Abstract
EphrinB2 regulates synaptic transmission and morphology however its role in memory formation is unknown. Here we show that deleting ephrinB2 from excitatory neurons in the basolateral amygdala (BLA) of male mice impairs long-term (LTM), but not short-term (STM), fear memory formation. Deleting ephrinB2 from astrocytes in the BLA impairs fear LTM but not STM. Removing ephrinB2 from astrocytes in the BLA reduces the level of the excitatory amino acid transporter 1 (EAAT1) in these cells. Inhibiting EAAT1 activity in the BLA during fear conditioning, by its specific inhibitor UCPH-101, impairs fear LTM showing that EAAT1 in the BLA is needed for fear LTM formation. The administration of ephrinB2 into the BLA during fear conditioning training enhances fear LTM. Moreover, ephrinB2 increases the ability of fear conditioning to activate cells in the BLA as detected by c-Fos labeling. EphrinB2 therefore determines the threshold for fear memory formation. In contrast to mature neurons, we show that ephrinB2 in neural stem cells (NSCs) is not needed for fear LTM. Our study shows that ephrinB2 in the BLA determines the strength of long-term memory consolidation.
Similar content being viewed by others
Introduction
In this study, we are interested in elucidating the molecular mechanisms that underlie memory formation. Ephrins and their cognate Eph receptors are attractive candidates to play a central role in memory formation as they are involved in cellular events believed to subserve memory formation, such as changes in synaptic transmission and neuronal morphology1,2,3,4. EphrinBs are tethered to the plasma membrane, activated by the Eph receptor and possess reverse signaling properties. On the other hand, ephrins can activate Eph receptors that transmit forward signals. Thus, the EphB-ephrinB system can function in a bidirectional contact-mediated fashion between two opposing cells. EphrinB2 regulates several key neuronal functions such as the regulation of synaptic transmission and morphology. For example, ephrinB2 activates EphB receptor forward signaling in primary cortical neurons and potentiates NMDA receptor-dependent influx of calcium5. Activation of ephrinB2 reverse signaling by Eph receptors leads to the stabilization of AMPA receptors in the synapse through ephrinB2 binding to the PDZ-containing protein GRIP6. Stimulation of ephrinB2 with the EphB receptor stabilizes dendritic spines7. Although ephrinB2 is intimately involved in regulating key neuronal functions believed to be involved in memory formation such as neuronal transmission and morphogenesis, its role in memory formation is poorly understood. In this study, we are aiming to explore the roles of ephrinB2 signaling in fear memory formation. Toward these ends, we utilized a combination of behavioral paradigms and advanced genetic and cellular approaches to explore the roles of ephrinB2 in excitatory neurons, astrocytes and neural stem cells, where it is expressed in the brain, in fear memory formation.
To examine the roles of ephrinB2 in memory formation we used auditory fear conditioning, a well-established behavioral paradigm. In this paradigm, an association is formed between the auditory stimulation (a tone conditioned stimulus (CS)) and an aversive mild footshock (unconditioned stimulus (US))8,9,10,11,12. The putative site of fear conditioning memory, the basolateral amygdala (BLA), has been identified8,12,13,14,15. Principle neurons and astrocytes are involved in fear conditioning memory formation and extinction in the BLA16,17,18,19.
Results
EphrinB2 in excitatory neurons in the basolateral amygdala is essential for long-term fear memory formation
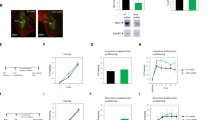
We were interested to study whether ephrinB2 in excitatory neurons in the basolateral amygdala (BLA) is needed for long-term memory formation. Toward that end, we utilized the B6.129S7-Efnb2tm2And/J mice that possess loxP sites flanking exon 1 of the ephrinB2 gene (Efnb2)20. To delete ephrinB2 in excitatory neurons in BLA, we injected AAV that contains the Cre recombinase gene downstream to CaMKII promoter (CaMKII promoter-mCherry-2A-Cre recombinase) (Fig. 1A) into the BLA. CaMKII is expressed only in excitatory neurons in the BLA21. AAV containing CaMKII promoter is used to express the DNA in pyramidal neurons in BLA18. Figure 1B shows that mCherry expression in the amygdala is colocalized with the NeuN neuronal marker (n = 5, 92.09 ± 1.31). CaMKII-Cre recombinase is expressed in BLA of Efnb2-floxed and wild-type (wt) littermate mice (Fig. 1C). We show that expression of the CaMKII-Cre recombinase in BLA-depleted ephrinB2 from excitatory neurons in BLA. The number of mCherry and ephrinB2 co-expressing excitatory neurons is significantly reduced (p < 0.001) in BLA of floxed-ephrinB2 (n = 11) when compared to the wt mice (n = 7) (Fig. 1D). The area of staining of ephrinB2 in mCherry cells is significantly (t(16) = 4.247, p < 0.001) reduced in BLA in floxed ephrinB2 (n = 11) compared to wt mice (n = 7) (Fig. 1D). Next, we tested whether the deletion of ephrinB2 proteins in excitatory neurons in BLA affects long-term fear memory formation (Fig. 1E). There is no difference in freezing during training between the groups (F(1,21) = 0.656, p = 0.427) and no treatment × tone trial interaction (F(1.428,29.992) = 0.438, p = 0.583) (Fig. 1F). We revealed that deleting ephrinB2 leads to impairment of long-term contextual fear conditioning (t(21) = 2.348, p = 0.029) (Fig. 1G). Deleting ephrinB2 in BLA (n = 9) impaired long-term auditory fear memory formation when compared to the wt control group (n = 14) (F(1,21) = 6.023, p = 0.023). There is no treatment × tone trial interaction (F(4,84) = 0.935, p = 0.448) (Fig. 1H). These results show that ephrinB2 is essential in excitatory neurons in BLA for long-term fear memory formation.
A Description of deletion of ephrinB2 in excitatory neurons of B6.129S7-Efnb2tm2And/J (floxed-ephrinB2) using the CaMKII promoter-mCherry-2A-Cre recombinase AAV (CaMKII-Cre). B mCherry expression in the amygdala is colocalized with NeuN neuronal marker (n = 5, 92.09 ± 1.31). C Photos that encompass mostly the BLA show that the CaMKII-Cre construct is expressed in BLA of wild-type (wt) and floxed-ephrinB2 littermate mice as detected by mCherry. D EphrinB2 is deleted in excitatory neurons in BLA of floxed-ephrinB2 by the AAV expressing Cre-recombinase. The number of mCherry and ephrinB2 co-expressing excitatory neurons is significantly reduced (p < 0.001) in BLA of floxed-ephrinB2 (n = 11) when compared to wt mice (n = 7). The area of staining of ephrinB2 in mCherry cells is significantly (t(16) = 4.247, p < 0.001) reduced in BLA in floxed ephrinB2 (n = 11) compared to wt mice (n = 7). E Long-term memory experiment: Mice were injected with AAV containing CaMKII-Cre. One month later the mice were trained for fear conditioning (schematic of fear conditioning used in this study is shown) and tested for contextual fear conditioning memory 24 h after fear conditioning and auditory fear memory 48 h after fear conditioning. F There is no difference in freezing during training between the groups (F(1,21) = 0.656, p = 0.427). G Deleting ephrinB2 leads to impairment of long-term contextual fear conditioning (t(21) = 2.348, p = 0.029). H Depleting ephrinB2 in BLA excitatory neurons of floxed-ephrinB2 (n = 9) significantly impaired auditory fear conditioning LTM tested 48 h after training when compared to wt mice (n = 14) (F(1,21) = 6.023, p = 0.023). There is no difference in treatment × tone trial interaction (F(4,84) = 0.935, p = 0.448). I Short-term memory experiment: Mice were injected with AAV containing CaMKII-Cre. One month later the mice were trained for fear conditioning and tested for contextual fear conditioning memory 1 h after fear conditioning and auditory fear memory 2 h after fear conditioning. J There is no difference in freezing during training between the groups (F(1,30) = 0.084, p = 0.773). K There is no difference in contextual fear conditioning STM between the groups (p = 0.59). L EphrinB2 deleted mice (n = 16) were not different from control mice (n = 16) in short-term auditory fear conditioning memory (F(1,30) = 0.811, p = 0.375). Data are presented as mean ± SEM.
Deleting ephrinB2 from excitatory neurons in the basolateral amygdala does not affect short-term fear memory formation
To test whether ephrinB2 in excitatory neurons is involved in controlling the ability to associate the CS and US or specifically suppress long-term memory formation, we tested the effects of ephrinB2 depletion in the excitatory neurons on short-term fear conditioning memory. B6.129S7-Efnb2tm2And/J mice were injected with CaMKII promoter-mCherry-2A-Cre recombinase AAV into the BLA. Controls are littermate wt animals injected with the AAV virus. A month later, they were trained for fear conditioning and tested 1 h afterward in the same context for contextual fear conditioning STM and 2 h later in a different context for auditory fear conditioning STM (Fig. 1I). There is no difference in freezing during training between the groups (F(1,30) = 0.084, p = 0.773) and no treatment × tone trial interaction (F(2,60) = 0.45, p = 0.64) (Fig. 1J). There is no difference in contextual fear conditioning STM between the groups (p = 0.59) (Fig. 1K). EphrinB2 deleted mice (n = 16) were not different from control mice (n = 16) in short-term auditory fear conditioning memory (F(1,30) = 0.811, p = 0.375) (Fig. 1L). There is no difference in treatment × tone trial interaction (F(4,120) = 1.818, p = 0.13). We, therefore, conclude that ephrinB2 in excitatory neurons is not involved in fear STM and the ability to associate the CS and US but rather specifically in the formation of long-term fear memory.
EphrinB2 in astrocytes in the basolateral amygdala is essential for long-term fear memory formation
Astrocytes have a key role in regulating synaptic transmission and neuronal morphology and are involved in memory formation22. However, the molecular mechanisms whereby they exert their effects on memory formation are not well understood. We were, therefore, interested to explore the roles of ephrinB2 in astrocytes in BLA in long-term fear memory formation. To delete ephrinB2 from astrocyte of B6.129S7-Efnb2tm2And/J mice we expressed the Cre-recombinase under the glial fibrillary acidic protein (GFAP) promoter using AAV (hGFAP-mCherry_iCre-WPRE-hGHp(A)) in BLA (Fig. 2A). The GFAP promoter is a useful promoter for selectively expressing constructs in astrocytes23. Moreover, the AAV we are using (v233 from VVF) was shown to specifically express iCre in GFAP-positive cells24. Figure 2B shows that mCherry expression in the amygdala is colocalized with the astrocytic marker GFAP (n = 5, 96.75 ± 0.35). Controls are littermate wt animals injected with the GFAP-Cre AAV virus. GFAP-Cre recombinase is expressed in the BLA of Efnb2-floxed and wild-type mice (Fig. 2C). Our results show that ephrinB2 is depleted in GFAP-expressing astrocytes when using the GFAP promoter-Cre-recombinase AAV in B6.129S7-Efnb2tm2And/J mice as significantly less mCherry expressing astrocytes in floxed-ephrinB2 mice (n = 11) also express ephrinB2 when compared to wt mice (n = 7) (t(16) = 5.107, p < 0.001) (Fig. 2D). The area of staining of ephrinB2 in mCherry cells is significantly (t(16) = 4.882, p < 0.001) reduced in BLA in floxed ephrinB2 (n = 11) compared to wt mice (n = 7) (Fig. 2D). Next, we tested whether the deletion of astrocytic ephrinB2 in BLA affects long-term fear memory formation (Fig. 2E). There is no difference in freezing during training between the groups (F(1,22) = 3.397, p = 0.079) and no treatment × tone trial interaction (F(1.59, 34.974) = 1.835, p = 0.18) (Fig. 2F). We revealed that deleting ephrinB2 leads to impairment of long-term contextual fear conditioning (p = 0.006) (Fig. 2G). Deleting ephrinB2 in astrocytes in BLA (n = 13) impaired long-term auditory fear memory formation when compared to the wt control group (n = 11) (F(1,22) = 9.243, p = 0.006). There is no treatment × tone trial interaction (F(4,88) = 1.668, p = 0.164) (Fig. 2H). These results show that ephrinB2 is essential in astrocytes in BLA in long-term fear memory formation.
A Description of deletion of ephrinB2 in astrocytes of B6.129S7-Efnb2tm2And/J (floxed-ephrinB2) using the hGFAP-mCherry_iCre-WPRE-hGHp(A) AAV (GFAP-Cre). B mCherry expression in the amygdala is colocalized with the GFAP astrocytic marker (n = 5, 96.75 ± 0.35). C Photos that encompass mostly the BLA show that the GFAP-Cre construct is expressed in BLA of wild-type and B6.129S7-Efnb2tm2And/J mice as detected by mCherry. D EphrinB2 is depleted in astrocytes in BLA of floxed-ephrinB2 by the AAV expressing Cre-recombinase. Less mCherry expressing astrocytes in floxed-ephrinB2 mice (n = 11) also express ephrinB2 when compared to wt mice (n = 7) (t(16) = 5.107, p < 0.001). The area of staining of ephrinB2 in mCherry cells is significantly (t(16) = 4.882, p < 0.001) reduced in BLA in floxed ephrinB2 (n = 11) compared to wt mice (n = 7). E Long-term memory experiment: Mice were injected with AAV containing GFAP-Cre. One month later the mice were trained for fear conditioning and tested for contextual fear conditioning memory 24 h after fear conditioning and auditory fear memory 48 h after fear conditioning. F There is no difference in freezing during training between the groups (F(1,22) = 3.397, p = 0.079). G Deleting ephrinB2 leads to impairment of long-term contextual fear conditioning (p = 0.006). H Deleting ephrinB2 in astrocytes in BLA (n = 13) impaired long-term auditory fear memory formation when compared to the wt control group (n = 11) (F(1,22) = 9.243, p = 0.006). There is no treatment × tone trial interaction (F(4,88) = 1.668, p = 0.164). I Short-term memory experiment: Mice were injected with AAV containing GFAP-Cre. One month later the mice were trained for fear conditioning and tested for contextual fear conditioning memory 1 h after fear conditioning and auditory fear memory 2 h after fear conditioning. J There is no difference in freezing during training between the groups (F(1,15) = 2.373, p = 0.144). K There is no difference in contextual fear conditioning STM between the groups (p = 0.236). L EphrinB2 deleted mice (n = 9) were not different from control mice (n = 8) in short-term auditory fear conditioning memory (F(1,15) = 1.134, p = 0.304). Data are presented as mean ± SEM.
EphrinB2 in astrocytes in the basolateral amygdala is not needed for short-term fear memory formation
To test whether ephrinB2 is involved in controlling the ability to associate the CS and US or whether it specifically suppresses long-term memory formation we tested the effects of ephrinB2 depletion in astrocytes on short-term fear conditioning memory. B6.129S7-Efnb2tm2And/J mice were injected into the BLA with AAV expressing the Cre-recombinase under the glial fibrillary acidic protein (GFAP) promoter (hGFAP-mCherry_iCre-WPRE-hGHp(A)). Controls are wild-type littermate animals injected with the GFAP-Cre AAV virus. A month later, they were trained for fear conditioning and tested 1 h afterward in the same context for contextual fear conditioning STM and 2 h later in a different context for auditory fear conditioning STM (Fig. 2I). There is no difference in freezing during training between the groups (F(1,15) = 2.373, p = 0.144) and no treatment × tone trial interaction (F(2,30) = 1.151, p = 0.33) (Fig. 2J). There is no difference in contextual fear conditioning STM between the groups (p = 0.236) (Fig. 2K). EphrinB2 deleted mice (n = 9) were not different from control mice (n = 8) in short-term auditory fear conditioning memory (F(1,15) = 1.134, p = 0.304) (Fig. 2L). There is no difference in treatment × tone trial interaction (F(4,60) = 0.714, p = 0.586). We, therefore, conclude that ephrinB2 in astrocytes is not involved in the formation of STM and the ability to form the association of CS and US but rather specifically in the formation of long-term memory.
EAAT1 glutamate transporter level in astrocytes in BLA is regulated by ephrinB2 and EAAT1 activity in BLA is needed for long-term memory formation
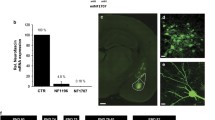
Glial glutamate transporters are known to regulate synaptic transmission by clearing glutamate from the synaptic cleft25,26. We were, therefore, intrigued to examine whether astrocytic ephrinB2 affects astrocytic glutamate transporters. Our results show by immunohistochemistry that the level of the glial glutamate transporter EAAT1 in astrocytes in B6.129S7-Efnb2tm2And/J mice injected with hGFAP-mCherry-iCre-WPRE-hGHp(A) (n = 6) is significantly reduced when compared to hGFAP-mCherry iCre-WPRE-hGHp(A) expressing cells in littermate wild-type mice (n = 6) (t(10) = 3.335, p = 0.008) (Fig. 3A, B). Similar results are shown using Western blot, which exhibits a significant reduction in the level of EAAT1 in astrocytic ephrinB2 deleted mice (n = 9) compared to wt mice (n = 9) (t(16) = 0.346, p = 0.01) (Fig. 3C, D). To examine whether EAAT1 activity is needed for long-term memory formation we injected UCPH-101, a specific EAAT1 inhibitor27, bilaterally into the BLA 30 minutes before fear conditioning (Fig. 3E, F). There is no difference in freezing during training between the groups (F(1,24) = 0.777, p = 0.387) and no treatment × tone trial interaction (F(2,48) = 0.022, p = 0.978) (Fig. 3G). Long-term contextual fear memory is not affected by UCPH-101 (n = 13) as there is no difference from the vehicle group (n = 13) (t(24) = 0.138, p = 0.892) (Fig. 3H). We show that long-term auditory fear conditioning is impaired in the UCPH-101 injected group (n = 13) compared to vehicle-injected mice (n = 13) (F(1,24) = 9.445, p = 0.005) (Fig. 3I). There is no difference in treatment × tone trial interaction (F(4,96) = 1.221, p = 0.307). Our results show that ephrinB2 KO in the BLA decreases the level of EAAT1 in astrocytes. In addition, these results show that ephrinB2 in astrocytes can mediate the ability of the animal to form long-term fear memory through controlling EAAT1 whose activity is essential for fear LTM formation.
A EAAT1 level in astrocytes that are depleted of ephrinB2 in B6.129S7-Efnb2tm2And/J mice injected with hGFAP-mCherry-iCre-WPRE-hGHp(A) (n = 6) is significantly reduced when compared to hGFAP-mCherry-iCre-WPRE-hGHp(A) expressing cells in wild-type mice (n = 6) (t(10) = 3.335, p = 0.008). B EAAT1 (green) in Cre-recombinase (red) expressing cells. C Western blot analysis shows a reduction in the level of EAAT1 in astrocytic ephrinB2-deleted mice (n = 9) compared to wt mice (n = 9) (t(16) = 0.346, p = 0.01). D A representative Western blot. E Mice were injected with UCPH-101 or vehicle into the BLA 30 min before fear conditioning. Contextual fear conditioning was tested 24 h after fear conditioning and auditory fear memory was tested 48 h after fear conditioning. F Cannula tip placements in the BLA. G There is no difference in freezing during training between the groups (F(1,24) = 0.777, p = 0.387). H Long-term contextual fear memory is not affected by UCPH-101 (n = 13) and there is no difference from the vehicle group (n = 13) (t(18) = 0.138, p = 0.892). I Long-term auditory fear conditioning is impaired in the UCPH-101 injected group (n = 13) compared to vehicle-injected mice (n = 13) (F(1,24) = 9.445, p = 0.005). Data are presented as mean ± SEM.
EphrinB2 in neural stem cells is not needed for long-term fear memory formation
Our observations show that ephrinB2 in mature neurons is needed for long-term memory formation in BLA. We next were curious to explore whether ephrinB2 in neural stem cells (NSC) is needed for fear memory formation. It was shown that neurogenic precursor cells are present in the mouse adult BLA, and can undergo neurogenesis in adults in the amygdala28,29,30,31.To examine the role of ephrinB2 in NSC in fear memory formation, we crossed ephrinB2-floxed (B6.129S7-Efnb2tm2And/J) with C57BL/6-Tg(Nes-cre/ERT2)KEisc/J and subjected them to 4-hydroxytamoxifen (4-OHT) to remove ephrinB2 in NSC (Fig. 4A). Control animals were wt animals crossed with ephrinB2-floxed mice that received 4-OHT. These animals were trained for fear conditioning and tested for long-term fear memory. There is no difference in freezing during training between the groups (F(1,16) = 0.007, p = 0.936) and no treatment × tone trial interaction (F(1.467,23.468) = 0.569, p = 0.522) (Fig. 4B). The ephrinB2-depleted mice (n = 8) were not different in contextual fear conditioning memory tested 24 h after training when compared to control mice (n = 10) (p = 0.897) (Fig. 4C). The animals were also not different in auditory fear conditioning memory tested 48 h after training (F(1,16) = 0.001, p = 0.971) (Fig. 4D). There is no treatment × tone trial interaction (F(4,64) = 0.998, p = 0.416). These results show that ephrinB2 in NPC is not needed for long-term memory formation.
A EphrinB2-floxed (B6.129S7-Efnb2tm2And/J) mice are crossed with C57BL/6-Tg(Nes-cre/ERT2)KEisc/J mice that are subjected to 4-OHT to remove ephrinB2 in NSCs. B There is no difference in freezing during training between the groups (F(1,16) = 0.007, p = 0.936). C The ephrinB2-depleted mice (n = 8) were not different in contextual fear conditioning memory tested 24 h after training when compared to control mice (p = 10) (p = 0.897). D The animals were not different in auditory fear conditioning memory tested 48 h after training (F(1,16) = 0.001, p = 0.971). E There is no difference in the number of cells labeled with DCX between the ephrinB2 KO (n = 8) and control (n = 7) animals (t(13) = 0.564, p = 0.582). F There is no effect on the number of NeuN-labeled cells between the ephrinB2 KO (n = 8) and control (n = 10) animals (t(16) = 0.037, p = 0.971). G DCX and NeuN labeled cells in Nes-CRE/floxed-ephrinB2 and Wt/floxed-ephrinB2 mice. Data are presented as mean ± SEM.
We examined the effects of deleting ephrinB2 in NSC on the amounts of immature neurons and mature neurons in BLA by monitoring doublecortin (DCX) and NeuN cells, respectively, after fear memory tests. We found no effect on the number of DCX-labeled cells between the ephrinB2 KO (n = 8) and control animals (n = 7) (t(13) = 0.564, p = 0.582) (Fig. 4E) and no effect on the number of NeuN-labeled cells between the ephrinB2 KO (n = 8) and control animals (n = 10) (t(16) = 0.037, p = 0.971) (Fig. 4F).
Increasing ephrinB2 in the basolateral amygdala enhances long-term fear memory formation
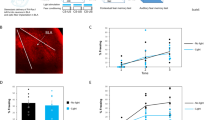
The previous observations show that removing ephrinB2 from excitatory neurons or astrocytes in BLA impaired the formation of long-term fear memory. We were, therefore, interested to explore the possibility that increasing ephrinB2 in BLA during learning will enhance the formation of long-term fear memory. Toward that end, we applied ephrinB2-Fc into the BLA 30 min before fear conditioning training and explored the effects on long-term fear memory (EphrinB2-Fc: EphrinB2-IgG1 chimera is typically used for ephrinB2 application5). There is no difference in freezing during training between the groups (F(1,17) = 0.132, p = 0.721) and no treatment × tone trial interaction (F(2,34) = 0.722, p = 0.493) (Fig. 5C). Our results show that injection of ephrinB2-Fc into BLA has no effect on long-term contextual fear conditioning when compared to Fc-injected animals (control group for injection of Fc per se) (t(17) = 1.194, p = 0.249) (Fig. 5D). In contrast, auditory fear conditioning memory was markedly enhanced in the ephrinB2-Fc group (n = 9) compared to the Fc-injected control group (n = 10) when tested 48 h after training (F(1,17) = 9.668, p = 0.006) (Fig. 5E). There is no treatment × tone trial interaction (F(4,68) = 0.267; p = 0.898). Thus, activation of the ephrinB2 system in the BLA during training enhances long-term fear memory formation.
A Mice were injected with ephrinB2-Fc or control Fc into the BLA 30 min before fear conditioning (0.1μg/μl, 0.5 μl in each BLA). Contextual fear conditioning was tested 24 h after fear conditioning and auditory fear memory was tested 48 h after fear conditioning. B Cannula tip placements in the BLA. C There is no difference in freezing during training between the groups (F(1,17) = 0.132, p = 0.721). D Injection of ephrinB2-Fc into BLA does not affect long-term contextual fear conditioning when compared to Fc-injected animals (t(17) = 1.194, p = 0.249). E Auditory fear memory is significantly enhanced after injection of ephrinB2-Fc (n = 9) into the BLA compared to Fc injection (n = 10) (F(1,17) = 9.668, p = 0.006). F Fear-conditioned mice injected with ephrinB2 into the BLA (n = 6) had a significantly increased number of c-Fos cells in the BLA 1.5 h after fear conditioning compared to fear-conditioned mice injected with Fc control (n = 6) (p = 0.026). G Representative labeling of c-Fos in BLA. Arrows show examples of cells labeled with c-Fos in BLA. H EphrinB2 per se did not increase the number of c-Fos in BLA of mice subjected to the conditioning box only (n = 7) when compared to mice subjected to the conditioning box only and injected with Fc (n = 6) (t(11) = 0.561, p = 0.586). Data are presented as mean ± SEM.
To further explore the effects of ephrinB2 in BLA on fear conditioning enhancement we monitored whether ephrinB2 can increase fear conditioning-induced cell activation in BLA. To monitor the number of active cells in BLA we measured c-Fos-labeled cells in BLA. c-Fos level is correlated with neuronal activity32. We revealed that fear-conditioned mice injected with ephrinB2-Fc in BLA (n = 6) had a significantly increased number of c-Fos cells in the BLA 1.5 h after conditioning compared to fear-conditioned mice injected with Fc control (n = 6) (p = 0.026; Fig. 5F, G). EphrinB2 per se did not increase the number of c-Fos in BLA of mice subjected to the conditioning box only (n = 7) when compared to mice subjected to the conditioning box only and injected with Fc (n = 6) (t(11) = 0.561, p = 0.586; Fig. 5H). These results show that ephrinB2 led to an increased ability of fear conditioning to activate cells in the BLA and set the threshold for fear memory formation in BLA.
Discussion
EphrinB2 is a key protein in neurons that is involved in the development of the brain, synaptic plasticity, and neuronal functions such as regulation of AMPA and NMDA receptors in the synapse, neuronal morphology, and neurogenesis5,6,7. However, its role in memory formation is not known. In this study, we were, therefore, interested to examine whether ephrinB2 is involved in fear memory formation. Toward that end, we removed ephrinB2 from excitatory neurons in the basolateral amygdala (BLA), a brain region needed for fear memory formation. Deleting ephrinB2 in excitatory neurons in the BLA impaired auditory and contextual long-term fear conditioning memory. EphrinB2 is also expressed in astrocytes. We, therefore, deleted it from astrocytes and revealed that removing ephrinB2 impaired contextual and auditory long-term fear conditioning memory. We further found that deleting ephrinB2 from astrocytes reduced the level of the glutamate transporter EAAT1 in astrocytes in BLA and that this transporter is needed in BLA for fear long-term memory formation. We next examined whether ephrinB2 is needed in neural stem cells (NSCs) for the formation of long-term fear conditioning memory. We found that knocking out ephrinB2 from NSCs does not affect the ability of the mice to form long-term memory and the levels of immature and mature cells in the BLA. We further found that increasing ephrinB2 in BLA during fear conditioning training enhanced the formation of long-term fear memory and the ability of fear conditioning to activate cells in BLA therefore setting the threshold for fear memory formation in BLA.
We show that knocking out ephrinB2 from excitatory cells impairs long-term, but not short-term, memory. EphrinB2 may affect several key processes in excitatory neurons that can affect memory formation. First, it affects glutamate receptors. For example, it was shown that ephrinB2 enhances AMPA receptors constitutive internalization and reduces synaptic transmission6. If such an effect on AMPA receptors occurs in our experimental setup in the BLA it does not cross the threshold needed for the mice to sense the CS and US properly and to associate between them as it does not affect short-term memory. AMPA receptors are needed for the formation of both short-term and long-term memories33. Another possibility for ephrinB2 effects is on dendritic spines morphology. Reverse signaling of ephrinBs is required for correct spine morphogenesise.g.7.It was shown that ephrinB2-mediated activation of the EphB receptors accelerates dendritic spine development34. In another study it was shown that in VEGFR2-ephrinB2 compound mice (Nes-cre Kdrlox/+ Efnb2lox/+) dendritic branching and spine head size are reduced35. In addition, VEGF stimulation of hippocampal neurons led to a strong increase in the number of spines an effect that was abolished in neurons where the ephrinB2 was knocked out. Astrocytic ephrinB1 negatively influences new spine formation in the hippocampus during learning and memory36,37. Fear conditioning induces spine morphogenesis38,39 and impairing long-term fear memory formation reverses dendritic spines changes40. Thus, it could be that ephrinB2 in excitatory neurons is needed for the alteration in morphology or formation of spines in BLA.
We show that deleting ephrinB2 in astrocytes impaired long-term fear memory but not short-term memory. These results show that ephrinB2 in astrocytes is needed for the consolidation of long-term fear memory in BLA. We further explored the molecular mechanism whereby ephrinB2 can regulate the ability of the animals to form long-term memory. Glial glutamate transporters are known to regulate synaptic transmission by clearing glutamate from the synaptic cleft25,26. We were, therefore, interested to explore whether deleting ephrinB2 in astrocytes affects EAAT1 level. We show that deleting astrocytic ephrinB2 in the BLA reduced the level of EAAT1 in astrocytes in the BLA. Reduction of glutamate transporters might have an effect on the brain faculties needed to associate the CS and the US caused by some adverse effects. However, we found that reduction of ephrinB2 in BLA has no effect on short-term memory which shows that deleting ephrinB2 does not affect the ability of the animal to perceive the CS and US stimuli and to associate between them. Since EAAT1 level is already reduced when the ephrinB2 KO animals are trained it infers that EAAT1 does not affect CS and US processing needed for their association. Next, we were interested to study whether EAAT1 glutamate transporter is needed for long-term fear conditioning memory formation. We, therefore, inhibited EAAT1 during fear conditioning and studied the effects on long-term fear memory. Inhibition of EAAT1 in BLA impaired long-term fear memory formation. Cumulatively, these observations argue that the role of EAAT1 in long-term memory formation can be caused by deficiencies in its acute activity during learning rather than its long-lasting effects induced by its reduced level. Thus, the clearance of glutamate from the perisynaptic and extrasynaptic area by astrocytic EAAT1 during learning and shortly afterward is needed for the proper formation of long-term memory. It implies that if glutamate is present in the perisynaptic area at higher concentration it impairs the proper encoding of long-term memory.
It would be interesting to explore also the roles of EAAT2 in fear memory formation in BLA. EAAT2 distribution and effects may be different from EAAT1. EAAT1 is the only glutamate transporter that appears to be selectively expressed in astroglial cells in the central nervous system whereas EAAT2 is found also in neurons41. EAAT2 is the major glutamate transporter in the mouse brain. Deletion of the EAAT2 gene in mice caused an almost complete loss (about 95%) of glutamate uptake activity42. EAAT1 and EAAT2 resemble each other as they have relatively short cycling times and fairly similar affinities. For example, the Km values reported for l-glutamate transport by EAAT1 is 20 ± 3 μM and for EAAT2 18 ± 3 μM in oocytes and 48 ± 10 μM, 97 ± 4 μM in mammalian cells43. Glutamate transporters are affected by ephrinA344,45. It would be interesting to explore the roles of ephrinA3 in BLA in regulating glutamate reuptake and fear memory.
The results of the study show that ephrinB2 deletion impairs long-term fear memory. We, therefore, were interested to explore whether injection of ephrinB2 into the BLA will enhance long-term fear memory formation. Injection of ephrinB2 into the BLA enhanced long-term fear memory formation. Interestingly, although deleting ephrinB2 from the BLA impaired both contextual and auditory fear conditioning, injection of ephrinB2 into the BLA enhanced only auditory, but not contextual, long-term fear conditioning memory. One possible reason for this is that the injection of ephrinB2 into the BLA is local whereas deleting ephrinB2 from neurons in BLA can affect targets beyond the BLA where these neurons project to and ephrinB2 is expressed in these projections in the normal condition. Removing ephrinB2 from these projections could lead to an impairment of contextual fear conditioning. It could be that ephrinB2 enhances memory formation through the potentiation of NMDA receptors as ephrinB2 activation of EphB in primary cortical neurons potentiates NMDA receptor-dependent influx of calcium5. Moreover, increased activation of cultured neurons is observed after the application of glutamate together with ephrinB2 and not by the application of ephrinB2 alone5. NMDA receptors in BLA are needed for fear memory formatione.g.46. We also observed that ephrinB2 enhanced the ability of fear conditioning to activate cells in the BLA. Moreover, we show that ephrinB2 per se cannot activate the neurons in BLA but only together with fear conditioning. EphrinB2 may potentiate BLA neurons to be more responsive to the incoming inputs as shown in primary cortical neurons5.
In summary, our study shows that ephrinB2 in excitatory neurons and astrocytes in the BLA is essential for long-term fear memory formation and that ephrinB2 sets the threshold for fear memory formation in the BLA. EphrinB2 is therefore a key molecule affecting fear memory formation in the BLA and can be involved in mediating fear-related diseases. It would be interesting to explore whether individuals with different levels of ephrinB2 in the amygdala have different sensitivities for fearful situations, are more resilient when ephrinB2 level is lower, or on the other hand prone to stress and fear-related disorders when ephrinB2 is at a higher level.
Methods
Animals
Adult (8 weeks of age) male C57BL/6 J mice weighing 22–28 g (ordered from Envigo, Israel) were used for the ephrinB2-Fc experiments. C57BL/6-Tg(Nes-cre/ERT2)KEisc/J and B6.129S7-Efnb2tm2And/J were obtained from The Jackson Laboratory (ME, USA) and were bred at the University of Haifa. C57BL/6-Tg(Nes-cre/ERT2)KEisc/J (11 weeks old) were injected with 4-hydroxytamoxifen (4-OHT; Sigma-Aldrich, Cat#H6278) (i.p. 50 mg/kg in 5 mg/ml) once a week before the experiment. Wild-type littermates served as the control mice for the genetically modified mice. Mice were housed at 22 °C in a 12 h light/dark cycle, with free access to food and water. Behavior was performed during the daytime. Behavioral experiments were approved by the University of Haifa Institutional Committee for animal experiments in accordance with National Institutes of Health guidelines.
AAV production
ssAAV-1/2-mCaMKIIα-mCherry_2A_iCre-WPRE-SV40p(A) (titer: 4.5 x 10E12 vg/ml) (v227-1) and ssAAV-1/2-hGFAP-mCherry_iCre-WPRE-hGHp(A) (titer: 6.1 x 10E12 vg/ml) (v233-1) were obtained from Viral Vector Facility (VVF, University of Zurich).
AAV microinjection
Animals were anesthetized with Medetomidine (Domitor) 1 mg/ml and Ketamine 100 mg/ml cocktail, diluted in sterile isotonic saline (administered doses: Ketamine 50 mg/kg; Domitor 0.5 mg/kg; 100 µl/10 gm of animal body weight). Dipyrone (50%) was injected for analgesia before surgery and consecutive 3 days after surgery. AAV particles were injected (0.5 µl/hemisphere, 0.1 µl/min) aimed at the BLA. The coordinates for performing stereotactic surgery were measured according to the mouse bregma location (coordinates are in reference to bregma: anteroposterior (AP), −1.4; lateral (L) ± 3.3; and dorsoventral (DV), −4.65) in the Mouse brain atlas. Animals were allowed to recuperate for 4 weeks before behavioral experiments. After behavioral or histological procedures, the animals were perfused and the localization of AAVs was examined. Only mice with expression of mCherry within the borders of the BLA were included in the data analysis. The BLA borders were defined according to Allen Mouse Brain Atlas.
Cannulation and microinjection
Mice were anesthetized as above. Guide stainless-steel cannulas (23 gauge) were implanted bilaterally by stereotaxic surgery and fixed in place with dental cement (Neurostar stereo drive). Mice were implanted with chronic cannulas bilaterally 1.5 mm above areas of injection (injection areas target coordinates are as in AAV injection above). On the day of surgery animals received the antibiotic Baytril (5 mg/kg; Enrofloxacin) and Dipyrone (50%) for analgesia and were also injected for 3 consecutive days after surgery. The animals recovered for at least 7 days before the behavioral training. On the day of microinjection, the stylus was removed from the guide cannula and a 28-gauge injection cannula, extending 1.5 mm from the tip of the guide cannula was carefully placed. The injection cannula was connected via PE20, tubing backfilled with saline with a small air bubble separating the saline from the compound solution to a 10 μl Hamilton micro-syringe, driven by a microinjection pump (CMA/100, Carnegie Medicine; or PHD 2000, Harvard Apparatus). The solution was injected bilaterally at a rate of 0.5 μl/minute. Following injection, the injection cannula was left for an additional 1 min before withdrawal to minimize dragging of injected liquid along the injection track. UCPH-101 was obtained from Abcam (ab120309) and DMSO, Dimethyl sulfoxide was from Sigma-Aldrich (D8418). The concentration of UCPH-101 in DMSO is 10nmol/µl. EphrinB2-Fc was obtained from R&D Systems (496-EB-200) and control anti-human IgG, Fc fragment specific from Jackson ImmunoResearch Laboratories, (109-001-008). The concentration of ephrinB2-Fc and Fc is 0.1μg/μl in PBS. We injected 0.5μl into each side of the brain area 30 min before fear conditioning.
Fear conditioning
On the day of training, mice were placed in a training chamber (Coulbourn Instruments)40,47. Mice were allowed to acclimate in the chamber for 2 min and then subjected to 3 pairs of tone (Conditioned stimulus (CS) - 20 s, 2.8 kHz, 85 dB) that co-terminated with a foot shock (Unconditioned stimulus (US) - 2 s, 0.8 mA). The inter-trial interval was 120 s. Mice were tested for contextual fear conditioning in the same context (for 9 min) 1 h after training for short-term memory or 24 h after training for long-term memory. Mice were tested for auditory fear conditioning in a different context 2 h after training for short-term memory or 48 h after training for long-term memory (2 min pre-tones followed by 5 CSs with inter-tone interval of 120 s) . The long-term and short-term memories tests were performed in different groups. Behavior was recorded and the video images were transferred to a computer equipped with an analysis program (FreezeFrame). The percentage of changed pixels between two adjacent 0.25 s images was used as a measure of activity.
Immunohistochemistry
Animals were anesthetized by inhalation of isoflurane and transcardially perfused with 50 ml of cold 0.01 M PBS solution, followed by 50 ml of 4% paraformaldehyde in 0.01 M PBS containing 5% sucrose. Brains were excised and postfixed in a fixative solution containing 30% sucrose and 1% paraformaldehyde in 0.01 M PBS for 48 h at 4°C. After postfixation, brains were frozen at −80°C until sectioning. Forty-five micrometer brain sections were sliced with a cooled cryostat (Leica, CM1900). Slices were washed with 0.01 M PBS and then blocked for 1 h at room temperature with 0.01 M PBS containing 3% BSA. For anti-NeuN experiment (Fig. 1) slices were permeabilized and blocked for 1 h at room temperature with PBS (1X) containing 0.3% Triton and 3% BSA. Sections were then incubated overnight at 4 °C with the anti-ephrinB2 (R&D Systems, AF496-SP1:100), anti-EAAT1 (Cell Signaling Technology, #5684; 1:100), anti-DCX (Cell Signaling Technology, #4604; 1:800), or anti-NeuN (Sigma-Aldrich, #ABN78; 1:500) in PBS. For anti-NeuN in Fig. 1, the antibody was incubated in the blocking solution. After three washes in 0.01 M PBS, the slices were subjected to Alexa-488 anti-rabbit or Alexa-488 anti-goat secondary antibody (1:500 Molecular Probes) for 1.5 h at room temperature. The slices were then washed twice with PBS 0.01 M and mounted on Super Frost-coated slides with Slow Fade antifade medium (Invitrogen). The level of labeling was calculated (area of antibody stain/area examined in mCherry labeled cells or the number of cells stained) in excitatory neurons or astrocytes using the Imaris software.
c-Fos immunohistochemistry
Brains were cut on the microtome to slices of 45 µm. The slices were washed in PBS (500 µL) 3 times for 10 min each on a shaker followed by 3 times PTx (500 µL) wash for 5 min each on a shaker. The slices were blocked for 2 h with blocking solution (500 µL 10% FBS, 150 µL 0.3% BSA, 4.35 ml 0.3% PTx). We then incubated the slices with anti-c-Fos antibody (Synaptic Systems # 226 008, 1:500) in blocking solution overnight at 4 °C. Slices were then washed 3 times with PTx for 5 min each. We then incubated with the secondary anti-rabbit antibody (Alexa Fluor 488; Invitrogen; 1:1000) in a blocking solution at RT for 1 h on a shaker. The slices were then washed 3 times with PBS for 5 min each. The slices were loaded on the microscope slides, dried in the dark for 10–30 min, and mounted with a cover slip. The number of labeled cells was calculated using the ImageJ.
GFAP immunohistochemistry
Slices were permeabilized for 30 minutes with 0.5% Triton in PBS (1X) and washed thrice for 5 min each. Slices were then blocked for 1 h at room temperature with PBS (1X) containing 3% BSA. Sections were then incubated overnight at 4 °C on a shaker with anti-GFAP (1:200; Sigma-Aldrich MAB360) in PBS (1X). The next day, after three washes in PBS (1X) for 5 min each, the slices were subjected to Alexa-488 anti-mouse (Thermo Fisher Scientific, # A-21202; 1:500) for 2 h at room temperature on the shaker, making sure that the well plate was protected against light. The slices were then washed thrice with PBS (1X) for 5 min each and mounted on slides with Slow Fade antifade medium.
Western blot analysis
After decapitation, forebrain tissue was sliced and the amygdala was collected from both hemispheres using a mouse tissue puncher. Samples were homogenized directly in SDS sample buffer (62.5 mM Tris–HCl, pH 6.8, 10% glycerol, 2.3% sodium dodecyl sulfate (SDS) and 5% β-mercaptoethanol). Samples were heated at 80 oC for 5 minutes, centrifuged for 5 min at 10,000 rpm and the supernatant liquid was stored at −20oC. The samples (10 µl) were subjected to SDS polyacrylamide gel electrophoresis (SDS–PAGE) followed by Western blot analysis. Blots were blocked in Tris-buffered saline solution containing 0.1% Tween 20 (TBST) and 5% BSA for 1 hour at room temperature. The blots were incubated with anti-EAAT1 (1:1000, Cell Signaling Technology) or anti-mCherry (1:1000; Novus Biologicals) overnight at 4 oC on a shaker. Blots were washed thrice with TBST and incubated for 1 h at room temperature with horseradish peroxidase anti-rabbit secondary antibody (1:10,000; Jackson ImmunoResearch Laboratories for anti-EAAT1) and anti-mouse secondary antibody (1:10,000; Jackson ImmunoResearch Laboratories for anti-mCherry). The blots were then washed thrice in TBST and exposed to enhanced chemiluminescence with the 20-500-120 EZ ECL kit (Biological Industries, Kibbutz Beit-Haemek, Israel). Blots were exposed in ChemiDox XRS (Bio-Rad), and analyzed by Quantity-One 4.5.0 software (Bio-Rad). The value of the mCherry band was normalized by subtracting it from 1 in all groups. The value of EAAT1 was divided by the results of the normalized mCherry.
Genotyping
Ear punches were added to 50 μl of Extraction Reagent (Quantabio, USA) and the samples were heated to 95 oC for 30 min. The samples were cooled to room temperature and 50μl of Stabilization Buffer was added. The samples were vortexed and the DNA was extracted for PCR. The reaction mixture for PCR contained 12.5 μl of Ready mix (Bio-ReadyMix, Bio-lab, Israel), 1μl of each primer (10uM), and finally nuclease-free water was added to make up the total volume to 25μl. The primers for Nes-Cre: ATG CAG GCA AAT TTT GGT GT and CGC CGC TAC TTC TTT TCA AC for the Transgene and AGT GGC CTC TTC CAG AAA TG and TGC GAC TGT GTC TGA TTT CC for Internal Positive Control. The primers for floxed-efnB2: AAG TTA TAA GCT TCA ACG CGT CC and GAG CCC CAG GTT CTA GAA TAA to detect the transgene and GCT GCC CGC GGC CGG TCC CAA CG and CCG TTA GTG GCA ACG TCC TCC GTC CTC to detect wild-type mice. The PCR cycle was: (1) 94 °C for 3 min, (2) 94 °C for 1 min, (3) 60 °C for 1 min, (4) 72 °C for 1 min, (5) Steps 2–4 repeated for 33 cycles, (6) 72 °C for 7 min, (7) hold at 4 °C. The reactions were analyzed by gel electrophoresis (1.5% agarose gel). Expected bands for B6.129S7-Efnb2tm2And/J mouse- Mutant = 350 bp, Heterozygote = 350 bp and 500 bp, Wild type = 500 bp and for the C57BL/6-Tg(Nes-cre/ERT2)KEisc/J mouse- Transgene = 150 bp, Internal positive control = 521 bp.
Statistics and reproducibility
Data are presented as mean ± SEM, with individual data points. All measurements were taken from distinct samples. Data were analyzed with repeated measures ANOVA for multiple tone presentation behavioral analysis and with t-test (2-tailed) or non-parametric Mann-Whitney U test (when not distributed normally; tested with Shapiro–Wilk test) for contextual fear conditioning, IHC and Western blot experiments with an α level of 0.05 using the PASW statistics 25.
Reporting summary
Further information on research design is available in the Nature Portfolio Reporting Summary linked to this article.
References
Klein, R. Bidirectional modulation of synaptic functions by Eph/ephrin signaling. Nat Neurosci. 12, 15–20 (2009).
Lai, K. O. & Ip, N. Y. Synapse development and plasticity: roles of ephrin/Eph receptor signaling. Curr Opin Neurobiol. 19, 275–283 (2009).
Hruska, M. & Dalva, M. B. Ephrin regulation of synapse formation, function and plasticity. Mol Cell Neurosci. 50, 35–44 (2012).
Henderson, N. T. & Dalva, M. B. EphBs and ephrin-Bs: Trans-synaptic organizers of synapse development and function. Mol Cell Neurosci. 91, 108–121 (2018).
Takasu, M. A., Dalva, M. B., Zigmond, R. E. & Greenberg, M. E. Modulation of NMDA receptor-dependent calcium influx and gene expression through EphB receptors. Science 295, 491–495 (2002).
Essmann, C. L. et al. Serine phosphorylation of ephrinB2 regulates trafficking of synaptic AMPA receptors. Nat Neurosci. 11, 1035–1043 (2008).
Segura, I., Essmann, C. L. & Weinges, S. Acker-Palmer A. Grb4 and GIT1 transduce ephrinB reverse signals modulating spine morphogenesis and synapse formation. Nat Neurosci. 10, 301–310 (2007).
Fanselow, M. S. & LeDoux, J. E. Why we think plasticity underlying Pavlovian fear conditioning occurs in the basolateral amygdala? Neuron. 23, 229–232 (1999).
LeDoux, J. E. Emotion circuits in the brain. Annu Rev Neurosci. 23, 155–184 (2000).
Davis, M. & Whalen, P. J. The amygdala: vigilance and emotion. Mol Psychiatry. 6, 13–34 (2001).
Sah, P., Faber, E. S., Lopez De Armentia, M. & Power, J. The amygdaloid complex: anatomy and physiology. Physiol Rev. 83, 803–834 (2003).
Maren, S. Synaptic mechanisms of associative memory in the amygdala. Neuron. 47, 783–786 (2005).
Schafe, G. E., Nader, K., Blair, H. T. & LeDoux, J. E. Memory consolidation of Pavlovian fear conditioning: a cellular and molecular perspective. Trends Neurosci. 24, 540–546 (2001).
Rodrigues, S. M., Schafe, G. E. & LeDoux, J. E. Molecular mechanisms underlying emotional learning and memory in the lateral amygdala. Neuron. 44, 75–91 (2004).
Johansen, J. P., Cain, C. K., Ostroff, L. E. & LeDoux, J. E. Molecular mechanisms of fear learning and memory. Cell. 147, 509–524 (2011).
Suthard, R. L. et al. Basolateral amygdala astrocytes are engaged by the acquisition and expression of a contextual fear memory. J Neurosci. 43, 4997–5013 (2023).
Shelkar, G. P., Liu, J. & Dravid, S. M. Astrocytic NMDA Receptors in the basolateral amygdala contribute to facilitation of fear extinction. Int J Neuropsychopharmacol. 24, 907–919 (2021).
Johansen, J. P. et al. Optical activation of lateral amygdala pyramidal cells instructs associative fear learning. Proc Natl Acad Sci USA. 107, 12692–12697 (2010).
d’Aquin, S. et al. Compartmentalized dendritic plasticity during associative learning. Science. 376, eabf7052 (2022).
Gerety, S. S. & Anderson, D. J. Cardiovascular ephrinB2 function is essential for embryonic angiogenesis. Development. 129, 1397–1410 (2002).
McDonald, A. J., Muller, J. F. & Mascagni, F. GABAergic innervation of alpha type II calcium/calmodulin-dependent protein kinase immunoreactive pyramidal neurons in the rat basolateral amygdala. J Comp Neurol. 446, 199–218 (2002).
Kol, A. & Goshen, I. The memory orchestra: the role of astrocytes and oligodendrocytes in parallel to neurons. Curr Opin Neurobiol. 67, 131–137 (2021).
Doron, A. et al. Hippocampal astrocytes encode reward location. Nature. 609, 772–778 (2022).
Castellanos-Jankiewicz, A. et al. Hypothalamic bile acid-TGR5 signaling protects from obesity. Cell Metab. 33, 1483–1492.e10 (2021).
Beart, P. M. & O’Shea, R. D. Transporters for L-glutamate: an update on their molecular pharmacology and pathological involvement. Br J Pharmacol. 150, 5–17 (2007).
Tzingounis, A. V. & Wadiche, J. I. Glutamate transporters: confining runaway excitation by shaping synaptic transmission. Nat Rev Neurosci. 8, 935–947 (2007).
Jensen, A. A. et al. Discovery of the first selective inhibitor of excitatory amino acid transporter subtype 1. J Med Chem. 52, 912–915 (2009).
Jhaveri, D. J. et al. Evidence for newly generated interneurons in the basolateral amygdala of adult mice. Mol Psychiatry. 23, 521–532 (2018).
Park, J. H., Cho, H., Kim, H. & Kim, K. Repeated brief epileptic seizures by pentylenetetrazole cause neurodegeneration and promote neurogenesis in discrete brain regions of freely moving adult rats. Neuroscience. 140, 673–684 (2006).
Roeder, S. S. et al. Evidence for postnatal neurogenesis in the human amygdala. Commun Biol. 5, 366 (2022).
Bernier, P. J., Bedard, A., Vinet, J., Levesque, M. & Parent, A. Newly generated neurons in the amygdala and adjoining cortex of adult primates. Proc Natl Acad Sci USA 99, 11464–11469 (2002).
Chung, L. A Brief Introduction to the Transduction of Neural Activity into Fos Signal. Dev Reprod. 19, 61–67 (2015).
Rumpel, S., LeDoux, J., Zador, A. & Malinow, R. Postsynaptic receptor trafficking underlying a form of associative learning. Science. 308, 83–88 (2005).
Henkemeyer, M., Itkis, O. S., Ngo, M., Hickmott, P. W. & Ethell, I. M. Multiple EphB receptor tyrosine kinases shape dendritic spines in the hippocampus. J Cell Biol. 163, 1313–1326 (2003).
Harde, E. et al. EphrinB2 regulates VEGFR2 during dendritogenesis and hippocampal circuitry development. Elife. 8, e49819 (2019).
Koeppen, J. et al. Functional consequences of synapse remodeling following astrocyte-specific regulation of ephrin-B1 in the adult hippocampus. J Neurosci. 38, 5710–5726 (2018).
Nguyen, A. Q. et al. Astrocytic Ephrin-B1 controls synapse formation in the hippocampus during learning and memory. Front Synaptic Neurosci. 12, 10 (2020).
Ostroff, L. E., Cain, C. K., Bedont, J., Monfils, M. H. & Ledoux, J. E. Fear and safety learning differentially affect synapse size and dendritic translation in the lateral amygdala. Proc Natl Acad Sci USA. 107, 9418–9423 (2010).
Heinrichs, S. C. et al. Dendritic structural plasticity in the basolateral amygdala after fear conditioning and its extinction in mice. Behav Brain Res. 248, 80–84 (2013).
Costa, J. F., Dines, M., Agarwal, K. & Lamprecht, R. Rac1 GTPase activation impairs fear conditioning-induced structural changes in basolateral amygdala neurons and long-term fear memory formation. Neuropsychopharmacology 48, 1338–1346 (2023)..
Danbolt, N. C., Furness, D. N. & Zhou, Y. Neuronal vs glial glutamate uptake: Resolving the conundrum. Neurochem Int. 98, 29–45 (2016).
Tanaka, K. et al. Epilepsy and exacerbation of brain injury in mice lacking the glutamate transporter GLT-1. Science. 276, 1699–1702 (1997).
Arriza, J. L. et al. Functional comparisons of three glutamate transporter subtypes cloned from human motor cortex. J Neurosci. 14, 5559–5569 (1994).
Filosa, A. et al. Neuron-glia communication via EphA4/ephrin-A3 modulates LTP through glial glutamate transport. Nat Neurosci. 12, 1285–1292 (2009).
Carmona, M. A., Murai, K. K., Wang, L. Roberts, A. J., & Pasquale, E. B. Glial ephrin-A3 regulates hippocampal dendritic spine morphology and glutamate transport. Proc. Natl Acad. Sci. USA 106, 12524–12529 (2009).
Rodrigues, S. M., Schafe, G. E. & LeDoux, J. E. Intra-amygdala blockade of the NR2B subunit of the NMDA receptor disrupts the acquisition but not the expression of fear conditioning. J Neurosci. 21, 6889–6896 (2001).
Alapin, J. M. et al. Activation of EphB2 forward signaling enhances memory consolidation. Cell Rep. 23, 2014–2025 (2018).
Acknowledgements
This work was supported by the Israel Science Foundation to R.L. We would like to thank Boris Shklyar from the Bioimaging Unit, Faculty of Natural Sciences, University of Haifa, for his help with the imaging.
Author information
Authors and Affiliations
Contributions
K.A. performed the experiments, analyzed the data and commented on the manuscript, A.F. performed the NeuN experiment in Fig. 1, and R.L. supervised the project analyzed the data and wrote the manuscript.
Corresponding author
Ethics declarations
Competing interests
The authors declare no competing interests.
Peer review
Peer review information
Communications Biology thanks the anonymous reviewers for their contribution to the peer review of this work. Primary Handling Editor: Benjamin Bessieres.
Additional information
Publisher’s note Springer Nature remains neutral with regard to jurisdictional claims in published maps and institutional affiliations.
Rights and permissions
Open Access This article is licensed under a Creative Commons Attribution-NonCommercial-NoDerivatives 4.0 International License, which permits any non-commercial use, sharing, distribution and reproduction in any medium or format, as long as you give appropriate credit to the original author(s) and the source, provide a link to the Creative Commons licence, and indicate if you modified the licensed material. You do not have permission under this licence to share adapted material derived from this article or parts of it. The images or other third party material in this article are included in the article’s Creative Commons licence, unless indicated otherwise in a credit line to the material. If material is not included in the article’s Creative Commons licence and your intended use is not permitted by statutory regulation or exceeds the permitted use, you will need to obtain permission directly from the copyright holder. To view a copy of this licence, visit http://creativecommons.org/licenses/by-nc-nd/4.0/.
About this article
Cite this article
Agarwal, K., Farhat, A. & Lamprecht, R. EphrinB2 in excitatory neurons and astrocytes in the basolateral amygdala controls long-term fear memory formation. Commun Biol 7, 1165 (2024). https://doi.org/10.1038/s42003-024-06844-9
Received:
Accepted:
Published:
DOI: https://doi.org/10.1038/s42003-024-06844-9
- Springer Nature Limited