Abstract
Cancer is the leading cause of death worldwide, accounting for nearly 10 million deaths every year. Immune checkpoint blockade approaches have changed the therapeutic landscape for many tumor types. However, current immune checkpoint inhibitors PD-1 or CTLA-4 are far from satisfactory, due to high immune-related adverse event incident (up to 60%) and the inefficiency in cases of “cold” tumor microenvironment. TNFR2, a novel hopeful tumor immune target, was initially proposed in 2017. It not only promotes tumor cell proliferation, but also correlates with the suppressive function of Treg cells, implicating in the development of an immunosuppressive tumor microenvironment. In preclinical studies, TNFR2 antibody therapy has demonstrated efficacy alone or a potential synergistic effect when combined with classical PD-1/ CTLA-4 antibodies. The focus of this review is on the characteristics, functions, and recent advancements in TNFR2 therapy, providing a new direction for the next generation of anti-tumor alternative therapy.
Similar content being viewed by others
Introduction
The cancer burden is a crucial global public health problem, which exerts tremendous physical, emotional and financial strain on people. According to the World Health Organization (WHO) estimates, cancer is the first or second leading cause of death before the age of 70 years in 112 of 183 countries and ranks third or fourth in a further 23 countries [1, 2]. In 2022, there were 20 million new cancer cases and 9.7 million deaths. Approximately, one in five people will develop cancer in their lifetime, and about one in nine men and one in 12 women will die from it [3]. The three predominant malignancies worldwide, namely colorectal cancer, lung cancer, and breast cancer, are characterized by aggressive disease progression, formidable therapeutic challenges, and discouraging survival rates [4]. Treatment usually includes surgery, radiotherapy, and/or systemic therapy (chemotherapy, hormonal treatments, targeted biological therapies).
Recently, the discovery of immune checkpoint inhibitors (ICIs) might has potentially ushered in a new era in cancer treatment, demonstrating remarkable advancements in prolonging the survival rates of patients with metastatic cancer [5]. In 2021, the sales of malignant melanoma and non-small cell lung cancer therapies reached $4.7 billion, and $24.1 billion, with ICIs attributing the majority (72% and 60%, respectively) [6, 7]. The world’s second approved PD-1 inhibitor Keytruda (Pembrolizumab) has overtaken Humira as the best-selling drug with $25 billion in annual sales. However, it is important to note that up to 60% of patients treated with ICIs, may experience immune-related adverse events (irAEs) such as pruritic diarrhea, myocarditis, colitis, panhypopituitarism, dermatitis, autoimmune hepatitis, autoimmune pituitary inflammation, and hepatitis [8]. This is on account of the wide expression of programmed death 1 (PD-1) and Cytotoxic T-lymphocyte-associated protein 4 (CTLA-4) receptors in different types of T cell populations in various parts of the body [9]. The lack of effector T cells or the presence of immunosuppressive cells may contribute to a “cold” tumor microenvironment, leading to poor response rates in certain patients undergoing immunotherapy [10].
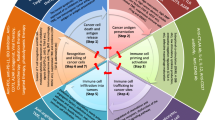
In comparison to the afore mentioned ICIs, tumor necrosis factor receptor II (TNFR2) offers a more significant advantage. On the one hand, TNFR2 is expressed on the surface of immunosuppressive cells and tumor cells as a crucial factor contributing to the “immune privilege” of tumors [11]. On the other hand, TNFR2 can promote the expression of programmed cell death-ligand 1 (PD-L1) on tumor cells to promote T cell depletion and tumor immune escape [12] (Fig. 1). Consequently, the development of TNFR2 target drugs serves as a useful supplement to the ICIs such as PD-1/PD-L1. Since TNFR2 was initially proposed to be a novel tumor immune target in 2017, various studies have provided substantial evidence of the significant effectiveness of TNFR2 antibodies in recent years for cancer treatment [13]. The TNFR2 monoclonal antibody has the ability to specifically bind to the extracellular domain of TNFR2 and effectively induce apoptosis in regulatory T cells (Tregs) infiltrating the tumor. Activation of CD8+ T cells enhance the immune response against tumors and facilitates direct cytotoxicity towards tumor cells both in vivo and in vitro [14, 15]. Promising results were observed in both PD-1-sensitive and PD-1-resistant models, demonstrating significant tumor suppression [12]. Furthermore, the combination of TNFR2 antibodies and PD-1/PD-L1 inhibitors has demonstrated superior therapeutic efficacy in multiple preclinical and clinical studies compared to either treatment alone [16,17,18].
Mechanism of action of PD-1/PD-L1 antibody combined with TNFR2 antibody. IL-6 and TNF-α synergistically induce the binding of signal transducer and activator of transcription 3 (STAT3) and TNFR2 promoter in tumor cells to promote the expression of TNFR2. Then, TNF-α-TNFR2 activates the expression of PD-L1 via NF-κB to induce T cell depletion, promote tumor cell growth and enhance the function of immunosuppressive cells. The combination of TNFR2 antibody and PD-1/PD-L1 antibody can inhibit the proliferation of Treg and tumor-associated macrophage (TAM), induce the apoptosis of tumor cells and the expression of PD-L1, and thus enhance the tumor-killing effect of T cells
Currently, approximately twenty TNFR2 antibodies are in the preclinical or phase I/II clinical stages with favorable profiles, indicating that TNFR2 drugs will be hopefully launched in the future. This review provides a comprehensive overview of the development of TNFR2 target drugs, with a specific focus on their mechanism, anti-tumor efficacy, safety profile and pharmacodynamics in both preclinical and clinical trials. Moreover, we discuss the remarkable effect and broad prospect of the combination of TNFR2 and PD-1/PD-L1 monoclonal antibodies, which provides a new direction for the future development of immune checkpoint inhibitors.
Expression, structure, and signal transduction of TNFR2
There are two different tumor necrosis factor receptors in the expression and structure, TNFR1 and TNFR2. TNFR1, also known as p55, is ubiquitously expressed across various cell types (excluding erythrocytes), while TNFR2 (p75) is mainly expressed in tumor cells and a variety of immunosuppressive cells, such as Tregs [19,20,21], myeloid-derived suppressor cells (MDSCs) [22], oligodendrocytes [23] and mesenchymal stem cells (MSCs) [24]. TNFR1 and TNFR2 are both type I transmembrane proteins, consisting of an N-terminal extracellular domain (ECD) of four cysteine-rich domains (CRDs), a transmembrane domain, and a cytoplasmic domain [25]. The CRD1 and CRD2 regions exhibit structural similarity, while displaying variations in domain sequence and conformation.TNFR2 consists of 461 amino acids, of which the first 256 amino acids form an extracellular structure containing four cysteine-rich motifs, 31 amino acids form a transmembrane domain, and the last 174 amino acids form an intracellular structure with TNF receptor-associated factor 2 (TRAF2) binding sites [26]. The TNFR2 receptor on the cell membrane exists in a dynamic equilibrium state, alternating between inactive monomers and double/trimer receptors. This receptor has the ability to self-assemble with transmembrane tumor necrosis factor (TNF), forming homologous trimers on the cell membrane and initiating downstream signal transduction [27, 28]. The other most determinative differences between TNFR1 and TNFR2 are shown in Fig. 2.
Signaling pathways involved in TNFR2. TNFR2 recruits TRAF2 and cIAP1/2 to activate NF-κB and AP1 transcription factors via distinct pathways, resulting in the transcription of target genes. This, in turn, promotes the development of tumor cells and angiogenesis. Moreover, TNFR2 can engage in crosstalk with TNFR1 to induce apoptosis
TNFR1 intracellular death domain (DD) can combined with TNF receptor-associated death domain (TRADD) and receptor-interacting serine/threonine protein kinase 1 (RIPK1) [29, 30]. The different ubiquitination states of RIPK1 activate different downstream response signaling pathways to mediate apoptosis or programmed necrosis [31]. Devoid of DD, TNFR2 cannot recruit TRADD but can interact directly with TRAF1 or TRAF2 via intracellular conserved TRAF binding domains to recruit cellular inhibitor of apoptosis protein 1 and 2 (cIAP1/2), thereby initiating downstream signaling [32]. K63-ubiquitin chin-bound cIAP1/2 can recruit linear ubiquitin chain assembly complex (LUBAC), while the M1-linked ubiquitin chains formed by LUBAC allow the recruitment of transforming growth factor-beta actived kinase 1 (TAK1) and ikappaB kinases (IKK) complexes [33, 34]. As in the case of TNFR1 signaling, this results lead to phosphorylation of IκB and degradation of ubiquitination proteasome to form a heterodimer of nuclear factor kappa-B (NF-κB) composed of p65 and p50 subunits, translocated into the nucleus, where it results in activation of NF-𝜅B-dependent transcription such as anti-apoptotic, and pro-survival genes [35].
Different from TNFR1, TNFR2 triggers non-regulated NF-κB signaling by promoting the activation of NF-κB-induced kinase (NIK) [36]. In the absence of stimulation, NIK is ubiquitinated by the intracellular TRAF/cIAPs complex and further degraded by the proteasome. TNF-α binds to TNFR2 to recruit the TRAF/cIAPs complex, which leads to NIK stabilization and activation. Activated NIK phosphorylates and induces the processing of p100, which is hydrolyzed by regulated proteins to form p52/RelB heterodimers, which enter the nucleus and regulate the transcription of target genes.
Furthermore, TNFR2 can activate the c-Jun N-terminal kinase (JNK) and Endothelial/Epithelial tyrosine kinase (Etk) pathways [37, 38]. TNFR2-induced activation of the JNK pathway occurs through a nonapoptotic TRAF2-dependent pathway [39, 40]. TRAF2 induced the activation of the ASK Interacting Protein 1 (AIP1), resulting in phosphorylation of apoptosis signal regulating kinase 1 (ASK1) and activation of ASK1-JNK signaling [35, 41,42,43]. TNFR2 involves the activation of Etk (also known as bone marrow X-linked kinase, Bmx) independently of TRAF2 [38, 44]. Etk mediates TNF-induced transactivation of vascular endothelial growth factor receptor 2 (VEGFR2), which in turn activates Etk. The mutual activation between Etk and VEGFR2 promotes the phosphorylation of Etk at Tyr-566 to mediate the recruitment of phosphatidyl inositol 3 kinase (PI3K), leading to the activation of Akt (also known as protein kinase B, PKB), promoting pro-survival and reparative cascades [38, 45].
In addition, TNFR2 can indirectly induce cell death through crosstalk with TNFR1. TNFR2 induces c-IAP1 with E3 ligase activity by labeling TRAF2 with ubiquitination, and then the proteasome degrades TRAF2. Depletion of TRAF2 inhibits TNFR1-mediated NF-kB and mitogen-activated protein kinase (MAPK) signaling pathways and facilitates the formation of death complexes [46, 47].
The membrane-bound TNFR2 (mTNFR2) can undergo proteolysis by TNF-α converting enzyme (TACE), resulting in the generation of soluble TNFR2 (sTNFR2) that is released into the bloodstream while retaining its ligand-binding functionality [48]. Notably, the immunosuppressive or immunostimulatory properties of mTNFR2 are primarily determined by the specific cell type expressing the receptor, while the shed sTNFR2 predominantly exhibits immunosuppressive effects [49]. The shedding of TNFR2 from the surface of CD8+T cells lead to a reduction in the number of membrane surface receptors, thereby attenuating the sensitivity of activated CD8+T cells towards TNF-α action [50]. Activated Tregs can release a substantial amount of sTNFR2, thereby antagonizing the effect of TNF and inhibiting the active immune response induced by the TNF-TNFR1 signaling pathway, consequently preventing the inflammatory impact of TNF [51]. Simultaneously, Tregs expressing membrane-bound TNFR2 can independently enhance the immunosuppressive behavior of Tregs.
Generally, the activation of TNFR2 signaling pathway in various cell types and plays important roles in several physiological functions. TNFR2 activation on Tregs promotes their proliferation, survival, and immunosuppressive properties, helping to maintain immune homeostasis and prevent excessive immune responses [52]. It has been shown to promote the expansion and activation of stem cells, which play a critical role in tissue repair and regeneration [53]. TNFR2 activation on stem cells can enhance their proliferation, differentiation, and tissue regenerative capacity [24]. Activation of TNFR2 on neurons has been shown to promote neuronal survival and protect against neurodegenerative diseases. TNFR2 signaling also contributes to synaptic plasticity, neural development, and maintenance of cognitive function [54, 55]. TNFR2 activation in endothelial cells promotes vascular repair and angiogenesis, contributing to cardiovascular tissue regeneration and maintaining vascular integrity [56, 57]. It also plays a role in regulating vascular inflammation. Currently, TNFR2 serves as a crucial regulator within the tumor microenvironment, making TNFR2 antibodies a central area of focus in ongoing research.
TNFR2 functions in tumor cell proliferation and oncogenesis
The function of TNFR2 in tumor cells
The malignant degree of the tumor progressively intensified as TNFR2 accumulated within the tumor [58]. Under the stimulation of IL-6 and TNF-α, STAT3 synergistically activates the TNFR2 promoter, leading to aberrant expression of TNFR2 on diverse tumor cell lines. Consequently, this event triggers AKT phosphorylation to facilitate tumor cell proliferation [59] and promotes Ki67-mediated DNA damage repair [60, 61], thereby safeguarding tumor cells from harm (Fig. 3A). Tumor necrosis factor-like weak inducer of apoptosis (TWEAK) / fibroblast growth biofluid factor - inducible 14 (Fn14) activation represents the predominant mechanism underlying TNFR2-mediated augmentation of tumor invasiveness and metastasis. The reciprocal interaction between TNFR2 and TWEAK/Fn14 triggers the activation of NF-κB signaling pathway, subsequently leading to matrix metalloproteinase-9 (MMP-9)-mediated degradation of extracellular matrix (ECM), thereby facilitating tumor invasion and metastasis [62, 63] (Fig. 3A). Additionally, the release and activation of vascular endothelial growth factor (VEGF) further contribute to the promotion of tumor metastasis.
The role of TNFR2 in tumor angiogenesis
The upregulation of PGRN is observed in malignant cells, resulting in the facilitation of VEGF-A, VEGF-C, and fibroblast growth factor 1 (FGF-1) expression through TNFR2/Akt and ERK signaling pathways [64,65,66]. Within endothelial cells, TNFR2 promotes the survival of endothelial progenitor cells (EPCs) and endothelial cell (ECs) via the NF-κB signaling pathway while stimulating activation of the Etk/Bmx pathway to enhance VEGFR2 expression in ECs [64, 67, 68] (Fig. 3B). Upon interaction between VEGFR and VEGF proteins, immediate phosphorylation occurs on tyrosine residues within the intracellular signal transduction region, thereby initiating downstream intracellular signaling pathways that ultimately drive vascular endothelial cell growth, proliferation, maturation, and neovascularization.
The role of TNFR2 in the tumor cell. (A) TNFR2 facilitates tumor cell proliferation and DNA repair through activation of NF-κB signaling. It also induces the secretion of MMPs and upregulates VEGF expression in tumor cells. (B) TNFR2 is associated with enhanced proliferation and VEGFR2 expression in endothelial cells, promoting angiogenesis and metastasis
The function of TNFR2 in tumor killer cells
During the initial stages of tumor growth, T cells are attracted to the tumor, exerting cytotoxic effects on cancerous cells and impeding their sustained proliferation. TNFR2 expression is implicated in the proliferation of CD8+ T effector cells during immune responses (Fig. 4A). In the early stages of immune response, TNFR2 functions as a co-stimulatory molecule for CD8+ T effector cells, promoting the production of IL-2, survivin, B-cell lymphoma-2 (Bcl-2), and Bcl-xL. This facilitates the survival and expansion of CD8+ T effector cells while also contributing to the regulation of T cell receptor (TCR)/CD28-mediated cellular activation [69, 70]. First, TNFR2 leads to NF-κB induction through AKT activation in response to TCR/CD28-mediated stimulation in early CD8+ T cells [71]. This lowers the activation threshold for T cell in vitro and provides an early co-stimulatory signal that promotes T cell activation, which has been demonstrated by the fact that Tnfr2−/− CD8+ T cells require a five-fold higher concentration of TCR agonists compared to normal CD8+ T cells. Second, activated CD8+ T cells possess the ability to produce pro-inflammatory cytokines including interferon (IFN-γ) and TNF-α, to clear antigens. TNFR2 is crucial for mediating the secretion of these cytokines by CD8+ effector T cells (Teffs), as evidenced by studies conducted in the tumor lymph nodes of Tnfr2−/− mice. The proportion of CD8+ T cells is significantly reduced, and tumor-specific CD8+ T cells exhibit diminished production of IL-2 and IFNγ [52, 70, 72]. In addition, TNFR2 plays a pivotal role in the regulation of activation-induced cell death (AICD) in activated CD8+ Teff [73, 74]. TNFR2 serves as the signal molecule that triggers apoptosis in CD8+ Teffs cells, while TRAF2 functions as a pro-survival signal, recruiting the apoptosis protein inhibitor cIAP-1/-2 and activates NF-κB to mediate its anti-apoptotic effects. However, TNFR2 stimulation induces mitochondrial hyperpolarization resulting in increased reactive oxygen species (ROS) production and causing DNA damage, thereby leading to degradation of TRAF2 survival signals and triggers programmed cell death in activated CD8+ T cells [75].
The interaction between dendritic cells (DCs) and natural killer (NK) cells also necessitates the TNF-TNFR2 interaction (Fig. 4A). Membrane-bound TNF-α expressed by DCs engages with TNFR2 on NK cells, thereby inducing NF-κB activation in NK cells and subsequently initiating the transcription of cytokine factors such as granulocyte-macrophage colony stimulating factor (GM-CSF). This leads to an augmented secretion of IL-12 and IFN-γ production in DCs, resulting in amplified proliferation and heightened cytotoxic activity of NK cells [76, 77]. Additionally, TNF-TNFR2 signaling can directly upregulate the expression of CD25 (IL-2Rα) and nutrient transporters in NK cells, promoting a metabolic shift towards aerobic glycolysis and facilitating the proliferation of NK cells [78].
The role of TNFR2 in the tumor microenvironment. (A) During tumor growth, both tumor cells and the TME release TNF-α, which triggers proliferation of CD8+T cells and NK cells within the tumor microenvironment to mount an effective immune response against cancerous cells. (B) Over time, the TME attracts an increasing number of immunosuppressive cells (including Tregs and MDSCs), leading to suppression of anti-tumor responses. Furthermore, prolonged exposure of CD8+T cells to TNF-α drives them towards AICD, enabling tumor cells to evade immune control mechanisms
Function of TNFR2 in immunosuppressive cells
As the tumor progresses, the tumor microenvironment (TME) progressively recruits an increasing number of immunosuppressive cells, such as Tregs and MDSC, resulting in suppression of the anti-tumor immune response and facilitating immune evasion by tumor cells (Fig. 4B). There is a significant research focus on the TNFR2-dependent proliferation and enhanced effects of Tregs, which are a subset of immunosuppressive cells responsible for maintaining immune homeostasis by inhibiting excessive Teffs proliferation and secreting inhibitory cytokines [79, 80]. In addition to CD4+ Tregs, a subset of CD8+ T cells also possess regulatory properties. Both CD4+ Tregs and CD8+ Tregs exhibit shared characteristics such as the expression of TNFR2, CD25, and Foxp3 markers, enabling them to suppress immune responses through cytokine induction [81, 82]. Nonetheless, the origin of CD8+ Tregs differs from that of CD4+ Tregs, which are induced by unknown factors within the TME. Multiple lines of evidence support this notion; specifically, CD8+ Tregs have been exclusively identified in tumor sites such as those found in breast, prostate, and colon cancers. Conversely, natural CD4+ Tregs (nTregs) and inducible CD4+ Tregs (iTregs) have been detected in various locations including the thymus, peripheral blood mononuclear cells (PBMC), and the TME [73, 83]. The accumulation and infiltration of numerous Tregs within the TME facilitate immune evasion, particularly by Tregs with elevated expression levels of TNFR2 that exhibit potent immunosuppressive activity [19,20,21]. Accumulation of highly suppressive TNFR2+ Tregs in the TME has been observed in LEwis lung and breast cancer mouse models, as well as in mouse liver and colon cancers [52, 60, 79, 84]. Furthermore TNFR2+ Tregs have been demonstrated to promote the infiltration and metastasis of tumor cells into the pleural cavity in lung cancer-induced malignant pleural effusion (MPE). These TNFR2+ Tregs possess polyclonal properties and display robust immunosuppressive functions [85]. Patients diagnosed with acute myeloid leukemia (AML) were observed to exhibit elevated levels of Tregs, concomitant with a significant augmentation in the abundance of TNFR2 on the surface of these cells. Importantly, this elevation demonstrates a positive correlation with disease severity [86]. In conclusion, tumor-infiltrating Tregs exhibit robust immunosuppressive capabilities.
The selective upregulation of TNFR2 in Tregs is facilitated through three subsequent pathways: NF-κB, MAPK, and PI3K-Akt signaling cascades [87]. Abundant evidence strongly supports a robust association between the activation, proliferation, and stability of Treg cells and the non-canonical NF-κB pathway induced by transmembrane TNF-α (tmTNF-α) binding to TNFR2. In stark contrast to TNFR1, TNFR2 lacks a death domain but can recruit the TRAF-2/cIAP-1/cIAP-2 complex, leading to the activation of both canonical and non-canonical NF-κB signaling pathways. Notably, cIAP exerts ubiquitin ligase activity while inhibiting cysteine aspartase and other apoptosis-inducing factors [85, 88,89,90,91]. Besides NF-κB, the signaling of TNFR2 also regulates the MAPK family members, which are crucial factors. The binding of TNF-α to TNFR2 triggers the activation of ERK, JNK, and p38, thereby promoting the proliferation and development of Tregs. This is further supported by the observed inhibition of TNF-induced expansion of Tregs upon treatment with a p38 inhibitor [92]. In contrast to NF-κB and MAPK, PI3K-Akt-mTOR pathway plays a crucial “tool” in maintaining the functional consistency and stability of Tregs [93]. Nonetheless, recent evidence suggests that NF-κB and MAPK directly regulate the expression of IL2, which enhances the expansion and stability of Tregs with exerting potent inhibition [88, 90, 94,95,96].
Function of TNFR2 in stem cell
ROS accumulation is the primary mechanism by which MDSCs and EPCs enhance their immunosuppressive function. Activation of tmTNF-α/TNFR2 induces a high level of arginase activity, NO synthetase transcription, secretion of NO, reactive oxygen species, IL-10 and TGF-β, as well as ROS and peroxynitrite accumulation. These lead to tyrosine nitration that impairs CD8+ T cell binding to phosphorylated MHC [22, 97,98,99,100], and result in loss of reactivity for CD8+ T and NK cells towards specific antigens. Additionally, secretions of IL-10 and TGF-β are also promoting factors in inducing amplification of Tregs.
TNFR2 is essential for MSC immunomodulatory [101], which facilitates MSC to increase the content of CD4+Foxp3+ T regs and CD8+Foxp3+ T regs. Previous studies suggested that 20% of CD8+ T cells expressed Foxp3, which were twice as much as those co-cultured with Tnfr2−/− MSCs when co-cultured with TNFR2+ MSCs. And TNFR2+ MSC-induced CD8+ Tregs were more immunosuppressive [24, 102, 103]. The tmTNF-α/TNFR2 signaling is crucial for conversion of bone marrow-MSC (BM-MSCs) into Tumor-MSC (T-MSCs). In addition to promoting expression of immunosuppressive factors and enhancing the proliferation, TNFR2 mediates the chemokine secretion in MSC, which leads the MSC localized in tumor [104, 105]. BM-MSCs tend to tumor and secret inflammatory cytokines to promote tumorigenesis, after stimulation with TNF-α [101, 104]. In TME, BM-MSCs differentiate into CAFs, which promote tumor cell proliferation, migration, and invasion by inducing epithelial-mesenchymal transition (EMT) and secreting matrix metalloproteinase (MMP) or growth factors [106]. Activation of the TNFR2-NF-κB-IRF1 signaling pathway induces CAFs to secrete IL-33, which enhances migration and invasion of gastric cancer cells via EMT [107].
Mechanism of TNFR2 antibody
Molecular mechanism of TNFR2 antibody
In normal circumstances, TNF and TNFR2 assemble into trimers to initiate downstream signaling pathways (Fig. 5A). However, TNFR2 antagonists selectively capture newly synthesized, unassembled TNFR2 proteins on rapidly proliferating cells prior to the trimerization of TNF occurs. By forming an anti-parallel dimeric structure, the antagonist becomes firmly locked in the dimer interface. In this conformation, the proximity of the TNFR2 binding region only allows for the accommodation of one Fab fragment, while the other Fab binds solely to an adjacent anti-parallel dimer within a hexagonal lattice arrangement [108].
TNFR2 comprises four cysteine-rich domains, CRD1, CRD2, CRD3 and CRD4 [109]. The CRD1, also known as pre-ligand assembly domain (PLAD), primarily involved in TNFR self-assembly on the cell surface. The CRD 2 and CRD3 domains are considered responsible for binding to tumor necrosis factor. Deletion of CRD4 could result in about a 10-fold reduction in affinity for TNF-α, indicating that CRD4 is important but not necessary for TNF binding. In the crystal structure of the TNF-TNFR2 complex (Fig. 5B), the CRD1 region is spatially separated by more than 30 Å distance and thus unable to interact; however, it is noteworthy that CRD1 is considered as the primary site for agonist binding. The coalescence of two adjacent TNFR2 molecules serves to stabilize the receptor and augment signaling [110]. Antibodies targeting TNFR2 engage with its CRD1 domain, occupying the binding site of TNFR2 agonists and impeding their signal-enhancing effects. Notably, TNFR2 antagonists exert their antagonistic role primarily through ligand blocking and agonist inhibition. Within the CRD2 domain, Cys71, Ser73, Cys74, and Arg77 of TNFR2 potentially form hydrogen bonds with Ala33, Arg32, Gln21, and Glu23 of TNF respectively. Moreover, Arg113 of TNFR2 may establish hydrogen bonds with Asp143 and Gln149 of TNF in close proximity [111].
In Fig. 5C, the TNFR2 antibody primarily binds to the CRD1, CRD2, and CRD3 domains of TNFR2. The Fab fragment of the TNFR2 antibody forms hydrogen bonds with Asp25, Gln26, Thr27, and Glu70 in the CRD2 domain while another Fab fragment binds to Asp81 in the CRD2 domain and Arg113 and Cys115 in the CRD3 domain through hydrogen bonding. Previous studies have identified Arg77 and Arg113 as crucial residues that bind to TNF [111]; thus, TNFR2 antibodies also occupy arginine residues at position 113 of TNFR2. The overlap between TNFR2 antagonists and TNF binding sites suggests that its antagonistic effect results from ligand blocking. Additionally, although it does not directly occupy the 77th amino acid site, the Fab of the TNFR2 antibody firmly fixes onto both CRD1 and CRD2 domains, effectively inhibiting the binding binding between TNF ligand.
Difference in the binding mode between the native TNF-TNFR2 and the antibody-TNFR2 complex. (A) TNFR2 monomers (shown in orange) form antiparallel dimers, and the combination of trimer and dimer symmetry leads to the formation of a hexagonal network, as shown on the right, where the hexagon can be repeated to form an infinite network. TNFR2 antagonists can lock the non-signaling state of the receptor in the antiparallel dimer state, thereby blocking ligand binding and signaling. TNFR2 binds to TNF to form a signaling complex that maintains hexagonal symmetry, and agonists can be attached in the middle of the complex to act as stabilizers and maintain signaling. (B) The native TNF-TNFR2 complex. The hydrogen bonds are depicted using yellow dashed lines (PDB entry: 3ALQ). (C) The TNFR2-TNFR2 biparatopic antibodies complex (PDB entry: 8HLB). The hydrogen bonds are depicted using yellow dashed lines
The anti-tumor effects of TNFR2 agonists and antagonists
It is very interesting that both TNFR2 agonists and antagonists can be promising candidates for anticancer therapy; however, their mechanisms of action differ.
Activation of TNFR2 to enhance the proliferation and cytotoxicity of T cells and NK cells may exert a favorable impact on tumors characterized by increased infiltration of T lymphocytes. CD8+ T cells play a crucial role in immune surveillance [112]. A favorable prognosis can be associated with a high presence of CD8+ T cells exhibiting potent cytotoxic abilities within tumor tissues. Promoting the augmentation of CD8+ T cells with cytotoxic capabilities in tumor tissues is beneficial for impeding tumor progression and potentially eradicating tumors altogether. The administration of TNFR2 agonist antibodies leads to the in vitro activation of CD8+ T and CD4+ T cells. Deficiency in CD8+ T cells and NK cells diminishes the activity of TNFR2 agonist antibodies. Remarkably, TNFR2 agonist antibodies still exhibit anti-tumor effects even in the absence of CD4+ T cells [52].
Blockade of TNFR2 can impede immunosuppressive function and ameliorate the tumor microenvironment. A high concentration of Treg cells within the tumor microenvironment can impede the activation of CD4+ and CD8+ T cells, thereby suppressing the anti-tumor immune response and facilitating tumor progression [113, 114]. Depletion of Treg in mice significantly impedes tumor growth. Amongst Treg subsets, TNFR2+Treg exhibits the most potent immunosuppressive properties [79]. Inhibiting TNFR2 with antagonists hampers Treg cell proliferation while promoting Teff cell expansion to exert an anti-tumor effect. Studies have shown that TNFR2 antagonist can significantly reduce ROS levels and inhibit AICD, and Tnfr2−/− CD8+ T cells are also highly resistant to AICD [115].
Research progress of TNFR2 antibody
Clinical trials of anti‑TNFR2 agents
Six TNFR2 agonists and antagonists registered in clinical trials are listed in Table 1. Among them, HFB200301, SIM-235 and NBL-020 are currently undergoing phase I clinical trial, while BI-1808, BI-1910, and LBL-019 have progressed in phase II clinical trial (Fig. 6). The mechanism of action of each anti-TNFR2 agent, as well as the therapeutic efficacy and safety of drug in preclinical/clinical trials, will be discussed in detail.
Agonist
BI-1910, a novel TNFR2 agonist, has been advanced to clinical phase II by Biolnvent Inc. It is currently undergoing trials for the treatment of non-small cell lung cancer, hepatocellular carcinoma, and some other solid tumors. Encouragingly, BI-1910 antibody exhibits extensive antitumor activity by co-stimulating with TNFR2 to activate T cells and NKs [116]. The specific research and development process is as follows: The preclinical data presented at the Society for Immunotherapy of Cancer (SITC) conference in November 2023 provided compelling evidence supporting the safety profile of BI-1910. Toxicological studies conducted on non-human primates (NHPs) demonstrated excellent tolerance to a dose of 25 mg/kg, absence of detrimental cytokine release, and significant T cell activation in a dose-dependent manner [117]. Currently, a clinical Phase I/IIa trial of the antibody is currently underway. In phase Ia, BI-1910 will be assessed for its safety, tolerability, and potential efficacy as a monotherapy in patients with advanced solid tumors. In phase Ib, the safety and tolerability of BI-1910 in combination with pembrolizumab will be evaluated in patients with advanced/metastatic solid tumors. In Phase IIa, pharmacokinetic (PK) and pharmacodynamic (PD) characteristics of BI-1910 will be assessed as a monotherapy or in combination with pembrolizumab [118].
HFB200301, developed by HiFiBiO Therapeutics, is under phase I clinical trials. It activates TNFR2, thereby inducing the activation of CD4+ T cells, CD8+ T cells, and NK cells to elicit potent anti-tumor immune responses. Single-cell sequencing analysis was employed to identify effector T cell subsets co-expressing TNFR2 and CD8A, exhibiting a preference for the action of TNFR2 agonists, leading to the selection of several cancer types as potential indications for phase I clinical trials. In phase I clinical trials, the identification of effector T cell subsets co-expressing TNFR2 and CD8A was achieved through the utilization of single-cell sequencing analysis, demonstrating a preference for the activity of TNFR2 agonists. This result has led to the selection of multiple cancer types as potential indications for phase I clinical trials aiming to evaluate the safety and efficacy of HFB200301 in combination with the PD-1 monoclonal antibody Tislelizumab, while also collecting preliminary data on pharmacokinetic parameters and anti-tumor effects [119, 120]. In addition, a phase II clinical trial NCT05238883 is ongoing.
Antagonist
BI-1808, a human IgG1 monoclonal antibody from Biolnvent Inc, is the first discovered TNFR2 antagonist. It is a very potential and has been advanced to clinical phase II. Generally speaking, BI-1808 exhibits potential for utilization as a monotherapy or in combination with pembrolizumab for patients afflicted with advanced malignancies. The mechanism of action involves inhibition of the interaction between TNFR2 and its ligand TNF-α, resulting in Treg depletion through FcγR dependence, thereby promoting the expansion of CD8+ T cells. Consequently, there is an increased proportion of CD8+/Treg cells leading to a cytotoxic effect on various tumor types [116]. In preclinical experiments, immunoactivity tumor-bearing mice with partial sensitivity to checkpoint blockade were administered a combination therapy comprising BI-1808 and an alternative anti-PD-1 antibody. Notably, all treated mice achieved complete remission, indicating promising synergistic effects. Non-human primate toxicology studies have demonstrated excellent tolerance to BI-1808 at doses up to 200 mg/kg, while in vitro studies using human cells have shown no evidence of detrimental cytokine release [121]. Pharmacokinetic investigations have revealed a predicted half-life of two weeks when receptor saturation is achieved, with sustained receptor occupancy over this duration being necessary for optimal therapeutic efficacy [122]. The Phase I Part b of the study aims to evaluate the safety and tolerability of co-administering BI-1808 with Keytruda [123]. The monotherapy cohort of BI-1808 demonstrated a favorable safety profile, with no observed dose-limiting toxicity and no maximum tolerated dose found. Recent scans revealed that patients receiving treatment with BI-1808 experienced a significant 48% reduction in tumor burden compared to their initial baseline measurements. Among the evaluated 21 patients, 7 cases showed stable disease, while comprehensive PK/ PD data facilitated the determination of an extensive dosage range capable of achieving complete target coverage without compromising safety [124]. The Phase IIa segment of the ongoing BI-1808 monotherapy trial will encompass a broader patient cohort, including individuals with lung cancer, ovarian cancer, and various subtypes of T-cell lymphoma.
LBL-019, discovered by Leads Biolabs, has been advanced to phase II clinical trials. It can hinder the interaction between TNF-α and TNFR2, due to its high-affinity binding to the CRD1 domain of TNFR2 (5.32 nM in humans). Furthermore, it exerts dose-dependent inhibition on tumor growth via two distinct mechanisms. Firstly, it enhances the activation of CD8+ T cells and CD4+ T cells while inducing IFN-γ release. Additionally, it upregulates the expression of activation maker such as CD25, PD-1, and 41BB. Secondly, LBL-019 effectively eliminates both Treg cells and tumor cells to reduce their inhibitory effects on CD4/CD8 activity. Notably, when combined with anti-PD-1 treatment, LBL-019 demonstrates significant efficacy in suppressing MC38 tumor growth in 80% of cases [125, 126]. A clinical Phase I/II trial of the antibody is currently underway to evaluate the safety, tolerability, pharmacokinetic profile, RO, immunogenicity, and efficacy of BLB-019 alone or in combination with PD-1 antibody in the treatment of advanced malignant tumors [127].
SIM-235, also namely SIM1811-03, was discovered by the Simcere Company. It is the first TNFR2 antibody to be tested in Phase I clinical trials in China. By specifically binding to TNFR2, SIM-235 effectively inhibits NFκB activation and impedes Treg proliferation. In this mechanism, SIM-235 can exhibit remarkable anti-tumor properties and enhance synergistic anti-tumor effects [128]. By April 18, 2023, a total of 22 patients received SIM1811-03 monotherapy in the dose-escalation segment without any observed instances of dose-limiting toxicity (DLT). The study phase did not reach the maximum tolerated dose. Adverse events were predominantly categorized as grade 1 or 2, with two patients experiencing serious adverse events (SAEs) associated with treatment. Furthermore, one patient exhibited peripheral edema while another presented an elevated platelet count. Upon reaching a dosage level of 15 mg/kg, the drug demonstrated an approximate half-life of 12 days. Multiple administrations at doses equal to or exceeding 15 mg/kg resulted in nearly saturated TNFR2 levels (> 95% RO) in peripheral blood. Preliminary PK and PD results provide support for the selection of a recommended dose of 40 mg/kg every 3 weeks in combination with PD(L)-1 inhibitors [129].
NBL-020, is a fully humanized monoclonal antibody from Novarock Biotherapeutics. It exhibits a favorable safety profile, high affinity towards target cells, and potent antitumor activity in preclinical studies. In both PD-1-sensitive and PD-1-resistant isogenic animal models, either as monotherapy or in combination with anti-PD-1 antibodies, it effectively suppressed tumor growth and significantly prolonged survival [130]. The ongoing Phase I clinical trial aims to evaluate the safety and tolerability of NBL-020 in patients with advanced malignancies, determine the maximum tolerated dose without inducing toxicity, and establish the recommended dosage for Phase II trials [131].
TNFR2 in preclinical studies
Previous studies suggested that reduced tumor burden and metastasis were found in TNFR2-deficient mice [98, 132]. Such as, TNFR2 has been identified as a potential star immune checkpoint molecule that inhibits tumor growth and even helps to enhance the therapeutic effect of immune checkpoint inhibitors [13]. In this section, we primarily consolidate the findings from preclinical studies that assess the potential of anti-TNFR2 therapy in treating tumors. Table 2 presents some of the main preclinical studies on TNFR2.
As shown in Table 2, TNFR2 antibodies have shown promising results in a variety of preclinical disease models. with colon cancer being the most extensively investigated model, thereby suggesting that TNFR2 antibodies may exhibit enhanced therapeutic efficacy against colon cancer cells among the three major malignancies. According to The Cancer Genome Atlas (TCGA) and Genotype-Tissue expression (GTEx) databases, the overall abundance of TNFR2 in multiple tumor tissues are significantly higher than that in normal tissues at the mRNA level. Furthermore, evaluation of immunohistochemical images from the human protein atlas HPA database demonstrates a conspicuous disparity in TNFR2 protein expression between multiple cancer types and normal tissues, with the former exhibiting markedly higher levels (Fig. 7). The abnormal expression of TNFR2 in a variety of tumor tissues also suggests that its antibodies may have a killing effect on a variety of tumors, Further investigation is warranted to determine the optimal application of TNFR2 antibodies, as clinical studies have demonstrated their broad efficacy against various solid tumors.
Tnfr2 expression levels in different cancer types. (A) The expression level of Tnfr2 mRNA between tumor and normal tissues in thirty-four cancer types based on the integrated database from TCGA and GTEx datasets. (B) The expression level of Tnfr2 mRNA between tumor and normal tissues in twenty-six cancer types based on the TCGA database. (C) Representative IHC images of Tnfr2 expression in various tumor tissues and normal tissues
Indications for TNFR2 antibodies
General eligibility of cancer patients for the administration of TNFR2 antibodies
The development of TNFR2-targeted drugs is still in the early stage with no TNFR2 drugs approved on the market so far. Therefore, there is no gold standard for the administration of TNFR2 antibodies to treat cancer patients. However, according to the clinical trials in Table 1 (NCT04752826, NCT06205706, NCT05238883, etc.), the general eligibility criteria can be described as follows: Cancer patients are 18 years old or older, with expected survival time ≥ 3 months (12 weeks). Measurable disease as determined by Response Evaluation Criteria in Solid Tumors (RECIST) 1.1 or modified RECIST (mRECIST). Eastern Cooperative Oncology Group performance status of 0 or 1. They do not have an active, known, or suspected autoimmune disease. And do not show Grade ≥ 3 autoimmune manifestations of previous immune checkpoint inhibitor treatments (e.g., anti-PD-1, anti-PD-L1, or anti-CTLA-4). Furthermore, they are not at high medical risk because of nonmalignant systemic disease including severe active infections on treatment with antibiotics, antifungals, or antivirals. Do not have known human immunodeficiency (HIV) and/or history of hepatitis B or C infections, or has a positive test for HIV antibody, hepatitis B antigen/hepatitis B virus DNA or hepatitis C antibody or RNA.
In addition, the immunosuppressive or immune-stimulating properties of mTNFR2 are primarily determined by the cell type expressing TNFR2. Activated Tregs cells release sTNFR2 after being stimulated by TNF or TNFR2-agonist antibodies, which can clear TNF and prevent its inflammatory effects, while maintaining Tregs’ immune suppression function against Teffs [110]. Therefore, the concentration of CD4+T and CD8+T cells in the tumor microenvironment as well as the level of sTNFR2, might also serve as potential indicators for cancer patients to receive TNFR2 antibody therapy.
Cancers that would benefit from TNFR2 as personalized therapy in the future
Colorectal cancer
There exists a significant positive correlation between the prognosis and sTNFR2 level in patients with colon cancer [51]. Elevated expression of sTNFR2 is associated with an increased risk of colon cancer in women [133], while higher pre-diagnosis levels of sTNFR2 are also significantly linked to elevated overall mortality among colon cancer patients [134]. CT26 colon cancer model tumors, which are infiltrated by Tregs, express the highest levels of TNFR2 [135]. The pivotal role of TNFR2 in the pathogenesis and progression of colorectal cancer renders this receptor an attractive therapeutic target for the treatment of colorectal cancer. TNFR2 antagonist antibodies have demonstrated efficacy in suppressing tumor growth across various mouse models of colon cancer, with the underlying mechanism believed to involve a reduction in immunosuppressive Treg cells within the TME, consequently augmenting the CD+8 Teff to Treg cell ratio [16, 135, 136].
Ovarian cancer
The expression of TNFR2 in the ascites of patients with ovarian tumors is elevated, indicating heightened immunosuppressive activity. Additionally, there is a significant increase in the levels of soluble TNFR2 within the ascites of patients with alviceant epithelial ovarian cancer (EOC), comparing to corresponding benign tumors [137]. Under the stimulation of EOC ascites, the expression of TNFR2 in T cell subsets will be upregulated, leading to an increased ratio of Tregs to Teffs [138]. Notably, it is noteworthy that compared to Tregs from healthy donors, antagonistic TNFR2-specific antibodies exhibit a heightened propensity to induce apoptosis in Tregs from ovarian cancer patients’ ascites [108].
Acute myeloid leukemia
In individuals with acute myeloid leukemia (AML), the immune system imbalance results in impaired effector T cell function and elevated levels of TNFR2+ Tregs, thereby creating an immunosuppressive microenvironment [139]. Furthermore, patients with AML exhibit a significant increase in the serum concentrations of TNF-α and sTNFR2. This aberrant elevation has the potential to promote Treg expansion through activation of the TNF-α-TNFR2 pathway. Additionally, a substantial reduction in TNFR2 expression level should be observed during AML symptom remission. The inhibitory effect of a TNFR2 antagonist antibody on the proliferation of Treg cells can counteract the stimulatory impact induced by TNF-α [86].
Breast cancer
The expression level of TNFR2 in breast cancer patients is significantly correlated with clinical stage [140], elevating levels of sTNFR2 in the blood of these patients compared to the control group. Notably, the level of sTNFR2 will decrease after surgical intervention [141]. TNFR2-specific antibody has been demonstrated to attenuate the proliferative capacity and suppressive function of regulatory T cells, remarkably extending the survival of tumor-bearing mice [19]. Chemotherapy can diminish the counts of TNFR2+Treg cells in the tumor microenvironments of patients with triple-negative breast cancer, while elevating the counts of TNFR2+CD8 + T cells. Consequently, TNFR2 agonists predominantly exhibit antitumor efficacy in the tumor tissues with chemotherapy, indicating that the agonists may offer enhanced therapeutic benefits [69].
Other ongoing studies on TNFR2 therapy
Currently, numerous clinical and preclinical studies have primarily focused on the synergistic effect between TNFR2 antibody and PD1/PDL1 antibody. However, some studies on TNFR2 antibodies in combination with other targeting drugs are also ongoing. First, in vivo study, combined inhibition of glucocorticoid-induced TNFR-related protein (GITR), OX40 and TNFR2 abrogated the development of Treg cells [142]. The expression of members of the TNFRSF on Treg cell progenitors translated strong T cell antigen receptor signals into molecular parameters that specifically promoted the development of Treg cells and shaped the Treg cell repertoire. Second, combinations with V-domain containing Ig suppressor of T cell activation (VISTA, PD-1 H) and lymphocyte activation gene 3 proteins (LAG-3) and the special TNFR2 antibodies might be also potential efficiency therapy in next future [143, 144]. Third, in combination with radiotherapy and/or chemotherapy, TNFR2 antibody can affect the balance between graft-versus-tumor and graft versus host disease, a life-threatening complication of hematopoietic cell transplantation. It promotes the function of immune cells endowed with effector functions, as well as that of immune cells with regulatory and suppressive properties. Promising studies in humanized mouse models are ongoing to clarify if targeting TNF-α/TNFR2 pathway could be useful to prevent or treat graft versus host disease [21].
Conclusions and perspectives
TNFR2, a member of the tumor necrosis factor receptor superfamily, is predominantly highly expressed in some malignant solid tumor cells, such as colon cancer cells. As a newly proposed immune checkpoint target since 2017, it might provide a potential alternative solution for these malignant cancers. This review provides a comprehensive overview of the distinct roles exhibited by TNFR2 agonists and antagonists against tumors. The signal pathways, functions for cancer immunotherapy of TNFR2, as well as binding modes of the antibody-TNFR2 complex have been discussed in detailed. Moreover, the latest research progress involving TNFR2 antibody therapy is also summarized.
Although the development of TNFR2-targeted drugs is still in the early stage with no TNFR2 drugs approved on the market so far, there are approximately twenty TNFR2 antibodies in preclinical or phase I/II clinical trials that show promising profiles. Ongoing clinical trials are currently assessing the efficacy of these agents either alone or in conjunction with anti-PD-1/PD-L1 drugs, offering a promising avenue for the treatment of various malignant cancer types. This fact suggests that TNFR2 agents are expected to be hopefully launched in the future, as an alternative anti-tumor therapeutic drugs. Given the enormous potential of TNFR2 agents for malignant cancers and their fewer side effects in immune-related adverse events, it will be intriguing to see who emerges as a frontrunner in this field.
Data availability
The datasets generated and analysed during the current study are available in the Human Protein Atlas repository, https://www.proteinatlas.org/ and SangerBox, http://sangerbox.com/.
Abbreviations
- AICD:
-
Activation-induced cell death
- AIP1:
-
Ask interacting protein 1
- AKT/PKB:
-
Protein kinase B
- AML:
-
Acute myeloid leukemia
- Bax:
-
Bcl2-associated X
- BM-MSC:
-
Bone marrow-MSC
- Bcl-2:
-
B-cell lymphoma-2
- cIAP:
-
Cellular inhibitor of apoptosis protein
- CRD:
-
Cysteine-rich domains
- CTLA-4:
-
Cytotoxic T lymphocyte antigen-4
- DC:
-
Dentritic cell
- DD:
-
Death domain
- EC:
-
Endothelial cell
- ECM:
-
Extracellular matrix
- EOC:
-
Epithelial ovarian cancer
- EPC:
-
Endothelial progenitor cell
- ERK:
-
Extracellular regulated protein kinase
- Etk:
-
Endothelial/epithelial tyrosine kinase
- FGF:
-
Fibroblast growth factor
- Fn14:
-
Fibroblast growth biofluid factor - inducible 14
- Foxp3:
-
Forkhead box protein P3
- GM-CSF:
-
Granulocyte-macrophage colony-stimulating factor
- GTEx:
-
Genotype-tissue expression
- HPA:
-
The human protein atlas
- ICI:
-
Immune checkpoint inhibitor
- IFN:
-
Interferon
- IHC:
-
Immunohistochemical staining
- IKK:
-
IkappaB kinases
- IL:
-
Interleukin
- irAE:
-
Immune-related adverse event
- iTreg:
-
Induce CD4+ Treg
- JNK:
-
C-Jun N-terminal kinase
- LUBAC:
-
Linear ubiquitin chain assembly complex
- MAPK:
-
Mitogen-activated protein kinase
- MDSC:
-
Myeloid-derived suppressor cell
- MHC:
-
Major histocompatibility complex
- MMP:
-
Matrix metalloproteinase
- MPE:
-
Malignant pleural effusion
- mRNA:
-
Messenger RNA
- MSC:
-
Mesenchymal stem cell
- mTNFR2:
-
Membrane-bound TNFR2
- NF-κB:
-
Nuclear factor kappa-B
- NHP:
-
Non-human primates
- NIK:
-
NF-κB-induced kinase
- NK:
-
Natural killer
- nTreg:
-
Nature CD4+ Treg
- PBMC:
-
Peripheral blood mononuclear cell
- PD:
-
Pharmacodynamic
- PD-1:
-
Programmed death-1
- PGRN:
-
Granulin precursors
- PI3K:
-
Phosphatidyl inositol 3 kinase
- PK:
-
Pharmacokinetic
- PLAD:
-
Pre-ligand assembly domain
- RIPK1:
-
Receptor-interacting serine/threonine protein kinase 1
- ROS:
-
Reactive oxygen species
- SAEs:
-
Serious adverse events
- SITC:
-
Society for Immunotherapy of Cancer
- SODD:
-
Silencer of death domains
- STAT3:
-
Signal Transducer and Activator of Transcription 3
- sTNFR2:
-
Soluble TNFR2
- sTNF-α:
-
Soluble TNF-α
- TACE:
-
TNF-α converting enzyme
- TAK1:
-
Transforming growth factor-beta actived kinase 1
- TAM:
-
Tumor-associated macrophage
- TCGA:
-
The cancer genome atlas
- TCR:
-
T-cell receptor
- Teff:
-
Effector T cell
- TGF-α:
-
Transforming growth factorα
- TGF-β:
-
Transforming growth factor-β
- TME:
-
Tumor microenvironment
- T-MSC:
-
Tumor-MSC
- tmTNF-α:
-
Transmembrane TNFα
- TNFR1:
-
Tumor necrosis factor receptor I
- TNFR2:
-
Tumor necrosis factor receptor II
- TNF-α:
-
Tumor necrosis factor-α
- TRADD:
-
TNF receptor-associated death domain
- TRAF:
-
TNF receptor-associated factors
- Treg:
-
Regulatory T cell
- TWEAK:
-
Tumor necrosis factor-like weak inducer of apoptosis
- VEGF:
-
Vascular endothelial growth factor
- VEGFR2:
-
Vascular endothelial growth factor receptor II
- WHO:
-
The World Health Organization
References
Qi J, Li M, Wang L, Hu Y, Liu W, Long Z, et al. National and subnational trends in cancer burden in China, 2005-20: an analysis of national mortality surveillance data. Lancet Public Health. 2023;8:e943–55.
Sung H, Ferlay J, Siegel RL, Laversanne M, Soerjomataram I, Jemal A, et al. Global Cancer statistics 2020: GLOBOCAN estimates of incidence and Mortality Worldwide for 36 cancers in 185 countries. CA Cancer J Clin. 2021;71:209–49.
Global cancer burden growing. amidst mounting need for services [https://www.who.int/news/item/01-02-2024-global-cancer-burden-growing--amidst-mounting-need-for-services]
Singh D, Vignat J, Lorenzoni V, Eslahi M, Ginsburg O, Lauby-Secretan B, et al. Global estimates of incidence and mortality of cervical cancer in 2020: a baseline analysis of the WHO Global Cervical Cancer Elimination Initiative. Lancet Glob Health. 2023;11:e197–206.
Tan S, Li D, Zhu X. Cancer immunotherapy: pros, cons and beyond. Biomed Pharmacother. 2020;124:109821.
Parra LM, Webster RM. The malignant melanoma market. Nat Rev Drug Discov. 2022;21:489–90.
Nawaz K, Webster RM. The non-small-cell lung cancer drug market. Nat Rev Drug Discov. 2023;22:264–5.
Kwok G, Yau TC, Chiu JW, Tse E, Kwong YL. Pembrolizumab (Keytruda). Hum Vaccin Immunother. 2016;12:2777–89.
Weinmann SC, Pisetsky DS. Mechanisms of immune-related adverse events during the treatment of cancer with immune checkpoint inhibitors. Rheumatology (Oxford). 2019;58:vii59–67.
Galon J, Bruni D. Approaches to treat immune hot, altered and cold tumours with combination immunotherapies. Nat Rev Drug Discov. 2019;18:197–218.
Mohd Salim NH, Mussa A, Ahmed N, Ahmad S, Yean Yean C, Hassan R, et al. The immunosuppressive effect of TNFR2 expression in the Colorectal Cancer Microenvironment. Biomedicines. 2023;11:173.
Zhang X, Lao M, Xu J, Duan Y, Yang H, Li M, et al. Combination cancer immunotherapy targeting TNFR2 and PD-1/PD-L1 signaling reduces immunosuppressive effects in the microenvironment of pancreatic tumors. J Immunother Cancer. 2022;10:e003982.
Vanamee ÉS, Faustman DL. TNFR2: a Novel Target for Cancer Immunotherapy. Trends Mol Med. 2017;23:1037–46.
Chen Y, Jia M, Wang S, Xu S, He N. Antagonistic antibody targeting TNFR2 inhibits Regulatory T cell function to promote Anti-tumor Activity. Front Immunol. 2022;13:835690.
Tam EM, Fulton RB, Sampson JF, Muda M, Camblin A, Richards J, et al. Antibody-mediated targeting of TNFR2 activates CD8(+) T cells in mice and promotes antitumor immunity. Sci Transl Med. 2019;11:eaax0720.
Guo Y, Xie F, Liu X, Ke S, Chen J, Zhao Y et al. Blockade of TNF-α/TNFR2 signalling suppresses colorectal cancer and enhances the efficacy of anti-PD1 immunotherapy by decreasing CCR8 + T regulatory cells. J Mol Cell Biol 2023: mjad067.
Case K, Tran L, Yang M, Zheng H, Kuhtreiber WM, Faustman DL. TNFR2 blockade alone or in combination with PD-1 blockade shows therapeutic efficacy in murine cancer models. J Leukoc Biol. 2020;107:981–91.
Zhou C, Sun BY, Zhou PY, Yang ZF, Wang ZT, Liu G, et al. MAIT cells confer resistance to Lenvatinib plus anti-PD1 antibodies in hepatocellular carcinoma through TNF-TNFRSF1B pathway. Clin Immunol. 2023;256:109770.
Fu Q, Shen Q, Tong J, Huang L, Cheng Y, Zhong W. Anti-tumor Necrosis factor receptor 2 antibody combined with Anti-PD-L1 therapy exerts Robust Antitumor effects in breast Cancer. Front Cell Dev Biol. 2021;9:720472.
Ghods A, Mehdipour F, Shariat M, Talei AR, Ghaderi A. Regulatory T Cells Express Tumor Necrosis Factor Receptor 2 with the highest intensity among CD4(+) T cells in the draining lymph nodes of breast cancer. Mol Immunol. 2021;137:52–6.
Mancusi A, Alvarez M, Piccinelli S, Velardi A, Pierini A. TNFR2 signaling modulates immunity after allogeneic hematopoietic cell transplantation. Cytokine Growth Factor Rev. 2019;47:54–61.
Polz J, Remke A, Weber S, Schmidt D, Weber-Steffens D, Pietryga-Krieger A, et al. Myeloid suppressor cells require membrane TNFR2 expression for suppressive activity. Immun Inflamm Dis. 2014;2:121–30.
Madsen PM, Motti D, Karmally S, Szymkowski DE, Lambertsen KL, Bethea JR, et al. Oligodendroglial TNFR2 mediates membrane TNF-Dependent repair in experimental autoimmune encephalomyelitis by promoting oligodendrocyte differentiation and remyelination. J Neurosci. 2016;36:5128–43.
Beldi G, Bahiraii S, Lezin C, Nouri Barkestani M, Abdelgawad ME, Uzan G, et al. TNFR2 is a crucial Hub Controlling Mesenchymal Stem Cell Biological and Functional properties. Front Cell Dev Biol. 2020;8:596831.
McMillan D, Martinez-Fleites C, Porter J, Fox D 3rd, Davis R, Mori P, et al. Structural insights into the disruption of TNF-TNFR1 signalling by small molecules stabilising a distorted TNF. Nat Commun. 2021;12:582.
Medler J, Kucka K, Wajant H. Tumor necrosis factor receptor 2 (TNFR2): an emerging target in Cancer Therapy. Cancers (Basel). 2022;14:2603.
Locksley RM, Killeen N, Lenardo MJ. The TNF and TNF receptor superfamilies: integrating mammalian biology. Cell. 2001;104:487–501.
Rhinn H, Tatton N, McCaughey S, Kurnellas M, Rosenthal A. Progranulin as a therapeutic target in neurodegenerative diseases. Trends Pharmacol Sci. 2022;43:641–52.
Hsu H, Huang J, Shu HB, Baichwal V, Goeddel DV. TNF-dependent recruitment of the protein kinase RIP to the TNF receptor-1 signaling complex. Immunity. 1996;4:387–96.
Hsu H, Shu HB, Pan MG, Goeddel DV. TRADD-TRAF2 and TRADD-FADD interactions define two distinct TNF receptor 1 signal transduction pathways. Cell. 1996;84:299–308.
Peltzer N, Darding M, Walczak H. Holding RIPK1 on the Ubiquitin Leash in TNFR1 Signaling. Trends Cell Biol. 2016;26:445–61.
Rothe M, Pan MG, Henzel WJ, Ayres TM, Goeddel DV. The TNFR2-TRAF signaling complex contains two novel proteins related to baculoviral inhibitor of apoptosis proteins. Cell. 1995;83:1243–52.
Borghi A, Haegman M, Fischer R, Carpentier I, Bertrand MJM, Libert C, et al. The E3 ubiquitin ligases HOIP and cIAP1 are recruited to the TNFR2 signaling complex and mediate TNFR2-induced canonical NF-κB signaling. Biochem Pharmacol. 2018;153:292–8.
Emmerich CH, Ordureau A, Strickson S, Arthur JS, Pedrioli PG, Komander D, et al. Activation of the canonical IKK complex by K63/M1-linked hybrid ubiquitin chains. Proc Natl Acad Sci U S A. 2013;110:15247–52.
Wajant, Pfizenmaier. Scheurich. Tumor necrosis factor signaling. Cell Death Differ. 2003;10:45–65.
Sun SC. Non-canonical NF-κB signaling pathway. Cell Res. 2011;21:71–85.
Reinhard C. Tumor necrosis factor alpha -induced activation of c-jun N-terminal kinase is mediated by TRAF2. EMBO J. 1997;16:1080–92.
Zhang R, Xu Y, Ekman N, Wu Z, Wu J, Alitalo K, Min W. Etk/Bmx transactivates vascular endothelial growth factor 2 and recruits phosphatidylinositol 3-Kinase to mediate the Tumor necrosis factor-induced angiogenic pathway. J Biol Chem. 2003;278:51267–76.
Natoli G, Costanzo A, Ianni A, Templeton DJ, Woodgett JR, Balsano C, et al. Activation of SAPK/JNK by TNF receptor 1 through a noncytotoxic TRAF2-Dependent pathway. Science. 1997;275:200–3.
Soo Y et al. Lee, and, Amy, Reichlin, TRAF2 Is Essential for JNK but Not NF-κB Activation and Regulates Lymphocyte Proliferation and Survival. Immunity. 1997; 275: 200-3.
Ichijo H, Nishida E. Induction of apoptosis by ASK1, a mammalian MAPKKK that activates SAPK/JNK and p38 signaling pathway. Science. 1997;7:7030–13.
Ji W, Li Y, Wan T, Wang J, Zhang H, Chen H, et al. Both internalization and AIP1 association are required for TNFR2-mediated JNK Signaling. Arterioscler Thromb Vascular Biology. 2012;32:2271.
Min W, Lin Y, Tang S, Yu L, Zhang H, Wan T, et al. AIP1 recruits phosphatase PP2A to ASK1 in Tumor Necrosis factor–Induced ASK1-JNK activation. Circul Res. 2008;102:840–8.
Qiu Y, Robinson D, Pretlow TG, Kung HJ. Etk/Bmx, a tyrosine kinase with a pleckstrin-homology domain, is an effector of phosphatidylinositol 3′-kinase and is involved in interleukin 6-induced neuroendocrine differentiation of prostate cancer cells. Proc Natl Acad Sci USA. 1998;5:3644–9.
Bradley JR. TNF-mediated inflammatory disease. J Pathol. 2010;214:149–60.
Fotin-Mleczek M, Henkler F, Samel D, Reichwein M, Hausser A, Parmryd I, et al. Apoptotic crosstalk of TNF receptors: TNF-R2-induces depletion of TRAF2 and IAP proteins and accelerates TNF-R1-dependent activation of caspase-8. J Cell Sci. 2002;115:2757–70.
Faustman D, Davis M. TNF receptor 2 pathway: drug target for autoimmune diseases. Nat Rev Drug Discov. 2010;9:482–93.
Iversen PL, Kipshidze N, Kipshidze N, Dangas G, Ramacciotti E, Kakabadze Z, et al. A novel therapeutic vaccine targeting the soluble TNFα receptor II to limit the progression of cardiovascular disease: AtheroVax™. Front Cardiovasc Med. 2023;10:1206541.
Chen X, Oppenheim JJ. Targeting TNFR2, an immune checkpoint stimulator and oncoprotein, is a promising treatment for cancer. Sci Signal. 2017;10:eaal2328.
DeBerge MP, Ely KH, Wright PF, Thorp EB, Enelow RI. Shedding of TNF receptor 2 by effector CD8⁺ T cells by ADAM17 is important for regulating TNF-α availability during influenza infection. J Leukoc Biol. 2015;98:423–34.
Kartikasari AER, Cassar E, Razqan MAM, Szydzik C, Huertas CS, Mitchell A, et al. Elevation of circulating TNF receptor 2 in cancer: a systematic meta-analysis for its potential as a diagnostic cancer biomarker. Front Immunol. 2022;13:918254.
Yang Y, Islam MS, Hu Y, Chen X. TNFR2: role in Cancer Immunology and Immunotherapy. Immunotargets Ther. 2021;10:103–22.
Zhao YP, Tian QY, Frenkel S, Liu CJ. The promotion of bone healing by progranulin, a downstream molecule of BMP-2, through interacting with TNF/TNFR signaling. Biomaterials. 2013;34:6412–21.
Jayaraman A, Htike TT, James R, Picon C, Reynolds R. TNF-mediated neuroinflammation is linked to neuronal necroptosis in Alzheimer’s disease hippocampus. Acta Neuropathol Commun. 2021;9:159.
Fischer R, Sendetski M, Del Rivero T, Martinez GF, Bracchi-Ricard V, Swanson KA, et al. TNFR2 promotes Treg-mediated recovery from neuropathic pain across sexes. Proc Natl Acad Sci U S A. 2019;116:17045–50.
Luo Y, Xu Z, Wan T, He Y, Jones D, Zhang H, et al. Endothelial-specific transgenesis of TNFR2 promotes adaptive arteriogenesis and angiogenesis. Arterioscler Thromb Vasc Biol. 2010;30:1307–14.
Wan T, Xu Z, Zhou HJ, Zhang H, Luo Y, Li Y, et al. Functional analyses of TNFR2 in physiological and pathological retina angiogenesis. Invest Ophthalmol Vis Sci. 2013;54:211–21.
Hamilton KE, Simmons JG, Ding S, Van Landeghem L, Lund PK. Cytokine induction of tumor necrosis factor receptor 2 is mediated by STAT3 in colon cancer cells. Mol Cancer Res Mcr. 2011;9:1718.
Li P, Yang Y, Yang X, Wang Y, Chou CK, Jiang M, et al. TNFR2 deficiency impairs the growth of mouse colon cancer. Int J Biol Sci. 2023;19:1024–35.
Chang LY, Lin YC, Chiang JM, Mahalingam J, Su SH, Huang CT, et al. Blockade of TNF-α signaling benefits cancer therapy by suppressing effector regulatory T cell expansion. OncoImmunology. 2015;4:e1040215.
Zhao T, Li H, Liu Z. Tumor necrosis factor receptor 2 promotes growth of colorectal cancer via the PI3K/AKT signaling pathway. Oncol Lett. 2017;13:342–6.
Tanimura Y, Kokuryo T, Tsunoda N, Yamazaki Y, Oda K, Nimura Y, et al. Tumor necrosis factor α promotes invasiveness of cholangiocarcinoma cells via its receptor, TNFR2. Cancer Lett. 2005;219:205–13.
Hu G, Liang L, Liu Y, Liu J, Tan X, Xu M, et al. TWEAK/Fn14 Interaction confers aggressive properties to cutaneous squamous cell carcinoma. J Invest Dermatol. 2019;139:796–806.
Yang D, Wang LL, Dong TT, Shen YH, Guo XS, Liu CY, et al. Progranulin promotes colorectal cancer proliferation and angiogenesis through TNFR2/Akt and ERK signaling pathways. Am J Cancer Res. 2015;5:3085–97.
Pan S, An P, Zhang R, He X, Yin G, Min W. Etk/Bmx as a tumor necrosis factor receptor type 2-specific kinase: role in endothelial cell migration and angiogenesis. Mol Cell Biol. 2002;22:7512–23.
Chen J, Yu M, Li X, Sun QF, Yang CZ, Yang PS. Progranulin promotes osteogenic differentiation of human periodontal ligament stem cells via tumor necrosis factor receptors to inhibit TNF-α sensitized NF-kB and activate ERK/JNK signaling. J Periodontal Res. 2020;55:363–73.
He Y, Luo Y, Tang S, Rajantie I, Salven P, Heil M, et al. Critical function of Bmx/Etk in ischemia-mediated arteriogenesis and angiogenesis. J Clin Invest. 2006;116:2344–55.
Al-Lamki RS, Sadler TJ, Wang J, Reid MJ, Warren AY, Movassagh M, et al. Tumor necrosis factor receptor expression and signaling in renal cell carcinoma. Am J Pathol. 2010;177:943–54.
Baram T, Erlichman N, Dadiani M, Balint-Lahat N, Pavlovski A, Meshel T, et al. Chemotherapy shifts the balance in Favor of CD8 + TNFR2 + TILs in Triple-negative breast tumors. Cells. 2021;10:1429.
Calzascia T, Pellegrini M, Hall H, Sabbagh L, Ono N, Elford AR, et al. TNF-alpha is critical for antitumor but not antiviral T cell immunity in mice. J Clin Invest. 2007;117:3833–45.
Kim EY, Priatel JJ, Teh SJ, Teh HS. TNF receptor type 2 (p75) functions as a costimulator for antigen-driven T cell responses in vivo. J Immunol. 2006;176:1026–35.
Kim EY, Teh HS. Critical role of TNF receptor type-2 (p75) as a costimulator for IL-2 induction and T cell survival: a functional link to CD28. J Immunol. 2004;173:4500–9.
Ye LL, Wei XS, Zhang M, Niu YR, Zhou Q. The significance of Tumor Necrosis factor receptor type II in CD8(+) Regulatory T cells and CD8(+) effector T cells. Front Immunol. 2018;9:583.
Twu YC, Gold MR, Teh HS. TNFR1 delivers pro-survival signals that are required for limiting TNFR2-dependent activation-induced cell death (AICD) in CD8 + T cells. Eur J Immunol. 2011;41:335–44.
Otano I, Alvarez M, Minute L, Ochoa MC, Migueliz I, Molina C, et al. Human CD8 T cells are susceptible to TNF-mediated activation-induced cell death. Theranostics. 2020;10:4481–9.
Tufa DM, Chatterjee D, Low HZ, Schmidt RE, Jacobs R. TNFR2 and IL-12 coactivation enables slanDCs to support NK-cell function via membrane-bound TNF-α. Eur J Immunol. 2014;44:3717–28.
Almishri W, Santodomingo-Garzon T, Le T, Stack D, Mody CH, Swain MG. TNFα augments Cytokine-Induced NK Cell IFNγ production through TNFR2. J Innate Immun. 2016;8:617–29.
Khan AUH, Ali AK, Marr B, Jo D, Ahmadvand S, Fong-McMaster C, et al. The TNFα/TNFR2 axis mediates natural killer cell proliferation by promoting aerobic glycolysis. Cell Mol Immunol. 2023;20:1140–55.
Chen X, Subleski JJ, Kopf H, Howard OM, Männel DN, Oppenheim JJ. Cutting edge: expression of TNFR2 defines a maximally suppressive subset of mouse CD4 + CD25 + FoxP3 + T regulatory cells: applicability to tumor-infiltrating T regulatory cells. J Immunol. 2008;180:6467–71.
Chen X, Bäumel M, Männel DN, Howard OM, Oppenheim JJ. Interaction of TNF with TNF receptor type 2 promotes expansion and function of mouse CD4 + CD25 + T regulatory cells. J Immunol. 2007;179:154–61.
Ceeraz S, Thompson CR, Beatson R, Choy EH. Harnessing CD8(+)CD28(-) Regulatory T cells as a Tool to treat Autoimmune Disease. Cells. 2021;10:2973.
Wang RF. CD8 + regulatory T cells, their suppressive mechanisms, and regulation in cancer. Hum Immunol. 2008;69:811–4.
Chakraborty S, Panda AK, Bose S, Roy D, Kajal K, Guha D, et al. Transcriptional regulation of FOXP3 requires integrated activation of both promoter and CNS regions in tumor-induced CD8(+) Treg cells. Sci Rep. 2017;7:1628.
Chen X, Yang Y, Zhou Q, Weiss JM, Howard OZ, McPherson JM, et al. Effective chemoimmunotherapy with anti-TGFβ antibody and cyclophosphamide in a mouse model of breast cancer. PLoS ONE. 2014;9:e85398.
Ye LL, Peng WB, Niu YR, Xiang X, Wei XS, Wang ZH, et al. Accumulation of TNFR2-expressing regulatory T cells in malignant pleural effusion of lung cancer patients is associated with poor prognosis. Ann Transl Med. 2020;8:1647.
Wang M, Zhang C, Tian T, Zhang T, Wang R, Han F, et al. Increased Regulatory T Cells in Peripheral blood of Acute myeloid leukemia patients rely on Tumor Necrosis factor (TNF)-α-TNF Receptor-2 pathway. Front Immunol. 2018;9:1274.
He T, Zhao Y, Zhao P, Zhao L, Zakaria J, Wang K. Signaling pathway(s) of TNFR2 required for the immunoregulatory effect of CD4(+)Foxp3(+) regulatory T cells. Int Immunopharmacol. 2022;108:108823.
Qu Y, Wang X, Bai S, Niu L, Zhao G, Yao Y, et al. The effects of TNF-α/TNFR2 in regulatory T cells on the microenvironment and progression of gastric cancer. Int J Cancer. 2022;150:1373–91.
Bai J, Ding B, Li H. Targeting TNFR2 in Cancer: all roads lead to Rome. Front Immunol. 2022;13:844931.
Lubrano di Ricco M, Ronin E, Collares D, Divoux J, Grégoire S, Wajant H, et al. Tumor necrosis factor receptor family costimulation increases regulatory T-cell activation and function via NF-κB. Eur J Immunol. 2020;50:972–85.
Wang J, Ferreira R, Lu W, Farrow S, Downes K, Jermutus L, et al. TNFR2 ligation in human T regulatory cells enhances IL2-induced cell proliferation through the non-canonical NF-κB pathway. Sci Rep. 2018;8:12079.
He T, Liu S, Chen S, Ye J, Wu X, Bian Z, et al. The p38 MAPK inhibitor SB203580 abrogates Tumor Necrosis factor-Induced proliferative expansion of mouse CD4(+)Foxp3(+) Regulatory T cells. Front Immunol. 2018;9:1556.
de Kivit S, Mensink M, Hoekstra AT, Berlin I, Derks RJE, Both D, et al. Stable human regulatory T cells switch to glycolysis following TNF receptor 2 costimulation. Nat Metab. 2020;2:1046–61.
Borghi A, Verstrepen L, Beyaert R. TRAF2 multitasking in TNF receptor-induced signaling to NF-κB, MAP kinases and cell death. Biochem Pharmacol. 2016;116:1–10.
Zhou Q, Wu X, Wang X, Yu Z, Pan T, Li Z, et al. The reciprocal interaction between tumor cells and activated fibroblasts mediated by TNF-α/IL-33/ST2L signaling promotes gastric cancer metastasis. Oncogene. 2020;39:1414–28.
Okuzono Y, Muraki Y, Sato S. TNFR2 pathways are fully active in cancer regulatory T cells. Biosci Biotechnol Biochem. 2022;86:351–61.
Zhao X, Rong L, Zhao X, Li X, Liu X, Deng J, et al. TNF signaling drives myeloid-derived suppressor cell accumulation. J Clin Invest. 2012;122:4094–104.
Ham B, Wang N, D’Costa Z, Fernandez MC, Bourdeau F, Auguste P, et al. TNF Receptor-2 facilitates an immunosuppressive microenvironment in the liver to promote the colonization and growth of hepatic metastases. Cancer Res. 2015;75:5235–47.
Xu X, Meng Q, Erben U, Wang P, Glauben R, Kühl AA, et al. Myeloid-derived suppressor cells promote B-cell production of IgA in a TNFR2-dependent manner. Cell Mol Immunol. 2017;14:597–606.
Hu X, Li B, Li X, Zhao X, Wan L, Lin G, et al. Transmembrane TNF-α promotes suppressive activities of myeloid-derived suppressor cells via TNFR2. J Immunol. 2014;192:1320–31.
Yan L, Zheng D, Xu RH. Critical role of Tumor necrosis factor signaling in mesenchymal stem cell-based therapy for Autoimmune and Inflammatory diseases. Front Immunol. 2018;9:1658.
Beldi G, Khosravi M, Abdelgawad ME, Salomon BL, Uzan G, Haouas H, et al. TNFα/TNFR2 signaling pathway: an active immune checkpoint for mesenchymal stem cell immunoregulatory function. Stem Cell Res Ther. 2020;11:281.
Razazian M, Khosravi M, Bahiraii S, Uzan G, Shamdani S, Naserian S. Differences and similarities between mesenchymal stem cell and endothelial progenitor cell immunoregulatory properties against T cells. World J Stem Cells. 2021;13:971–84.
Ren G, Zhao X, Wang Y, Zhang X, Chen X, Xu C, et al. CCR2-dependent recruitment of macrophages by tumor-educated mesenchymal stromal cells promotes tumor development and is mimicked by TNFα. Cell Stem Cell. 2012;11:812–24.
Wu D, Guo X, Su J, Chen R, Berenzon D, Guthold M, et al. CD138-negative myeloma cells regulate mechanical properties of bone marrow stromal cells through SDF-1/CXCR4/AKT signaling pathway. Biochim Biophys Acta. 2015;1853:338–47.
Chan TS, Shaked Y, Tsai KK. Targeting the interplay between Cancer fibroblasts, mesenchymal stem cells, and Cancer Stem cells in desmoplastic cancers. Front Oncol. 2019;9:688.
Tang W, Lu Y, Tian QY, Zhang Y, Guo FJ, Liu GY, et al. The growth factor progranulin binds to TNF receptors and is therapeutic against inflammatory arthritis in mice. Science. 2011;332:478–84.
Torrey H, Butterworth J, Mera T, Okubo Y, Wang L, Baum D, et al. Targeting TNFR2 with antagonistic antibodies inhibits proliferation of ovarian cancer cells and tumor-associated Tregs. Sci Signal. 2017;10:eaaf8608.
Chan FK, Chun HJ, Zheng L, Siegel RM, Bui KL, Lenardo MJ. A domain in TNF receptors that mediates ligand-independent receptor assembly and signaling. Science. 2000;288:2351–4.
Torrey H, Kühtreiber WM, Okubo Y, Tran L, Case K, Zheng H, et al. A novel TNFR2 agonist antibody expands highly potent regulatory T cells. Sci Signal. 2020;13:eaba9600.
Mukai Y, Nakamura T, Yoshikawa M, Yoshioka Y, Tsunoda S, Nakagawa S, et al. Solution of the structure of the TNF-TNFR2 complex. Sci Signal. 2010;3:ra83.
St Paul M, Ohashi PS. The roles of CD8(+) T cell subsets in Antitumor Immunity. Trends Cell Biol. 2020;30:695–704.
Onda M, Kobayashi K, Pastan I. Depletion of regulatory T cells in tumors with an anti-CD25 immunotoxin induces CD8 T cell-mediated systemic antitumor immunity. Proc Natl Acad Sci U S A. 2019;116:4575–82.
Paluskievicz CM, Cao X, Abdi R, Zheng P, Liu Y, Bromberg JS. T Regulatory Cells and priming the suppressive Tumor Microenvironment. Front Immunol. 2019;10:2453.
Moatti A, Cohen JL. The TNF-α/TNFR2 pathway: targeting a Brake to release the Anti-tumor Immune Response. Front Cell Dev Biol. 2021;9:725473.
Mårtensson L, Cleary K, Semmrich M, Kovacek M, Holmkvist P, Svensson C, et al. Abstract 936: Targeting TNFR2 for cancer immunotherapy: Ligand blocking depletors versus receptor agonists. Cancer Res. 2020;80:936.
Martensson L, Holmkvist P, Kovacek M, Svensson C, Bodén M, Cleary K, et al. 1368 preclinical development of an agonistic anti-TNFR2 antibody (BI-1910) for cancer immunotherapy. J Immunother Cancer. 2023;11:A1524.
BI-1910 as. A single Agent and in Combination with Pembrolizumab for the treatment of Advanced Solid Tumors
Spira AI, Naing A, Babiker HM, Borad MJ, Garralda E, Leventakos K, et al. Phase I study of HFB200301, a first-in-class TNFR2 agonist monoclonal antibody in patients with solid tumors selected via drug Intelligent Science (DIS). J Clin Oncol. 2022;40:TPS2670.
Wei S, Fulton R, Lu Y-Y, Zhang Q, Zhou H, Raue A, et al. Abstract 1883: mechanism of action and biomarker strategy for HFB200301, an anti-TNFR2 agonist antibody for the treatment of cancer. Cancer Res. 2021;81:1883.
Mårtensson L, Cleary K, Holmkvist P, Kovacek M, Svensson C, Semmrich M, et al. Abstract 4156: BI-1808 - a first in class ligand-blocking αTNFR2 antibody for cancer immunotherapy. Cancer Res. 2022;82:4156.
Mårtensson L, Kovacek M, Holmkvist P, Semmrich M, Svensson C, Blidberg T, et al. 725 pre-clinical development of TNFR2 ligand-blocking BI-1808 for cancer immunotherapy. J Immunother Cancer. 2020;8:A434.
BI-1808 as. A single Agent and with Pembrolizumab in Treatment of Advanced malignancies(Keynote-D20)
Rohrberg KS, Papai Z, Carneiro A, Lang I, Yachnin J, Lim S, et al. 757 phase 1/2a clinical trial of BI-1808, a monoclonal antibody to tumor necrosis factor receptor 2 (TNFR2) as single agent and in combination with pembrolizumab. J Immunother Cancer. 2023;11:A853.
Wang B, Wang H, Li T, Sun J, Huang X, Lin H, et al. LBL-019, a novel TNFR2 agonist antibody, shows potent anti-tumor efficacy through preferentially activating CD8 + T cells and alleviating the suppressive effect of Treg cells. J Immunother Cancer. 2023;11:A939.
Lin H, Huang X, Qin Y, Dang Y, Zhang P, Sun J et al. Abstract 5523: LBL-019, a novel anti-TNFR2 antibody, shows a potent anti-tumor efficacy in a mouse MC38 model. Cancer Research. 2022; 82: 5523.
Nanjing Leads Biolabs Co. L. evaluation of LBL-019 monotherapy or combined with Anti-PD-1 antibody in the treatment of Advanced Malignant tumors. 2024. https://clinicaltrials.gov/study/NCT05223231
SIM0235. aTNFR2 monoclonal antibody independently developed by Simcere received IND approval by the U.S. FDA
Liu F. SIM1811-03 in participants with advanced solid tumor and cutaneous T cell lymphomas: preliminary results from an on-going first-in-human phase I trial in China. [https://www.simcere.com/attached/upload/file/20231023/6383365169940589434856358.pdf]
Dongchen C,.S.NBL-020 for the treatment of advanced solid tumors obtains clinical trial approval in the U; 2022.[https://en.e-cspc.com/news/nbl-020-for-the-treatment-of-advanced-solid-tumors-obtains-clinial-trial-approval-in-china.html]
A Study of NBL-020. Injection in Subjects With Advanced Malignant Tumors. [https://clinicaltrials.gov/study/NCT05877924]
Chopra M, Riedel SS, Biehl M, Krieger S, von Krosigk V, Bäuerlein CA, et al. Tumor necrosis factor receptor 2-dependent homeostasis of regulatory T cells as a player in TNF-induced experimental metastasis. Carcinogenesis. 2013;34:1296–303.
Chan AT, Ogino S, Giovannucci EL, Fuchs CS. Inflammatory markers are associated with risk of colorectal cancer and chemopreventive response to anti-inflammatory drugs. Gastroenterology. 2011;140:799–808.
Babic A, Shah SM, Song M, Wu K, Meyerhardt JA, Ogino S, et al. Soluble tumour necrosis factor receptor type II and survival in colorectal cancer. Br J Cancer. 2016;114:995–1002.
Williams GS, Mistry B, Guillard S, Ulrichsen JC, Sandercock AM, Wang J, et al. Phenotypic screening reveals TNFR2 as a promising target for cancer immunotherapy. Oncotarget. 2016;7:68278–91.
Jing Q, Wan Q, Nie Y, Luo J, Zhang X, Zhu L, et al. Ansofaxine hydrochloride inhibits tumor growth and enhances Anti-TNFR2 in murine colon cancer model. Front Pharmacol. 2023;14:1286061.
Nomelini RS, Borges Júnior LE, de Lima CA, Chiovato AFC, Micheli DC, Tavares-Murta BM, et al. TNF-R2 in tumor microenvironment as prognostic factor in epithelial ovarian cancer. Clin Exp Med. 2018;18:547–54.
Govindaraj C, Scalzo-Inguanti K, Madondo M, Hallo J, Flanagan K, Quinn M, et al. Impaired Th1 immunity in ovarian cancer patients is mediated by TNFR2 + tregs within the tumor microenvironment. Clin Immunol. 2013;149:97–110.
Govindaraj C, Madondo M, Kong YY, Tan P, Wei A, Plebanski M. Lenalidomide-based maintenance therapy reduces TNF receptor 2 on CD4 T cells and enhances immune effector function in acute myeloid leukemia patients. Am J Hematol. 2014;89:795–802.
Yang F, Zhao Z, Zhao N. Clinical implications of tumor necrosis factor receptor 2 in breast cancer. Oncol Lett. 2017;14:2393–8.
Jablonska E. Release of soluble IL-6 receptor (IL-6sR) in comparison with release of soluble TNF receptors (sTNF-Rs) by PMNs and WBC derived from breast cancer patients. Cancer Lett. 1997;119:79–85.
Mahmud SA, Manlove LS, Schmitz HM, Xing Y, Wang Y, Owen DL, et al. Costimulation via the tumor-necrosis factor receptor superfamily couples TCR signal strength to the thymic differentiation of regulatory T cells. Nat Immunol. 2014;15:473–81.
Nguyen LT, Ohashi PS. Clinical blockade of PD1 and LAG3–potential mechanisms of action. Nat Rev Immunol. 2015;15:45–56.
Saito T. Molecular Dynamics of Co-signal molecules in T-Cell activation. Adv Exp Med Biol. 2019;1189:135–52.
Sampson JF, Kurella VB, Paragas V, Kumar S, Lulo JE, Qiu JA, et al. Abstract 555: a novel human TNFR2 antibody (MM-401) modulates T cell responses in anti-cancer immunity. Cancer Res. 2019;79:555.
Richards J, Wong C, Koshkaryev A, Fulton R, Camblin A, Sampson J et al. Abstract 4846: MM-401, a novel anti-TNFR2 antibody that induces T cell co-stimulation, robust anti-tumor activity and immune memory. Cancer Research. 2019; 79: 4846.
Moatti A, Debesset A, Pilon C, Beldi-Ferchiou A, Leclerc M, Redjoul R, et al. TNFR2 blockade of regulatory T cells unleashes an antitumor immune response after hematopoietic stem-cell transplantation. J Immunother Cancer. 2022;10:e003508.
Yang Y, Cao S, Zhang J, Shao Z, Jiang X, Duan Q, et al. Abstract 2907: 1C3, a Novel non-blocking anti-human TNFR2 antibody generated from RenMice, exhibits promising anti-tumor activity and safety in syngeneic tumor models in humanized TNFR2 mice. Cancer Res. 2022;82:2907.
Nie Y, He J, Shirota H, Trivett AL, Yang D, Klinman DM, et al. Blockade of TNFR2 signaling enhances the immunotherapeutic effect of CpG ODN in a mouse model of colon cancer. Sci Signal. 2018;11:eaan0790.
Chen J, Qiao YD, Li X, Xu JL, Ye QJ, Jiang N, et al. Intratumoral CD45(+)CD71(+) erythroid cells induce immune tolerance and predict tumor recurrence in hepatocellular carcinoma. Cancer Lett. 2021;499:85–98.
Chen Y, Jia M, Xu S, Zhao Y, Chan E, Zhang M, et al. Abstract 1451: AN3025: a novel anti-human TNFR2 antibody that exhibits immune activation and strong anti-tumor activity in vivo. Cancer Res. 2021;81:1451.
Filbert E, Krishnan S, Alvarado R, Huang G, Bahjat F, Yang X. APX601, a Novel TNFR2 antagonist antibody for cancer immunotherapy. J Immunother Cancer. 2020;8:A417.
Krishnan S, Alvarado R, Huang G, Yang X, Filbert EL, Abstract. APX601, a potent TNFR2 antagonist as a Novel and Promising Approach to reverse Tumor Immune suppression. Cancer Res. 2021;LB175:81: LB175.
Torrey H, Khodadoust M, Tran L, Baum D, Defusco A, Kim YH, et al. Targeted killing of TNFR2-expressing tumor cells and T(regs) by TNFR2 antagonistic antibodies in advanced Sézary syndrome. Leukemia. 2019;33:1206–18.
Acknowledgements
Not applicable.
Funding
This work was supported by The National Natural Science Foundation of China (No.82273844, No. 42076094 and No.81773627), The National Major Scientific and Technological Special Projects for “Significant New Drugs Innovation and Development” (No. 2019ZX09301119), the Shanghai Science and Technology Innovation Action Plan (Grant No.21S11902400).
Author information
Authors and Affiliations
Contributions
L.L. and R.Y. designed and wrote the manuscript. Y.L. and H.P. prepared the table and figures. Y.L. and S.H. reviewed and revised the manuscript. All authors listed have made a substantial contribution to the work. All authors have read and approved the article.
Corresponding authors
Ethics declarations
Ethics approval and consent to participate
Not applicable.
Consent for publication
Not applicable. Our manuscript does not contain data from any individual person. All authors consent for publication of this review.
Competing interests
The authors declare that they have no known competing financial interests or personal relationships that could have appeared to influence the work reported in this paper. The authors declare no conflict of interest.
Additional information
Publisher’s note
Springer Nature remains neutral with regard to jurisdictional claims in published maps and institutional affiliations.
Rights and permissions
Open Access This article is licensed under a Creative Commons Attribution-NonCommercial-NoDerivatives 4.0 International License, which permits any non-commercial use, sharing, distribution and reproduction in any medium or format, as long as you give appropriate credit to the original author(s) and the source, provide a link to the Creative Commons licence, and indicate if you modified the licensed material. You do not have permission under this licence to share adapted material derived from this article or parts of it. The images or other third party material in this article are included in the article’s Creative Commons licence, unless indicated otherwise in a credit line to the material. If material is not included in the article’s Creative Commons licence and your intended use is not permitted by statutory regulation or exceeds the permitted use, you will need to obtain permission directly from the copyright holder. To view a copy of this licence, visit http://creativecommons.org/licenses/by-nc-nd/4.0/.
About this article
Cite this article
Li, L., Ye, R., Li, Y. et al. Targeting TNFR2 for cancer immunotherapy: recent advances and future directions. J Transl Med 22, 812 (2024). https://doi.org/10.1186/s12967-024-05620-x
Received:
Accepted:
Published:
DOI: https://doi.org/10.1186/s12967-024-05620-x