Abstract
Background
The association between marital status and gallbladder cancer (GBC) remains uncertain. This study aimed to verify the relationship between marital status and GBC and construct a prognostic nomogram to predict the impact of marital status on GBC patients.
Method
GBC patients were divided into married and unmarried groups using data from the Surveillance, Epidemiology, and End Results (SEER) database. We employed competing risk analyses, propensity score matching (PSM), and Kaplan-Meier survival analyses. The relationship between marital status and GBC was then verified, and the predicted nomogram was constructed.
Results
A total of 3913 GBC patients were obtained from the SEER database, and an additional 76 GBC patients from Hangzhou Traditional Chinese Medicine Hospital were selected as the external validation group. The competing risk analysis revealed a significant disparity in the 5-year cumulative incidence of cancer-specific death (CSD) between the two cohorts (59.1% vs. 65.2%, p = 0.003). Furthermore, the multivariate competing hazards regression analysis identified a significant association (HR, 1.17; 95% CI, 1.04–1.31; p = 0.007) between marital status and CSD. To assess the 1-, 3-, and 5-year risks of CSD, a comprehensive competing event nomogram was constructed using factors derived from the multivariate analysis. The area under the receiver operating characteristic curve (AUC) values for the 1-, 3-, and 5-year training cohorts were 0.806, 0.785, and 0.776, respectively. In the internal validation cohort, these values were 0.798, 0.790, and 0.790, while the external validation cohort exhibited AUC values of 0.748, 0.835, and 0.883 for the corresponding time intervals. Furthermore, calibration curves demonstrated a commendable level of concordance between the observed and predicted probabilities of CSD.
Conclusion
Marriage was a protective factor for GBC patients after taking competing risk into consideration. The proposed nomogram demonstrated exceptional predictive power.
Similar content being viewed by others

Introduction
Socioeconomic factors have been actively investigated for their impact on cancer prognosis and their potential to facilitate novel public health interventions. Among these factors, marital status has been identified as a critical factor related to survival outcomes in multiple malignancies, including colorectal [1], gastric [2], pancreatic [3], hepatic [4], and breast cancers [5]. Studies have reported that married individuals exhibit a more favorable prognosis and a lower mortality rate compared to those who are single, separated, widowed, or divorced [1, 2, 4]. The positive role of marriage in tumor prognosis may be attributed to the associated pleasure and positive mentality. Additionally, marriage positively affects endocrinology, and adequate social support may help reduce cortisol levels, which have been linked to the survival rates of cancer patients [6, 7].
Gallbladder cancer (GBC) is the predominant malignancy within the biliary tract, ranking as the sixth most prevalent neoplasm within the digestive system [8]. This malignancy is characterized by a median survival time of less than one year and a five-year overall survival (OS) rate below 5% [9, 10]. Identifying prognostic factors associated with GBC is crucial for patient management.
The effect of marital status on survival of GBC patients has been studied, however, the results remain equivocal. Several studies have shown the positive role of marriage in survival, with widowed individuals having a higher likelihood of dying from the disease [11,12,13,14]. According to Bai’s study, widowed patients faced the highest risk of death compared to other groups, and marital status was identified as a significant predictive risk factor for survival in GBC patients who underwent surgical resection [12]. In Li’s research, GBC patients in TNM stages I, II, and IV had a higher survival rate when they were married [13]. However, Randi et al. suggested that a substantial correlation between GBC risk and marital status was not discernible, and there was no consistent excess risk for divorced or widowed individuals [15]. The inconsistent results in these studies might be due to a lack of consideration for competing risks, which can obscure the observation of the event of interest during follow-up. In other words, cancer-related deaths may compete with deaths from other causes, affecting the results of traditional prognostic analyses.
GBC is a type of aggressive malignancy. Establishing a survival prediction model for GBC patients will be of great significance for their treatment selection and follow-up. However, in previously published literature, survival prediction models for GBC did not take into account the impact of competitive risk, which could affect the model’s application in clinical practice. Therefore, we proposed a competing event nomogram in our research, which considers the impact of competitive risk on survival and is expected to provide higher prediction accuracy.
Materials and methods
Data source and patient selection
During the interval from 2010 to 2015, relevant patient data were extracted from the SEER 18 Regs Custom Data Set. Additionally, for external validation, data from January 2012 to December 2018 were obtained from the Hangzhou Traditional Chinese Medicine (TCM) Hospital.
The inclusion criteria were defined as follows: GBC patients were identified based on the third edition of the International Classification of Diseases for Oncology (ICD-10: C23.051). Eligible patients needed to meet four specific conditions: (1) be over eighteen years old; (2) have GBC as their initial or only cancer diagnosis; (3) have a minimum survival duration of more than one month. Exclusion criteria included patients with a history of other malignancies, those who died from other cancers, and those with incomplete demographic, clinicopathological, therapeutic, or follow-up data. Figure 1 illustrates the systematic procedure employed for patient selection.
Clinicopathological variables
Clinical parameters encompassed a range of variables, including marital status, age, gender, race, grade, American Joint Committee on Cancer (AJCC) stage, pathology, T stage, N stage, M stage, tumor size, surgery, radiotherapy, and chemotherapy. Marital status was categorized into married and unmarried groups, with the latter further subdivided into single, separated, divorced, and widowed. The transition of the AJCC TNM staging system from the 7th to the 8th edition was conducted in accordance with data sourced from the SEER database.
Statistical analysis
The chi-square test was used to compare categorical variables. Kaplan-Meier survival analyses were employed to assess both OS and cancer-specific survival (CSS). To thoroughly examine the potential influence of competing risk factors, the patient cohort was stratified into three distinct endpoints: survival, cancer-specific death (CSD) and other caused death (OCD). Fine and Grey’s proportional subdistribution hazard model was created, and cumulative incidence function analysis was executed using the R package “cmprsk” [16].
Propensity score matching (PSM) is a contemporary statistical methodology aimed at mitigating the impact of confounding variables in research [17]. Using the nearest-neighbor technique, a 1:1 match ratio was established for patients within the married and unmarried groups, with a 0.1 caliper applied. The research included a comprehensive set of factors for matching purposes, such as age, gender, race, grade, AJCC stage, pathology, TNM stage, tumor size, surgery, radiotherapy, and chemotherapy. The SD was employed as a metric to illustrate variations in variables before and after PSM, with optimal balance achieved when SD ≤ 0.1 following the matching process [18]. Implementation of the PSM technique was executed utilizing the “Matching” R package.
Subsequently, a randomized division assigned patients into two cohorts: a training set (50%) and a validation set (50%). Utilizing prognostic parameters inherent to the competing risk model, a 1-, 3-, and 5-year CSD nomogram was meticulously developed for the training dataset, drawing insights from Zhang’s comprehensive study as a foundational reference [19]. The model’s predictive performance was evaluated using calibration plots and the assessment of the area under the receiver operating characteristic curve (AUC). Calibration curves, generated through 1000 bootstrap resamples, facilitated a comparative analysis between observed and expected survival probabilities. Additionally, receiver operating characteristic (ROC) curves were employed to compute AUC values, effectively illustrating the model’s predictive power.
The R statistical analysis and visualization software, specifically version 4.0.3 (http://www.r-project.org), constituted the analytical framework for all investigations. Significance in statistical outcomes was determined by a two-tailed p-value of less than 0.05.
Results
Patients’ characteristics
From 2010 to 2015, the SEER database documented 3193 patients diagnosed with GBC, while the external validation cohort comprised 76 individuals from Hangzhou TCM Hospital. Among these patients, 1689 were identified as married, leaving 1504 categorized as unmarried. Significant differences were observed between the two cohorts in terms of age, gender, AJCC stage, N stage, M stage, radiation, and chemotherapy (all p < 0.05). Notably, individuals in the married group exhibited higher proportions of receiving radiation and chemotherapy, elevated AJCC, N, and M stages, as well as a tendency towards a younger age demographic.
PSM was employed to mitigate the disparities in baseline data between the groups. The SD for most metrics, as illustrated in Figure S1, were consistently below 0.1, confirming the effectiveness of the balancing procedure. Ultimately, two cohorts, each comprising 1091 patients, were delineated: the married group (n = 1091) and the unmarried group (n = 1091). Comprehensive depictions of baseline characteristics before and after PSM are presented in Table 1; Fig. 1.
Survival analysis
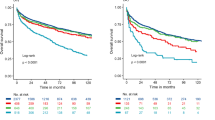
Before PSM, CSS didn’t differ between married and unmarried patients (P = 0.061) (Fig. 2A). After PSM, unmarried patients had worse survival outcomes than married patients in OS and CSS (all P < 0.001) (Fig. 2C and S2C). Then the unmarried patients were stratified into widows, singles, separated individuals, and divorced individuals. The survival analysis indicated that married patients had the best prognosis, while widowed individuals had the worst prognosis in terms of OS and CSS both before and after PSM (all P < 0.05) (Fig. 2B and D, S2B, and S2D).
Considering the existence of competing risk, we further performed competing risk analysis. Before PSM, the cumulative incidence of death curves revealed no significant differences in CSD between married and unmarried patients in the overall population (P = 0.265). However, after PSM, unmarried patients suffered higher CSD than married patients (P = 0.003). Subgroup analyses demonstrates that unmarried patients had significantly elevated CSD in the age, grade II, grade III, and surgery categories (all p < 0.05) (Fig. 3). In the multivariate competing risks regression analysis, a statistically significant association between marital status and CSD was affirmed (HR, 1.17; 95% CI, 1.04–1.31; p = 0.007) (Table S1). This result indicated that marital status remained a significant prognostic factor for GBC patients after considering competing risk.
Univariate and multivariate analysis
An additional independent analysis was undertaken, wherein patients were randomly assigned to two groups for the development of a prognostic model: a training cohort (50%, n = 1091) and a validation cohort (50%, n = 1091). Through univariate analysis, the 1-, 3-, and 5-year cumulative incidence function (CIF) values of CSD were determined in the training cohort. Variables such as tumor size, grade, T stage, N stage, and M stage, as well as chemotherapy and surgery, exhibited significant associations with CSD. Subsequently, the multivariate evaluation using the Fine-Gray proportional subdistribution hazards model was employed to identify crucial factors (p < 0.05). In the multivariate competing risk analysis, it was discerned that grade, T stage, N stage, M stage, tumor size, surgery, and chemotherapy independently predicted CSD in patients with GBC (Table 2).
Constructing and verifying the nomogram
After the multivariate analysis, a competing event nomogram was devised to ascertain the probabilities of CSD at 1, 3, and 5 years, integrating pertinent variables (Fig. 4). Tumor size, grade, T stage, N stage, M stage, surgery, and chemotherapy were included in the predictive model. The cumulative total points, derived by summing the scores corresponding to each patient’s prognostic features, provide clinicians with a tool to assess the likelihood of CSD at distinct time intervals.
Both internal and external validation cohorts were employed to evaluate the model’s performance. In the training cohort, the 1-, 3-, and 5-year AUC values were 0.806, 0.785, and 0.776, respectively. Correspondingly, the internal validation cohort exhibited AUC values of 0.798, 0.790, and 0.790, while the external validation cohort demonstrated values of 0.748, 0.835, and 0.883. These results, depicted in Fig. 5A-C, collectively underscore the model’s robust discrimination ability. Calibration plots were used to evaluate the model’s prediction accuracy, revealing a commendable consistency between expected and observed probabilities of CSD across the three datasets (Fig. 5D-F). The comprehensive findings affirm the strong predictability and high credibility of our developed nomogram.
Discussion
In this study, we performed competing risk analysis and Kaplan-Meier survival analysis using data from 3193 GBC patients sourced from the SEER database. The results revealed that marital status conferred a survival benefit in terms of OS; however, its influence on CSS was not evident in these patients. The study underscores the significance of CSS as a more reliable metric for gauging the true impact of marital status, given that OS may be influenced by OCD [20]. Additionally, the presence of OCD may introduce complexities in the accurate diagnosis of CSD [21]. Complementary competing risk analyses were conducted to explore potential competing risks of mortality in this context. After meticulous 1:1 PSM, a notable discrepancy emerged in the 5-year cumulative incidence of CSD between the two cohorts (59.1% vs. 65.2%, p = 0.003). Conversely, a comparable cumulative incidence of OCD was observed (12.8% vs. 13.5%, p = 0.601). In-depth subgroup analyses and multivariate competing hazards regression analysis substantiated a significant association between marital status and CSD. This suggests that the variance in OCD between the two groups may be partially explicable by unaccounted comorbidities not detailed in the SEER database and a lower prevalence of high-risk factors in the unmarried cohorts. It is noteworthy that prolonged survival times are inherently linked to an elevated risk of OCD, alongside other cancer types and cardiovascular conditions.
Based on our findings, unmarried patients exhibited notably elevated CSD rates in the age, grade II, grade III, and surgical categories compared to their married counterparts. Notably, at grade IV, there was no significant difference in CSD between the two groups (p = 0.515), possibly due to the relatively smaller sample size. Subsequent classification of unmarried patients into widowed, divorced, single, and separated groups revealed that widowed individuals experienced the lowest CSS. Our observations align with Song et al.‘s investigation [11], suggesting an increased risk of cancer-specific events among bereaved individuals and highlighting marital status as a predictive factor for clinical outcomes in GBC patients. Additionally, Bai et al.‘s research further substantiates these findings [12], indicating that bereaved patients exhibited lower 5-year CSS compared to individuals who were married, never married, or divorced/separated. The fact that the marital status might have changed during the follow-up but wasn’t recorded is another limatition. In summary, married GBC patients have a better prognosis. We can help GBC patients live longer by promoting marital status, improving health education before discharge, and working together.
We introduced a nomogram tailored for individual patient survival estimations, derived from the outcomes of competing risk analysis. The predictive model integrated grade, tumor size, surgery, chemotherapy, and T, N, M stages. Examination of the maximum points assigned to the combined parameters identified T stage and grade as high-risk variables. Notably, prior research, such as the study conducted by Li et al., [13] has highlighted the correlation between these risk factors and GBC. In that study, multivariate analysis using Cox regression identified age, race, grade, histologic type, AJCC stage, SEER stage, and marital status as independent predictive factors for GBC survival. Furthermore, Li et al.‘s investigation on prognostic estimation for patients over 45 undergoing gallbladder adenocarcinoma resection identified seven independent survival predictors associated with OS [14]. These predictors, as determined by multivariate Cox regression analysis, included age, marital status, grade, T stage, M stage, tumor size, and the logarithm of positive lymph nodes. We observed that our findings were fundamentally consistent, differing mainly in the variables included for analysis. Similar to our study, the multivariate competing risk analysis identified elevated tumor diameter, increased grade, advanced TNM stage, absence of treatment, and lack of surgery as independent risk factors for CSD. In comparison to conventional multivariate regression models, the nomogram offers a graphical representation of the individual probabilities of 1-, 3-, and 5-year CSD. Additionally, the model was validated in an independent cohort, affirming its robust reliability and commendable predictive capacity. This nomogram can be used to forecast survival times for GBC patients and has demonstrated that chemotherapy and surgery can extend survival, which is highly beneficial for clinical diagnosis and treatment.
Earlier investigations had established a correlation between marital status and cancer outcomes; however, the underlying mechanisms remained elusive. Married individuals tend to have better access to standard healthcare and receive more social support compared to their single counterparts. A study by Hoskins KF et al. on US women uncovered racial disparities in social determinants of health and markers indicative of aggressive tumor biology, including genetic biomarkers, which elucidated the observed survival gap in early-stage, estrogen receptor-positive breast cancer [22]. Additionally, a review by Coughlin SS highlighted the association of social variables with cancer stage and survival [23]. Prolonged exposure to chronic stress has been shown to alter the regulation of both the immunological and endocrine systems, consequently increasing susceptibility to cancer and influencing survival rates [24, 25]. Cortisol, a commonly used biomarker, serves to gauge the immune response and the functionality of the hypothalamic-pituitary-adrenal axis [26, 27]. Notably, cancer patients who receive psychosocial support exhibit lower cortisol levels, which enhances their prospects [28]. Scholarly investigations suggest that psychosocial therapies represent a promising approach for improving immune-related health, given their consistent association with enhanced immune system functionality [29]. Marital union, as previously noted, is considered a major source of social support. The diminished survival outcomes observed in unmarried patients may be attributed to the lack of psychological support that is often provided by marriage. Consequently, we propose that unmarried GBC patients may require increased social support and psychological interventions.
Limitation
The study exhibits several limitations. Firstly, as is inherent in retrospective studies, selection bias remains a significant constraint. Secondly, the nomogram is applicable only to patients with characteristics similar to those in the propensity score analysis, as determined by the application of PSM. Additionally, the relatively modest size of the external validation sample highlights the need for further external multicenter prospective validations.
Conclusion
By employing 1:1 PSM and competing risk analysis, our study reveals potential advantages associated with marital status in GBC patients. Specifically, married GBC patients demonstrate improved CSS compared to their unmarried counterparts. The nomogram, which shows significant predictive accuracy, assists in estimating individualized probabilities of 1-, 3-, and 5-year CSD for these patients. Although our results suggest a beneficial influence of marital status, further validation through RCTs and additional research is essential to confirm these findings.
Data availability
The datasets generated and analyzed in the course of this inquiry are accessible within the SEER database, accessible at https://seer.cancer.gov/. For further inquiries, the corresponding author can be reached for additional information.
References
Lee S, et al. Marital status, living arrangement, and Cancer recurrence and survival in patients with stage III Colon Cancer: findings from CALGB 89803 (Alliance). Oncologist. 2022;27:e494–505.
Li B, Hu X. Time-varying effects of Marital Status on gastric Cancer: a Population-based study. Cancer Manag Res. 2019;11:10949–55.
Zhou H, et al. Marital status is an independent prognostic factor for pancreatic neuroendocrine tumors patients: an analysis of the Surveillance, Epidemiology, and end results (SEER) database. Clin Res Hepatol Gastroenterol. 2017;41:476–86.
Zhang W, et al. Prognostic value of marital status on stage at diagnosis in hepatocellular carcinoma. Sci Rep. 2017;7:41695.
Martínez ME, et al. Prognostic significance of marital status in breast cancer survival: a population-based study. PLoS ONE. 2017;12:e0175515.
Alyabsi M, Ramadan M, Algarni M, Alshammari K, Jazieh AR. The effect of marital status on stage at diagnosis and survival in saudis diagnosed with colorectal cancer: cancer registry analysis. Sci Rep. 2021;11:8603.
Rachidi S, Deng Z, Sullivan DY, Lipson EJ. Shorter survival and later stage at diagnosis among unmarried patients with cutaneous melanoma: a US national and tertiary care center study. J Am Acad Dermatol. 2020;83:1012–20.
Lee M-H, et al. A Metallomic Approach to Assess associations of serum metal levels with gallstones and Gallbladder Cancer. Hepatol Baltim Md. 2020;71:917–28.
Hundal R, Shaffer EA. Gallbladder cancer: epidemiology and outcome. Clin Epidemiol. 2014;6:99–109.
Shen H, et al. PLEK2 promotes gallbladder cancer invasion and metastasis through EGFR/CCL2 pathway. J Exp Clin Cancer Res CR. 2019;38:247.
Song W, Miao D-L, Chen L. Survival rates are higher in married patients with biliary tract cancer: a population-based study. Oncotarget. 2018;9:9531–9.
Bai D-S, Chen P, Qian J-J, Jin S-J, Jiang G-Q. Effect of marital status on the survival of patients with gallbladder cancer treated with surgical resection: a population-based study. Oncotarget. 2017;8:26404–13.
Li X, et al. The influence of marital status on survival of gallbladder cancer patients: a population-based study. Sci Rep. 2017;7:5322.
Li P, Song L. Prognostic Evaluation for Patients over 45 Years Old with Gallbladder Adenocarcinoma Resection: A SEER-Based Nomogram Analysis. BioMed Res. Int 2020;1–11.
Randi G, et al. Marital status and cancer risk in Italy. Prev Med. 2004;38:523–8.
T F, Jm AL. B. & A, K. An R function to non-parametric and piecewise analysis of competing risks survival data. Comput Methods Programs Biomed. 100, (2010).
Austin PC. An introduction to Propensity score methods for reducing the effects of confounding in Observational studies. Multivar Behav Res. 2011;46:399–424.
Austin PC. Balance diagnostics for comparing the distribution of baseline covariates between treatment groups in propensity-score matched samples. Stat Med. 2009;28:3083–107.
Zhang Z, Geskus RB, Kattan MW, Zhang H, Liu T. Nomogram for survival analysis in the presence of competing risks. Ann Transl Med. 2017;5:403.
Fu J, et al. De-escalating chemotherapy for stage II colon cancer? Ther Adv Gastroenterol. 2019;12:1756284819867553.
Scrucca L, Santucci A, Aversa F. Regression modeling of competing risk using R: an in depth guide for clinicians. Bone Marrow Transpl. 2010;45:1388–95.
Hoskins KF, et al. Association of Social Determinants and Tumor Biology with racial disparity in Survival from Early-Stage, hormone-dependent breast Cancer. JAMA Oncol. 2023;9:536–45.
Coughlin SS. Social determinants of breast cancer risk, stage, and survival. Breast Cancer Res Treat. 2019;177:537–48.
Glaser R, Kiecolt-Glaser JK. Stress-induced immune dysfunction: implications for health. Nat Rev Immunol. 2005;5:243–51.
Batty GD, Russ TC, Stamatakis E, Kivimäki M. Psychological distress in relation to site specific cancer mortality: pooling of unpublished data from 16 prospective cohort studies. BMJ. 2017;356:j108.
Figueira JA, et al. Predisposing factors for increased cortisol levels in oral cancer patients. Compr Psychoneuroendocrinology. 2022;9:100110.
Lee DY, Kim E, Choi MH. Technical and clinical aspects of cortisol as a biochemical marker of chronic stress. BMB Rep. 2015;48:209–16.
Mészáros Crow E, et al. Psychosocial interventions reduce cortisol in breast cancer patients: systematic review and meta-analysis. Front Psychol. 2023;14:1148805.
Shields GS, Spahr CM, Slavich GM. Psychosocial interventions and Immune System function: a systematic review and Meta-analysis of Randomized clinical trials. JAMA Psychiatry. 2020;77:1031–43.
Acknowledgements
Not applicable.
Funding
Funding for this research came from the Hangzhou Agricultural and Social Development Research Guide Project (20220919Y116) and the Science and Technology Program of Traditional Chinese Medicine in Zhejiang Province (2023ZL112).
Author information
Authors and Affiliations
Contributions
WJ-C, DW-D, and HM-J initiated and played pivotal roles in conceptualizing and designing the manuscript. JF-T, HJ-J and BB-L also contributed to the manuscript. Statistical analysis and database organization were handled by YY-X. Each author thoroughly reviewed and approved the submitted version of the paper, actively participating in the editing process.
Corresponding author
Ethics declarations
Ethics approval and consent to participate
sGiven that the patient data sourced from the SEER database is publicly accessible, the necessity for ethical clearance was obviated. The Ethics Committee of Hangzhou TCM Hospital meticulously scrutinized and sanctioned the experiment involving human subjects. In compliance with national legislation and institutional guidelines, the procurement of written informed consent was deemed unnecessary for participation in this study.
Consent for publication
Not applicable.
Competing interests
The authors declare no competing interests.
Additional information
Publisher’s Note
Springer Nature remains neutral with regard to jurisdictional claims in published maps and institutional affiliations.
Electronic supplementary material
Below is the link to the electronic supplementary material.
Rights and permissions
Open Access This article is licensed under a Creative Commons Attribution-NonCommercial-NoDerivatives 4.0 International License, which permits any non-commercial use, sharing, distribution and reproduction in any medium or format, as long as you give appropriate credit to the original author(s) and the source, provide a link to the Creative Commons licence, and indicate if you modified the licensed material. You do not have permission under this licence to share adapted material derived from this article or parts of it. The images or other third party material in this article are included in the article’s Creative Commons licence, unless indicated otherwise in a credit line to the material. If material is not included in the article’s Creative Commons licence and your intended use is not permitted by statutory regulation or exceeds the permitted use, you will need to obtain permission directly from the copyright holder. To view a copy of this licence, visit http://creativecommons.org/licenses/by-nc-nd/4.0/.
About this article
Cite this article
Jin, H., Du, D., Xie, Y. et al. The role of marital status in gallbladder cancer: a real-world competing risk analysis. BMC Gastroenterol 24, 276 (2024). https://doi.org/10.1186/s12876-024-03364-y
Received:
Accepted:
Published:
DOI: https://doi.org/10.1186/s12876-024-03364-y