Abstract—
Variability in infection rates of trematodes Diplostomum pseudospathaceum in 0+ rainbow trout Oncorhynchus mykiss under different hydraulic regimes was studied in the experimental setup with regulated flow rates and environmental heterogeneity. The average infection rate in the conditions of the current was 1.5 times less than that in the stagnant water. An increase of the flow rate from 3.2 to 11.3 cm/s did not result in lower infection rates, while the interindividual variability in the infection rate (coefficient of variation) tended to increase along with changing the still-water conditions (20%) to the high flow rate environments (40%) within the experiment. A decrease in the average infection rate and an increase in the variability within the heterogenous flow of water could indicate the fact that some fish effectively avoid infection. It may be caused by redistribution of cercariae suspended in the water column across microhabitats with different flow rates together with fish behavior which allows them to choose sites with low parasite concentration.
Similar content being viewed by others
Avoid common mistakes on your manuscript.
INTRODUCTION
Small-scale structures of flows along with the other abiotic factors form a mosaic of physical heterogeneity of environments influencing vital functions of hydrobionts and their interactions (Hughes and Dill, 1990; Wiens, 2002; Mikheev, 2006). Many studies on ecology and behavior of juvenile salmonids in the fresh-water phase of their life paid attention to the hydraulic and topographic structure of habitats, availability of refuges and food, intra- and interspecies competition, and impacts of predators (Chapman, 1966; Parker and Barnes, 2014; Lusardi et al., 2018). When studying interactions between biotic and abiotic factors, researchers more often focused on the role of currents in the migratory and feeding behavior (Fausch, 1984; Grant, 1990; Pavlov and Mikheev, 2017) as well as the physical shelters which provide defense from predators (Wilzbach, 1985; Anholt and Werner, 1995; Mikheev et al., 2010).
Parasites as an important factor affecting the behavior and the biotic relationships have been recently taken into consideration (Barber et al., 2000; Moore, 2002). Parasites along with predators are assigned to the category of “natural enemies” in ecology (Raffel et al., 2008; Koprivnikar and Penalva, 2015). However, the effects of parasites and predators are more frequently considered separately, despite there are reasons to consider their interactons. An animal under the parasite impact may be more (Lafferty, Morris, 1996; Seppälä et al., 2004; Mikheev et al., 2010) or less (Weinreich et al., 2013; Gopko et al., 2015) available for a predator. In presence of a predator, an animal spending time and energy on defense behavior may become more available for parasites (Gopko and Mikheev, 2017).
Currents represent a most important factor affecting the distribution of small hydrobionts with poor swimming capacity. The researchers of fish ecology and behavior are usually interested in the passive displacement or “drift” of such small hydrobionts as a factor affecting the availability of food items for fish, Salmonidae juveniles included (Hughes and Dill, 1990; Fausch, 1993). It is no wonder that the vast majority of publications on the topic is associated with rivers and streams.
It has been previously shown that cercariae of trematodes Diplostomum pseudospathaceum, which use fish as an intermediate host, predominantly penetrate the fish host through the gills via the ventilatory current of water entering its mouth (Mikheev et al., 2014). This mechanism is very important for a parasite, since cercariae exhibit active locomotion in attempts to penetrate the fish only if they are not further than 3–8 mm from the fish (Haas et al., 2008). The fish ventilatory water current serves efficiently as a mechanism of parasite transmission to a fish in still water environments.
However, it poses challenges as to whether the efficiency of this mechanism tends to vary in the running water environments and whether the rate of a flow and its heterogeneity in the presence of obstacles can play any role in it.
In order to answer these challenges, the experiments in the setup with regulated flow rate and shelter availability for fish (yearlings of the rainbow trout Oncorhynchus mykiss) were conducted. The cercariae D. pseudospathaceum were used as parasites. Working hypotheses suggested changes in the infection rate in the running water environments: (1) an increase in the infection rate because of parasite aggregation in low-current sites which fish use as shelters and (2) a decrase in the infection rate if fish chose habitats with high-rate flows, where the parasite concentration and their locomotory performance are lower.
MATERIALS AND METHODS
The study was performed at the Konnevesi biological station, University of Jyväskylä (Finland). The yearlings O. mykiss (average fork length ± SD 6.97 ± 0.71 cm) were obtained from the fish farm, where they were kept in artesian water to prevent infestation with parasites before the experiments. The fish were kept in a 150-L running-water reservoir at 14–15°С, subjected to the photoperiod of 15 : 9 h (15 h light : 9 h dark). The fish were fed twice a day with pelleted food of appropriate size. Cercariae of D. pseudospathaceum were obtained from 20 mollusks Lymnaea stagnalis collected from Lake Konnevesi. The mollusks were kept in dark in the fridge at the temperature of 7°C. For 3–4 h before the experiment, the mollusks were exposed to light at the temperature of 20°C to stimulate cercaria release. Cercaria concentration comprised 150 ind/L across all the experiments. For each replicate, three randomly chosen fish were acclimated to the conditions of the experiment in the free of parasites water for 15 min. Thereafter, added cercariae were evenly distributed across the experimental tank. This procedure was conducted in both standing and running waters. In the latter case, parasites were added into standing water just before the initiation of water current. The experimental procedures to study the relationships in the “O. mykiss–D. pseudospathaceum” system were previously reported in detail (Seppälä et al., 2004; Mikheev et al., 2010).
The experiments were conducted in the closed not flow-through circular channels of 78 cm length, 20 cm width, and 13 cm depth each for 15 min, The channels were filled with 24 L lake water filtered through a plankton sieve (with mesh size of 150 μm). The channel bottom and walls were dark grey in color. Shelters, ceramic plates of 12 × 12 cm in size with edges based on the rounded stones of ~4 cm in diameter, were put on the channel bottom in half of the experiments. Two speed water-running modes (3.2 and 11.3 cm/s) were provided with the mode adjustable fan placed 5 cm above the water surface. The flow rate on the surface was measurd with a drifter. In preliminary observations with the fully developed flow in the channel (it took ~5 min), we qualitatively assessed flow rate gradients. Two most distinct gradients were observed (1) from the surface to the bottom (slowest at the bottom) and (2) from the external wall to the internal wall of a channel (the flow rate decreasing at the internal wall and fully disappeared at the bottom). In the experiments with shelters, the formation of locations with almost stagnant water was revealed downstream from the stones. Therefore, hydraulic heterogeneity was observed in both experimental variants tending to become more complicated in the presence of shelters.
Overall, six experiments with different combinations of certain hydraulic conditions and shelter presence/absence were carried out: in still water, at low flow rate of 3.2 cm/s, and at high flow rate of 11.3 cm/s. Each mode involved the variants with and without a shelter. After the experiment, fish from each replicate were kept in separate flow-through tanks, where they were fed as previously. Two days later, when parasites reached their area of localization (fish eye lenses), fish were killed with MS-222 to calculate the number of metacercariae under the microscope. Six replicates per experiment for a set of 6 experiments were performed. Overal, 108 fish specimens were examined. All of the examined fish were found infected at different infection rates. Distribution of data on the mean infection rate (mean total number of metacercariae in both eyes per fish) was tested for normality with the Shapiro–Wilks W-test for each of six experimental groups. The values for all of the samples met the criteria for normality, which allowed us to use the two-way ANOVA test for subsequent analyses followed by the pairwise comparisons between the samples (the Tukey test).
RESULTS
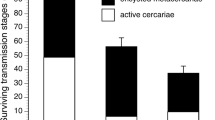
The mean infection rate in fish under both low and high flow rates decreased by a factor of 1.5 compared to that in the still water experiments (Fig. 1). The difference between fish infection rates in standing and running water environments was highly significant (Two-way ANOVA: F = 34.5, p < 0.0001). The influence of the shelter effect was not significant (p = 0.63). However, a significant interaction between these (flow and shelter) factors was revealed (p = 0.012). It means that the shelter effects on the infection rate in the standing and running water environments were multidirectional. The pairwise comparison between the infection rates in all of the “shelter-no shelter” variants under different hydraulic conditions did not reveal any significant differences (the Tukey test)
Along with the results on the mean infection rate, changes in the coefficient of variation under different hydraulic conditions proved to be no less interesting and unexpected (Fig. 2). The lowest coefficient of variation value (~20%) was recorded in fish, which were exposed to parasites in the still water. Variability in the infection rate increased along with increasing the flow rate. Thus, it comprised ~30 and ~40% under low and high flow rates, respectively. Considering such a high variability and a rather low mean level of infection, some fish in the high flow-rate environments were poorly infected, containing one to seven metacercariae per fish at the mean values of about 20 (Fig. 1).
Coefficient of variation (CV) of the mean infection rate of Diplostomum pseudospathaceum metacercariae in yearlings of the rainbow trout Oncorhynchus mykiss in running and standing waters. Designations as in Fig 1.
DISCUSSION
The rate at which metacercariae D. pseudospathaceum invaded the yearlings of the rainbow trout O. mykiss in flowing environments was significantly lower than that in standing water. This effect did not depend on the flow rate, since the average number of parasites in the fish eye lenses at both the low (3.2 cm/s) and high (11.3 cm/s) flow rates was 1.5 times less than in the still water. Another trend was revealed for the other important population parameter, individual variability in the infection rate. An increase in the flow rate induced a steady increase in the coefficient of variation in the infection rate from 20% in the still water to 40% in the high-flow environment. Two possible mechanisms are considered herein to explain these results. The first concept supposes that under the effect of water flow, a disruption in ventilatory water current produced by the fish which carry suspended parasites to the gills may occur. The key role of D. pseudospathaceum cercariae penetrating the fish-host through the gills was previously shown (Mikheev et al., 2014). If the disruption in the ventilatory water current played a key role in formation of the infection pattern, the observed effect would most probably depend on the flow rate. Another probable mechanism of a decrease in the infection rate under the flow conditions is associated with the formation of heterogeneous hydraulic structure of a flow. Sites with high and low flow rates are interspersed, and even stable stagnant water areas could occur close to the bottom. Suspended parasites may accumulate theredecreasing their concentration in the other habitats with higher flow rates. Fish juveniles feeding in the water flow choose habitats with optimal flow rates, where the potential prey are plentiful (Fausch, 1984; Hughes and Dill, 1990; Mikheev, 2006). When choosing such areas, fish may not only collect more food but also reduce parasite infection.
Hydraulic heterogeneity tends to increase along with an increased flow rate, which makes the pattern of spatial distribution of areas with different flow rates more contrast. This fact can explain a significant increase in the individual infection rate variability with increased flow rate. Depending on the social status, the impact of predators, and the feeding motivation, some fish may spend more time under the higher flow conditions, while others prefer the environments with lower current. The mean infection rate depends on the number of fish in a group, attracted to certain microhabitats.
Another important aspect of the habitat physical heterogeneity is availability of landmarks and shelters due to their significant effect on the fish behavior (Mikheev, 2006; Mikheev et al., 2010), which, in turn, may affect susceptibility of fish to parasite infection (Mikheev et al., 2020). No significant effect of a shelter onthe infection rate was found under any of the tested hydraulic regimes. It may be explained by short duration of experiments (15 min), while the territorial relationships between fish which affect infection rate, need more time to develop (at least 30 min) (Mikheev et al., 2020). Nevertheless, a significant interaction between the “flow” and “shelter” factors suggests a certain role of shelters in the fish-parasite relationship even in the short-term experiments. An increase in the infection rate in the stagnant water with the shelter suggests that in this case fish faster explore the environment and begin competing for shelters, which increases the ventilatory currents and parasite transmission. Competition for a shelter in stagnant water has previously been shown to cause higher infection rates (Mikheev et al., 2020). It probably requires more time for Exploration of the novel environment in the water flow under complicated and changeable conditions needs more time before the start of the competition. In order to test this assumption, the long-term experiments with more replicates are required.
The obtained results show that small-scale water heterogeneity based mainly on the flow pattern is important not only in the migratory, defense, and feeding behavior of fish. Hydraulic structure of a habitat may affect fish infection rate through (1) the modification of fish behavior and spatial distribution, and (2) the parasite heterogenous distribution, which can provide the fish with opportunity to choose sites with lower risk of infection. Both the solitary and shoaling fish can avoid the parasite aggregations, while the latter can do it more successfully (Mikheev et al., 2013). The effects associated with the hydraulic heterogeneity of environments may be important in the “parasite–host” interactions in both the lotic (Hockley et al., 2014) and limnetic ecosystems, for instance, in the shallow waters of lakes and reservoirs, where the currents are primarily driven by winds. Despite little attention focused on the currents in the shallow-water habitats in water bodies of a lake type compared with rivers, we consider their ecological role underestimated. This role may be important in not only “predator–prey” but also “host–parasite” interactions. The latter may be especially important, since the shallow-water habitats in lakes and reservoirs are characterized by considerable invertebrate biodiversity, in particular, mollusks, which serve as intermediate hosts for many macroparasites.
CONCLUSIONS
Search and choice of an optimal habitat, which provides fish with maximum fitness includes favorable cost/benefit ratio and reliable defense against predators. Our results suggest that behavior minimizing the risk of parasite infection is also important as a part of the habitat choice strategy. Small-scale heterogeneity of environment, which is most distinctly expressed by flow regimes, can provide fish with opportunity to choose an optimal location relative to their status and needs, distribution of resources and threats within a habitat. Besides avoiding the high risk of parasite infection by uninfected fish in certain habitats, the problem of choosing the optimal habitat also exists for the fish already infected. Many parasites including D. pseudospathaceum are capable of manipulating the behavior of their host making it more available for the next host, the fish-eating birds. Vulnerability of infected fish for birds in a hydraulically heterogeneous habitat may depend on fish choosing an area with certain flow rate and shelter availability. When checking this suggestion, one has to take into account fish infection rate and the age of metacercariae, which is associated with their capability to manipulate the host behavior.
REFERENCES
Anholt, B.R. and Werner, E.E., Interaction between food availability and predation mortality mediated by adaptive behavior, Ecology, 1995, vol. 76, no. 7, pp. 2230−2234. https://doi.org/10.2307/1941696
Barber, I., Hoare, D., and Krause, J., Effects of parasites on fish behaviour: A review and evolutionary perspective, Rev. Fish Biol. Fish., 2000, vol. 10, no. 2, pp. 131–165. https://doi.org/10.1023/A:1016658224470
Chapman, D.W., Food and space as regulators of salmonid populations in streams, Am. Natur., 1966, vol. 100, no. 913, pp. 345–357. https://doi.org/10.1086/282427
Fausch, K.D., Profitable stream positions for salmonids: Relating specific growth rate to net energy gain, Can. J. Zool., 1984, vol. 62, no. 3, pp. 441–445. https://doi.org/10.1139/z84-067
Fausch, K.D., Experimental analysis of microhabitat selection by juvenile steelhead (Oncorhynchus mykiss) and coho salmon (O. kisutch) in a British Columbia stream, Can. J. Fish. Aquat. Sci., 1993, vol. 50, no. 6, pp. 1198–1207. https://doi.org/10.1139/F93-136
Gopko, M.V., and Mikheev, V.N., Parasitic manipulations of the host phenotype: Effects in internal and external environments, Biol. Bull. Rev., 2019, vol. 9, no. 1, pp. 1–28.
Gopko, M.V., Mikheev, V.N., and Taskinen, J., Changes in host behaviour caused by immature larvae of the eye fluke: Evidence supporting the predation suppression hypothesis, Behav. Ecol. Sociobiol., 2015, vol. 69, no. 10, pp. 1723–1730. https://doi.org/10.1007/s00265-015-1984-z
Grant, J.W.A., Aggressiveness and foraging behavior of young-of-the-year brook charr, Salvelinus fontinalis, Can. J. Fish. Aquat. Sci., 1990, vol. 47, no. 5, pp. 915–920. https://doi.org/10.1139/F90-105
Haas, W., Beran, B., and Loy, C., Selection of the host’s habitat by cercariae: From laboratory experiments to the field, J. Parasitol., 2008, vol. 94, no. 6, pp. 1233–1238. https://doi.org/10.1645/GE-1192.1
Hockley, F.A., Wilson, C.A.M.E., Graham, N., and Cable, J., Combined effects of flow condition and parasitism on shoaling behaviour of female guppies Poecilia reticulate, Behav. Ecol Sociobiol., 2014, vol. 68, no. 9, pp. 1513–1520. https://doi.org/10.1007/s00265-014-1760-5
Hughes, N.F. and Dill, L.M., Position choice by drift-feeding salmonids: model and test for Arctic grayling (Thymallus arcticus) in subarctic mountain streams, interior Alaska, Can. J. Fish. Aquat. Sci., 1990, vol. 47, no. 10, pp. 2039–2048. https://doi.org/10.1139/F90-228
Koprivnikar, J. and Penalva, L., Lesser of two evils? Foraging choices in response to threats of predation and parasitism, PLoSOne, 2015, vol. 10, no. 1, Article e0116569. https://doi.org/10.1371/journal.pone.0116569
Lafferty, K.D. and Morris, A.K., Altered behaviour of parasitized killifish increases susceptibility to predation by bird final hosts, Ecology, 1996, vol. 77, no. 5, pp. 1390–1397. https://doi.org/10.2307/2265536
Lusardi, R.A., Jeffres, C.A., and Moyle, P.B., Stream macrophytes increase invertebrate production and fish habitat utilization in a California stream, River Res. Applic., 2018, vol. 34, no. 8, pp. 1003–1012. https://doi.org/10.1002/rra.3331
Mikheev, V.N., Neodnorodnost’ sredy i troficheskie otnosheniya u ryb (Habitat Heterogeneity and Trophic Relationships in Fish), Moscow: Nauka, 2006.
Mikheev, V.N., Afonina, M.O., and Pavlov, D.S., Habitat heterogeneity and fish behavior: Units of heterogeneity as a resource and as a source of information, J. Ichthyol., 2010, vol. 50, no. 5, pp. 386–395. https://doi.org/10.1134/S0032945210050048
Mikheev, V., Pasternak, A., Taskinen, J., and Valtonen, E.T., Parasite induced aggression and impaired contest ability in a fish host, Parasites Vectors, 2010, vol. 3, Article 17. https://doi.org/10.1186/1756-3305-3-17
Mikheev, V.N., Pasternak, A.F., Taskinen, J., and Valtonen, E.T., Grouping facilitates avoidance of parasites by fish, Ibid., 2013, vol. 6, Article no. 301. https://doi.org/10.1186/1756-3305-6-301
Mikheev, V.N., Pasternak, A.F., Taskinen, J., and Valtonen, E.T., Increased ventilation by fish leads to a higher risk of parasitism, Ibid., 2014, vol. 7, Article 281. https://doi.org/10.1186/1756-3305-7-281
Mikheev, V.N., Pasternak, A.F., Morozov, A.Yu., and Taskinen, J., Innate antipredator behavior can promote infection in fish even in the absence of predators, Behav. Ecol., 2020, vol. 31, no. 1, pp. 267–276. https://doi.org/10.1093/beheco/arz188
Moore, J., Parasites and the Behaviour of Animals, Oxford: Oxford Univ. Press, 2002.
Parker, T.M. and Barnes, M.E., Rearing velocity impacts on landlocked fall Chinook Salmon (Oncorhynchus tshawytscha) growth, condition, and survival, Open J. Anim. Sci., 2014, vol. 4, no. 5, pp. 244–252. https://doi.org/10.4236/OJAS.2014.45031
Pavlov, D.S. and Mikheev, V.N., Downstream migration and mechanisms of dispersal of young fish in rivers, Can. J. Fish. Aquat. Sci., 2017, vol. 74, no. 8, pp. 1312–1323. https://doi.org/10.1139/cjfas-2016-0298
Raffel, T.R., Martin, L.B., and Rohr, J.R., Parasites as predators: Unifying natural enemy ecology, Trends Ecol. Evol., 2008, vol. 23, no. 11, pp. 610–618. https://doi.org/10.1016/j.tree.2008.06.015
Seppälä, O., Karvonen, A., and Valtonen, E.T., parasite-induced change in host behaviour and susceptibility to predation in an eye fluke-fish interaction, Anim. Behav., 2004, vol. 68, no. 2, pp. 257–263. https://doi.org/10.1016/j.anbehav.2003.10.021
Weinreich, F., Benesh, D.P., and Milinski, M., Suppression of predation on the intermediate host by two trophically-transmitted parasites when uninfective, Parasitology, 2013, vol. 140, no. 1, pp. 129–135. https://doi.org/10.1017/S0031182012001266
Wiens, J.A., Riverine landscapes: Taking landscape ecology into the water, Freshw. Biol., 2002, vol. 47, no. 4, pp. 501–515. https://doi.org/10.1046/j.1365-2427.2002.00887.x
Wilzbach, M.A., Relative roles of food abundance and cover in determining the habitat distribution of stream-dwelling cutthroat trout (Salmo clarki), Can. J. Fish. Aquat. Sci., 1985, vol. 42, no. 10, pp. 1668–1672. https://doi.org/10.1139/F85-208
Funding
The study was carried out with a financial support of the Russian Foundation for Basic Research (project 20-04-00239, data processing and analysis) and the Russian Science Foundation (project 19-14-00015-P, article writing) and within the State Task FMWE-2021-0007.
Author information
Authors and Affiliations
Corresponding author
Ethics declarations
Conflict of interests. The authors declare that they have no conflict of interests.
Statement on the welfare of animals. All applicable international, national, and/or institutional guidelines for the care and use of animals were followed.
Additional information
Translated by O. Zhiryakova
Rights and permissions
Open Access. This article is licensed under a Creative Commons Attribution 4.0 International License, which permits use, sharing, adaptation, distribution and reproduction in any medium or format, as long as you give appropriate credit to the original author(s) and the source, provide a link to the Creative Commons license, and indicate if changes were made. The images or other third party material in this article are included in the article’s Creative Commons license, unless indicated otherwise in a credit line to the material. If material is not included in the article’s Creative Commons license and your intended use is not permitted by statutory regulation or exceeds the permitted use, you will need to obtain permission directly from the copyright holder. To view a copy of this license, visit http://creativecommons.org/licenses/by/4.0/.
About this article
Cite this article
Mikheev, V.N., Pasternak, A.F. & Taskinen, J. Hydraulic Habitat Structure Impacts Risk of Trematode Infection. J. Ichthyol. 62, 1190–1195 (2022). https://doi.org/10.1134/S0032945222060170
Received:
Revised:
Accepted:
Published:
Issue Date:
DOI: https://doi.org/10.1134/S0032945222060170




 )—without the shelter, (
)—without the shelter, ( )—with the shelter, and
(
)—with the shelter, and
( )—standard error.
)—standard error.