Abstract
This study was intended to investigate the macular vascular and photoreceptor changes for diabetic macular edema (DME) at the early stage. A total of 255 eyes of 134 diabetes mellitus patients were enrolled and underwent an ophthalmological and systemic evaluation in this cross-sectional study. Early DME was characterized by central subfoveal thickness (CST) value between 250 and 325 μm, intact ellipsoid zone, and an external limiting membrane. While non-DME was characterized by CST < 250 μm with normal retinal morphology and structure. Foveal avascular zone (FAZ) area ≤ 0.3 mm2 (P < 0.001, OR = 0.41, 95% CI 0.26–0.67 in the multivariate analysis) and HbA1c level ≤ 8% (P = 0.005, OR = 0.37, 95% CI 0.19–0.74 in multivariate analysis) were significantly associated with a higher risk of early DME. Meanwhile, no significant differences exist in cone parameters between non-DME and early DME eyes. Compared with non-DME eyes, vessel diameter, vessel wall thickness, wall-to-lumen ratio, the cross-sectional area of the vascular wall in the upper side were significantly decreased in the early DME eyes (P = 0.001, P < 0.001, P = 0.005, P = 0.003 respectively). This study suggested a vasospasm or vasoconstriction with limited further photoreceptor impairment at the early stage of DME formation. CST ≥ 250 μm and FAZ ≤ 0.3 mm2 may be the indicator for early DME detection.
Similar content being viewed by others
Explore related subjects
Discover the latest articles, news and stories from top researchers in related subjects.Introduction
The global population with diabetes mellitus (DM) was estimated to be 463 million in 2019 and 700 million in 20451. Among which, 34.6% individuals suffered from diabetic retinopathy (DR) and 6.8% experienced diabetic macular edema (DME)2. DR has been the leading cause of preventable blindness in adult population at working age3,4. DME is one of the major causes of vision impairment in DR patients5. Therefore, timely detection and early intervention of DME is of great importance.
Optical coherence tomography (OCT) is a safe, non-invasive, convenient method for DME screening. Central subfoveal thickness (CST), provided by OCT, is suggested as the most reliable objective measure of DME6. Recently, a grading system was established to show the progression and severity of DME. Based on spectral domain optical coherence tomography (SD-OCT) results, DME is classified into four stages: early DME (small intraretinal cysts, well-recognizable and detectable inner retinal layers, intact ellipsoid zone and external limiting membrane, and increase in CST ≤ 30% of upper normal values), advanced DME (macrocysts and/or multiple intraretinal cystoid spaces and CST > 30% of maximum normal values), severe DME (advanced DME characteristics with mostly undetactable inner retinal segmentation and/or ellipsoid zone and/or the external limiting membrane), and atrophic maculopathy (complete disruption of the outermost retinal layers and disorganization of the inner retinal layers)7.
Previous studies indicated that retinal thickness values are different using different OCT devices8,9. Using Zeiss stratus OCT (Carl Zeiss Meditec, Dublin, California, USA), mean CST was 209 ± 18 μm for man and 194 ± 23 μm for women10. Previous studies frequently defined CST values over 250 μm as an indicative of macular edema8,10,11,12. Using Heidelberg Spectralis OCT (Heidelberg Engineering, Inc., Heidelberg, Germany), averaged CST was 278 ± 23 μm for man and 262 ± 22 μm for women. CST values ≥ 320 μm for man and 305 μm for women are proposed as DME13. Therefore, early DME could be defined as CST value between 250 and 325 μm on Zeiss stratus OCT; 320–415 μm for men and 305–397 μm for women on Heidelberg Spectralis OCT8,10,11,14.
Optical coherence tomography angiography (OCTA) is an objective, non-invasive, convenient method to visualize microvasculature without intravenous dye injection. It provides depth-resolved information about morphology, structure, vessel density, perfusion, microangiopathy of retinal and capillary vessels. Multiple details can be quantified using OCTA images, including vessel density (VD), the size of foveal avascular zone (FAZ), number of microaneurysms and intraretinal hyperreflective foci (iHRF), integrity of retinal pigment epithelium (RPE) and ellipsoid zone (EZ). Previous studies used these quantitative OCTA metrics to indicate the severity of DR and prognosticate the development of DME6,15,16,17,18,19. Recent studies laid emphasis on OCTA metric changes in clinically significant macular edema (CSME) patients post anti-VEGF treatment20,21. Most researchers focused on advanced and severe DME with retinal thickness exceeding 300 μm, rather than early DME, because early DME typically presents with small or no observable cysts, and early DME patients show no obvious clinical symptoms22.
The adaptive optics (AO) scanning laser ophthalmoscope (AOSLO) is an advanced non-invasive method to visualize microcirculation and retinal structures like cone cells. In 2023, Kupis et al.23 demonstrated significant differences in cone density, mosaic arrangement, and vascular morphology between healthy and diabetic patients. Zaleska-Żmijewska et al.24 used AOSLO imaging to identify decreased cone regularity and density in non-proliferative diabetic retinopathy (NPDR) patients and arteriolar dysfunction in DR patients. MONA II (Robotrak Techologies, China) was used to visualize and assess photoreceptors in laser-induced retinopathy25. It improves the quality of obtained images through real-time human eye-tracking technology26,27. The maximum field of view is 5° × 5°, which allows the imaging of blood velocity and flow across a broader range of vessel diameters28,29. The results of AO imaging may detect the pathological changes of the retina in advance30,31. To our knowledge, there are no studies assessing cones and vascular changes with the MONA II AO device in DME patients. This study was a cross-sectional observational study to investigate the macular vascular and photoreceptor changes for early DME. The association between risk factors and early DME was also investigated to indicate the possible pathogenesis at the onset of DME.
Methods
Study design
From November 2021 to August 2022, a cross-sectional observational study was performed at the First affiliated hospital with Nanjing Medical University. This research was approved by the Clinical Research Ethics Committee of the First Affiliated Hospital with Nanjing Medical University (NO. 2021-SE-406).
Early DME was defined as CST value between 250 and 325 μm; small (highest vertical size < 200 µm) or less obvious intraretinal cysts; well-recognizable and detectable inner retinal layers; intact EZ and external limiting membrane (ELM). Non-DME was defined as CST < 250 μm, normal inner retinal structure, intact RPE layer, EZ, and ELM32.
A total of 255 eyes of 134 DM patients were enrolled and informed consents were written. When comparing demographic variables and systematic evaluation results, such as sex, age and HbA1c levels among patients, we categorized 134 patients into two groups: early diabetic macular edema patients (early DME group, 69 subjects) and patients without DME (non-DME group, 65 subjects). A patient was assigned to the early DME group upon diagnosis in at least one eye. For the comparison of ophthalmological examination results, including OCT, OCTA, and AOSLO outcomes, a total of 255 eyes were classified into two groups: early DME (114 eyes with CST ≥ 250 μm and < 325 μm) and non-DME eyes (141 eyes with CST < 250 μm). The correlation of CST of two groups was implemented with intraclass correlation coefficient (ICC, Supplementary Table S14)33. Generalized estimating equations (GEE) model was applied to account for the inter-eye correlation in both-eyes data34.
Criteria for inclusion included patients aged 18 years old or older; type I or II diabetes; CST value ≤ 325 μm by Zeiss stratus OCT. Exclusion criteria were proliferative diabetic retinopathy (PDR) according to the ETDRS Retinopathy Severity Scale, media opacity including vitreous hemorrhage and cataract; poor-quality OCTA images with signal strength index < 6/10; a recent history of intraocular surgery (< 4 months), such as vitreous hemorrhage or cataract; a history of focal macular laser or panrentinal photocoagulation; a history of intravitreal therapy including anti-VEGF and steroids; the presence of any other retinal disorder that leads to macular edema including uveitis, high myopia, epiretinal membrane, vitreomacular traction syndrome, ocular trauma, systemic disorders such as chronic renal insufficiency.
Study protocol
All participants underwent an ophthalmological evaluation, including best-correlated visual acuity (BCVA; logMAR visual acuity), axis length, intraocular pressure (IOP; non-contact tonometer), slit-lamp biomicroscopy, color fundus photography, OCT, OCTA and AOSLO. OCT was performed using Zeiss stratus OCT (Carl Zeiss Meditec, Dublin, California, USA) with a scanning area of 6 mm × 6 mm (512 A-scans × 128 B-scans, 68,000 A-scans per second) centered on the fovea, generating a macular thickness map of 6 × 6 mm213. CST values in the 1-mm-diameter ring were collected using a manually assisted technique of the program contained within the system software (Cirrus™ HD-OCT 5000, version 9.5.2.19038, http://www.zeiss.com). OCTA (Optovue RTVueXR, Fremont, CA, USA) was conducted using the 6mm × 6mm (400 × 400 A-scans, 70,000 A-scans per second) Amgio Retina model centered at fovea, and a series of quantitative OCTA metrics were analyzed using XR Avanti software (version 2018.1.1.63, http://www.optovue.com)35. We used 6mm × 6mm OCTA images to observe continuous high-reflective lines on the first and second high-reflective bands of the four outermost layers, and to assess the integrity of the EZ, and ELM, respectively. The software automatically separated capillary plexuses into superior capillary plexus (SCP), which is embedded in the ganglion cell layer and nerve fiber layer, the deep capillary plexus (DCP) in the inner nuclear layer, the outer retina (OR) in the RPE layer, and the choriocapillaris. The vessel density and FAZ area were measured automatically by software. Data including glycated hemoglobin A1c (HbA1c), total cholesterol, triglycerides, blood urea, blood pressure, proteinuria, serum creatinine, and estimated glomerular filtration rate (eGFR) was collected. Age, sex, diabetes duration, body mass index (BMI), history of medication, and insulin dosage were recorded.
AO camera-MONA II (Robotrak Technologies, China) was used to acquire images of cone and vascular characteristics from the fovea center. A total of 28 patients followed macular cone detection (21 patients had both eyes detected and 7 patients had one eye detected), and 11 patients followed vascular detection (8 patients had both eyes detected and 3 patients had one eye detected) using AOSLO. Retinal artery parameters were collected from 11 non-DME eyes and 8 early DME eyes. Cone parameters were collected from 28 non-DME eyes and 22 early DME eyes. Non-DME and early DME patients required 1% tropicamide to dilate the pupil before examination. For detecting photoreceptors, each MONA II examination captured scans of the four perifoveal areas of the retina, 3° off the center of the fovea, with a standardized sampling window size of 2.4° × 2.4°. Using the in-built software (Robotrak Mona II a, version 1.00.01.230714, https://www.robotrak.cn/#/home/zh), the data of cone density (the number of cones per square millimeter), cone spacing (the average distance between a cell and its nearest neighbor cell), cone dispersion (the ratio of the mean inter-cell distance to the variance of inter-cell distances), cone regularity ratio (the mean percentage of hexagonal cones), and hexagonal cones ratio (the mean percentage of cones showing optimal hexagonal) were automatically calculated and collected. For detecting arteies, images were taken from the upper side (4° superior, 3° nasal) and lower side (4° inferior, 3° nasal) of fovea. In order to achieve the highest quality results, we performed 3–4 scans on cone regions and arterioles for our research, then selected the highest quality data. By manually drawing a line perpendicular to the labeled vessel wall, and accurately positioning the inner and outer edges of the vessel wall, we obtained the numerical values for vessel diameter (VeD), vessel wall thickness (WT), lumen diameter (LD). The wall-to-lumen ratio (WLR) is calculated as WT/LD, and the cross-sectional area of the vascular wall (WCSA) is calculated as π(VeD/2)2 − π(LD/2)2. Each retinal arteriole with a size between 70 and 130 μm was measured at least three times, and the average of these measurements was recorded36,37.
Statistical analysis
The analysis presented continuous variables as the mean accompanied by the standard deviation (SD) or as the median with an interquartile range (Q1-Q3), depending on their distribution. The Student t-test was applied for normally distributed data, while the Mann–Whitney test was employed for data with non-normal distributions. Categorical variables were represented as counts and percentages. The statistical significance of categorical variables was assessed using Pearson's chi-square test or Fisher's exact test.
A GEE multivariate logistic regression was used to test the relationship between clinical variables and the presence of diabetic macular edema in the patient. The model was adjusted for variates with a P value of 0.10 or less. In addition, variates that were clinically relevant to the primary endpoint were included in the model regardless of their P value.
The outcomes were presented as adjusted odds ratios (ORs) accompanied by 95% confidence intervals (95% CIs). All hypothesis tests were two-sided, and statistical significance was defined as a P value < 0.05. Stata SE (version 13, Serial number 401306302851, https://www.stata.com/), R software (version 3.6.1, http://cran.r-project.org/), EmpowerStats (version 2.0, www.empowerstats.com), and GraphPad Prism (version 8.0.2, https://www.graphpad.com/scientific-software/prism/) were applied for the data analysis.
Results
Patient characteristics
The mean age of the 134 DM patients was 51.34 ± 14.4 (18–87) without significant age difference between groups (P = 0.998 as shown in Supplementary Table S1). As shown in Table 1, the mean best correlated visual acuity (BCVA) in the LogMAR scale was 0.16 ± 0.17 for right eyes (oculus dexter, OD) and 0.18 ± 0.24 for left eyes (oculus sinister, OS). The mean BCVA of the early DME group and the non-DME group showed no significant difference between groups (P = 0.363). Besides, IOP (P = 0.606) and axis length (P = 0.406) were not significantly different between groups. These results indicated that there was limited vision loss or no obvious clinical symptom in early DME patients.
In this study, 69.40% of the participants were male. Compared to non-DME patients, early DME patients were more likely to have lower HbA1c levels (P = 0.007). The treatment method (P = 0.902), cumulative insulin dosage (P = 0.722), and diabetes duration (P = 0.382) were not significantly different between groups. Besides, there was no significant difference between groups in their serum creatinine (P = 0.272), systolic blood pressure (SBP; P = 0.517), diastolic blood pressure (DBP; P = 0.614), BMI (P = 0.530), total cholesterol (P = 0.472), triglycerides (P = 0.529), blood urea (P = 0.728), eGFR (P = 0.387), proteinuria (P = 0.205), as shown in Supplementary Table S1. Comparison of characteristics among different types of DM are presented in Supplementary Table S2.
OCTA parameter feature of early DME and non-DME patients
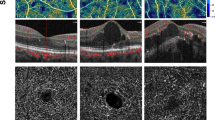
The mean CST was 266.41 ± 13.1 μm (OD) and 267.44 ± 13.5 μm (OS) in the early DME group, 230.98 ± 12.69 μm (OD), and 230.55 ± 18.09 μm (OS) in the non-DME group. FAZ area was significantly decreased in the early DME group than in the non-DME group (P < 0.001). Superficial vessel density (P = 0.714 for whole superficial VD, P = 0.504 for superficial parafovea VD, P = 0.821 for superficial perifovea VD), deep vessel density (P = 0.419 for whole deep VD, P = 0.483 for deep parafovea VD, P = 0.489 for deep perifovea VD) were not significantly different between groups (Table 1 and Fig. 1). The averaged data of both eyes and OCTA features among different types of DM are presented in Supplementary Tables S3 and S4.
OCTA images of a non-DME and an early DME eyes. In this figure, the eye with early DME showed increased central subfoveal thickness (CST, 321 μm), small intraretinal cysts, well-recognizable inner retinal layers, intact ellipsoid zone (EZ), and external limiting membrane (ELM). In the early DME eye, the whole vessel density of superficial capillary plexus (SCP) and deep capillary plexus (DCP) was 40.2% and 52.0%, and the foveal avascular zone (FAZ) area was 0.295 mm2. The eye with non-DME showed normal CST (235 μm), retinal morphology and structure. In the non-DME eye, the whole vessel density of SCP and DCP was 43.9% and 47.9%, and the FAZ area was 0.394 mm2.
Associations between risk factors and the presence of early DME in DM patients
Table 2 presents the relationships between DME-related risk factors and early DME development. In the univariate analysis, HbA1c level ≤ 8% (P = 0.005; odds ratio [OR] = 0.39; 95% confidence interval [95% CI] 0.21–0.75), FAZ area ≤ 0.3 mm2 (P = 0.001; OR = 0.41; 95% CI 0.27–0.65) were significantly associated with a higher risk of early DME. The associations remained significant for HbA1c level (P = 0.005; OR = 0.37; 95% CI 0.19–0.74) and FAZ area (P < 0.001; OR = 0.41; 95% CI 0.26–0.67) in the multivariate analysis.
GEE linear regression of FAZ area
GEE linear regression analysis results showed that FAZ area was correlated with sex (P = 0.047; coefficient 0.0399; 95% CI 0.0005–0.0793) but was not correlated with cumulative insulin dosage (P = 0.653), age (P = 0.421), HbA1c level (P = 0.095), as shown in Table 3.
Cone parameters among early DME and non-DME patients
There was no significant difference in the average cone density in all four retinal locations between early DME and non-DME patients: 11,676.21 ± 2899.8 vs. 14,194.64 ± 2625.05 cells/mm2 (OD) and 14,630.62 ± 4909.16 vs. 13,385.44 ± 2206.45 cells/mm2 (OS) in the inferior (P = 0.481), 12,602.57 ± 3012.96 vs. 12,849.5 ± 2164.08 cells/mm2 (OD) and 14,172.88 ± 3468.95 vs. 13,471.56 ± 3231.61 cells/mm2 (OS) in the superior (P = 0.294), 14,628.36 ± 3077.04 vs. 14,934.83 ± 2379.2 cells/mm2 (OD) and 15,459.38 ± 5907.4 vs. 15,986.94 ± 3206.94 cells/mm2 (OS) in the temporal (P = 0.792), 13,457.14 ± 4322.94 vs. 15,379.00 ± 3503.42 cells/mm2 (OD) and 15,624.75 ± 2766.39 vs. 15,660.5 ± 3114.64 cells/mm2 (OS) in the nasal quadrant (P = 0.346). In addition, The cone regularity, spacing, dispersion, and hexagonal cone ratio were additionally assessed with no significant results in the temporal, nasal, inferior, and superior quadrants in the two groups (Supplementary Table S5 and Fig. 2).
OCTA and AOSLO images of a non-DME and an early DME eyes. In the OCTA images of the macula from both non-DME and early DME eyes, intact external limiting membrane (ELM, red arrow) and ellipsoid zone (EZ, blue arrow) can be observed. Besides, the foveal avascular zone (FAZ) area of the early DME eye (0.287 mm2) is smaller than the FAZ area of the non-DME eye (0.372 mm2). Retinal artery images were taken from the upper side (4°superior, 3°nasal) and lower side (4°inferior, 3°nasal) of fovea (green dotted squares). The vessel diameter (VeD, 78.74 μm), wall thickness (WT, 49.40 μm), wall-to-lumen ratio (WLR, 0.39), and the cross-sectional area of the vascular wall (WCSA, 1794.40 mm2) in the upper side of the early DME eye was decreased than the non-DME eye (VeD, 79.02 μm; WT, 60.14 μm; WLR, 3.18; WCSA, 4624.46 mm2). Lumen diameter (LD) are labeled in this figure. Photoreceptor images were captured at 3°offset from the center of the fovea (indicated by the red dotted circle) in the nasal, superior, temporal and inferior quadrants (blue dotted squares).
Retinal artery parameters among early DME and non-DME patients
As shown in Table 4 and Fig. 2, the mean vessel diameter in the upper side was significantly decreased in the early DME group than in the non-DME group (82.31 ± 23.22 vs. 93.12 ± 20.46 μm in OD, 78.74 vs. 88.60 ± 16.28 μm in OS, P = 0.001). Meanwhile, the WT, WLR, and WCSA in the upper side were also significantly decreased in the early DME group than in the non-DME group (for WT 32.36 ± 26.95 vs. 59.17 ± 10.18 μm in OD, 19.34 vs. 24.31 ± 5.95 μm in OS, P < 0.001; for WLR 0.76 ± 0.83 vs. 1.94 ± 0.83 in OD, 0.39 vs. 0.38 ± 0.05 in OS, P = 0.005; for WCSA 3552.06 ± 3208.31 vs. 6054.01 ± 2609.92 μm2 in OD, 1794.4 vs. 3011.77 ± 1283.76 μm2 in OS, P = 0.003). However, there were no significant differences in the total vessel diameter, wall thickness, or wall-to-lumen ratio between the two groups on the lower side. The mean lumen diameter was not significantly different between both groups in the upper side (P = 0.071) and lower side (P = 0.871).
Correlation between cone and artery parameters and associated factors
The relationships between changes in cone and artery parameters and factors, including Hba1c, BCVA, sex, superficial and deep vessel density were determined with Spearman correlation analysis (Supplementary Tables S6–S13). Our results showed that the cone density in the superior quadrant was weekly correlated with HbA1c level (r = 0.317, P = 0.025). The vessel wall thickness on the upper side was correlated with superficial parafovea VD (r = 0.543, P = 0.048), deep VD (r = 0.556, P = 0.042), deep perifovea VD (r = 0.565, P = 0.038). The wall-to-lumen ratio on the upper side was correlated with superficial parafovea VD (r = 0.541, P = 0.046) and deep VD (r = 0.616, P = 0.019).
Discussion
While most researchers laid focus on CSME and DME at advanced and severe stages6,16,17,19,20,21, this study unveiled the macular vascular change and cone features at the beginning of DME formation, with no obvious clinical symptom.
The vessel density of the SCP, DCP, and FAZ areas indicate macular perfusion. Hyperreflective foci or dots within retinal layers (iHRF/iHRD) are complex mixture containing migrating RPE and lipid material that indicate the breakdown of the inner blood-retinal barrier (BRB) and microvascular damage6,38. Microaneurysms in the inner nuclear layer indicate microvascular lesions and microaneurysms leakage. Among the above OCTA metrics, only FAZ area and microaneurysm numbers were found to be significantly different between early DME and non-DME patients. What’s more, FAZ area ≤ 0.3 mm2 was found to be associated with a higher risk of early DME. Firstly, early DME patients showed intact ELM, EZ, and RPE in OCT and OCTA images, and there was no significant iHRF/iHRD difference when compared with non-DME patients. So, BRB was supposed to be intact in early DME participants. Secondly, it has been widely accepted that the FAZ area in DR and DME patients was larger in comparison with non-DME patients35,39,40,41. However, these studies focused on advanced and severe DME in CSME. Our findings suggest that although there are no obvious clinical symptoms at the onset of DME, there may be a compensatory increase of macular perfusion, which manifests as FAZ area reduction. As DME progresses, the FAZ area increases, and macular perfusion reduces. Our results also suggested that CST ≥ 250 μm and < 325 μm and FAZ ≤ 0.3 mm2 may be an indicator for early DME detection during DR screening.
Our data showed no correlation between FAZ area and cumulative insulin dosage, age, sex, HbA1c level, and diabetes duration. This result is supported by a previous study showing no correlation between FAZ area and age, diabetic duration, HbA1c level, BCVA, CST, and DR severity in DR patients42. Another study reported a significant correlation between the FAZ area and diabetic duration in DR patients18. In normal eyes, the FAZ area was found to correlate with age43. Further prospective studies with large samples are needed to investigate the factors associated with FAZ area reduction.
It has been reported that an increase in HbA1c level was associated with a higher risk of CSME44,45. What’s more, lower glycosylated hemoglobin at baseline was associated with retinopathy improvement46. A previous study showed that in DM patients with minimum or no diabetic retinopathy, no significant CST difference was found between patients with HbA1c < 8% and HbA1c ≥ 8%10. In this study, an inverse relation was found between HbA1c level and early DME development: DM patients with HbA1c ≤ 8% were more likely to have early DME than those with HbA1c > 8%. The first interpretation is that hyperglycemia, as measured by HbA1c, might show a relative “protective” effect at the onset of DME development by increasing plasma osmolarity and inducing dehydration. The second possible reason is that this result may be influenced by sample size and study design.
In this study, we analyzed retinal photoreceptor (cone) features and vascular change with AOSLO in early DME patients, which has not been previously investigated37,47,48. Previous researches reported a significant decreased cone density in DM, moderate and severe NPDR, PDR patients when compared with healthy participants49,50,51. In adolescents, no significant difference in cone density was observed between DM patients and healthy participants52. What’s more, cone density was not significantly different between prediabetic and healthy participants37. Our findings revealed no significant differences in cone parameters between early DME and non-DME patients. Also, we found that cone density in the superior quadrant was correlated with HbA1c level. This result indicates that at the early stage of DME formation, high glucose-induced photoreceptor impairment at the macula did not progress further.
However, our results showed obvious signs of vascular anatomical changes that may lead to functional alterations of vessels in the early DME group with significantly decreased vessel diameter, WT, WLR, and WCSA in the upper side (superotemporal region). Previous studies showed that WT, WLR and WCSA in DM patients with and without DR was significantly higher than healthy people53,54. Narrowing of arterioles lumen was observed in prediabetes and DM patients, which indicates smooth muscle cell proliferation and vascular fibrosis55. In this study, no significant differences in vessel parameters were observed in the lower sides (inferotemporal region) of early DME and non-DME eyes. The retinal arteries in the upper side were branches of superotemporal retinal artery. A previous study indicated that the vessel diameter was slightly lower and the average velocity was slightly higher in the arteries of the superotemporal region of retina compared to the inferotemporal region in healthy human subjects56. At the onset of DME, hyperglycemia induces excessive production of mitochondrial reactive oxygen species (ROS), leading to the production of angiotensin, which causes vasoconstriction and promotes inflammation57,58. With a smaller vessel diameter, constriction and dysregulation of the superotemporal retinal arteries may cause more sever ischemic and hypoxic condition than the inferotemporal retinal arteries. In addition, we cannot exclude that this result is due to the small sample size. Obtaining high-quality AOSLO images is challenging in patients with DME and DR, because of the requirement for patients to have good fixation ability and prolonged cooperation during examinations. Our study suggested a vascular spasm or vasoconstriction in the superotemporal region of the macula at the early stage of DME formation, which was consistent with FAZ area results.
There are some limitations of this study. Central subfovea is nourished by diffusion of choroidal circulation. So, it would be better if chorioidal parameters were investigated. However, current automated and manual segmentation methods of choriocapillaris layer were still inaccurate and unconvincing59,60,61. Other limitations include a relatively small sample size and the cross-sectional study design.
Part of this manuscript has been preprinted62. After being made public through a preprint server, comments from specialists in ophthalmology and endocrinology were received. Compliments were made to find the characterized OCTA metrics as non-invasive and convenient indicators for early DME detection. Suggestions were given for using the GEE model in statistical analysis, according to which we revised this manuscript.
In summary, this study indicated that the primary macular vascular change at the onset of DME formation was vascular spasm or vasoconstriction with FAZ area and vessel diameter reduction. Glucose-associated photoreceptor damage was limited at the onset of DME formation. CST ≥ 250 μm and FAZ ≤ 0.3 mm2 may be the indicators for early DME detection and could be applied in DR screening.
Data availability
The raw data of this study is available to access upon reasonable request.
Abbreviations
- DM:
-
Diabetes mellitus
- DR:
-
Diabetic retinopathy
- DME:
-
Diabetic macular edema
- CSME:
-
Clinically significant macular edema
- OCT:
-
Optical coherence tomography
- SD-OCT:
-
Spectral domain optical coherence tomography
- OCTA:
-
Optical coherence tomography angiography
- AO:
-
Adaptive optics
- AOSLO:
-
Adaptive optics scanning laser ophthalmoscope
- SBP:
-
Systolic blood pressure
- DBP:
-
Diastolic blood pressure
- BMI:
-
Body mass index
- HbA1c:
-
Glycated hemoglobin A1c
- eGFR:
-
Estimated glomerular filtration rate
- BCVA:
-
Best-correlated visual acuity
- SCP:
-
Superficial capillary plexus
- DCP:
-
Deep capillary plexus
- CST:
-
Central subfoveal thickness
- iHRF/iHRD:
-
Intraretinal hyperreflective foci or dots
- FAZ:
-
Foveal avascular zone
- VD:
-
Vessel density
- RPE:
-
Retinal pigment epithelium
- EZ:
-
Ellipsoid zone
- ELM:
-
External limiting membrane
- BRB:
-
Blood-retinal barrier
- VeD:
-
Vessel diameter
- WT:
-
Vessel wall thickness
- LD:
-
Lumen diameter
- WLR:
-
Wall-to-lumen ratio
- WCSA:
-
Cross-sectional area of the vascular wall
- OD:
-
Oculus dexter
- OS:
-
Oculus sinister
- GEE:
-
Generalized estimating equations
- OR:
-
Odds ratio
- SD:
-
Standard deviation
- 95% CI:
-
95% Confidence intervals
References
Teo, Z. et al. Global prevalence of diabetic retinopathy and projection of burden through 2045: Systematic review and meta-analysis. Ophthalmology 128, 1580–1591 (2021).
Yau, J. et al. Global prevalence and major risk factors of diabetic retinopathy. Diabet. Care 35, 556–564 (2012).
Bourne, R. et al. Causes of vision loss worldwide, 1990–2010: A systematic analysis. Lancet Glob. Health 1, e339-349 (2013).
Leasher, J. et al. Global estimates on the number of people blind or visually impaired by diabetic retinopathy: A meta-analysis from 1990 to 2010. Diabet. Care 39, 1643–1649 (2016).
Cheung, N., Mitchell, P. & Wong, T. Diabetic retinopathy. Lancet (London, England). 376, 124–136 (2010).
Sen, S., Ramasamy, K. & Sivaprasad, S. Indicators of visual prognosis in diabetic macular oedema. J. Pers. Med. 11, 449 (2021).
Panozzo, G. et al. An optical coherence tomography-based grading of diabetic maculopathy proposed by an international expert panel: The European School for Advanced Studies in Ophthalmology classification. Eur. J. Ophthalmol. 30, 8–18 (2020).
Hanumunthadu, D. et al. Agreement between spectral-domain and swept-source optical coherence tomography retinal thickness measurements in macular and retinal disease. Ophthalmol. Ther. 10, 913–922 (2021).
Friedman, S. et al. Topical nepafenec in eyes with noncentral diabetic macular edema. Retina. 35, 944–956 (2015).
Bressler, N. et al. Retinal thickness on Stratus optical coherence tomography in people with diabetes and minimal or no diabetic retinopathy. Am. J. Ophthalmol. 145, 894–901 (2008).
Mahajan, V. et al. Management of sympathetic ophthalmia with the fluocinolone acetonide implant. Ophthalmology. 116, 552-557.e551 (2009).
Konstantina, S. et al. Comparison of SDOCT scan types for grading disorganization of retinal inner layers and other morphologic features of diabetic macular edema. Transl. Vis. Sci. Technol. 9, 45 (2020).
Chalam, K. et al. Retinal thickness in people with diabetes and minimal or no diabetic retinopathy: Heidelberg Spectralis optical coherence tomography. Investig. Ophthalmol. Vis. Sci. 53, 8154–8161 (2012).
Sampani, K. et al. Comparison of SDOCT scan types for grading disorganization of retinal inner layers and other morphologic features of diabetic macular edema. Transl. Vis. Sci. Technol. 9, 45 (2020).
Dimitrova, G., Chihara, E., Takahashi, H., Amano, H. & Okazaki, K. Quantitative retinal optical coherence tomography angiography in patients with diabetes without diabetic retinopathy. Investig. Ophthalmol. Vis. Sci. 58, 190–196 (2017).
Sun, Z. et al. OCT angiography metrics predict progression of diabetic retinopathy and development of diabetic macular edema: A prospective study. Ophthalmology. 126, 1675–1684 (2019).
Han, R., Gong, R. & Liu, WXu. G. Optical coherence tomography angiography metrics in different stages of diabetic macular edema. Eye Vis. 9, 14 (2022).
Fernández-Espinosa, G. et al. Retinal vascularization abnormalities studied by optical coherence tomography angiography (OCTA) in type 2 diabetic patients with moderate diabetic retinopathy. Diagnostics 12, 379 (2022).
Mirshahi, R. et al. Differentiating features of OCT angiography in diabetic macular edema. Sci. Rep. 11, 23398 (2021).
Ayman, G. E., Alia, M. N., Ahmed, A.A.-K., Osama, A. S. & David, J. R. Optical coherence tomography angiography biomarkers predict anatomical response to bevacizumab in diabetic macular edema. Diabet. Metab. Syndr. Obes. 15, 395–405 (2022).
Wei, L. et al. Microvascular changes after conbercept intravitreal injection of PDR with or without center-involved diabetic macular edema analyzed by OCTA. Front. Med. 9, 797087 (2022).
Neelakshi, B., Ruben, A. G., Arthur, T. & Marco, A. Z. Diabetic macular edema: Pathogenesis and treatment. Surv. Ophthalmol. 54, 1–32 (2009).
Kupis, M., Wawrzyniak, Z. M., Szaflik, J. P. & Zaleska-Zmijewska, A. Retinal photoreceptors and microvascular changes in the assessment of diabetic retinopathy progression: A two-year follow-up study. Diagnostics 13, 2513 (2023).
Zaleska-Zmijewska, A., Wawrzyniak, Z. M., Dabrowska, A. & Szaflik, J. P. Adaptive optics (rtx1) high-resolution imaging of photoreceptors and retinal arteries in patients with diabetic retinopathy. J. Diabet. Res. 2019, 9548324 (2019).
Fang, D. et al. Morphologic and functional assessment of photoreceptors in laser-induced retinopathy using adaptive optics scanning laser ophthalmoscopy and microperimetry. Am. J. Ophthalmol. 265, 61–72 (2024).
Chui, T. Y. P. et al. Human retinal microvascular imaging using adaptive optics scanning light ophthalmoscopy. Int. J. Retina Vitreous 2, 11 (2016).
Karst, S. G. et al. Characterization of in vivo retinal lesions of diabetic retinopathy using adaptive optics scanning laser ophthalmoscopy. Int. J. Endocrinol. 2018, 7492946 (2018).
Burns, S. A., Elsner, A. E., Sapoznik, K. A., Warner, R. L. & Gast, T. J. Adaptive optics imaging of the human retina. Prog. Retin. Eye Res. 68, 1–30 (2019).
Chui, T. Y., Gast, T. J. & Burns, S. A. Imaging of vascular wall fine structure in the human retina using adaptive optics scanning laser ophthalmoscopy. Investig. Ophthalmol. Vis. Sci. 54, 7115–7124 (2013).
Nesper, P. L., Scarinci, F. & Fawzi, A. A. Adaptive optics reveals photoreceptor abnormalities in diabetic macular ischemia. PLoS One. 12, e0169926 (2017).
Torm, M. E. W. et al. Detection of capillary abnormalities in early diabetic retinopathy using scanning laser ophthalmoscopy and optical coherence tomography combined with adaptive optics. Sci. Rep. 14, 13450 (2024).
Giacomo, P. et al. An optical coherence tomography-based grading of diabetic maculopathy proposed by an international expert panel: The European School for Advanced Studies in Ophthalmology classification. Eur. J. Ophthalmol. 30, 8–18 (2019).
Richard, A. A. Statistical guidelines for the analysis of data obtained from one or both eyes. Ophthalmic Physiol. Opt. 33, 7–14 (2012).
Ying, G., Maguire, M., Glynn, R. & Rosner, B. Tutorial on biostatistics: Longitudinal analysis of correlated continuous eye data. Ophthalmic Epidemiol. 28, 3–20 (2021).
Samara, W. et al. Quantification of diabetic macular ischemia using optical coherence tomography angiography and its relationship with visual acuity. Ophthalmology. 124, 235–244 (2017).
Anna, Z. -Ż, Zbigniew, M. W., Anna, D. & Jacek, P. S. Adaptive optics (rtx1) high-resolution imaging of photoreceptors and retinal arteries in patients with diabetic retinopathy. J Diabetes Res. 2019, 9548324 (2019).
Anna, Z. -Ż et al. Retinal photoreceptors and microvascular changes in prediabetes measured with adaptive optics (rtx1™): A case-control study. J. Diabet. Res. 2017, 4174292 (2017).
Serena, F. et al. Significance of hyperreflective foci as an optical coherence tomography biomarker in retinal diseases: Characterization and clinical implications. J. Ophthalmol. 2021, 6096017 (2021).
Bhanushali, D. et al. Linking retinal microvasculature features with severity of diabetic retinopathy using optical coherence tomography angiography. Investig. Ophthalmol. Vis. Sci. 57, OCT519–OCT525 (2016).
AttaAllah, H., Mohamed, A. & Ali, M. Macular vessels density in diabetic retinopathy: Quantitative assessment using optical coherence tomography angiography. Int. Ophthalmol. 39, 1845–1859 (2019).
Hsieh, Y. et al. OCT angiography biomarkers for predicting visual outcomes after ranibizumab treatment for diabetic macular edema. Ophthalmol. Retina 3, 826–834 (2019).
Durbin, M. et al. Quantification of retinal microvascular density in optical coherence tomographic angiography images in diabetic retinopathy. JAMA Ophthalmol. 135, 370–376 (2017).
Iafe, N., Phasukkijwatana, N., Chen, X. & Sarraf, D. Retinal capillary density and foveal avascular zone area are age-dependent: Quantitative analysis using optical coherence tomography angiography. Investig Ophthalmol. Vis. Sci. 57, 5780–5787 (2016).
Tan, G., Cheung, N., Simó, R., Cheung, G. & Wong, T. Diabetic macular oedema. Lancet. Diabet. Endocrinol. 5, 143–155 (2017).
Lachin, J. et al. Effect of intensive diabetes therapy on the progression of diabetic retinopathy in patients with type 1 diabetes: 18 years of follow-up in the DCCT/EDIC. Diabetes. 64, 631–642 (2015).
Klein, R., Klein, B., Moss, S. & Cruickshanks, K. The Wisconsin Epidemiologic Study of Diabetic Retinopathy: XVII. The 14-year incidence and progression of diabetic retinopathy and associated risk factors in type 1 diabetes. Ophthalmology. 105, 1801–1815 (1998).
Marco, L., Sebastiano, S., Nicholas, D., Mariacristina, P. & Giuseppe, L. Adaptive optics technology for high-resolution retinal imaging. Sensors 13, 334–366 (2012).
Marco, L. et al. Analysis of retinal capillaries in patients with type 1 diabetes and nonproliferative diabetic retinopathy using adaptive optics imaging. Retina. 33, 1630–1639 (2013).
Mohamed Kamel, S. et al. High-resolution imaging of parafoveal cones in different stages of diabetic retinopathy using adaptive optics fundus camera. PLoS One 11, e0152788 (2016).
Marco, L. et al. Investigation of adaptive optics imaging biomarkers for detecting pathological changes of the cone mosaic in patients with type 1 diabetes mellitus. PLoS One. 11, e0151380 (2016).
Marco, L. et al. Adaptive optics imaging of parafoveal cones in type 1 diabetes. Retina. 34, 546–557 (2013).
Wylie, T. et al. Cone-photoreceptor density in adolescents with type 1 diabetes. Investig. Ophthalmol. Vis. Sci. 56, 6339–6343 (2015).
Arichika, S. et al. Correlation of retinal arterial wall thickness with atherosclerosis predictors in type 2 diabetes without clinical retinopathy. Br. J. Ophthalmol. 101, 69–74 (2017).
Wojciech, M., Katarzyna, G.-N., Joanna, M. H. & Elżbieta, B.-S. Evaluation of morphological changes in retinal vessels in type 1 diabetes mellitus patients with the use of adaptive optics. Biomedicines. 10, 1926 (2022).
Allen, C. C. & Sven-Erik, B. Retinal blood flow in diabetes. Microcirculation. 14, 49–61 (2007).
Otero-Marquez, O. et al. Retinal blood flow biomarkers in healthy human subjects measured with clinical doppler optical coherence tomography. Investig. Ophthalmol. Vis. Sci. 63, 3323-F0132 (2022).
Tomomi, M., Masamitsu, S. & Hideaki, H. Retinal diseases associated with oxidative stress and the effects of a free radical scavenger (Edaravone). Oxid. Med. Cell Longev. 2017, 9208489 (2017).
Stefan, S., Janna, K. & Albert, J. A. Pathophysiology of macular edema. Ophthalmologica 224, 8–15 (2010).
Devarajan, K. et al. Optical coherence tomography angiography for the assessment of choroidal vasculature in high myopia. Br. J. Ophthalmol. 104, 917–923 (2020).
Ang, M. et al. Imaging in myopia: Potential biomarkers, current challenges and future developments. Br. J. Ophthalmol. 103, 855–862 (2019).
Zhang, L. et al. Automated segmentation of the choroid from clinical SD-OCT. Investig. Ophthalmol. Vis. Sci. 53, 7510–7519 (2012).
Shen, H., Gu, Q., Cheng, R., Cheng, P. & Liu, Q. Associated factors and macular vascular perfusion change for diabetic macular edema at early stage: A cross-sectional observational study. (2023).
Acknowledgements
This research was approved by the Clinical Research Ethics Committee of the First Affiliated Hospital with Nanjing Medical University (NO. 2021-SE-406) on 24th November 2021. All methods were carried out in accordance with relevant guidelines and regulations. Informed consent was obtained from all subjects and/or their legal guardian(s).We thank colleagues from the Ethics Committee of Jiangsu Provincial People’s Hospital for their support and supervision. We appreciate Advanced Ophthalmology Laboratory (AOL), Robotrak Technologies, Nanjing, China, for generously providing AOSLO instruments free of charge and assisting with the analysis.
Funding
This study was supported by the National Natural Science Foundation of China (82101151, 82271100); Natural Science Foundation of Jiangsu Province (BK20210972).
Author information
Authors and Affiliations
Contributions
Conceptualization and methodology were carried out by H.S., Q.H.L., and P.C.; investigation was performed by H.S., Q.Y.G., T.P., J.L.H., and K.Z.; original draft writing and formal analysis were conducted by H.S. and R.W.C.; manuscript review and editing were performed by H.S. and Y.Y.; statistical analysis was conducted by Y.Y. and J.Y.Z.; funding was acquired by H.S. and Q.H.L. All authors have seen and approved the final version of the manuscript for publication. All authors reviewed the manuscript.
Corresponding authors
Ethics declarations
Competing interests
The authors declare no competing interests.
Additional information
Publisher's note
Springer Nature remains neutral with regard to jurisdictional claims in published maps and institutional affiliations.
Supplementary Information
Rights and permissions
Open Access This article is licensed under a Creative Commons Attribution-NonCommercial-NoDerivatives 4.0 International License, which permits any non-commercial use, sharing, distribution and reproduction in any medium or format, as long as you give appropriate credit to the original author(s) and the source, provide a link to the Creative Commons licence, and indicate if you modified the licensed material. You do not have permission under this licence to share adapted material derived from this article or parts of it. The images or other third party material in this article are included in the article’s Creative Commons licence, unless indicated otherwise in a credit line to the material. If material is not included in the article’s Creative Commons licence and your intended use is not permitted by statutory regulation or exceeds the permitted use, you will need to obtain permission directly from the copyright holder. To view a copy of this licence, visit http://creativecommons.org/licenses/by-nc-nd/4.0/.
About this article
Cite this article
Gu, Q., Pan, T., Cheng, R. et al. Macular vascular and photoreceptor changes for diabetic macular edema at early stage. Sci Rep 14, 20544 (2024). https://doi.org/10.1038/s41598-024-71286-6
Received:
Accepted:
Published:
DOI: https://doi.org/10.1038/s41598-024-71286-6
- Springer Nature Limited