Abstract
Background
Culex mosquitoes are the primary vectors of West Nile virus (WNV) across the USA. Understanding when these vectors are active indicates times when WNV transmission can occur. This study determined the proportion of female Culex mosquitoes that were in diapause during the fall and winter and when they terminated diapause and began blood feeding in the spring.
Methods
Mosquitoes were collected from parks using various traps and/or aspirated from culverts in Franklin County, Ohio, from October to mid-May from 2019 to 2022. Culex mosquitoes were morphologically identified to species, and the ovaries of females were dissected to determine their diapause and parity statuses.
Results
By early October 2021, roughly 95% of Culex pipiens collected in culverts were in diapause and 98% of Cx. erraticus were in diapause. Furthermore, gravid and blood-fed Culex salinarius, Cx. pipiens, and Cx. restuans were collected in late November in 2019 and 2021 in standard mosquito traps. In the winter of 2021, the proportions of non-diapausing Culex decreased within culverts. The last non-diapausing Cx. erraticus was collected in late December 2021 while the final non-diapausing Cx. pipiens was collected in mid-January 2022, both in culverts. Roughly 50% of Cx. pipiens terminated diapause by mid-March 2022, further supported by our collections of gravid females in late March in all 3 years of mosquito collection. In fact, male mosquitoes of Cx. pipiens, Cx. restuans, and Cx. territans were collected by the 1st week of May in 2022, indicating that multiple species of Culex produced a second generation that reached adulthood by this time.
Conclusions
We collected blood-fed and gravid Culex females into late November in 2 of the 3 years of our collections, indicating that it might be possible for WNV transmission to occur in late fall in temperate climates like Ohio. The persistence of non-diapausing Cx. pipiens and Cx. erraticus throughout December has important implications for the winter survival of WNV vectors and our overall understanding of diapause. Finally, determining when Culex terminate diapause in the spring may allow us to optimize mosquito management programs and reduce the spread of WNV before it is transmitted to humans.
Graphical Abstract

Similar content being viewed by others
Background
West Nile virus (WNV) is the most common mosquito-borne pathogen in the continental USA [1]. Birds are the primary reservoirs of WNV, and transmission typically occurs after a mosquito consumes a blood meal from an infected avian host and then then bites another bird or human. In the US in 2022 there were 1035 confirmed human cases of WNV [2], and in the state of Ohio there were 7 confirmed cases, which unfortunately resulted in 1 death [2].
Human cases of WNV typically occur in mid-June to late October in Ohio, with peaks during mid-August [3]. As illness onset occurs anywhere from 2 to 14 days after a bite from an infected mosquito, residents of Ohio and other states at similar latitudes are at the greatest risk of becoming infected with WNV in early June through mid-October [3]. This seasonal distribution of WNV transmission is influenced by at least three important biological aspects of the mosquito vectors: competence for carrying and transmitting WNV, host preferences, and diapause.
Although many genera of mosquitoes have been found to transmit WNV and may serve as important bridge vectors (e.g. Aedes vexans and Coquillettidia perturbans [4,5,6,7]), Culex mosquitoes are the major vectors throughout the US [8,9,10]. During the spring and summer, Cx. pipiens and Cx. restuans are likely the primary vectors in the Northeastern US of WNV [9]. This is because both species are highly competent vectors of WNV [11, 12] and because both species primarily bite birds but will opportunistically bite mammals [13].
In addition to Cx. pipiens and Cx. restuans, three species of Culex are routinely collected in central Ohio: Cx. salinarius, Cx. erraticus, and Cx. territans [14]. The role of these remaining species in WNV transmission is currently unclear. Although Cx. salinarius may not play a large role in WNV transmission because of their lower vector competence [15], they may serve as an important bridge vector as they bite both birds and mammals [16]. Similarly, Cx. erraticus bite both birds and mammals [17], making them another potential bridge vector for WNV, especially as Cx. erraticus are frequently collected later in the year [18]. Previously, researchers collected WNV-infected Cx. erraticus from the field [19], even when overwintering [18]. However, these mosquitoes are often less abundant and therefore account for a lower proportion of WNV-infected mosquitoes relative to Cx. pipiens, Cx. restuans, and Cx. salinarius in the Northeastern US [9]. Culex territans primarily bite amphibians, and infrequently bite reptiles or mammals, and are therefore unlikely to transmit WNV to humans [17].
Diapause is an overwintering strategy common among adult Culex females in temperate areas that results in physiological and behavioral changes [20, 21]. Female larvae of Cx. pipiens subjected to short daylengths and low temperatures enter diapause as adults [22, 23]. Semi-field studies have found that 50% of Cx. pipiens initiate diapause around mid-September in Ames, Iowa (latitude 32.0°N; [24]) and at higher latitudes, diapause initiation occurs earlier with 50% of the population entering diapause around mid-July in Guelph, Ontario, Canada (latitude 43.4°N; [25]). One field study in Boston, Massachusetts (latitude 42.4°N; [26]) found that adult Cx. pipiens began entering diapause in mid-August and diapause incidence reached 50% by early September. While in diapause, adult female mosquitoes arrest ovarian development and store glycogen and lipids [27, 28]. Behaviorally, diapausing Culex females no longer seek vertebrate hosts [29], take blood meals [30], or lay eggs [31] but instead remain in secluded shelters (e.g. sewage tunnels/culverts, and caves; [32, 33]). However, not all diapausing Culex females survive harsh winter conditions, causing populations to reach their lowest abundance shortly after mosquitoes terminate diapause in the spring [33,34,35,36]. Therefore, diapause initiation corresponds to the cessation of WNV transmission in autumn while diapause termination allows WNV transmission to resume in the spring, especially because a small proportion of female Cx. pipiens and Cx. erraticus overwinter infected with WNV [18, 32, 37,38,39].
A better understanding of when Culex species initiate and terminate diapause will allow us to accurately predict seasonal cycles of WNV transmission and pinpoint when mosquito surveillance and control will be most effective. Therefore, our goal was to characterize how long Culex remain reproductively active throughout the fall and when they terminate diapause in the spring. Although previous semi-field studies have characterized how photoperiod and temperature induce diapause in larvae of Cx. pipiens [24, 25] and another study characterized the diapause incidence of female Cx. pipiens collected in a single overwintering site from August to mid-November [26], our study is unique in that it determined how long non-diapausing and reproductively active mosquitoes persist in the field across multiple sites. Additionally, by continuously collecting mosquitoes throughout the winter and spring, we were able to determine when Cx. pipiens terminate diapause and resume blood feeding over a 3-year period. We were also able to make strong inferences on when Cx. erraticus and Cx. restuans initiate and terminate their overwintering dormancies. Based on previous findings, we expected the majority of Cx. pipiens to cease host-seeking by October [24,25,26] and terminate diapause by mid-April [33, 39]. We found that most Cx. pipiens initiated diapause by October, yet a small proportion of Cx. pipiens along with many other Culex spp. remained reproductively active until later in the year. We also found that Cx. pipiens and Cx. restuans terminate diapause by mid-March.
Methods
Field sites and mosquito collection
Collections took place over a fall to spring period for 3 years. During the fall 2019 to spring 2020 collection season, gravid traps (upright-style CDC Gravid Trap Model 1712.11) baited with “stinky water” (a mixture of fermented grass clippings, milk protein, and yeast brewed outdoors for 4 days until November, when it then was brewed indoors) were placed at five field sites consisting of wooded parks and meadows around Franklin County, Ohio (40.0° N) and were collected roughly 24 h afterwards (Additional file 1: Fig. S1a). Traps were placed weekly from September 16th, 2019, until November 7th, 2019; then, on November 21st, December 12th, and once every 2 weeks from January 10th, 2020, to May 14th, 2020. Additionally, homemade resting box and floral bait traps were placed at these five sites from March 13, 2020, through May 14, 2020.
During the fall 2020 to spring 2021 collection season, gravid traps baited with stinky water (brew methods same as above), floral-baited BG Sentinel traps (Model 2883.11 baited with 1:1 linalool and nonanal), and resting box traps (Mosquito Resting Trap Model 2799 from BioQuip) were placed weekly at six lightly wooded parks from October 2nd, 2020, through November 12th, 2020. After four additional park sites were identified in November, mosquitoes were collected from a total of 10 lightly wooded parks once every 2 weeks from November 19th, 2020, through February 25th, 2021, using gravid, floral-baited BG Sentinel traps and resting box traps (Additional file 1: Fig. S1b). All three trap types were then placed weekly at all 10 parks from February 25th, 2021, through May 14th, 2021. Additionally, mosquitoes were aspirated from six culvert sites once every 2 weeks from April 2 through May 14th, 2021.
During the fall 2021 to spring 2022 collection season, gravid traps baited with stinky water (brewed in an environmental chamber at 27 °C for 3 days) and BG Sentinel traps baited with CO2 and human scent lures of hexanoic acid were placed at nine park sites (Fig. 1a and b) and collected ~ 24 h afterwards. Traps were placed weekly from October 6th, 2021, through November 30th, 2021, and then once every 2 weeks until February 23rd, 2022. From March 2nd, 2022, until May 13th, 2022, gravid, BG Sentinel traps, and CDC light traps baited with CO2 and light were placed weekly at each park site. In addition to the nine park sites, mosquitoes were collected from nine culverts using mouth/mechanical aspirators on the same dates that mosquitoes were collected from park sites (Fig. 1a and c; Additional file 1: Fig. S1c).
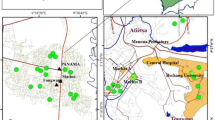
Distribution and examples of collection sites. A All 2021–2022 collection sites designed using ArcGIS pro [66]. Mosquito traps were placed in lightly wooded parks which are referred to as park sites (n = 5). Mosquitoes were aspirated from culvert sites (n = 5). Additionally, mosquitoes were collected from locations that contained both a park and culvert site (n = 4). B Example of a lightly wooded park site. C Example of a culvert site
Species identification
Mosquito specimens were identified morphologically to species using keys in Craker and Collins [40]. The level of confidence in the morphological identification of mosquitoes was categorized as either “high” or “low.” PCR-based assays were used to confirm the morphological identifications of Cx. pipiens, Cx. restuans, Cx. erraticus, and Cx. salinarius (n = 36, 20, 7, and 10, respectively). In brief, genomic DNA was isolated from mosquito tissues using Phire Animal Tissue Direct or GeneJET Genomic DNA Purification kits. PCR assays were prepared using a universal reverse primer and three species-specific forward primers developed by Crabtree et al. [41] to distinguish Cx. pipiens, Cx. restuans, and Cx. salinarius while primers developed by Williams and Savage [42] were used to distinguish Cx. erraticus. PCRs were run using standard protocols for Phire Animal Tissue Direct PCR assays and DreamTaq Green Master Mix (2x; Additional file 1). Using this method, we determined that morphological identifications of 97.22% of Cx. pipiens (35/36), 100% of Cx. restuans (20/20), 100% of Cx. erraticus (7/7), and 50% of Cx. salinarius (5/10) were accurate. Additional PCR identifications were needed for samples identified to Culex or mosquitoes that were aspirated from the culverts and had initially been identified as Cx. restuans (Additional file 1).
Diapause and parity status
Given the high number of samples we collected, we chose to assess the diapause and parity status of a subset of individuals. We first excluded female mosquitoes that were too damaged to be morphologically identified to species and those with missing thoraces or abdomens (n = 532 mosquitoes, leaving 3605 intact and morphologically identified Culex females). Then, we selected a maximum of 20 females that were collected from each site, collection date, and collection method. We preferentially selected female mosquitoes that had high confidence morphological identifications. If fewer than 20 females were captured with a particular collection method within a site/date, all females were dissected regardless of identification confidence. In total, we dissected 2480 female Culex mosquitoes (n = 425 for fall 2019–spring 2020; n = 133 for fall 2020–spring 2021; n = 1922 for fall 2021–spring 2022).
Ovaries were dissected from female mosquitoes under a stereomicroscope. To determine whether mosquitoes were parous, or had previously lain a batch of eggs, the tracheoles within to the ovaries were observed, where coiled tracheoles indicated that the mosquito was nulliparous and had not lain a batch of eggs, while uncoiled tracheoles indicated that the female was parous [39]. Mosquitoes that were parous with high certainty were classified as non-diapausing, regardless of egg follicle length. To determine the diapause status of nulliparous female mosquitoes, the lengths of the five largest primary egg follicles were measured using an inverted microscope (Nikon) at 200 × magnification. If the average length of the primary egg follicles was < 75 µm, the female mosquito was categorized as “diapausing” [43]. If the average egg follicle length was > 90 µm, female mosquitoes were classified as “non-diapausing” [43]. Those who were nulliparous and had egg follicle measurements between 75 µm and 90 µm were classified as “intermediate” [43].
Data analysis
All analyses were conducted using R, version 4.1.2 [44], in RStudio [45]. The maps package [46] was used to generate maps of Culex abundance by species and collection site.
To determine when Cx. pipiens terminated diapause in the spring, we first grouped the Cx. pipiens samples collected from each culvert site and collection date. The proportions of Cx. pipiens in diapause in each culvert site was calculated as the ratio of diapausing Cx. pipiens divided by all Cx. pipiens that were dissected (e.g. diapausing, intermediate, and non-diapausing). Next, we plotted our diapause proportions from each site and each collection week and used a dose response curve to determine the time at which 50% of the Cx. pipiens terminated diapause (DTT or diapause termination time) and 95% CI using the effective dose function in the drc package in R [47].
Sample collection dates, mosquito abundance by genus, month, or collection method, species identifications for all mosquitoes collected including non-Culex genera abundances, dissection results, and coding for data analyses can all be found in the GitHub Repository in Availability of data and materials section.
Results
Culex species counts and seasonal distribution
Of the 4137 female Culex collected, 3605 female Culex were identified to species (Table 1; 122 male Culex collected; 83 male Culex were identified to species). During the fall 2019 to spring 2020 collection season, female and male Culex were collected almost exclusively from gravid traps (n = 845) and one female from a resting trap (n = 1), where the last female Culex were collected in late November and the first in mid-March (Table 2). The fall 2020 to spring 2021 collection season yielded far fewer Culex (Table 1); only 49 females were collected from gravid traps, 5 females from resting traps, 1 female from floral-baited BG Sentinel traps, and 98 from aspirating mosquitoes from culverts in the winter and spring. Notably, the last female Culex collected in park sites were collected in early November 2020 and the first in late March 2021 (Table 2). Most Culex were collected during the fall 2021 to spring 2022 collection season (Table 1); 664 female and male Culex were collected from park sites in traditional mosquito traps (Table 3) and were collected as late as mid-November and as early as mid-March. We collected 2596 female and male Culex from culverts during the 2021–2022 collection season (Table 3).
The duration of female reproductive activity varied depending on the collection year. Non-diapausing Cx. pipiens were collected in park sites as late as November in each collection season and as early in the spring as mid- to late March, depending on the collection season (Table 2). Culex pipiens were collected from culverts for the entire duration of the 2021 to 2022 collection season (Table 3).
Aspirating mosquitoes from the culverts yielded far higher collection numbers of Cx. erraticus than the BG Sentinel and gravid traps placed at the park sites in the fall 2021–spring 2022 collection season (Table 3). Non-diapausing Cx. erraticus were collected in park sites as late as early to mid-October from the 2019 and 2021 seasons and as early in the spring as early May in 2020 (Table 2). Furthermore, we only collected one non-diapausing female Cx. erraticus from a culvert in late April and one additional non-diapausing female Cx. erraticus in May (Additional file 1: Table S1).
Culex pipiens and Cx. erraticus were commonly found in the same culvert sites throughout the fall 2021 to spring 2022 collection season yet differed in population abundance across sites (Fig. 2d and f). Culex pipiens were more abundant than Cx. erraticus at all collection sites except one, a train underpass, where 170 Cx. pipiens and 244 Cx. erraticus were collected. In fact, more Culex mosquitoes were collected from culverts than from traps in park sites throughout the 2021–2022 collection season until the first week of May (Fig. 3).
Counts of Cx. pipiens (A), Cx. restuans (B), and Cx. erraticus (C) collected across time and relative abundance of Cx. pipiens (D), Cx. restuans (E), and Cx. erraticus (F) at various collection sites in Franklin County, Ohio, during the 2021 to 2022 collection season. For panels D–F the size of the point at each site is proportional to the number of mosquitoes collected at that site
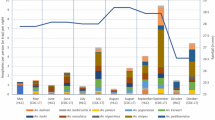
Counts of all Culex collected from traditional mosquito traps placed at park sites (blue) and counts of mosquitoes aspirated from culvert sites (red) during the 2021–2022 collection season. Mosquitoes were initially collected from park sites using gravid trap and BG Sentinel traps from October–February; starting in March, CDC light traps were also used weekly. Mosquitoes were collected from culverts using aspirators throughout the collection season
Like other species, the seasonal abundance and distribution of Cx. restuans varied among collection sites (Fig. 2c and e). Non-diapausing Cx. restuans were collected in park sites as late as mid-November during the 2019 and 2021 seasons and as early in the spring as mid-March to mid-April (Table 2). Culex restuans could not be collected year-round from culverts, and the few females collected from culverts in the fall of 2021 were non-diapausing (n = 9 collected; 5 dissected with 100% non-diapause; Table 3). Culex restuans were less abundant than Cx. pipiens or Cx. erraticus throughout the 2021–2022 collection season until the 1st week of May (Fig. 2ac).
Culex territans and Cx. salinarius were less abundant than the other Culex species (Table 1). No non-diapausing Cx. territans were collected from park sites during the fall or winter in any collection season, yet diapausing females were collected in BG Sentinel traps as late as 21 October in fall 2021 and diapausing females of Cx. territans were collected throughout the fall and winter 2021 within culverts. The only months when female Cx. territans were not collected from culverts were February and March. Gravid Cx. territans were collected in culverts in mid-April. Culex salinarius were collected from mid-October to mid-November, depending on the collection season. In fall 2021, only 1 female of Cx. salinarius was collected within a culvert while 12 females were collected from BG Sentinel traps. Across all collection seasons only one female of Cx. salinarius was ever collected in the spring. This sample was collected on May 2, 2020, from a gravid trap.
Culex reproductive activity
Culex pipiens populations exhibited low rates of reproductive activity throughout the fall that ceased in the early winter. Culex pipiens then resumed reproductive activity in early spring. Non-diapausing Cx. pipiens were collected in November during each collection season (Table 2). Non-diapausing Cx. pipiens were also found within culverts in October, November, and December 2021 and January 2022. In fact, roughly 6% of Cx. pipiens collected from culverts in October 2021 that we dissected were in a non-diapause or intermediate state (Fig. 4a). The proportion of non-diapausing Cx. pipiens within culverts decreased during November and December 2021, such that by the 2nd week of January 2022, 100% of Cx. pipiens were in a diapause state (Fig. 4a). Interestingly, the non-diapausing Cx. pipiens from December showed no higher rates of Cx. pipiens form molestus introgression than diapausing Cx. pipiens in December (Additional file 1: Supplemental Materials and Methods and Supplemental Results). Diapause incidence reached 100% by the 2nd week of January in 2022 and remained 100% until the first week of March 2022 (Fig. 4), at which point a single non-diapausing female of Cx. pipiens was collected within a culvert (Table 2). Our analyses indicate 50% of the Cx. pipiens within culverts terminated diapause by March 24, 2022 (dose-response curve; SE = 4.83 days; df = 151; Additional file 1: Fig. S2). The proportion of diapausing Cx. pipiens in culverts remained consistently low throughout April and May (Fig. 4a; ranged from 0 to 26.3% between weeks). Additionally, the proportion of Cx. pipiens in diapause varied among individual culverts (Additional file 1: Fig. S3).
The proportions of diapausing Cx. pipiens (A) and Cx. erraticus (B) collected from culverts in fall 2021 through spring 2022. The size of each point is proportional to the number of culverts where Culex were collected. Gray shading highlights dates between March 1, 2022, and April 21, 2022, when there was a rapid decrease in the proportion of mosquitoes in diapause. Although all nine culverts were sampled during each collection event, Cx. pipiens and/or Cx. erraticus were not necessarily collected from every culvert on every collection date
The proportion of diapausing Cx. erraticus in the fall was higher than for Cx. pipiens, where only 1.2% of female Cx. erraticus collected from the culverts in October 2021 were in a non-diapause or intermediate state (Fig. 4b). Additionally, the proportion of diapausing Cx. erraticus within culverts varied (Additional file 1: Fig. S3b). During November 2021, only one non-diapausing female was collected from a park trap, and the last non-diapausing Cx. erraticus was collected from a culvert on December 31, 2021 (parous sample with average egg follicle length = 66 µm; Table 2). As collection numbers substantially decreased throughout the winter and did not rebound in early spring, it is unclear when females of Cx. erraticus terminated diapause and became reproductively active (Fig. 4b). However, two non-diapausing Cx. erraticus were collected from culverts in the spring of 2022; the first was collected on April 28th (gravid female) and the second on May 12th (nulliparous female with an average egg follicle length of 105 µm).
Non-diapausing females of Cx. restuans were collected until mid to late November during the 2019 and 2021 collection seasons (Table 2). The first non-diapausing females of Cx. restuans were collected in mid-March to early April depending on the collection season (Table 2). In fact, the first Cx. restuans collected in spring 2022 was a gravid female who was collected from a gravid trap (Table 3). The abundance of Cx. restuans substantially increased over the spring (Fig. 2b). By the 1st week of May 2022, 196 non-diapausing females of Cx. restuans were collected in gravid traps (averaging 21.78 per site) and 32 non-diapausing female Cx. restuans were collected from culverts (averaging 3.55 per site).
The presence of blood-fed and gravid females of Cx. pipiens and Cx. restuans further provide evidence of reproductive activity. In the fall, blood-fed females of both species were collected until November 10th in 2021 (Table 4). The last gravid Cx. restuans was found on November 22nd in 2019 whereas the last gravid Cx. pipiens was collected on December 31st in 2021 (Table 4). In the spring, the first gravid Cx. restuans was collected on March 17th in 2022 whereas the first gravid Cx. pipiens was collected by late March for all three collection seasons (Table 4).
As male Culex mosquitoes do not overwinter, the presence of males in early spring also indicates when the first generation of mosquitoes produced by post-diapause Culex females reaches reproductive maturity. Notably, no male Culex were found in any culvert or park sites after early December 2021, indicating that all males had perished by this time. However, by the 1st week of May 2022, four adult males of Cx. pipiens were collected from two sites (2 collected from a gravid trap; 2 collected from a culvert). Similarly, three males of Cx. restuans and two males of Cx. territans were collected in a culvert site. Subsequently, by the 2nd week of May, we found 4 males of Cx. pipiens from 4 sites (1 in a CDC light trap; 1 from three different culverts), 10 males of Cx. restuans from 3 separate culvert sites (n = 6, 3, and 1 for each culvert site), and 1 male Cx. territans from a culvert. Collectively, this indicates that by early May, multiple species of Culex had completed at least one full life cycle.
Discussion
By continuously collecting mosquitoes from multiple field sites over a 3-year period, we were able to uncover several interesting results that have important implications for WNV transmission. First, several species of Culex, including Cx. pipiens, Cx. restuans, and Cx. salinarius, are reproductively active into late November, although this varied slightly among collection years. Despite differences in collection methods, we consistently collected reproductively active Cx. pipiens in gravid traps until November. This demonstrates that reproductively active mosquitoes persist through the fall. Second, both Cx. pipiens and Cx. erraticus overwinter within culverts, and a low proportion of non-diapausing female Cx. pipiens persist until mid-January. Third, the proportion of diapausing Cx. pipiens dramatically decreased throughout the month of March 2022, and gravid Cx. pipiens were collected by late March in gravid traps across all three collection seasons, indicating that Cx. pipiens can consistently be collected in early spring using a common surveillance trap.
Previous studies have also reported low incidences of non-diapausing Cx. pipiens in late fall and early winter [39, 48, 49]. A closely related and interfertile subspecies of Cx. pipiens, Cx. pipiens form molestus, hereafter Cx. p. molestus, is commonly found underground and does not enter diapause [50,51,52,53]. We were curious whether the non-diapausing females of Cx. pipiens that we had collected in December and early January belonged to this sub-species and/or showed higher rates of Cx. p. molestus ancestry, especially as other investigators have reported this phenomenon [49]. However, we found no evidence of higher rates of Cx. p. molestus introgression in our non-diapausing samples (Additional file 1: Supplemental Materials & Methods and results; [54]). Therefore, we propose three possible explanations for the presence of non-diapausing Cx. pipiens within culverts in December and January. First, these Cx. pipiens females that had not entered diapause may simply be holdouts that emerged earlier in the year. As they had not initiated diapause and lacked resources to survive the winter, this would explain why these females were not collected after late January. Notably, other studies that collected overwintering Cx. pipiens [39, 48, 49] report a similar trend and reached this same conclusion. A second possibility is that a small proportion of local Cx. pipiens that emerge in September or October may not enter diapause and similarly eventually perish during hard winters. Notably, Batz et al. [55] report that a low proportion of Aedes albopictus do not enter diapause under strong diapause-inducing conditions, and we also observed this in our laboratory colony of Cx. pipiens [56]. This small proportion of the Cx. pipiens that averts diapause may do so as a bet-hedging mechanism, enabling these females to avoid the reproductive costs of entering diapause and increase the mean geometric fitness of the population in the event of a mild winter [57,58,59]. Finally, the small proportion of the population that averts diapause could be caused by human-mediated changes to the urban environment such as the urban heat island effect (Fyie et al. accepted) and artificial light at night [60]. Notably, none of these possibilities is mutually exclusive, and all three could explain why we collected non-diapausing Cx. pipiens in culverts through mid-January.
Our collection of male Cx. restuans, Cx. territans, and Cx. pipiens by the 1st week of May 2022 indicates that at least three species of Culex had terminated diapause and completed at least one generation before most mosquito districts began their adult surveillance efforts (week 20; FCPH). Notably, Ciota et al. [33] uncovered annual variation when Cx. pipiens terminated diapause in New York, and therefore surveillance over multiple years may be necessary to determine which environmental factors influence diapause termination in multiple Culex spp. so that we can accurately predict when cycles of WNV transmission are likely to reinitiate. Although previous studies have demonstrated that the overwintering survivorship of Cx. pipiens can vary, the literature consistently demonstrates that populations of Cx. pipiens are lowest in early spring [33, 48, 61]. Additionally, our data showcase how rapidly mosquito populations can increase during the spring and are consistent with Helbing et al.'s [62] finding that populations of Cx. pipiens and especially Cx. restuans rapidly increase throughout May in Lucas County, Ohio. Therefore, identifying when Culex mosquitoes terminate diapause and emerge from their hibernacula in the spring can be applied to improve integrated pest management. This control method would target mosquito populations when they are at their lowest, hopefully leading to reduced WNV transmission throughout the year.
We found several interesting differences in the abundance and species composition of Culex mosquitoes within culverts and above-ground sites throughout the year in Franklin County, Ohio. Two vectors of WNV overwinter within culverts, Cx. pipiens and Cx. erraticus, whereas Cx. restuans and Cx. salinarius do not. Although Nasci et al. [32] collected low numbers of Cx. restuans from various humanmade overwintering sites in Queens, NY, we did not collect any diapausing or non-diapausing Cx. restuans within culverts during winter. Therefore, the mystery of where Cx. restuans overwinter in the field remains. Culex salinarius were almost exclusively collected from BG Sentinel traps in the fall, and only one female was collected in a culvert during the winter. A previous study indicates that Cx. salinarius can be collected from animal burrows and other natural habitats as opposed to humanmade sites [63]. It is also unclear whether Cx. salinarius enter a true reproductive diapause or whether they stop host-seeking and enter an overwintering quiescence in direct response to low temperatures [64, 65]. Unfortunately, our results are similarly unable to address this question. In the future, however, better characterizing the diapause response of multiple Culex species and identifying where they overwinter will allow us to better understand the relative contribution of each species to the seasonal dynamics of WNV transmission.
Unlike traditional mosquito traps that collect host-seeking and/or gravid mosquitoes, aspirating mosquitoes from culverts allowed us to collect Cx. pipiens, Cx. erraticus, and, to a lesser extent, Cx. territans year-round. Using these collection methods, future studies can determine which environmental cues are most relevant for diapause termination and specifically whether variations in microclimate between culverts influence when mosquitoes exit from hibernacula. Moreover, future studies can assess the proportion of overwintering Cx. pipiens or Cx. erraticus that are infected with WNV and to what extent diapausing mosquitoes serve as reservoir for this and other arboviruses [18, 32, 37,38,39]. Future studies that collect mosquitoes from both rural and urban areas at similar latitudes will allow researchers to determine the impact of human-mediated changes to the environment on the timing and duration of diapause in Culex and predict how urbanization alters the seasonal dynamics of WNV transmission.
Conclusion
The overall objective of this study was to determine when different species of Culex were reproductively active. We specifically focused on when Cx. pipiens, the region’s primary vector of WNV, ceased host-seeking and reproducing in the fall and terminated its diapause in the spring. In doing so, we have found that many different Culex species and other mosquitoes remain reproductively active and can be collected with traditional mosquito traps (e.g. gravid and BG Sentinel traps) as late as November in the fall and as early as late March in the spring in Franklin County, Ohio. However, these traps failed to collect the mosquitoes through late November and mid-March, indicating that Culex mosquitoes are largely inactive and are likely in diapause during this time. In contrast, three species, Cx. pipiens, Cx. erraticus, and Cx. territans, could be collected only from culverts throughout the fall, winter, and early spring. By dissecting and examining the ovaries of female mosquitoes collected throughout the fall, winter, and spring, we determined that most females of Cx. pipiens terminated diapause by mid-March. Our findings and those of other studies indicate that Culex populations are lowest early in spring [33, 35]. Therefore, our results suggest that targeted pesticide applications in early spring could potentially provide a novel opportunity to effectively control mosquitoes before they are able to transmit deadly pathogens to birds, humans, and other animals.
Availability of data and materials
Please visit our GitHub Repository for additional information: https://github.com/AldenDSiperstein/Culex-Surveillance-P-V-Data-Availability. Any additional information is available from the corresponding author upon request.
Abbreviations
- WNV:
-
West Nile virus
- CDC:
-
Center for Disease Control
- BG:
-
BioGents®
- FCPH:
-
Franklin county public health
References
CDC. West Nile Virus [Internet]. Centers for Disease Control and Prevention. 2022. https://www.cdc.gov/ncezid/dvbd/media/westnilevirus.html
West Nile Virus Disease Cases by State 2022 | West Nile Virus | CDC. 2023. https://www.cdc.gov/westnile/statsmaps/preliminarymapsdata2022/disease-cases-state-2022.html
West Nile Virus | Ohio Department of Health. https://odh.ohio.gov/know-our-programs/zoonotic-disease-program/diseases/west-nile-virus
Goddard LB, Roth AE, Reisen WK, Scott TW. Vertical transmission of West Nile virus by three California Culex (Diptera: Culicidae) species. J Med Entomol. 2003. https://doi.org/10.1603/0022-2585-40.6.743.
Sardelis MR, Turell MJ, Dohm DJ, O’Guinn ML. Vector competence of selected North American Culex and Coquillettidia mosquitoes for West Nile virus. Emerg Infect Dis. 2001;7:1018–22.
Molaei G, Andreadis TG. Identification of avian—and mammalian-derived bloodmeals in Aedes vexans and Culiseta melanura (Diptera: Culicidae) and its implication for West Nile virus transmission in connecticut USA. J Med Entomol. 2006;43:1088–93.
Tiawsirisup S, Kinley JR, Tucker BJ, Evans RB, Rowley WA, Platt KB. Vector competence of Aedes vexans (Diptera: Culicidae) for West Nile virus and potential as an enzootic vector. J Med Entomol. 2008;45:452–7.
Hamer GL, Kitron UD, Brawn JD, Loss SR, Ruiz MO, Goldberg TL, et al. Culex pipiens (Diptera: Culicidae): A bridge vector of West Nile virus to humans. J Med Entomol. 2008;45:125–8.
Andreadis TG. The contribution of Culex pipiens complex mosquitoes to transmission and persistence of West Nile Virus in North America. J Am Mosq Control Assoc. 2012;28:137–51.
Kilpatrick AM, Kramer LD, Campbell SR, Alleyne EO, Dobson AP, Daszak P. West Nile virus risk assessment and the bridge vector paradigm. Emerg Infect Dis. 2005;11:425–9.
Davis LE, DeBiasi R, Goade DE, Haaland KY, Harrington JA, Harnar JB, et al. West Nile virus neuroinvasive disease. Ann Neurol. 2006;60:286–300.
Kilpatrick AM, Fonseca DM, Ebel GD, Reddy MR, Kramer LD. Spatial and temporal variation in vector competence of Culex pipiens and Cx restuans mosquitoes for West Nile virus. Am Soc Trop Med Hyg. 2010;83:607–13.
Hamer GL, Kitron UD, Goldberg TL, Brawn JD, Loss SR, Ruiz MO, et al. Host selection by Culex pipiens mosquitoes and west nile virus amplification. Am Soc Trop Med Hyg. 2009;80:268–78.
ODH. Vectorborne Surveillance in Ohio. 6, 2023.
Anderson JF, Main AJ, Cheng G, Ferrandino FJ, Fikrig E. Horizontal and vertical transmission of West Nile virus genotype NY99 by Culex salinarius and genotypes NY99 and WN02 by Culex tarsalis. Am J Trop Med Hyg. Am Soc Trop Med Hyg. 2012;86:134–9.
Molaei G, Andreadis TG, Armstrong PM, Anderson JF, Vossbrinck CR. Host feeding patterns of Culex mosquitoes and West Nile virus transmission Northeastern United States. Emerg Infect Dis. 2006;12:468–74.
Burkett-Cadena ND, Graham SP, Hassan HK, Guyer C, Eubanks MD, Katholi CR, et al. Blood feeding patterns of potential arbovirus vectors of the genus Culex targeting ectothermic hosts. Am J Trop Med Hyg. 2008;79:809–15.
Cupp EW, Hassan HK, Yue X, Oldland WK, Lilley BM, Unnasch TR. West Nile virus infection in mosquitoes in the mid-South USA, 2002–2005. J Med Entomol. 2007;44:117–25.
Hribar L, Stark L, Stoner R, Demay D, Nordholt A, Hemmen M, et al. Isolation of West Nile virus from mosquitoes (Diptera Culicidae) in the Florida Keys Carib. J Sci. 2004. https://doi.org/10.1603/0022-2585-40.3.361.
Denlinger DL, Armbruster PA. Mosquito diapause. Annu Rev Entomol. 2014;59:73–93.
Denlinger DL, Armbruster PA. Chapter Eleven—molecular physiology of mosquito diapause. In: Raikhel AS, editor. Adv in Insect Phys. Cambridge: Academic Press; 2016.
Sanburg LL, Larsen JR. Effect of photoperiod and temperature on ovarian development in Culex pipiens pipiens. J Insect Phys. 1973;19:1173–90.
Spielman A, Wong J. Environmental control of ovarian diapause in Culex pipiens. Ann Entomol Soc Am. 1973;66:905–7.
Field EN, Shepard JJ, Clifton ME, Price KJ, Witmier BJ, Johnson K, et al. Semi-field and surveillance data define the natural diapause timeline for Culex pipiens across the United States. Commun Biol Nature Publ Group. 2022;5:1–12.
Madder DJ, Surgeoner GA, Helson BV. Induction of diapause in Culex pipiens and Culex restuans (Diptera: Culicidae) in Southern Ontario. Can Entomol. 1983;115:877–83.
Spielman A. Structure and seasonality of Nearctic Culex pipiens populations. Ann N Y Acad Sci. 2001;951:220–34.
Zhou G, Miesfeld RL. Energy metabolism during diapause in Culex pipiens mosquitoes. J Insect Phys. 2009;55:40–6.
Sim C, Denlinger DL. A shut-down in expression of an insulin-like peptide, ILP-1, halts ovarian maturation during the overwintering diapause of the mosquito Culex pipiens. Insect Mol Biol. 2009;18:325–32.
Bowen MF, Davis EE, Haggart DA. A behavioural and sensory analysis of host-seeking behaviour in the diapausing mosquito Culex pipiens. J Insect Phys. 1988;34:805–13.
Mitchell CJ, Briegel H. Inability of diapausing Culex pipiens (Diptera: Culicidae) to use blood for producing lipid reserves for overwinter survival. J Med Entomol. 1989;26:318–26.
Spielman A. Effect of synthetic juvenile hormone on ovarian diapause of Culex pipiens mosquitoes. J Med Entomol. 1974;11:223–5.
Nasci RS, Savage HM, White DJ, Miller JR, Cropp BC, Godsey MS, et al. West Nile virus in overwintering Culex mosquitoes, New York City, 2000. Emerg Infect Dis. 2001;7:742–4.
Ciota AT, Drummond CL, Drobnack J, Ruby MA, Kramer LD, Ebel GD. Emergence of Culex pipiens from overwintering hibernacula. J Am Mosq Control Assoc. 2011;27:21–9.
Onyeka JOA, Boreham PFL. Population studies, physiological state and mortality factors of overwintering adult populations of females of Culex pipiens L. (Diptera: Culicidae). Bull Entomol Res. 1987;77:99–111.
Barker CM, Eldridge BF, Reisen WK. Seasonal abundance of Culex tarsalis and Culex pipiens complex mosquitoes (Diptera: Culicidae) in California. J Med Entomol. 2010;47:759–68.
Lalubin F, Delédevant A, Glaizot O, Christe P. Temporal changes in mosquito abundance (Culex pipiens), avian malaria prevalence and lineage composition. Parasit Vectors. 2013;6:307.
Bugbee LM, Forte LR. The discovery of West Nile virus in overwintering Culex pipiens (Diptera: Culicidae) mosquitoes in lehigh county Pennsylvania. J Am Mosq Control Assoc. 2004;20:326–7.
Farajollahi A, Crans WJ, Bryant P, Wolf B, Burkhalter KL, Godsey MS, et al. Detection of west nile viral RNA from an overwintering pool of Culex pipens pipiens (Diptera: Culicidae) in New Jersey, 2003. J Med Entomol. 2005;42:490–4.
Andreadis TG, Armstrong PM, Bajwa WI. Studies on hibernating populations of Culex pipiens from a West nile virus endemic focus in New York City: parity rates and isolation of west nile Virus. J Am Mosq Control Assoc. 2010;26:257–64.
Craker CLE, Collins FH. The Mosquitoes of the Ohio River Basin
Crabtree MB, Savage HM, Miller BR. Development of a species-diagnostic polymerase chain reaction assay for the identification of Culex vectors of St. Louis Encephalitis virus based on interspecies sequence variation in ribosomal DNA spacers. Am J Trop Med Hyg. 1995;53:105–9.
Williams MR, Savage HM. Development of multiplexed species specific polymerase chain reaction assays for identification of the Culex (Melanoconion) Species (Diptera: Culicidae) of the Southeastern United States based on rDNA. J Med Entomol. 2011;48:961–6.
Meuti ME, Short CA, Denlinger DL. Mom matters: diapause characteristics of Culex pipiens–Culex quinquefasciatus (Diptera: Culicidae) hybrid mosquitoes. J Med Entomol. 2015;52:131–7.
R Core Team. A language and environment for statistical computing. Vienna: R Foundatio for Statistical Computing; 2013.
RStudio Team. RStudio: Integrated development for R. Boston, MA: RStudio, PBC; 2020. http://www.rstudio.com/.
Becker RA, Wilks AR, Brownrigg R, Minka TP, Deckmyn A. Maps: Draw geographical maps. 2022. https://CRAN.R-project.org/package=maps.
Ritz C, Baty F, Streibig JC, Gerhard D. Dose-response analysis using R. PLOS ONE Public Library Sci. 2015;10:e0146021.
Slaff ME, Crans WJ. Parous rates of overwintering Culex pipiens pipiens in New Jersey. Mosq News. 1977;37:11–4.
Jumars PA, Murphey FJ, Lake RW. Can blood-fed Culex pipiens L. overwinter? Proc Annu N J Mosq Exterm Assoc. 1969;1969:219–25.
Roubaud E. Sur I’existence de race biologiques genetiquement distinctes chez moustiques commun Culex pipiens. C R Acad Sci. 1930;191:1386–8.
Marshall JF, Staley J. Exhibition of Autogenous and Stenogamous Characteristics by Theobaldia subochrea, Edwards (Diptera, Culicidæ). Nature. 1936;137:580–1.
Gao Q, Su F, Zhou Y-B, Chu W, Cao H, Song L-L, et al. Autogeny, fecundity, and other life history traits of Culex pipiens molestus (Diptera: Culicidae) in Shanghai. China J Med Entomol. 2019;56:656–64.
Fritz ML, Walker ED, Miller JR, Severson DW, Dworkin I. Divergent host preferences of above- and below-ground Culex pipiens mosquitoes and their hybrid offspring. Med Vet Entomol. 2015;29:115–23.
Batz ZA, Clemento AJ, Fritzenwanker J, Ring TJ, Garza JC, Armbruster PA. Rapid adaptive evolution of the diapause program during range expansion of an invasive mosquito. Evolution. 2020;74:1451–65.
Meuti ME, Stone M, Ikeno T, Denlinger DL. Functional circadian clock genes are essential for the overwintering diapause of the Northern house mosquito. Culex pipiens J Exp Biol. 2015;218:412–22.
Starrfelt J, Kokko H. Bet-hedging—a triple trade-off between means, variances and correlations. Biol Rev. 2012;87:742–55.
Rajon E, Desouhant E, Chevalier M, Débias F, Menu F. The evolution of bet hedging in response to local ecological conditions. Am Nat. 2014;184:1–15.
Peffers CS, Pomeroy LW, Meuti ME. Critical photoperiod and its potential to predict mosquito distributions and control medically important pests. J Med Entomol. 2021;58:1610–8.
Fyie LR, Gardiner MM, Meuti ME. Artificial light at night alters the seasonal responses of biting mosquitoes. J Insect Phys. 2021;129:104194.
Bailey CL, Faran ME, Gargan II TP, Hayes DE. Winter survival of blood-fed and nonblood-fed Culex pipiens L. 1982. https://apps.dtic.mil/sti/citations/ADA123092
Helbing CM, Moorhead DL, Mitchell L. Population dynamics of Culex restuans and Culex pipiens (Diptera: Culicidae) related to climatic factors in Northwest Ohio. Environ Entomol. 2015;44:1022–8.
Murphy FJ. The Bionomics of Culex salinarius, Coquillet [Ph.D.]. [United States—Delaware]: University of Delaware; 1961. https://www.proquest.com/docview/302079125/citation/CD5AF56D6B184D84PQ/1.
Eldridge BF, Bailey CL, Johnson MD. A preliminary study of the seasonal geographic distribution and overwintering of Culex restuans Theobald and Culex salinarius Coquillet (Diptera: Culicidae). J Med Entomol Oxford Acad. 1972;9:233–8.
Slaff M, Crans WJ. The activity and physiological Status of pre- and posthibernating Culex salinarius (Diptera: Culicidae) populations. J Med Entomol Oxford Academic. 1981;18:65–8.
Bahnck CM, Fonseca DM. Rapid assay to identify the two genetic forms of Culex (Culex) pipiens L (Diptera: Culicidae) and hybrid populations. Am J Trop Med Hyg. 2006. https://doi.org/10.4269/ajtmh.2006.75.2.0750251.
ArcGIS Pro. 3.0.0, Esri Inc. 2022.
Acknowledgements
We thank Matthew Wolkoff for his help troubleshooting laboratory protocols, Chase McManning for providing assistance in data visualization and coding assistance, Leeanne Garrett for providing mosquito traps and baits, and Rosalie Hendon for providing permits for us to collect mosquitoes from Columbus area parks. We also thank Rebecca Robich and Joseph Rinehart for their support and advice on finding culverts around Franklin County, Ohio, for mosquito collection.
Funding
This research was funded by National Science Foundation, grant number IOS-1944324, awarded to MM, NIH grant R21-AI144266 awarded to MM, and by state and federal funds appropriated to The Ohio State University, College of Food, Agricultural, and Environmental Sciences to MM, AS and LWP. This project was further supported by the USDA National Institute of Food and Agriculture, Hatch Multi-State project NE 1943.
Author information
Authors and Affiliations
Contributions
AS designed and implemented collection methods alongside SR, LS, HD, TL, and MM. SR, HD, and AS identified mosquitoes to species. AS, LS, and MM performed PCR assays to confirm morphological IDs and measure the introgression with Culex p. molestus. AS, TL, and HD dissected female Culex mosquitoes to assess their diapause status. AS, LWP, and LF analyzed the data and prepared figures and tables. AS wrote the original draft of the manuscript, which was edited by MM, LWP, HD, and AS, LWP, and MM received funding for the project. MM supervised all aspects of the project. All authors read and approved the final maunscript.
Corresponding author
Ethics declarations
Ethics approval and consent to participate
This research did not involve human participants, human material, or human data and has no need of ethics approval. Additionally, no vertebrates were used in this study and therefore it did not require approval from OSU’s Institutional Animal Use and Care Committee.
Consent for publication
All authors of this manuscript have read and agreed to the content within it. Furthermore, the contents of this article are original, and we agree with the BioMed Central Copyright and License Agreement.
Competing interests
The authors declare no competing interests.
Additional information
Publisher's Note
Springer Nature remains neutral with regard to jurisdictional claims in published maps and institutional affiliations.
Supplementary Information
Additional file 1:
Methods. Figure S1. The location of collection sites used in each collection season. A Fall 2019–spring 2020; B fall 2020–spring 2021; C fall 2021–spring 2022. Figure S2. Dose-response curve fitted onto the proportion of Culex pipiens that were in diapause collected from each culvert site and each week from the 2021–2022 collection season. The gray line indicates the 95% confidence interval. Our analyses indicate that 50% of Cx. pipiens had terminated diapause by March 24 in 2022. Figure S3. The proportion of Culex pipiens A and Cx. erraticus B in diapause over time varied across nine different culvert sites. Lines plotted are smoothing splines with three degrees of freedom. Each of the nine culverts are plotted in a different color. Table S1. The number of female Culex mosquitoes by diapause status that were collected each month during each collection season. Note that “D” refers to diapausing, “ND” refers to non-diapausing, “Int.” refers to intermediate, and “Undis.” refers to undissected mosquito samples.
Rights and permissions
Open Access This article is licensed under a Creative Commons Attribution 4.0 International License, which permits use, sharing, adaptation, distribution and reproduction in any medium or format, as long as you give appropriate credit to the original author(s) and the source, provide a link to the Creative Commons licence, and indicate if changes were made. The images or other third party material in this article are included in the article's Creative Commons licence, unless indicated otherwise in a credit line to the material. If material is not included in the article's Creative Commons licence and your intended use is not permitted by statutory regulation or exceeds the permitted use, you will need to obtain permission directly from the copyright holder. To view a copy of this licence, visit http://creativecommons.org/licenses/by/4.0/. The Creative Commons Public Domain Dedication waiver (http://creativecommons.org/publicdomain/zero/1.0/) applies to the data made available in this article, unless otherwise stated in a credit line to the data.
About this article
Cite this article
Siperstein, A., Pomeroy, L.W., Robare, S. et al. Characterizing seasonal changes in the reproductive activity of Culex mosquitoes throughout the fall, winter, and spring in Ohio. Parasites Vectors 16, 173 (2023). https://doi.org/10.1186/s13071-023-05806-0
Received:
Accepted:
Published:
DOI: https://doi.org/10.1186/s13071-023-05806-0