytes during embryonic blood vessel formation in the mouse. Development 126, 3047–3055 (1999).
Gerhardt, H. et al. VEGF guides angiogenic sprouting utilizing endothelial tip cell filopodia. J. Cell Biol. 161, 1163–1177 (2003).
Heldin, C. H. Targeting the PDGF signaling pathway in tumor treatment. Cell Commun. Signal 11, 97 (2013).
Onimaru, M. et al. VEGF-C regulates lymphangiogenesis and capillary stability by regulation of PDGF-B. Am. J. Physiol. Heart Circ. Physiol. 297, H1685–H1696 (2009).
Shang, Q. et al. Expression and purification of functional PDGF receptor beta. Biochem Biophys. Res. Commun. 489, 353–359 (2017).
Chung, S. W. et al. LHT7, a chemically modified heparin, inhibits multiple stages of angiogenesis by blocking VEGF, FGF2 and PDGF-B signaling pathways. Biomaterials 37, 271–278 (2015).
Klinghoffer, R. A., Hamilton, T. G., Hoch, R. & Soriano, P. An allelic series at the PDGFalphaR locus indicates unequal contributions of distinct signaling pathways during development. Dev. Cell 2, 103–113 (2002).
Nystrom, H. C. et al. Platelet-derived growth factor B retention is essential for development of normal structure and function of conduit vessels and capillaries. Cardiovasc. Res. 71, 557–565 (2006).
Bar-Sagi, D. & Feramisco, J. R. Induction of membrane ruffling and fluid-phase pinocytosis in quiescent fibroblasts by ras proteins. Science 233, 1061–1068 (1986).
Seger, R. & Krebs, E. G. The MAPK signaling cascade. FASEB J. 9, 726–735 (1995).
Hu, Q., Klippel, A., Muslin, A. J., Fantl, W. J. & Williams, L. T. Ras-dependent induction of cellular responses by constitutively active phosphatidylinositol-3 kinase. Science 268, 100–102 (1995).
Berridge, M. J. Inositol trisphosphate and calcium signalling mechanisms. Biochim. Biophys. Acta 1793, 933–940 (2009).
Kundra, V. et al. Regulation of chemotaxis by the platelet-derived growth factor receptor-beta. Nature 367, 474–476 (1994).
Egawa-Tsuzuki, T. et al. The PDGF B-chain is involved in the ontogenic susceptibility of the developing rat brain to NMDA toxicity. Exp. Neurol. 186, 89–98 (2004).
Ferrara, N. Vascular endothelial growth factor. Arterioscler Thromb. Vasc. Biol. 29, 789–791 (2009).
Folkman, J. & Kaipainen, A. Genes tell lymphatics to sprout or not. Nat. Immunol. 5, 11–12 (2004).
Jussila, L. & Alitalo, K. Vascular growth factors and lymphangiogenesis. Physiol. Rev. 82, 673–700 (2002).
Karkkainen, M. J. et al. Vascular endothelial growth factor C is required for sprouting of the first lymphatic vessels from embryonic veins. Nat. Immunol. 5, 74–80 (2004).
Dellinger, M. T., Hunter, R. J., Bernas, M. J., Witte, M. H. & Erickson, R. P. Chy-3 mice are Vegfc haploinsufficient and exhibit defective dermal superficial to deep lymphatic transition and dermal lymphatic hypoplasia. Dev. Dyn. 236, 2346–2355 (2007).
Jeltsch, M. et al. Hyperplasia of lymphatic vessels in VEGF-C transgenic mice. Science 276, 1423–1425 (1997).
Lohela, M., Helotera, H., Haiko, P., Dumont, D. J. & Alitalo, K. Transgenic induction of vascular endothelial growth factor-C is strongly angiogenic in mouse embryos but leads to persistent lymphatic hyperplasia in adult tissues. Am. J. Pathol. 173, 1891–1901 (2008).
Mandriota, S. J. et al. Vascular endothelial growth factor-C-mediated lymphangiogenesis promotes tumour metastasis. EMBO J. 20, 672–682 (2001).
Yao, L. C. et al. Pulmonary lymphangiectasia resulting from vascular endothelial growth factor-C overexpression during a critical period. Circ. Res. 114, 806–822 (2014).
Joukov, V. et al. A novel vascular endothelial growth factor, VEGF-C, is a ligand for the Flt4 (VEGFR-3) and KDR (VEGFR-2) receptor tyrosine kinases. EMBO J. 15, 1751 (1996).
Joukov, V. et al. Proteolytic processing regulates receptor specificity and activity of VEGF-C. EMBO J. 16, 3898–3911 (1997).
Coso, S., Bovay, E. & Petrova, T. V. Pressing the right buttons: signaling in lymphangiogenesis. Blood 123, 2614–2624 (2014).
Fevurly, R. D., Hasso, S., Fye, A., Fishman, S. J. & Chan, J. Novel zebrafish model reveals a critical role for MAPK in lymphangiogenesis. J. Pediatr. Surg. 47, 177–182 (2012).
Salameh, A., Galvagni, F., Bardelli, M., Bussolino, F. & Oliviero, S. Direct recruitment of CRK and GRB2 to VEGFR-3 induces proliferation, migration, and survival of endothelial cells through the activation of ERK, AKT, and JNK pathways. Blood 106, 3423–3431 (2005).
Yu, J. et al. Slit2N and Robo4 regulate lymphangiogenesis through the VEGF-C/VEGFR-3 pathway. Cell Commun. Signal 12, 25 (2014).
Matsumura, K. et al. Modulation of VEGFR-2-mediated endothelial-cell activity by VEGF-C/VEGFR-3. Blood 101, 1367–1374 (2003).
Engelmann, D. et al. E2F1 promotes angiogenesis through the VEGF-C/VEGFR-3 axis in a feedback loop for cooperative induction of PDGF-B. J. Mol. Cell Biol. 5, 391–403 (2013).
Dias, S., Choy, M., Alitalo, K. & Rafii, S. Vascular endothelial growth factor (VEGF)-C signaling through FLT-4 (VEGFR-3) mediates leukemic cell proliferation, survival, and resistance to chemotherapy. Blood 99, 2179–2184 (2002).
Zhao, T. et al. VEGF-C/VEGFR-3 pathway promotes myocyte hypertrophy and survival in the infarcted myocardium. Am. J. Transl. Res. 7, 697–709 (2015).
Du, H. T. & Liu, P. Matrix metalloproteinase 14 participates in corneal lymphangiogenesis through the VEGF-C/VEGFR-3 signaling pathway. Exp. Ther. Med. 12, 2120–2128 (2016).
Su, J. L. et al. Further evidence for expression and function of the VEGF-C/VEGFR-3 axis in cancer cells. Cancer Cell 13, 557–560 (2008).
Su, J. L. et al. The role of the VEGF-C/VEGFR-3 axis in cancer progression. Br. J. Cancer 96, 541–545 (2007).
Alitalo, K., Tammela, T. & Petrova, T. V. Lymphangiogenesis in development and human disease. Nature 438, 946–953 (2005).
Stacker, S. A. et al. Lymphangiogenesis and lymphatic vessel remodelling in cancer. Nat. Rev. Cancer 14, 159–172 (2014).
Lee, E. et al. Breast cancer cells condition lymphatic endothelial cells within pre-metastatic niches to promote metastasis. Nat. Commun. 5, 4715 (2014).
min, Y. et al. C/EBP-delta regulates VEGF-C autocrine signaling in lymphangiogenesis and metastasis of lung cancer through HIF-1alpha. Oncogene 30, 4901–4909 (2011).
Christianson, D. R. et al. Ligand-directed targeting of lymphatic vessels uncovers mechanistic insights in melanoma metastasis. Proc. Natl. Acad. Sci. USA 112, 2521–2526 (2015).
He, Y. et al. Suppression of tumor lymphangiogenesis and lymph node metastasis by blocking vascular endothelial growth factor receptor 3 signaling. J. Natl. Cancer Inst. 94, 819–825 (2002).
Lin, J. et al. Inhibition of lymphogenous metastasis using adeno-associated virus-mediated gene transfer of a soluble VEGFR-3 decoy receptor. Cancer Res. 65, 6901–6909 (2005).
Shimizu, K. et al. Suppression of VEGFR-3 signaling inhibits lymph node metastasis in gastric cancer. Cancer Sci. 95, 328–333 (2004).
Roberts, N. et al. Inhibition of VEGFR-3 activation with the antagonistic antibody more potently suppresses lymph node and distant metastases than inactivation of VEGFR-2. Cancer Res. 66, 2650–2657 (2006).
Chen, Z. et al. Down-regulation of vascular endothelial cell growth factor-C expression using small interfering RNA vectors in mammary tumors inhibits tumor lymphangiogenesis and spontaneous metastasis and enhances survival. Cancer Res. 65, 9004–9011 (2005).
Nusse, R. & Clevers, H. Wnt/beta-catenin signaling, disease, and emerging therapeutic modalities. Cell 169, 985–999 (2017).
van Amerongen, R. Alternative Wnt pathways and receptors. Cold Spring Harb. Perspect. Biol. 4, https://doi.org/10.1101/cshperspect.a007914 (2012).
McNeill, H. & Woodgett, J. R. When pathways collide: collaboration and connivance among signalling proteins in development. Nat. Rev. Mol. Cell Biol. 11, 404–413 (2010).
Baarsma, H. A., Konigshoff, M. & Gosens, R. The WNT signaling pathway from ligand secretion to gene transcription: molecular mechanisms and pharmacological targets. Pharmacol. Ther. 138, 66–83 (2013).
Ishitani, T. et al. The TAK1-NLK mitogen-activated protein kinase cascade functions in the Wnt-5a/Ca(2+) pathway to antagonize Wnt/beta-catenin signaling. Mol. Cell Biol. 23, 131–139 (2003).
Mikels, A. J. & Nusse, R. Purified Wnt5a protein activates or inhibits beta-catenin-TCF signaling depending on receptor context. PLoS Biol. 4, e115 (2006).
Katoh, M. & Katoh, M. Comparative integromics on non-canonical WNT or planar cell polarity signaling molecules: transcriptional mechanism of PTK7 in colorectal cancer and that of SEMA6A in undifferentiated ES cells. Int J. Mol. Med. 20, 405–409 (2007).
Zhu, Y. et al. Dvl2-dependent activation of Daam1 and RhoA regulates Wnt5a-induced breast cancer cell migration. PLoS ONE 7, e37823 (2012).
Liu, J. et al. PI3K/Akt-dependent phosphorylation of GSK3beta and activation of RhoA regulate Wnt5a-induced gastric cancer cell migration. Cell Signal 25, 447–456 (2013).
Wang, C. et al. C-Jun N-terminal kinase (JNK) mediates Wnt5a-induced cell motility dependent or independent of RhoA pathway in human dental papilla cells. PLoS ONE 8, e69440 (2013).
Weeraratna, A. T. et al. Wnt5a signaling directly affects cell motility and invasion of metastatic melanoma. Cancer Cell 1, 279–288 (2002).
Nusse, R., van Ooyen, A., Cox, D., Fung, Y. K. & Varmus, H. Mode of proviral activation of a putative mammary oncogene (int-1) on mouse chromosome 15. Nature 307, 131–136 (1984).
Peters, G., Lee, A. E. & Dickson, C. Activation of cellular gene by mouse mammary tumour virus may occur early in mammary tumour development. Nature 309, 273–275 (1984).
Zhan, T., Rindtorff, N. & Boutros, M. Wnt signaling in cancer. Oncogene 36, 1461–1473 (2017).
Serafini, T. et al. Netrin-1 is required for commissural axon guidance in the developing vertebrate nervous system. Cell 87, 1001–1014 (1996).
Mehlen, P. & Furne, C. Netrin-1: when a neuronal guidance cue turns out to be a regulator of tumorigenesis. Cell Mol. Life Sci. 62, 2599–2616 (2005).
Mehlen, P. & Mazelin, L. The dependence receptors DCC and UNC5H as a link between neuronal guidance and survival. Biol. Cell 95, 425–436 (2003).
Rajasekharan, S. & Kennedy, T. E. The netrin protein family. Genome Biol. 10, 239 (2009).
Serafini, T. et al. The netrins define a family of axon outgrowth-promoting proteins homologous to C. elegans UNC-6. Cell 78, 409–424 (1994).
Wang, H., Copeland, N. G., Gilbert, D. J., Jenkins, N. A. & Tessier-Lavigne, M. Netrin-3, a mouse homolog of human NTN2L, is highly expressed in sensory ganglia and shows differential binding to netrin receptors. J. Neurosci. 19, 4938–4947 (1999).
Yin, Y., Sanes, J. R. & Miner, J. H. Identification and expression of mouse netrin-4. Mech. Dev. 96, 115–119 (2000).
de Wit, J. & Verhaagen, J. Proteoglycans as modulators of axon guidance cue function. Adv. Exp. Med. Biol. 600, 73–89 (2007).
Barallobre, M. J., Pascual, M., Del Rio, J. A. & Soriano, E. The Netrin family of guidance factors: emphasis on Netrin-1 signalling. Brain Res. Brain Res. Rev. 49, 22–47 (2005).
Llambi, F., Causeret, F., Bloch-Gallego, E. & Mehlen, P. Netrin-1 acts as a survival factor via its receptors UNC5H and DCC. EMBO J. 20, 2715–2722 (2001).
Ly, N. P. et al. Netrin-1 inhibits leukocyte migration in vitro and in vivo. Proc. Natl. Acad. Sci. USA 102, 14729–14734 (2005).
Mehlen, P. & Guenebeaud, C. Netrin-1 and its dependence receptors as original targets for cancer therapy. Curr. Opin. Oncol. 22, 46–54 (2010).
Ramesh, G., Berg, A. & Jayakumar, C. Plasma netrin-1 is a diagnostic biomarker of human cancers. Biomarkers 16, 172–180 (2011).
Layne, K., Ferro, A. & Passacquale, G. Netrin-1 as a novel therapeutic target in cardiovascular disease: to activate or inhibit? Cardiovasc. Res. 107, 410–419 (2015).
Ramesh, G., Kwon, O. & Ahn, K. Netrin-1: a novel universal biomarker of human kidney injury. Transpl. Proc. 42, 1519–1522 (2010).
Barclay, A. N. Membrane proteins with immunoglobulin-like domains-a master superfamily of interaction molecules. Semin. Immunol. 15, 215–223 (2003).
Moore, S. W., Tessier-Lavigne, M. & Kennedy, T. E. Netrins and their receptors. Adv. Exp. Med. Biol. 621, 17–31 (2007).
Imamura, T. Physiological functions and underlying mechanisms of fibroblast growth factor (FGF) family members: recent findings and implications for their pharmacological application. Biol. Pharm. Bull. 37, 1081–1089 (2014).
Ornitz, D. M. & Itoh, N. Fibroblast growth factors. Genome Biol. 2, REVIEWS3005 (2001).
Aimi, F. et al. Endothelial Rictor is crucial for midgestational development and sustained and extensive FGF2-induced neovascularization in the adult. Sci. Rep. 5, 17705 (2015).
Herriges, J. C. et al. FGF-regulated ETV transcription factors Control FGF-SHH feedback loop in lung branching. Dev. Cell 35, 322–332 (2015).
Fernandes-Freitas, I. & Owen, B. M. Metabolic roles of endocrine fibroblast growth factors. Curr. Opin. Pharmacol. 25, 30–35 (2015).
Yang, X., Dormann, D., Munsterberg, A. E. & Weijer, C. J. Cell movement patterns during gastrulation in the chick are controlled by positive and negative chemotaxis mediated by FGF4 and FGF8. Dev. Cell 3, 425–437 (2002).
Goetz, R. & Mohammadi, M. Exploring mechanisms of FGF signalling through the lens of structural biology. Nat. Rev. Mol. Cell Biol. 14, 166–180 (2013).
Hedrick, S. M., Hess Michelini, R., Doedens, A. L., Goldrath, A. W. & Stone, E. L. FOXO transcription factors throughout T cell biology. Nat. Rev. Immunol. 12, 649–661 (2012).
Allen, B. L., Filla, M. S. & Rapraeger, A. C. Role of heparan sulfate as a tissue-specific regulator of FGF-4 and FGF receptor recognition. J. Cell Biol. 155, 845–858 (2001).
Mendez, M. J. et al. Functional transplant of megabase human immunoglobulin loci recapitulates human antibody response in mice. Nat. Genet. 15, 146–156 (1997).
Mills, L. et al. Fully human antibodies to MCAM/MUC18 inhibit tumor growth and metastasis of human melanoma. Cancer Res. 62, 5106–5114 (2002).
McGary, E. C. et al. A fully human antimelanoma cellular adhesion molecule/MUC18 antibody inhibits spontaneous pulmonary metastasis of osteosarcoma cells in vivo. Clin. Cancer Res. 9, 6560–6566 (2003).
Lin, Y. et al. A novel antibody AA98 V(H)/L directed against CD146 efficiently inhibits angiogenesis. Anticancer Res. 27, 4219–4224 (2007).
Luo, Y. et al. Recognition of CD146 as an ERM-binding protein offers novel mechanisms for melanoma cell migration. Oncogene 31, 306–321 (2012).
Zheng, C. et al. Endothelial CD146 is required for in vitro tumor-induced angiogenesis: the role of a disulfide bond in signaling and dimerization. Int. J. Biochem. Cell Biol. 41, 2163–2172 (2009).
Zhuang, J. et al. NADPH oxidase 4 mediates reactive oxygen species induction of CD146 dimerization in VEGF signal transduction. Free Radic. Biol. Med. 49, 227–236 (2010).
Acknowledgements
This work was supported by the following grants: the National Key Technology Research and Development Program of the Ministry of Science and Technology of China (No. 2018ZX10101004002004), the National Natural Science Foundation of China (No. 31770793), and the Beijing Municipal Natural Science Foundation, China (No. 7192123).
Author information
Authors and Affiliations
Contributions
X.Y. conceived the idea for the review. Z.W. and Q.X. searched the literature, wrote the paper, and generated the figures. N.Z., X.D., and G.X. participated in writing the paper.
Corresponding authors
Ethics declarations
Competing interests
The authors declare no competing interests.
Rights and permissions
Open Access This article is licensed under a Creative Commons Attribution 4.0 International License, which permits use, sharing, adaptation, distribution and reproduction in any medium or format, as long as you give appropriate credit to the original author(s) and the source, provide a link to the Creative Commons license, and indicate if changes were made. The images or other third party material in this article are included in the article’s Creative Commons license, unless indicated otherwise in a credit line to the material. If material is not included in the article’s Creative Commons license and your intended use is not permitted by statutory regulation or exceeds the permitted use, you will need to obtain permission directly from the copyright holder. To view a copy of this license, visit http://creativecommons.org/licenses/by/4.0/.
About this article
Cite this article
Wang, Z., Xu, Q., Zhang, N. et al. CD146, from a melanoma cell adhesion molecule to a signaling receptor. Sig Transduct Target Ther 5, 148 (2020). https://doi.org/10.1038/s41392-020-00259-8
Received:
Revised:
Accepted:
Published:
DOI: https://doi.org/10.1038/s41392-020-00259-8
- Springer Nature Limited
This article is cited by
-
Exosomes multiplex profiling, a promising strategy for early diagnosis of laryngeal cancer
Journal of Translational Medicine (2024)
-
Unveiling heterogeneity in MSCs: exploring marker-based strategies for defining MSC subpopulations
Journal of Translational Medicine (2024)
-
CD146+CAFs promote progression of endometrial cancer by inducing angiogenesis and vasculogenic mimicry via IL-10/JAK1/STAT3 pathway
Cell Communication and Signaling (2024)
-
Mcam stabilizes a luminal progenitor-like breast cancer cell state via Ck2 control and Src/Akt/Stat3 attenuation
npj Breast Cancer (2024)
-
Mcam inhibits macrophage-mediated development of mammary gland through non-canonical Wnt signaling
Nature Communications (2024)